Abstract
Small proline-rich repeat protein 1A (SPRR1A) is expressed in dorsal root ganglion (DRG) neurons following peripheral nerve injury but it is not known whether SPRR1A is differentially expressed following injury to peripheral versus central DRG projections and a detailed characterisation of expression in sensory neuron sub-populations and spinal cord has not been performed. Here we use immunocytochemical techniques to characterise SPRR1A expression following sciatic nerve, dorsal root and dorsal column injury in adult mice. SPRR1A was not detected in naïve spinal cord, DRG or peripheral nerves and there was minimal expression following injury to the centrally projecting branches of DRG neurons. However, following peripheral (sciatic) nerve injury, intense SPRR1A immunoreactivity was observed in the dorsal horn and motoneurons of the spinal cord, in L4/5 DRG neurons and in the injured nerve. A time-course study comparing expression following sciatic nerve crush and transection revealed maximum SPRR1A levels at day 7 in both models. However, while SPRR1A was down-regulated to baseline by 30 days post-lesion following crush injury, it remained elevated 30 days after transection. Cell-size and double-labelling studies revealed that SPRR1A was expressed by DRG cells of all sizes and co-localized with classical markers of DRG subpopulations and their primary afferent terminals. High co-expression of SPRR1A with activating transcription factor-3 and growth-associated protein-43 was observed, indicating that it is expressed by injured and regenerating neurons. This study supports the hypothesis that SPRR1A is a regeneration-associated gene and that SPRR1A provides a valuable marker to assess the regenerative potential of injured neurons.
Keywords: nerve injury and repair, axonal regeneration, immunolabelling, primary afferents, dorsal root ganglia, regeneration-associated genes
Introduction
Neurons injured in the adult mammalian central nervous system (CNS) typically fail to regenerate, in contrast to the long-distance growth and successful target re-innervation that often occurs following injury to the peripheral nervous system (PNS). The robust regenerative response following PNS injury corresponds with the expression of a number of genes thought to aid rapid regeneration (Broude et al., 1997; Chong et al., 1994; Schreyer and Skene, 1993; Snider et al., 2002) and it is thought that the enhanced regeneration is due to the co-ordinated expression of these genes, which are largely absent following CNS injury (Bareyre and Schwab, 2003; Plunet et al., 2002).
A number of these regeneration-associated genes (RAGs) have now been identified and they encode a diverse group of molecules (Costigan et al., 2002; Fu and Gordon, 1997; Gillen et al., 1995; Skene, 1989; Tanabe et al., 1999; Vogelaar et al., 2003). The most widely known RAGs are the immediate early gene c-Jun (Broude et al., 1997; Jenkins et al., 1993) and growth-associated protein-43 (GAP-43) (Skene and Willard, 1981). GAP-43 is highly expressed in development during target innervation, is down-regulated in the adult and its induction following injury is correlated with regeneration (Chong et al., 1992; Fitzgerald et al., 1991; Meiri et al., 1986; Skene et al., 1986; Tetzlaff et al., 1989; Van der Zee et al., 1989).
For example, sensory neurons in the dorsal root ganglia (DRG) are capable of a robust regenerative response when injured in their peripherally projecting branch, in contrast to the delayed and slower rate of regeneration following injury to the dorsal root and the largely abortive regeneration when injured within the spinal dorsal columns (Bradbury et al., 2000). This correlates with the robust and sustained up-regulation of GAP-43 observed in virtually all DRG neurons following peripheral nerve injury (Chong et al., 1992; Schreyer and Skene, 1993; Verge et al., 1990), and largely unchanged levels of GAP-43 following dorsal root (Chong et al., 1994; Schreyer and Skene, 1993) or dorsal column (Bradbury et al., 2002) injury. Similarly, while c-Jun is substantially up-regulated in DRG neurons following peripheral axotomy, a much smaller percentage of DRG neurons express c-Jun following central injury (Broude et al., 1997).
Experimental strategies to promote regeneration can induce increased RAG expression, both in DRG neurons following injury to their central projections and in injured CNS neurons. For example, treatment with the enzyme chondroitinase ABC can promote regeneration of injured dorsal column axons and an associated increase in GAP-43 expression in large diameter DRG neurons whose axons project in the dorsal columns (Bradbury et al., 2002). Furthermore, following dorsal root injury, treatment with neurotrophic factors leads to increased regeneration through the dorsal root entry zone and functional reinnevation of the dorsal horn and corresponds with an up-regulation of GAP-43 in DRG neurons (Ramer et al., 2000; Ramer et al., 2001). Similarly, increased expression of c-Jun has been observed in DRG cell bodies when rhizotomised dorsal roots were placed into a hemisection site containing foetal spinal cord transplants, which was associated with in-growth of primary afferent fibres into the tissue graft (Broude et al., 1997). The de novo expression of RAGs has also been observed in injured CNS neurons. For example, Chaisuksunt et al. (Chaisuksunt et al., 2000) compared the ability of different populations of injured CNS neurons to successfully grow axons into peripheral nerve grafts and found the expression of L1, CHL1, c-Jun and GAP-43 mRNA to be correlated with regenerative capacity. Furthermore, stimulation of GAP-43 and T 1-tubulin mRNA following neurotrophin treatment has been correlated with an increased regenerative capacity of rubrospinal neurons (Kobayashi et al., 1997; Kwon et al., 2002).
1-tubulin mRNA following neurotrophin treatment has been correlated with an increased regenerative capacity of rubrospinal neurons (Kobayashi et al., 1997; Kwon et al., 2002).
Here we focus on small proline-rich repeat protein 1A (SPRR1A) which has recently been proposed as a RAG (Bonilla et al., 2002). SPRR1A belongs to the multigene SPRR family of keratinocyte differentiation markers (Gibbs et al., 1993; Kartasova and van de Putte, 1988) and neuronal expression of SPRR1A was first detected in a microarray analysis of genes induced during successful sciatic nerve regeneration in the mouse (Bonilla et al., 2002). Unlike GAP-43, which is expressed during development and constitutively by some cells in the adult (Benowitz and Routtenberg, 1997; Chong et al., 1992; Skene, 1989; Van der Zee et al., 1989), there is no expression of SPRR1A during development or in naïve adult tissue (Bonilla et al., 2002), except in a small percentage of neurons in the neocortex (Marklund et al., 2006). Since SPRR1A is not normally present but following peripheral nerve injury becomes de novo expressed in sensory neurons at a time corresponding to rapid regeneration, and when over-expressed in cultured embryonic DRG neurons can increase neurite outgrowth (Bonilla et al., 2002), it has been proposed as a truly regeneration-specific protein (Snider et al., 2002). A detailed characterisation of SPRR1A expression has not been carried out. It is not known what proportion of sensory neurons express SPRR1A following injury, whether SPRR1A is differentially expressed in different DRG subpopulations, where it is localised in the spinal cord or whether SPRR1A is differentially expressed in DRG neurons following peripheral versus central injury. Here we use immunohistochemistry to compare SPRR1A expression following injury to the peripheral nerve, dorsal root and dorsal columns. We have also studied the time course of SPRR1A expression following peripheral nerve crush or transection to determine whether this correlates with the known period of regeneration and reinnervation in these two injury models. Finally, we have used cell size analysis and double labeling techniques to characterise the expression of SPRR1A in the different subtypes of lumbar DRG neurons and in their central terminals within the spinal cord following peripheral nerve injury. Understanding the time course, extent and localisation of SPRR1A expression following injury may prove useful when assessing interventions aimed at enhancing peripheral nerve regeneration or promoting regeneration following CNS injury.
Materials and Methods
Animals and surgery
Adult male C57BL/6 mice (Harlan UK Ltd., 20-25g, 6-8 weeks old, n = 44) were used in these studies. All surgical procedures were performed in accordance with U.K. Home Office regulations (European Communities Council Directive of 24 November 1986 (86/609/EEC)) and sterile precautions were used throughout.
Sciatic nerve crush or transection injury
Mice were anaesthetised with 5% isoflurane (Abbott Laboratories Ltd) in oxygen, and maintained with 2% isoflurane. Mice were prepared for surgery by shaving and disinfecting the left flank and leg. The sciatic nerve was exposed following incision of the skin between the knee and thigh. The nerve was either crushed (n = 16) or transected (n = 16) immediately distal to the sciatic notch. The crush injury was made with no. 5 Dumont Watchmakers forceps (InterFocus, Fine Science Tools), which were held in place for 30 seconds, and the crush site was marked with charcoal powder. For the transection injury the sciatic nerve was tied off with a 4-0 Vicryl suture (Ethicon, Johnson & Johnson Intl) and then cut with iridectomy scissors (InterFocus, Fine Science Tools) distal to the tie. The muscles and the skin were closed with 5-0 Vicryl sutures before the animals were returned to their home cage. Naïve mice (n = 4) did not receive a lesion.
Dorsal root and dorsal column crush surgery
Mice were anaesthetised with Avertin mouse anaesthetic (1.25g 2,2,2-tribromoethanol/100ml tert-amyl alcohol; both Sigma, Poole, U.K.; diluted to 20mg/ml with saline administered i.p. (0.5ml/20g), with re-administration as a half dose as required) and prepared for surgery by shaving and disinfecting the skin of the back. Dorsal root crush surgery (n=4) was as described previously in rats (Ramer et al., 2000; Wong et al., 2006) and adapted for mice (Cafferty et al., 2007). Briefly, a hemilaminectomy was carried out over the cervical spinal cord to reveal the C6 and C7 dorsal roots. Using fine forceps (Interfocus, Fine Science Tools), each of these roots was crushed (held for 10 s) between the DRG and dorsal root entry zone then muscle and skin layers were sutured with 5-0 Vicryl sutures. Dorsal column crush surgery (n=4) was adapted from methods described previously for rats (Bradbury et al., 1999; Bradbury et al., 2002). Briefly, muscle tissue was cleared from the dorsal processes of the lower thoracic vertebrae and a laminectomy performed to expose the dorsal surface of the T12 spinal cord. Gelfoam soaked with lignocaine (2%, B Braun Medical Ltd, UK) was placed on the spinal cord for 2mins, the dura was opened, lignocaine was reapplied and then the exposed dorsal columns were crushed to a depth of 0.4mm into the cord using a pair of fine forceps (held for 10 s). Muscle and skin layers were sutured as above. All mice were placed on a heated blanket during recovery, before being returned to their home cage.
Cholera toxin (CTB) Injections
Cholera toxin β (CTB) subunit binding was used to identify myelinated DRG afferents. CTB is a transganglionic tracer that when injected into a peripheral nerve is transported to the cell bodies of myelinated DRG neurons and their central projections in the spinal cord. Four days before sacrifice, mice (n = 3) that had received a sciatic nerve crush were anaesthetised with isoflurane, as above. The sciatic nerve was re-exposed proximal to the crush site and 2% lignocaine applied, followed by unilateral intraneural injection of CTB (List Biological Labs, CA, USA; 1.5μl of a 1% solution delivered over 1 minute) just proximal to the lesion site. The muscles and the skin were closed with 5-0 Vicryl sutures before the animals were returned to their home cage.
Tissue preparation
Naïve mice, dorsal rhizotomy and dorsal column-injured mice (n = 4 per group) were sacrificed at day 7 post-injury; those receiving a sciatic nerve crush or transection were sacrificed at days 1, 3, 7 or 30 post-injury (for each injury model n = 3 per time point plus a further n=4 at day 7 for co-localisation studies). Mice were deeply anaesthetised with pentobarbitone (80mg/kg i.p., Merial Animal Health Ltd., Essex, UK) and transcardially perfused with 10ml saline followed by 50ml paraformaldehyde (4% in 0.1M phosphate buffer, pH 7.4). For dorsal root crush tissue the cervical spinal cords with ipsilateral and contralateral DRGs still attached were removed; for dorsal column injury, the thoracic spinal cord and L4-L6 DRGs were removed; and for peripheral nerve injury, the lumbar spinal cord plus ipsilateral and contralateral L4 and L5 DRG and sciatic nerves were removed. A small pin (Agar Scientific, 0.1mm) was inserted along the ventral white matter of the contralateral side of the lumbar spinal cord for identification. Tissue was post-fixed in 4% paraformaldehyde (2 hours at 4oC) and transferred to 20% sucrose (in 0.1M phosphate buffer) for two days at 4oC. The tissue was then blocked in OCT embedding compound (BDH, Essex, UK) and stored at −70°C. Serial transverse sections of lumbar spinal cord at level L4 and L5 were cut at a thickness of 20 m; sciatic nerves were cut longitudinally at a thickness of 15
m; sciatic nerves were cut longitudinally at a thickness of 15 m; lumbar DRG were cut at a thickness of 15
m; lumbar DRG were cut at a thickness of 15 m. All tissue was mounted onto Superfrost Plus microscope slides (BDH Laboratory Supplies, UK).
m. All tissue was mounted onto Superfrost Plus microscope slides (BDH Laboratory Supplies, UK).
For co-localization studies in lumbar DRG from sciatic nerve crush injured mice, tissue was gelatin-embedded and vibratome sectioned to allow for greater morphological resolution. Following post-fixing (4% paraformaldehyde, 2 hours at 4oC) L4 and L5 DRG were stored in PBS + 0.1% NaN3 (Sigma-Aldrich Inc.) solution overnight at 4°C. Tissue was then embedded in 10% gelatin (300 bloom, Sigma-Aldrich Inc.), the gelatin blocks hardened in 4% paraformaldehyde for 6 hours at 4oC then transferred to a solution of PBS + 0.1% NaN3 and stored at 4°C until sectioning. Free-floating serial sections were cut on a vibratome at 15 um and stored in PBS + 0.1% NaN3 at 4oC until immunofluorescence staining.
Immunofluorescence
SPRR1A
SPRR1A immunofluorescence staining was amplified using tyramide signal amplification (TSA) (Michael et al., 1997). As detailed below, in some instances TSA of anti-SPRR1A was followed by indirect immunofluorescence with another polyclonal primary antibody. Therefore, appropriate control experiments were carried out to validate that there was no cross-reactivity with this double-labelling technique. In these control experiments each antibody was tested separately and revealed staining patterns identical to those after the double-immunofluorescence staining (data not shown). Additionally, in control singlestaining experiments using indirect labelled immunofluorescence to visualise SPRR1A immunoreactivity, we were unable to detect positive staining at the primary antibody dilutions used in the TSA protocol. This protocol for double-immnuofluorescence staining, using two primary antibodies from the same species, has been used previously (Michael et al., 1997), showing that the lack of cross-reactivity is because the TSA procedure allows the first primary antibody (in this case anti-SPRR1A) to be used at a dilution that is too weak to be detected by the fluorescence conjugated secondary antibody which binds to the second primary antibody. Tissue sections were washed in PBS six times for 5 minutes each. Both cryostat sections and free-floating gelatin-embedded sections were incubated in the following (with three PBS washes between each stage): rabbit anti-SPRR1A (kind gift from Professor Stephen Strittmatter; diluted in PBS + 0.2% Triton X-100 (BDH, UK) + 0.1% NaN3 (Sigma-Aldrich Inc.); 1:20,000 for cryostat sections and 1:7000 for free-floating gelatin sections), at room temperature overnight; goat anti-rabbit biotin (1:400, in PBS + 0.2% Triton X-100 + 0.1% NaN3) at room temperature for 1.5 hours; ABC reagent (1:250, in PBS, Vector Laboratories Ltd., UK) at room temperature for 30 minutes; biotinyl tyramide (1:75 in amplification diluent, PerkinElmer Life Sciences Products, Boston, MA) at room temperature for 10 minutes; extra-avidin FITC (Jackson labs, 1:500 in PBS + 0.2% Triton X-100 + 0.1% NaN3) at room temperature for 2 hours. Free-floating sections were mounted onto Superfrost slides and all slides were coverslipped using Vectashield fluorescent mounting medium (Vector Laboratories Ltd., UK).
Double labelling with other cell markers
The two sets of antisera were applied sequentially, the SPRR1A TSA protocol being followed by indirect immunofluorescence for the other markers. DRG sections were double immunofluorescence stained with a series of markers specific for DRG subpopulations (calcitonin gene related peptide, CGRP, isolectin B4, IB4, and neurofilament 200, NF200), markers of injured (activating transcription factor-3, ATF-3) or regenerating (GAP-43) neurons and Β(III) tubulin to identify all cells (for calculating percentages of positive cells and cell size analysis). Spinal cord sections were double immunofluorescence stained for CGRP, IB4, CTB, GAP-43 and PKC©. All antibodies were diluted in PBS + 0.2% Triton X- 100 + 0.1% NaN3. In each case, with the exception of IB4 labelling, tissue was incubated with the primary antibody (Table 1) at room temperature overnight followed by three washes in PBS then incubation with the secondary antibody at room temperature for 4 hours. In the case of IB4 immunofluorescence, incubation with IB4 Lectin (10μg/ml, Sigma-Aldrich Inc. Cat # L5391, Lot # 110K4031, at room temperature overnight) was followed by goat anti-IB4 (at room temperature overnight) and then the secondary antibody. The following secondary antibodies were used: donkey anti-rabbit TRITC (1:200, Jackson ImmunoResearch Laboratories, Inc., USA, Cat #711-026-152, Lot #58091), donkey anti-goat TRITC (1:200, Jackson ImmunoResearch Laboratories Inc., USA, Cat #705-025-147, Lot #49945) and donkey anti-mouse TRITC (1:200, Jackson ImmunoResearch Laboratories Inc., USA, Cat #715-025-150, Lot #48332). Following immunostaining the free-floating sections were mounted onto Superfrost Plus slides and all slides were coverslipped using Vectashield fluorescent mounting medium.
Table 1.
List of Antibodies
| Antibody (name and species) |
Source, catalog. & lot no. |
Immunogen | Dilution |
|---|---|---|---|
| Rabbit anti-CGRP | Sigma-Aldrich Inc. Cat. # C8198 Lot # 65K4804 |
Synthetic CGRP (rat) conjugated to KLH (CGRP-KLH). This antibody specifically recognizes the CGRP C-terminal segment (amino acids 24-37) of rat CGRP. |
1:4000 |
| Rabbit anti-ATF-3 | Santa Cruz Biotechnology Cat # sc-188 Lot # F2006 |
The antibody was raised against human ATF-3 (accession No. P18847), the antibody epitope was amino acids 163- 181 in the C-terminus. The antibody was raised against a peptide mapping at the C- terminus of ATF-3 of human origin. Genetic locus: Atf3 (human) mapping to 1q32.3; Atf3 (mouse) mapping to 1 H6. |
1:400 |
| Mouse anti-Neurofilament 200 |
Sigma-Aldrich Inc. Cat. # N0142 Lot # 073K4834 |
The antibody was raised against the carboxyterminal tail segment of enzymatically dephosphorylated pig neurofilament H-subunit. |
1:400 |
| Mouse anti-β (III) Tubulin | Promega, UK Cat # G7121 Lot # 197688 |
Peptide (EAQGPK) corresponding to the C-terminus of β III tubulin. |
1:6000 |
| Rabbit anti-SPRR1A | Prof. Stephen Strittmatter, Yale University Antisera ID. Mocha Bleed #2 |
SPRR1A cDNA (AA230988) was sub- cloned into pTrc-His. Recombinant SPRR1A protein with N terminal His6 tag was purified from transformed E. coli. Rabbits were immunized with SPRR1A- His protein (Bonilla et al., 2002). |
1:7000 (gelatin) 1:20,000 (cryostat) |
| Rabbit anti-PKCγ | Santa Cruz Biotechnology Cat # sc-211 Lot # A0704 |
An oligopeptide corresponding to the carboxyl-terminal end region of 13I PKC (residues 661-671; Ser-Tyr-Thr-Asn-Pro- Glu-Phe-Val-Ile-Asn-Val) was synthesized and used as an immunogen. |
1:200 |
| Rabbit anti-GAP-43 | Dr. G. P. Wilkin, Imperial College London. |
A 610-bp fragment encoding amino acids 17-219 of rat GAP-43 was cloned into the pUR 292 expression vector and expressed in E. coli. The resulting protein was purified and rabbits were inoculated with approx. 100μg of protein in Freund’s complete adjuvant and boosted 1 month later with a further 100 mg in incomplete adjuvant. |
1:2000 |
| Goat anti-IB4 | Vector Labs Ltd., UK Cat # AS-2104 Lot # N0913 |
The antibody was raised in goat against whole lectin 1 of Griffonia simplicifolia. |
1:1000 |
Antibody Specificity
For antibody catalogue, immunogen and dilution details see Table 1.
Rabbit anti-CGRP
Details of the characterization and controls for staining with this antibody have been published previously (Fasanella et al., 2008; Lorenzo et al., 2008). Specifically, the antiserum was raised in rabbit against a synthetic whole rat -CGRP conjugated to keyhole limpet hemocyanin (KLH). The antiserum was shown to be reactive to rat CGRP and human CGRP as well as with -CGRP, when conjugated to bovine serum albumin, in a dot blot immunoassay. The antiserum showed no cross-reactivity with substance P, vasoactive intestinal peptide, neuropeptide Y, calcitonin, or somatostatin (manufacturer’s technical information). Staining was completely abolished when the antiserum was preadsorbed with rat CGRP. When the primary antibodies were replaced with preimmune serum from the same source species, no staining was observed (Lorenzo et al., 2008). Staining patterns in the current study are consistent with previously published articles (Bennett et al., 1996; Ramer et al., 2003).
Rabbit anti-ATF3
This antibody has been used extensively in previous studies (Averill et al., 2004; Hyatt et al., 2007; Kool et al., 2003; Walker et al., 2003; Young et al., 2008) and details of its characterization have previously been published. Specifically, Western blot analysis showed that the antibody detects ATF-3 in extracts of axotomized superior cervical ganglia but not in undamaged ganglia of sham animals (Hyatt et al., 2007). This was later confirmed using extracts from cultured guinea pig cardiac ganglia where a single band with the appropriate molecular weight (the antiserum stains a single band of 21kDa, manufacturer’s technical information) was detected by Western blot analysis with the anti-ATF-3 antiserum (Young et al., 2008). Antibody specificity for cells in the guinea pig cardiac ganglia was also determined by the suppression of immunostaining by preadsorption of the antibody with a blocking peptide sc-188P (Santa Cruz Biotechnology) (Young et al., 2008). Analysis of the peptide containing solution indicated that the solution contained multiple peptide fragments located between amino acids 163 and 181 of the C-terminal domain of human ATF-3 (Young et al., 2008). Staining patterns in the current study are consistent with previously published articles (Averill et al., 2004; Shortland et al., 2006; Tsujino et al., 2000).
Mouse anti-Neurofilament 200
Anti-NF200 was raised against the carboxyterminal tail segment of enzymatically dephosphorylated pig neurofilament H-subunit and shows a broad species cross-reactivity, including the rat (manufacturer’s data). This antibody has also been extensively characterized and controlled in previous studies. Specifically Western blot analysis for antibody characterization as described previously (Fukuoka et al., 2008) and as a result this antibody stained a single band at the expected position (200 kDa). All neuronal staining was abolished when 1 ml of the diluted primary antibody was pre-incubated with 100 μg of porcine NF200 and no staining was seen without the primary antibody (Fukuoka et al., 2008). The antibody does not cross-react with the other intermediate filament proteins (manufacturer’s information) and is well established as a marker of myelinated DRG neurons (Hammond et al., 2004; Lawson and Waddell, 1991). Staining patterns in the current study are consistent with previously published articles (Bradbury et al., 1998; Debus et al., 1983; Ruscheweyh et al., 2007).
Mouse anti-Β(III) Tubulin
Anti-β(III)tubulin antibody from clone 5G8 was originally produced by Dr. Anthony Frankfurter (manufacturer’s information). The antibody used in this study is derived from the published peptide sequence (Lee et al., 1990) and it has been confirmed that this antibody exhibits all the same properties as the published clone (manufacturer’s information). On Western blot analysis of mouse spinal cord and brain homogenates, the antibody detects a single band migrating at the appropriate molecular weight of 50kDa (Spittaels et al., 2000; Carter et al., under revision). Neuronal specificity of staining has been demonstrated in formalin-fixed mouse brain paraffin sections (Vig et al., 2000) and Staining patterns in the current study are consistent with previously published articles (Bradbury et al., 2002).
Rabbit anti-SPRR1A
The SPRR1A polyclonal antibody used in this study was a kind gift from Professor Stephen Strittmatter (Yale University School of Medicine, New Haven). Characterisation of the antibody specificity has been previously described (Bonilla et al., 2002). Specifically, COS-7 cells both with and without SPRR1A transfection were tested. The transfected cells were shown to produce the appropriate size band by immunoblot, and the immunofluorescent signal was shown to be dependent on the expression of SPRR1A (Bonilla et al., 2002). Immunostaining carried out on injured DRG was also blocked by excess recombinant SPRR1A protein antigen. The same results were obtained with serum and affinity-purified anti-SPRR1A antibody (personal communication). Immunologic controls included incubation with pre-immune serum and blockade with the protein antigen (Bonilla et al., 2002).
Rabbit anti-PKCγ
The preparation of this polyclonal antibody has been described in detail in previous studies (Hosoda et al., 1989; Mori et al., 1990; Saito et al., 1989). The specificity of the antibody was confirmed by Western blot analysis using several PKC subspecies, which were separately expressed in COS-7 cells transfected with each cDNA-containing plasmid (Mori et al., 1990). Full details of the characterisation of this antibody have been previously published (Hosoda et al., 1989). The antiserum stains a single band of 77 kDa molecular weight on Western blot (manufacturer’s technical information). The antibody has previously shown similar staining patterns to those reported here (Bradbury et al., 2002; Kerr et al., 2000; Malmberg et al., 1997; Mori et al., 1990).
Rabbit anti-GAP-43
This antibody has been described and fully characterised in previous publications (Curtis et al., 1991; Curtis et al., 1993). Western blot analysis of mouse spinal cord, cerebellum and whole brain homogenates shows a single immunoreactive protein band migrating at 43kDa, and specifically, co-migrating with GAP-43 purified from neonatal rat brain. No band was detected upon omission of the antiserum or incubation with pre-immune serum, (Curtis et al., 1993). Staining patterns in the current study are consistent with previously published articles (Bradbury et al., 2002; Doubell and Woolf, 1997; Ramer et al., 2001).
Goat anti-IB4
This antibody has been used in previous studies (Brumovsky et al., 2005; Kim et al., 2008; Lu et al., 2003; Sandelin et al., 2004; Shehab et al., 2004), and its characterization and specificity have been widely confirmed (Fang et al., 2006; Hwang et al., 2005; Hwang et al., 2001; Shehab et al., 2004). The antibody, raised against whole lectin 1 of Griffonia simplicifolia, yields identical recognition when tested through immunodiffusion (manufacturer’s information). The antibody was purified using affinity chromatography on lectin-specific columns (details from manufacturer). Solid-phase binding assays using ELISA were carried out, specifically the lectin was plated and the antibody used to detect it (manufacturer’s technical information). In previous studies, sections incubated with the anti-IB4 antibody without prior incubation with IB4 or with normal goat serum showed no specific staining (Kim et al., 2008). Staining patterns in the current study are consistent with previously published articles (Averill et al., 2008; Bennett et al., 1998; Michael et al., 1997).
Photomicrograph Production
Photomicrograph images in figures 1, 2, and 5 were taken with a Zeiss Axiocam HRm camera fitted to a Carl Zeiss Axioplan 2 Imaging fluorescence microscope. Photomicrograph images in figure 4 (high power DRG co-localisation images) were taken using the ApoTome Imaging System, since with conventional fluorescence microscopy illumination of structures outside the focal plane can lead to poor contrast and resolution in images acquired from thick tissue sections. The Carl Zeiss ApoTome uses a grid projection to achieve optical sections through the specimen, removing out-of-focus information. Use of ApoTome allows DRG cells to be visualised within their correct spatial context and colocalisation of neurochemical markers can be assessed reliably, without artefact from out-of-focus information. Photomontages (Fig. 1 all panels except D-G, Fig. 2 A and B, and Fig. 5 all panels) were prepared in Adobe Photoshop 7.0 which was also used for annotating the images.
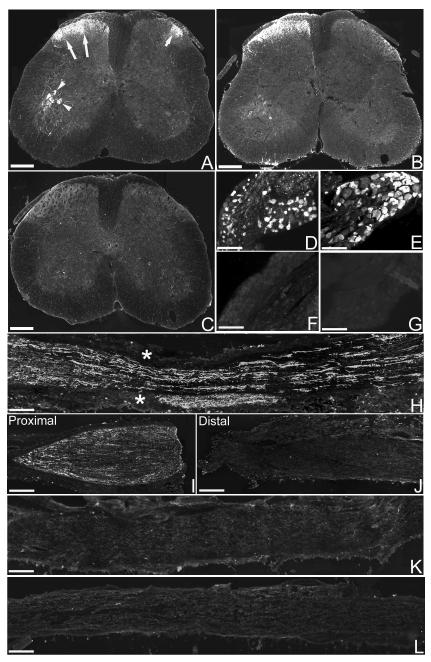
Figure 1. Expression of SPRR1A in spinal cord, DRG and peripheral nerve following sciatic nerve injury.
In the mouse lumbar spinal cord one week after sciatic nerve crush (A) or transection (B), SPRR1A immunoreactivity is expressed in the ipsilateral (left) side within the superficial dorsal horn (long arrows), and in the motoneurons in the ventral horn (arrowheads). SPRR1A immunoreactivity is also expressed in a small lateral area of the dorsal horn on the contralateral (right) side (short arrows). C, in the lumbar spinal cord of a naïve mouse no SPRR1A-positive immunofluorescence is present. One week after sciatic nerve crush (D) or transection (E) SPRR1A immunofluorescence is seen in a high proportion of cells in the ipsilateral lumbar DRG. No positive immunofluorescence for SPRR1A is observed in lumbar DRG contralateral to nerve injury (F) or in naïve lumbar DRG (G). H, one week after sciatic nerve crush SPRR1A immunofluorescence was observed along the entire length of the nerve (asterisks mark the site of crush injury). One week after sciatic nerve transection, SPRR1A immunofluorescence is apparent in the proximal (I) but not distal (J) portion of the nerve. No SPRR1A immunofluorescence is apparent in the sciatic nerve contralateral to the injury (K) or in the sciatic nerve from a naïve mouse (L). Scale bar = 300 um (A, B & C), 100 um (D, E, F & G), 200 um (H, I, J, K & L).
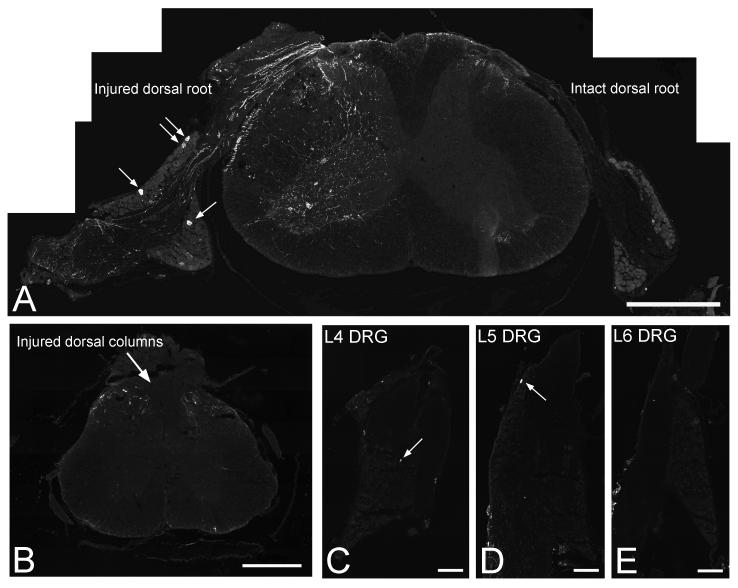
Figure 2. Expression of SPRR1A in spinal cord and DRG following injury to the central projections of DRG neurons.
In the mouse cervical spinal cord one week after unilateral dorsal root crush injury (A, open arrow points to crush site) SPRR1A is expressed in some regenerating axons in the injured dorsal root as they approach the dorsal root entry zone (demarcated by dashed line), in some fibres within the ipsilateral spinal cord and in a small number of cells in the ipsilateral DRG (arrows point to 4 immunopositive neurons in the attached DRG); no positive immunofluorescence for SPRR1A is observed in the contralateral DRG. One week after a T12 thoracic dorsal column crush injury (B-E), only a few fine fibres are observed in the superficial dorsal horns (B) at the level of the lesion and virtually no SPRR1A immunofluorescence is apparent in the lumbar DRG; arrows point to the single immunopositive neuron observed in an entire section of the L4 (C) and L5 (D) DRG, and no positive cells are apparent in the L6 DRG (E). Scale bar = 500 um (A,B), 400 um (C-E).
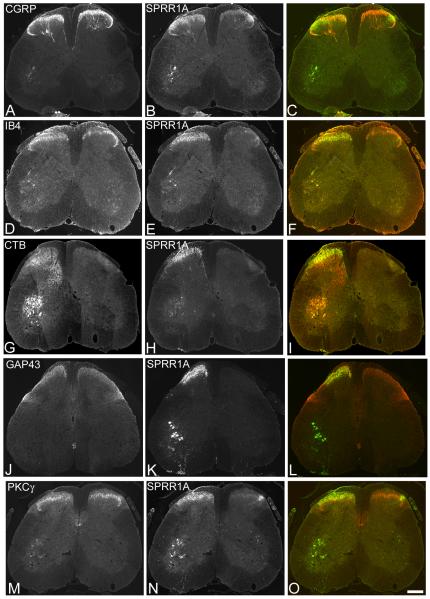
Figure 5. Double labelling for SPRR1A with other markers in the spinal cord.
Transverse sections of the lumbar spinal cord one week after sciatic nerve crush double immunostained for SPRR1A (B,E,H,K,N) and several other markers (A,D,G,J,M) to determine co-localisation (merged image, C,F,I,L,O). A, CGRP immunoreactivity is apparent in laminae I and II of the dorsal horn of the spinal cord. B, in the same section SPRR1A immunoreactivity is apparent in the ipsilateral (left) side as a band of axon terminals extending mediolaterally across laminae I and II, of the dorsal horn. C, there is some colocalisation of CGRP (red) with SPRR1A (green) in lamina II (outer) of the ipsilateral dorsal horn (yellow). D, IB4 immunoreactivity is apparent in lamina II (inner) of the dorsal horn. E,F, SPRR1A immunoreactivity in the same section reveals co-localisation of IB4 (red) with SPRR1A (green) in the mediolateral portion of lamina II (inner) of the ipsilateral dorsal horn (yellow). G, CTB immunoreactivity is apparent in central terminals in the deep dorsal horn and motoneurons in the ventral horn. H,I, SPRR1A immunoreactivity in the same section reveals co-localisation of CTB (red) with SPRR1A (green) in the motoneurons and at the ipsilateral lamina II-III border (yellow). J, GAP-43 immunoreactivity is apparent predominantly in laminae I and II and the dorsolateral funiculus. K,L, SPRR1A in the same section reveals co-localisation of GAP-43 (red) and SPRR1A (green) in the ipsilateral superficial dorsal horn (yellow). M, PKC© immunoreactivity is apparent in lamina II and III interneurons and the corticospinal tract located in the ventral portion of the dorsal funiculus. N,O, SPRR1A immunoreactivity in the same section reveals co-localisation of PKCγ (red) and SPRR1A (green) within the ipsilateral lamina II-III border (yellow). Scale bar = 200 um. Supplementary figure 2 shows these images in magenta-green.
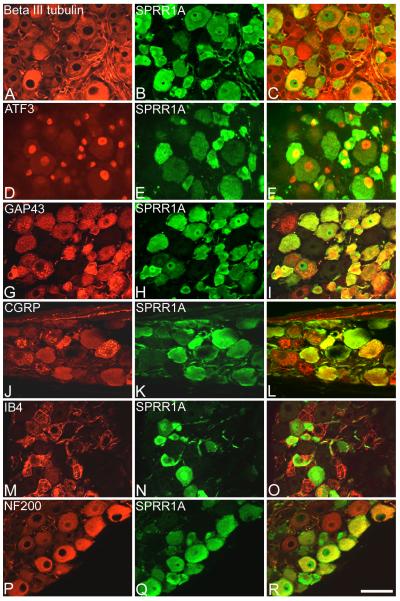
Figure 4. Double labelling for SPRR1A and classical markers of injury, regeneration or DRG sub populations.
DRG sections harvested one week after sciatic nerve crush, double immunostained for SPRR1A (green, B,E,H,K,N,Q) and several other markers (red, A,D,G,J,M,P) to determine co-localisation (merged image, C,F,I,L,O,R). A, diffuse labelling of the pan neuronal marker ® (III) tubulin is apparent in all cells. B, immunostaining for SPRR1A in the same section reveals intense immunoreactivity in many cells of small and large diameter. C, merged image shows that SPRR1A is expressed in a high proportion of all cells. D, immunostaining for ATF-3 is observed in the nuclei of injured cells. E, SPRR1A immunostaining in the same section reveals that SPRR1A is up-regulated in a high proportion of injured cells (ATF-3 and SPRR1A merged, F). G, GAP-43 immunostaining is apparent as granular staining in both large- and small-diameter cells following injury. H, SPRR1A immunostaining in the same section reveals a high degree of co-expression with the well-established regeneration-associated marker (GAP-43 and SPRR1A merged, I). J, CGRP immunoreactivity appears as granular staining in a proportion of small and medium diameter cells. K, SPRR1A immunostaining in the same section reveals that SPRR1A is expressed in a proportion of nociceptive, peptidergic cells (CGRP and SPRR1A merged, L). M, IB4 immunoreactivity appears as granular staining in a proportion of small diameter cells. N, SPRR1A immunostaining in the same section reveals that SPRR1A is expressed in a proportion of nociceptive, non-peptidergic cells (IB4 and SPRR1A merged, O). P, NF200 immunostaining is present throughout the cytoplasm of large diameter cells. Q, SPRR1A immunostaining in the same section reveals that SPRR1A is expressed in a proportion of the non-nociceptive large diameter cells (NF200 and SPRR1A merged, R). Scale bar = 50 um. Supplementary figure 1 shows these images in magenta-green.
Analysis
Cell size analysis
Images of DRG immunostained for SPRR1A and ® (III) tubulin were selected (4 sections per animal, 3 randomly selected fields of view per section, n = 4 animals each for crush and transection injuries at 7 days). Two images were captured for each field of view, one with the FITC (green) filter to show the SPRR1A positive cells and one with the TRITC (red) filter to show the Β(III) tubulin positive cells (all images were captured under identical brightness, contrast and exposure settings). Cell outlines of nucleus-containing Β(III) tubulin sections were traced using SigmaScan Pro 4 software (SPSS Inc., Chicago, IL, USA) to obtain the profile diameter (in μm) and profile areas (in μm2) of all DRG somata. This created a template, which was then overlaid onto the equivalent SPRR1A image and used to measure staining intensity (in grey level units) for each SPRR1A stained cell profile. The areas for each individual cell profile were thus recorded, along with their corresponding SPRR1A staining intensity (the intensity values of just-positive and just-negative cells were recorded for each section and used to calculate the intensity threshold for positive cells). Data were therefore obtained for the total number of cell body profiles within each field of view, the number of these profiles that were SPRR1A-positive, plus the diameter and area of each cell body profile. Cell counts were corrected for cell size using the Abercrombie method (Abercrombie, 1946; for discussion see Guillery, 2002). Histograms were drawn using SigmaPlot (Systat Software UK Limited). Statistical tests were carried out with the SigmaStat 3.0.1 (SPSS Inc.) statistical package. Two-sample Kolmogorov-Smirnov tests were carried out to determine whether the cell size frequency distributions differed between SPRR1A positive cells and the total cell population.
Co-localisation
To calculate the percentage of SPRR1A co-localisation with other markers 7 days after nerve crush injury, the number of cells positive for SPRR1A, the number of cells positive for a second marker (i.e. ATF-3, CGRP, IB4, NF200 or GAP-43) and the number of cells double immunostained (i.e. positive for both SPRR1A and the second marker) were counted in 3-4 whole cross-sections per animal (n=4). Cell counts were corrected for cell size differences using the Abercrombie method (Abercrombie, 1946) and the results were expressed as mean percentage ± s.e.m.
Results
SPRR1A expression in spinal cord, DRG and sciatic nerve one week after crush or transection of the sciatic nerve
One week after sciatic nerve crush (Fig. 1A) or transection (Fig. 1B) there was positive immunofluorescence staining for SPRR1A ipsilateral to the injury in the superficial laminae of the dorsal horn and in ventral horn motoneurons (Fig. 1A & B); SPRR1A immunofluorescence staining in the contralateral side was apparent in a small lateral area of the superficial dorsal horn (Fig. 1A & B). In naïve lumbar spinal cord no positive staining for SPRR1A was observed in any region (Fig. 1C). In ipsilateral lumbar (L4/5) DRG, SPRR1A was expressed by a high proportion of cells one week after sciatic nerve crush (Fig. 1D) or transection (Fig. 1E). The immunofluorescence staining for SPRR1A was evenly distributed throughout the cytoplasm of SPRR1A-positive DRG cell bodies (Fig. 1D & E). No positive immunofluorescence staining for SPRR1A was seen in the contralateral lumbar DRG one week after sciatic nerve crush (Fig. 1F) or transection (data not shown), or in lumbar DRG from naïve (uninjured) animals (Fig. 1G). SPRR1A positive immunofluorescence staining was observed both proximal and distal to the crush site in regenerating fibres along the entire length of the sciatic nerve one week after a crush injury (Fig. 1H). One week after sciatic nerve transection there was positive immunofluorescence staining for SPRR1A in the proximal portion of the transected nerve (Fig. 1I) but not in the distal portion (Fig. 1J). The accumulation of SPRR1A in the proximal nerve indicates that SPRR1A is transported from the cell body. No positive immunofluorescence staining for SPRR1A was observed in the contralateral sciatic nerve one week after sciatic nerve crush (Fig. 1K) or transection (data not shown), or in the sciatic nerve of naïve (uninjured) animals (Fig. 1L).
SPRR1A expression in spinal cord and DRG following injury to the central projections of DRG neurons
One week after unilateral dorsal root crush injury (Fig. 2A) there was positive immunofluorescence staining for SPRR1A ipsilateral to the injury in regenerating fibres in the dorsal root and in some fine fibres and motoneurons within the spinal cord. Only a small proportion of neurons in the ipsilateral DRG expressed SPRR1A. In this injury model few fibres cross the dorsal root entry zone to enter the spinal cord and thus, having reached the inhibitory barrier of the DREZ, regeneration is largely abortive. No positive immunofluorescence staining for SPRR1A was observed in the contralateral spinal cord or DRG (Fig. 2A). One week after a T12 dorsal column crush injury (Fig. 2B-E), at the level of the injury site (Fig. 2B), there was very little expression of SPRR1A within the spinal cord, with positive immunofluorescence staining apparent in only a few fine fibres in the superficial dorsal horns. In this injury model there is no regeneration of ascending dorsal column axons; correspondingly, there was virtually no expression of SPRR1A apparent in lumbar (L4/5) DRG cell bodies (Fig. 2C-E) whose axons project in the dorsal columns. Therefore, SPRR1A induction correlates with regenerative capacity.
SPRR1A expression in lumbar DRG following sciatic nerve injury
The time course of SPRR1A expression in lumbar DRG following sciatic nerve crush and transection injuries was studied using dual colour immunofluorescence for SPRR1A and β(III) tubulin to obtain cell counts and cell size distributions. The data from L4 and L5 DRGs were combined. One day after sciatic nerve crush 8.6 ± 3.9% of all cells were positive for SPRR1A, whereas after sciatic nerve transection 4.5 ± 1.8% of all cells were positive (Fig. 3A & 3B, respectively); by day 3 after injury this value had risen to 42.8 ± 3.5% following crush and 45.8 ± 3.0% following transection (Fig. 3A & 3B, respectively). For both injuries, maximal levels of expression were observed at 7 days with 80.6 ± 4.7% positive following crush and 82.8 ± 10.7% positive following transection (Fig. 3A & 3B, respectively). However, while SPRR1A expression was reduced to negligible levels by 30 days after crush injury (1.4 ± 0.5%; Fig. 3A), SPRR1A was still expressed by a significant proportion of DRG neurons following transection (13.6 ± 2.1%; Fig. 3B).
Figure 3. Time course and cell size distribution of SPRR1A expression in lumbar DRG following crush or transection of the sciatic nerve.
Following sciatic nerve crush (A) or transection (B) the time course of SPRR1A expression in L4/5 DRG neurons follows a similar pattern, becoming apparent by 1 day post lesion, rising to maximal levels by day 7 and then decreasing. However, at the earliest time point examined (1 d) SPRR1A expression is greater in the DRG of crush injured mice compared to transection. Conversely, at the latest time point examined (30 d), while SPRR1A expression is virtually absent in the DRG of crush injured mice it remains elevated following transection. Cell size distributions of SPRR1Aimmunopositive cells (SPRR1A +ve) compared to all cells in the L4 and L5 DRG (all cells) at the time point of maximal expression (7 d) reveal that a high proportion of cells in all size ranges are SPRR1A + ve following sciatic nerve crush (C) or transection (D). Cumulative frequency size distributions confirm no significant differences in the cell size distributions of SPRR1A+ve neurons compared to all neurons 7 days after sciatic nerve crush (E) or transection (F) (two-sample Kolmogorov-Smirnov test, P > 0.05).
Cell size distribution analysis following crush or transection injury revealed that at the time point of maximal expression (7 days) the size distribution of SPRR1A-positive DRG cells was identical to that of all cells in the lumbar DRG, albeit with fewer cells in each bin range (Fig. 3C & 3D, respectively). This expression has a mode between 300-500μm2 and a ‘tail’ of larger cells up to 2000μm2. Most SPRR1A-positive DRG cell bodies were approximately 400μm2, which is the most common cell size in mouse DRG. Thus, there was no significant difference in the cell size distribution between SPRR1A-positive DRG cells and all DRG cells following either crush (Fig. 3C) or transection (Fig. 3D) of the sciatic nerve. Cumulative frequency distributions confirmed that the cell size distribution of SPRR1A-positive DRG neurons was almost identical to the size distribution of all DRG neurons following either crush or transection of the sciatic nerve (two-sample Kolmogorov-Smirnov test, P > 0.05; Fig. 3E & 3F, respectively).
Co-expression of SPRR1A with DRG markers after sciatic nerve injury
SPRR1A immunoreactivity was examined in relation to a number of widely used markers of subpopulations of DRG neurons. ® (III) tubulin is a pan-neuronal marker and thus was observed in all DRG neurons, apparent as diffuse staining throughout the cytoplasm (Fig. 4A). As described above, a high proportion of these DRG neurons also showed SPRR1A immunoreactivity (Fig. 4B,C). Figures 4A and 4B are examples of images that were used in the cell size analysis.
ATF-3 and GAP-43
ATF-3 is widely used as a marker of injured cells (Tsujino et al., 2000). Therefore, we used ATF-3 expression to identify neurons whose axons were injured by sciatic nerve crush. ATF-3 expression was observed in the nuclei of most cells of both large and small diameter (Fig. 4D) and showed a high degree of co-localisation with SPRR1A immunoreactivity (Fig. 4D-F). Following adult nerve injury GAP-43 is expressed and rapidly transported along the axons of neurons that successfully regenerate (Skene and Willard, 1981). Therefore, we used GAP-43 expression as a regeneration-associated marker. GAP-43 expression was observed as punctate staining in the cytoplasm of large and small diameter cells (Fig. 4G) and, in a high proportion of these cells, co-localised with SPRR1A immunoreactivity (Fig. 4G-I).
Quantification of ATF-3 double labelling with SPRR1A in the L4/5 DRG confirmed that 1 week after either crush or transection injury to the sciatic nerve a high proportion of SPRR1A-positive neurons also express ATF-3 (83% and 95%, respectively; Table 2). Quantification of SPRR1A and GAP-43 co-expression revealed that following sciatic nerve crush or transection approximately 65% and 50%, respectively, of SPRR1A-positive neurons also express GAP-43 (Table 2).
Table 2.
Co-localisation of SPRR1A with injury and regeneration markers following sciatic nerve crush and transection injury
| Double staining combinations | Mean % of SPRR1A expressing other (± s.e.m) |
Mean % of other expressing SPRR1A (± s.e.m) |
|---|---|---|
| SPRR1A and ATF-3 (crush) | 83.2 ± 3.4 (552/654) | 63.4 ± 3.9 (552/912) |
| SPRR1A and ATF-3 (transection) | 95.3 ± 1.9 (474/498) | 92.5 ± 0.4 (474/512) |
| SPRR1A and GAP-43 (crush) | 65.7 ± 3.6 (595/951) | 60.8 ± 5.6 (595/941) |
| SPRR1A and GAP-43 (transection) |
49.8 ± 5.6 (81/200) | 60.7 ± 5.0 (81/131) |
The figures in brackets indicate the total number of cells counted, after applying an Abercrombie correction for cell size.
CGRP, IB4 and NF200
The small diameter cells in L4/L5 DRG can be subdivided into two subpopulations. The numerically larger group consists of nociceptive, peptidergic cells which express the neuropeptide CGRP. CGRP expression was observed in small to medium sized cells, appearing granular throughout the cytoplasm (Fig. 4J) and some degree of colocalisation with SPRR1A immunoreactivity was apparent (Fig. 4J-L). The numerically smaller group of small diameter DRG neurons consists of nociceptive, non-peptidergic cells that can bind the lectin IB4. IB4 binding was observed in the plasma membrane, in smallcalibre fibres and as granular staining in the cytoplasm of small diameter cells (Fig. 4M) and there was some degree of co-localization with SPRR1A immunoreactivity (Fig. 4M-O). NF200 is a marker of large diameter non-nociceptive A fibres. NF200 immunoreactivity was observed as intense staining throughout the cytoplasm of large cells (Fig. 4P) and again there was some degree of co-localization with SPRR1A immunoreactivity (Fig. 4P-R).
Quantification of SPRR1A double-labelling with markers of DRG subtypes 7 days after crush injury revealed that approximately 11% of SPRR1A-positive neurons also express CGRP, 27% also express IB4 and 40% express NF200 (Table 3). Taken together these results indicate that approximately 40% of the cells expressing SPRR1A following sciatic nerve injury are small diameter nociceptive DRG neurons and approximately 40% of SPRR1A expressing cells are large-diameter neurons.
Table 3.
Co-localisation of SPRR1A with classical markers of DRG sub-populations following sciatic nerve crush injury
| Double-staining combinations | % of SPRR1A expressing other (± s.e.m) |
% of other expressing SPRR1A (± s.e.m) |
|---|---|---|
| SPRR1A and CGRP | 11.4 ± 1.5 (119/903) | 15.0 ± 2.7 (119/725) |
| SPRR1A and IB4 | 27.2 ± 1.7 (264/1010) | 36.2 ± 5.7 (264/830) |
| SPRR1A and NF200 | 40.5 ± 3.8 (76/208) | 24.6 ± 4.7 (76/295) |
The figures in brackets indicate the total number of cells counted after applying an Abercrombie correction for cell size.
Co-expression of SPRR1A with spinal cord markers after sciatic nerve injury
SPRR1A expression was also investigated in the lumbar spinal cord where the central axons of lumbar DRG neurons project and terminate. Figure 5 shows the lumbar spinal cord doubleimmunostained for SPRR1A and several other spinal cord markers. SPRR1A was colocalised to some extent with each marker used. Positive immunofluorescence staining for CGRP was observed in laminae I and II of the dorsal horn of the spinal cord (Fig. 5A), with some co-localisation with SPRR1A apparent in lamina II outer of the ipsilateral dorsal horn (Fig. 5A-C). IB4 positive immunofluorescence staining was apparent in lamina II inner (Fig. 5D) and there was some co-expression of SPRR1A with IB4 in the medio-lateral portion of lamina II of the ipsilateral dorsal horn (Fig. 5D-F). CTB labelling was observed ipsilateral to the tracer injection in central terminals in the deep dorsal horn and motoneurons in the ventral horn (Fig. 5G). There was some co-localisation of CTB with SPRR1A in motoneurons and in the lamina II-III border (Fig. 5G-I). GAP-43 immunofluorescence staining was most apparent in laminae I and II, with increased expression ipsilateral to injury (Fig. 5J) and there was colocalisation with SPRR1A in the superficial ipsilateral dorsal horn (Fig. 5J-L). Finally, positive immunofluorescence for PKCγ was observed in interneurons in laminae II and III and in the corticospinal tract in the dorsal funiculus (Fig. 5M) and some co-localisation of PKCγ with SPRR1A was observed ipsilaterally in lamina II (Fig. 5M-O).
Discussion
We have characterised SPRR1A expression in the DRG and spinal cord of adult mice following injury to the peripheral and central projections of DRG neurons and found that SPRR1A induction correlates with regenerative capacity, with virtually all injured DRG neurons expressing SPRR1A one week after sciatic nerve injury and little or no expression one week after dorsal rhizotomy or dorsal column injury, respectively. Furthermore, a time course study comparing expression following transection or crush of the peripheral (sciatic) nerve reveals that the down regulation of SPRR1A expression corresponds with the time course of reinnervation known to occur in these models. Finally, we performed a detailed characterisation of expression in the sensory neuron subpopulations and their primary afferent projections following peripheral nerve injury and found SPRR1A to be expressed by the three main sub-populations, as well as being co-localised with well-known markers of injury and regeneration. Thus, SPRR1A becomes de novo expressed by the main subpopulations of DRG neurons following an injury to their peripheral, but not central, projections. These findings support the hypothesis that SPRR1A is a regeneration-associated protein and may be valuable in future studies investigating intervention strategies aimed at promoting enhanced regeneration following peripheral or central nervous system injury.
SPRR1A expression following peripheral versus central injury
Primary sensory neurons are capable of successful regenerative growth in response to peripheral nerve but not dorsal root or dorsal column injury (Bradbury et al., 2000; Wujek and Lasek, 1983). Therefore, as SPRR1A has been proposed as a RAG, we studied whether there was a differential expression of SPRR1A in DRG neurons following injury to their peripheral and central branches. Following peripheral nerve injury, SPRR1A was expressed in a high proportion of DRG neurons (approximately 80%) at the time point of maximal expression (day 7). This proportion is presumably because a number of L4/L5 DRG neurons, possibly as many as 20-25%, remain uninjured since they do not project as far as the point of injury at the sciatic notch (Shi et al., 2001). Thus, our data indicate that the majority of injured DRG neurons express SPRR1A following peripheral nerve injury. In contrast, following injury to the central projections of DRG neurons, only a very small proportion of DRG neurons expressed SPRR1A, with very few immunopositive neurons apparent in the DRG adjacent to dorsal root injury and virtually no expression following dorsal column injury. These data are similar to earlier observations of GAP-43 expression in the adult rat following injury, where virtually all injured DRG neurons are known to up-regulate GAP-43 following peripheral nerve injury, but levels do not change following injury to central DRG projections (Bradbury et al., 2002; Chong et al., 1992; Chong et al., 1994; Schreyer and Skene, 1993). Thus, as previously shown for GAP-43, our findings show a differential expression of SPRR1A following central versus peripheral injury and that SPRR1A expression is correlated with regenerative potential. However, SPRR1A expression in DRG neurons is completely de novo following injury, unlike GAP-43 which is highly expressed during development and remains constitutively expressed by some DRG neurons in the adult (Chong et al., 1992; Fitzgerald et al., 1991; Meiri et al., 1986; Skene et al., 1986). This supports the notion that SPRR1A is indeed a truly regeneration-specific protein (Snider et al., 2002) and the failure to induce SPRR1A expression may contribute to the poor regenerative response initiated by dorsal root or dorsal column injury.
Following dorsal root injury, the majority of SPRR1A immunopositive fibres were observed within the dorsal root where they accumulated at the inhibitory DREZ, where regenerating axons are prevented from entering the spinal cord. Fewer SPRR1A-positive axons were apparent on the DRG side of the crush, presumably due to the degree of tissue damage caused by the close proximity of the lesion to the ganglion. A similar pattern of SPRR1A immunopositive axons has previously been reported in rhizotomised adult mice (Cafferty et al., 2007). Some SPRR1A immunopositive motoneurons and fine fibres were also observed within the spinal cord parenchyma following dorsal root rhizotomy. These may represent a local sprouting response from neurons that may have been denervated or damaged due to their close proximity to the injury. This is similar to previous observations where SPRR1A induction was observed within motoneurons of the same segmental level following rhizotomy in the adult mouse and was attributed to surgical traction on ventral roots (Cafferty et al., 2007). Limited SPRR1A expression has previously been observed rostral and caudal to a spinal cord hemisection where it may reflect a propensity for regenerative attempts and/or plastic rearrangements of spinal circuitry in a small number of CNS neurons, a phenomenon that can be increased by regeneration-promoting strategies (Kim et al., 2004; Li and Strittmatter, 2003).
Time course of SPRR1A expression in lumbar DRG following peripheral nerve crush or transection
We examined the time course of SPRR1A induction following peripheral (sciatic) nerve injury comparing SPRR1A expression following nerve crush and transection. SPRR1A expression follows a similar pattern in both injury models, becoming apparent by 1 day post lesion, rising to maximal levels by day 7 with decreased levels at 30 days post-injury. However, expression appears to be more robust early post-injury following crush, with slightly higher levels observed at 1 day. Conversely, by the late post-injury time point (30 days) SPRR1A expression is virtually absent in the DRG of crush-injured mice but remains elevated following transection. Evidence from studies of other RAGs suggests that the down-regulation of SPRR1A expression may be related to successful target re-innervation. For example, the expression of GAP-43 is rapidly down-regulated when regenerating axons reach their targets (Chaisuksunt et al., 2000; Chong et al., 1992; Chong et al., 1994). Activation of c-Jun has also been correlated with target deprivation, with expression remaining high until target re-innervation occurs and persisting in most DRG cells for up to 15 months following transection (Broude et al., 1997; Jenkins et al., 1993; Leah et al., 1991). Similarly, following sciatic nerve crush, Reg-2 is expressed for the entire period of regeneration (Livesey et al., 1997), again suggesting that its expression is regulated by target innervation. Target innervation is also thought to control the expression of the cytoskeletal proteins SCG10 and CAP-23 (Mason et al., 2002) and the cell adhesion molecule CHL1 (Zhang et al., 2000), which are down-regulated upon target innervation but remain highly expressed when regeneration, and therefore target innervation, are prevented. Thus, similar to previous findings for RAG expression in adult rats, our observations of SPRR1A expression correspond to the period of target reinnervation and functional recovery observed in adult mice following sciatic nerve injury, where permanent impairments are observed following transection but recovery to normal function is observed by 3-4 weeks post-injury following crush (Baptista et al., 2007). This provides further evidence that SPRR1A is a regeneration-associated protein. Although these findings demonstrate that SPRR1A expression correlates with regenerative potential, it is perhaps surprising that SPRR1A levels declined at all in the transection model and did not remain highly elevated at the 30 day time point. However, similar findings have been reported for GAP-43 when it was observed that six weeks after transection of the sciatic nerve, expression of GAP-43 was still elevated but at a reduced level compared to that observed at early post-injury time points (Chen et al., 2001). Similarly, GAP-43 expression was still elevated, but again at a reduced level, four weeks after transection of the facial nerve where successful regeneration had not occurred (Kohmura et al., 1999). ATF-3 expression, however, remains highly expressed in DRG neurons at late post-injury time points, although expression in Schwann cells in the distal nerve segment has been shown to decrease over time, suggesting that the successful regeneration of axons is not entirely responsible for the down-regulation of ATF-3 expression (Kataoka et al., 2007).
Co-expression of SPRR1A with markers of sensory neuron subtypes
It is known that the response of DRG neurons to injury is not uniform, suggesting that the subpopulations may differ in their capacity to up-regulate regeneration-associated genes (Broude et al., 1997). Thus, we used neurochemical markers to analyse SPRR1A expression in DRG subpopulations following injury. Using double immunofluorescence to show the extent of co-expression of SPRR1A with CGRP, IB4 and NF200 we found that SPRR1A is co-localised to some extent with all three DRG cell populations, although we may have underestimated the number of small diameter cells expressing SPRR1A since expression of CGRP and IB4 is reduced following peripheral nerve injury (Bennett et al., 1998; Shi et al., 2001; Verge et al., 1995). SPRR1A expression was also determined in the spinal cord one week after crush injury and was found to be expressed in the terminals of DRG neurons in the dorsal horn and in motoneurons in the ventral horn. In the dorsal horn SPRR1A was predominantly localised in a thick band of axon terminals extending mediolaterally and spanning laminae I and II as well as the lamina II/III border and, as observed in the DRG, there was some degree of co-localisation of SPRR1A with other spinal cord markers. Thus, SPRR1A is expressed by a proportion of all sensory neuron subtypes and their central projections within the spinal cord following peripheral nerve injury.
Co-expression of SPRR1A with markers of injury and regeneration in the DRG
As SPRR1A has been proposed to be a RAG (Bonilla et al., 2002), we characterised the expression of SPRR1A in relation to GAP-43. GAP-43 is highly expressed in development during target innervation and following injury to adult peripheral nerves is re-expressed in most DRG cell bodies, reaching peak levels by 2 weeks and being reduced upon target reinnervation (Bisby, 1988; Chong et al., 1992; Van der Zee et al., 1989; Woolf et al., 1990). We found that following crush injury a large proportion of SPRR1A-immunopositive cells also expressed GAP-43 at 7 days (approximately 65%), with a lower percentage following transection (approximately 50%), presumably due to the greater propensity for regeneration after crush injury. We also examined SPRR1A co-expression with ATF-3 which is known to be induced in a number of tissue types in response to stress (Chen et al., 1996; Hai et al., 1999; Yin et al., 1997) and is widely used as a marker of injured neurons (Averill et al., 2004; Tsujino et al., 2000; Tsuzuki et al., 2001). Approximately 83% of SPRR1A expressing cells co-expressed ATF-3 following a crush injury, with the effect more pronounced following transection, where approximately 95% of SPRR1A immunopositive cells were co-labelled, probably due to the greater damage that occurs following transection injury. Thus, our data show a greater degree of SPRR1A co-localisation with ATF-3 than with GAP-43. This raises the possibility that SPRR1A may in fact be an injury-associated, rather than regeneration-associated protein. Previous work has identified a role for SPRR1A as a stress-inducible protein, regulated by the gp130 signalling pathway in the heart (Pradervand et al., 2004). In support of this, SPRR1A has also been found to be present in non-regenerating neurons in the cortex that have been injured following a moderate brain injury (Marklund et al., 2006). Conversely, ATF-3 may play a role in the regeneration process following peripheral nerve injury. Indeed, recent evidence shows that over-expression of ATF-3 in cultured DRG neurons enhances neurite outgrowth (Seijffers et al., 2006) and transgenic mice which constitutively express ATF-3 display enhanced peripheral nerve regeneration (Seijffers et al., 2007). This suggests a similar growth-associated role for ATF-3 as that initially proposed for SPRR1A, when expression of SPRR1A in DRG neurons via recombinant HSV infection enabled neurite outgrowth on inhibitory substrates (Bonilla et al., 2002). Interestingly, differential expression of GAP-43 and SPRR1A was observed in ATF3-expressing transgenic mice, with increased transcript levels of SPRR1A, but not GAP-43, apparent in the noninjured ATF-3-expressing mice, despite them both being increased following peripheral nerve injury (Seijffers et al., 2007). It would be interesting to determine whether SPRR1A is upregulated in other models where a regenerative response has not been associated with GAP-43, such as the observation of a vigorous motoneuron sprouting response in the absence of GAP-43 expression following intramuscular injection of Botulinum toxin (Bisby et al., 1996; Osborne et al., 2007; Siegel et al., 2000). Furthermore, the findings that motor function and motoneuron survival are not completely abolished in mice that lack CNTF, LIF and CT-1 (Holtmann et al., 2005) suggests that there may be additional factors involved in promoting the survival of motoneurons. Since these cytokines share gp130 as their common signaling subunit, and SPRR1A is involved in the regulation of gp130 signalling (Pradervand et al., 2004), it would be interesting to investigate whether SPRR1A is upregulated in these triple knockout mice. Thus, it remains arguable whether SPRR1A should be classified as an injury- or regeneration-associated protein. However, it is at least clear from previous data, as well as the current study, that SPRR1A is expressed de novo by sensory neurons capable of undergoing a robust regenerative response following nerve injury, and is largely absent in sensory neurons under non-regenerative conditions, following injury to their central projections. Moreover, SPRR1A is not present in the majority of injured CNS neurons and has recently been used as an indicator of increased capacity for regeneration following spinal cord injury and anti-NogoA therapy (Cafferty et al., 2007; Kim et al., 2004; Li and Strittmatter, 2003). Finally, a recent study reported reduced expression of SPRR1A in the lumbar DRG of p110deltaPI3-kinase inactive mice that demonstrated impaired recovery of function following sciatic crush injury (Eickholt et al., 2007). These findings support the hypothesis that SPRR1A is indeed a regeneration-associated protein.
Up-regulation of SPRR1A in the contralateral dorsal horn following peripheral nerve injury
A surprising observation from the present study was the up-regulation of SPRR1A in a small lateral portion of the contralateral dorsal horn. Contralateral effects following unilateral lesions of the sciatic nerve have previously been reported, see (Koltzenburg et al., 1999) for review. For example, following sciatic nerve crush, immunoreactivity for the neuropeptide Y receptor was observed in the contralateral sciatic nerve and dorsal root (Brumovsky et al., 2002). Contralateral changes have also been observed following sciatic nerve ligation, with increased binding sites for bradykinin apparent at 2 days post-lesion in contralateral L4 and L5 DRG cell bodies (Eckert et al., 1999; Petersen et al., 1998). Furthermore, basic fibroblast growth factor immunoreactivity was observed in the dorsal and ventral horns of the contralateral spinal cord following sciatic nerve cryo-lesions (DeLeo et al., 1997). Unilateral sciatic nerve transection has also been associated with contralateral effects. For example, CGRP expression was up-regulated in contralateral spinal cord motoneurons, with the same temporal pattern as that in ipsilateral motoneurons but at lower expression levels (Piehl et al., 1991). Low-level expression of p75 has also been demonstrated in contralateral L5 DRG post-lesion (Zhou et al., 1996) and GAP-43 expression, though transient, has also been observed in undamaged motoneurons of the contralateral motoneuron pool in mice (Booth and Brown, 1993; Linda et al., 1992). It has been suggested that contralateral effects may be due to bilateral increases in trophic factors, or to bilateral peripheral innervation of the target or transmedial sprouting (Koltzenburg et al., 1999). Alternatively, the effects could involve immune interactions as nerve injury is known to cause the accumulation of macrophages in contralateral, as well as ipsilateral, DRG (Dubovy et al., 2007; Zhou et al., 1996). Another suggestion is that contralateral effects could result from a transneuronal mechanism involving the dorsal horn due to crossed collaterals of damaged afferent axons (Zhou et al., 1996). This mechanism is the most likely to underlie contralateral SPRR1A expression observed in the current study, rather than effects in peripheral target tissue, since increased contralateral expression of SPRR1A was only observed in the lumbar dorsal horn, with no SPRR1A expression observed in the contralateral DRG or peripheral nerves.
Conclusions
We have performed a detailed immunohistochemical characterisation of the expression of the SPRR1A protein in the DRG and spinal cord of adult mice and found that SPRR1A induction correlates with regenerative capacity, being highly expressed in the cell bodies and central terminals of primary sensory neurons following peripheral nerve, but not central, injury. SPRR1A co-localises with classic markers of sensory neuron subpopulations and expression corresponds with the period of target reinnervation that occurs following peripheral nerve injury. A cascade of molecular changes is known to occur in sensory neuron cell bodies following peripheral nerve injury and regeneration is associated with the co-ordinated expression of a diverse group of molecules involved in survival and growth. However, our findings provide support for the hypothesis that unlike many growth-associated molecules, SPRR1A induction is regeneration-specific and distinct from developmental programs of gene expression. These findings may be important for the future use of SPRR1A as a regeneration-specific marker in CNS and PNS injury studies in mice.
Supplementary Material
Acknowledgements
This work was supported by the UK Medical Research Council and the International Spinal Research Trust. The authors would like to thank Prof. Stephen Strittmatter and Dr. Iris Bonilla for the kind gift of the SPRR1A antibody, Dr. G. P. Wilkin for the kind gift of the GAP-43 antibody, Prof. Patrick Doherty for invaluable input and Carl Hobbs and John Grist for technical support.
Support: this work was supported by the UK Medical Research Council (Grant sponsor: E. J.
Bradbury, grant code: G120/818) and the International Spinal Research Trust (Grant sponsors: S. B. McMahon, P. Doherty, E. J. Bradbury, grant code: NRB054).
Abbreviations
- ATF-3
activating transcription factor-3
- CGRP
calcitonin gene related peptide
- CNS
central nervous system
- CTB
cholera toxin β subunit
- DRG
dorsal root ganglion
- GAP-43
growth associated protein-43
- IB4
isolectin B4
- NF200
neurofilament 200
- PBS
phosphate buffered saline
- PKCγ
protein kinase Cγ
- PNS
peripheral nervous system
- RAG
regeneration-associated gene
- SPRR1A
small proline-rich repeat protein 1A
References
- Abercrombie M. Estimation of nuclear population from microtome sections. The Anatomical Record. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Averill S, Inglis JJ, King VR, Thompson SW, Cafferty WB, Shortland PJ, Hunt SP, Kidd BL, Priestley JV. Reg-2 expression in dorsal root ganglion neurons after adjuvant-induced monoarthritis. Neuroscience. 2008;155:1227–1236. doi: 10.1016/j.neuroscience.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Averill S, Michael GJ, Shortland PJ, Leavesley RC, King VR, Bradbury EJ, McMahon SB, Priestley JV. NGF and GDNF ameliorate the increase in ATF3 expression which occurs in dorsal root ganglion cells in response to peripheral nerve injury. Eur J Neurosci. 2004;19:1437–1445. doi: 10.1111/j.1460-9568.2004.03241.x. [DOI] [PubMed] [Google Scholar]
- Baptista AF, Gomes JR, Oliveira JT, Santos SM, Vannier-Santos MA, Martinez AM. A new approach to assess function after sciatic nerve lesion in the mouse - adaptation of the sciatic static index. J Neurosci Methods. 2007;161:259–264. doi: 10.1016/j.jneumeth.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Schwab ME. Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci. 2003;26:555–563. doi: 10.1016/j.tins.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Bisby MA. Dependence of GAP43 (B50, F1) transport on axonal regeneration in rat dorsal root ganglion neurons. Brain Res. 1988;458:157–161. doi: 10.1016/0006-8993(88)90509-4. [DOI] [PubMed] [Google Scholar]
- Bisby MA, Tetzlaff W, Brown MC. GAP-43 mRNA in mouse motoneurons undergoing axonal sprouting in response to muscle paralysis of partial denervation. Eur J Neurosci. 1996;8:1240–1248. doi: 10.1111/j.1460-9568.1996.tb01292.x. [DOI] [PubMed] [Google Scholar]
- Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CM, Brown MC. Expression of GAP-43 mRNA in mouse spinal cord following unilateral peripheral nerve damage: is there a contralateral effect? Eur J Neurosci. 1993;5:1663–1676. doi: 10.1111/j.1460-9568.1993.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Khemani S, Von R, King, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB, Ramer MS. Keeping in touch: sensory neurone regeneration in the CNS. Trends Pharmacol Sci. 2000;21:389–394. doi: 10.1016/s0165-6147(00)01536-4. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Broude E, McAtee M, Kelley MS, Bregman BS. c-Jun expression in adult rat dorsal root ganglion neurons: differential response after central or peripheral axotomy. Exp Neurol. 1997;148:367–377. doi: 10.1006/exnr.1997.6665. [DOI] [PubMed] [Google Scholar]
- Brumovsky P, Stanic D, Shuster S, Herzog H, Villar M, Hokfelt T. Neuropeptide Y2 receptor protein is present in peptidergic and nonpeptidergic primary sensory neurons of the mouse. J Comp Neurol. 2005;489:328–348. doi: 10.1002/cne.20639. [DOI] [PubMed] [Google Scholar]
- Brumovsky PR, Shi TJ, Matsuda H, Kopp J, Villar MJ, Hokfelt T. NPY Y1 receptors are present in axonal processes of DRG neurons. Exp Neurol. 2002;174:1–10. doi: 10.1006/exnr.2001.7845. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ. The YFP-H mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair following spinal cord injury. J Neurosci. doi: 10.1523/JNEUROSCI.2217-08.2008. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisuksunt V, Zhang Y, Anderson PN, Campbell G, Vaudano E, Schachner M, Lieberman AR. Axonal regeneration from CNS neurons in the cerebellum and brainstem of adult rats: correlation with the patterns of expression and distribution of messenger RNAs for L1, CHL1, c-jun and growth-associated protein-43. Neuroscience. 2000;100:87–108. doi: 10.1016/s0306-4522(00)00254-2. [DOI] [PubMed] [Google Scholar]
- Chen BP, Wolfgang CD, Hai T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol Cell Biol. 1996;16:1157–1168. doi: 10.1128/mcb.16.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Chai YF, Cao L, Lu CL, He C. Glial cell line-derived neurotrophic factor enhances axonal regeneration following sciatic nerve transection in adult rats. Brain Res. 2001;902:272–276. doi: 10.1016/s0006-8993(01)02395-2. [DOI] [PubMed] [Google Scholar]
- Chong MS, Fitzgerald M, Winter J, Hu-Tsai M, Emson PC, Wiese U, Woolf CJ. GAP-43 mRNA in Rat Spinal Cord and Dorsal Root Ganglia Neurons: Developmental Changes and Re-expression Following Peripheral Nerve Injury. Eur J Neurosci. 1992;4:883–895. doi: 10.1111/j.1460-9568.1992.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Chong MS, Reynolds ML, Irwin N, Coggeshall RE, Emson PC, Benowitz LI, Woolf CJ. GAP-43 expression in primary sensory neurons following central axotomy. J Neurosci. 1994;14:4375–4384. doi: 10.1523/JNEUROSCI.14-07-04375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, Averill S, Priestley JV, Wilkin GP. The distribution of GAP-43 in normal rat spinal cord. J Neurocytol. 1993;22:39–50. doi: 10.1007/BF01183974. [DOI] [PubMed] [Google Scholar]
- Curtis R, Hardy R, Reynolds R, Spruce BA, Wilkin GP. Down-regulation of GAP-43 During Oligodendrocyte Development and Lack of Expression by Astrocytes In Vivo: Implications for Macroglial Differentiation. Eur J Neurosci. 1991;3:876–886. doi: 10.1111/j.1460-9568.1991.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Colburn RW, Rickman AJ. Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res. 1997;759:50–57. doi: 10.1016/s0006-8993(97)00209-6. [DOI] [PubMed] [Google Scholar]
- Doubell TP, Woolf CJ. Growth-associated protein 43 immunoreactivity in the superficial dorsal horn of the rat spinal cord is localized in atrophic C-fiber, and not in sprouted A-fiber, central terminals after peripheral nerve injury. J Comp Neurol. 1997;386:111–118. doi: 10.1002/(sici)1096-9861(19970915)386:1<111::aid-cne10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Dubovy P, Tuckova L, Jancalek R, Svizenska I, Klusakova I. Increased invasion of ED-1 positive macrophages in both ipsi- and contralateral dorsal root ganglia following unilateral nerve injuries. Neurosci Lett. 2007;427:88–93. doi: 10.1016/j.neulet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Eckert A, Segond von BG, Sopper S, Petersen M. Spatio-temporal pattern of induction of bradykinin receptors and inflammation in rat dorsal root ganglia after unilateral nerve ligation. Pain. 1999;83:487–497. doi: 10.1016/S0304-3959(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Eickholt BJ, Ahmed AI, Davies M, Papakonstanti EA, Pearce W, Starkey ML, Bilancio A, Need AC, Smith AJ, Hall SM, Hamers FP, Giese KP, Bradbury EJ, Vanhaesebroeck B. Control of Axonal Growth and Regeneration of Sensory Neurons by the p110delta PI 3-Kinase. PLoS ONE. 2007;2:e869. doi: 10.1371/journal.pone.0000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, McMullan S, Berry C, Waxman SG, Okuse K, Lawson SN. Intense isolectin-B4 binding in rat dorsal root ganglion neurons distinguishes C-fiber nociceptors with broad action potentials and high Nav1.9 expression. J Neurosci. 2006;26:7281–7292. doi: 10.1523/JNEUROSCI.1072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanella KE, Christianson JA, Chanthaphavong RS, Davis BM. Distribution and neurochemical identification of pancreatic afferents in the mouse. J Comp Neurol. 2008;509:42–52. doi: 10.1002/cne.21736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, Reynolds ML, Benowitz LI. GAP-43 expression in the developing rat lumbar spinal cord. Neuroscience. 1991;41:187–199. doi: 10.1016/0306-4522(91)90209-7. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Kobayashi K, Yamanaka H, Obata K, Dai Y, Noguchi K. Comparative study of the distribution of the alpha-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J Comp Neurol. 2008;510:188–206. doi: 10.1002/cne.21786. [DOI] [PubMed] [Google Scholar]
- Gibbs S, Fijneman R, Wiegant J, van Kessel AG, van de PP, Backendorf C. Molecular characterization and evolution of the SPRR family of keratinocyte differentiation markers encoding small proline-rich proteins. Genomics. 1993;16:630–637. doi: 10.1006/geno.1993.1240. [DOI] [PubMed] [Google Scholar]
- Gillen C, Gleichmann M, Spreyer P, Muller HW. Differentially expressed genes after peripheral nerve injury. J Neurosci Res. 1995;42:159–171. doi: 10.1002/jnr.490420203. [DOI] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- Hammond DL, Ackerman L, Holdsworth R, Elzey B. Effects of spinal nerve ligation on immunohistochemically identified neurons in the L4 and L5 dorsal root ganglia of the rat. J Comp Neurol. 2004;475:575–589. doi: 10.1002/cne.20209. [DOI] [PubMed] [Google Scholar]
- Holtmann B, Wiese S, Samsam M, Grohmann K, Pennica D, Martini R, Sendtner M. Triple knock-out of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function. J Neurosci. 2005;25:1778–1787. doi: 10.1523/JNEUROSCI.4249-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K, Saito N, Kose A, Ito A, Tsujino T, Ogita K, Kikkawa U, Ono Y, Igarashi K, Nishizuka Y. Immunocytochemical localization of the beta I subspecies of protein kinase C in rat brain. Proc Natl Acad Sci U S A. 1989;86:1393–1397. doi: 10.1073/pnas.86.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Oh JM, Valtschanoff JG. The majority of bladder sensory afferents to the rat lumbosacral spinal cord are both IB4- and CGRP-positive. Brain Res. 2005;1062:86–91. doi: 10.1016/j.brainres.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Pagliardini S, Rustioni A, Valtschanoff JG. Presynaptic kainate receptors in primary afferents to the superficial laminae of the rat spinal cord. J Comp Neurol. 2001;436:275–289. [PubMed] [Google Scholar]
- Hyatt SH, Schreiber RC, Shoemaker SE, Sabe A, Reed E, Zigmond RE. Activating transcription factor 3 induction in sympathetic neurons after axotomy: response to decreased neurotrophin availability. Neuroscience. 2007;150:887–897. doi: 10.1016/j.neuroscience.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Jenkins R, McMahon SB, Bond AB, Hunt SP. Expression of c-Jun as a response to dorsal root and peripheral nerve section in damaged and adjacent intact primary sensory neurons in the rat. Eur J Neurosci. 1993;5:751–759. doi: 10.1111/j.1460-9568.1993.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Kartasova T, van de Putte P. Isolation, characterization, and UV-stimulated expression of two families of genes encoding polypeptides of related structure in human epidermal keratinocytes. Mol Cell Biol. 1988;8:2195–2203. doi: 10.1128/mcb.8.5.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Kanje M, Dahlin LB. Induction of activating transcription factor 3 after different sciatic nerve injuries in adult rats. Scand J Plast Reconstr Surg Hand Surg. 2007;41:158–166. doi: 10.1080/02844310701318288. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Cafferty WB, Gupta YK, Bacon A, Wynick D, McMahon SB, Thompson SW. Galanin knockout mice reveal nociceptive deficits following peripheral nerve injury. Eur J Neurosci. 2000;12:793–802. doi: 10.1046/j.1460-9568.2000.00967.x. [DOI] [PubMed] [Google Scholar]
- Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kim YS, Paik SK, Cho YS, Shin HS, Bae JY, Moritani M, Yoshida A, Ahn DK, Valtschanoff J, Hwang SJ, Moon C, Bae YC. Expression of P2X3 receptor in the trigeminal sensory nuclei of the rat. J Comp Neurol. 2008;506:627–639. doi: 10.1002/cne.21544. [DOI] [PubMed] [Google Scholar]
- Kobayashi NR, Fan DP, Giehl KM, Bedard AM, Wiegand SJ, Tetzlaff W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmura E, Yuguchi T, Yoshimine T, Fujinaka T, Koseki N, Sano A, Kishino A, Nakayama C, Sakaki T, Nonaka M, Takemoto O, Hayakawa T. BDNF atelocollagen mini-pellet accelerates facial nerve regeneration. Brain Res. 1999;849:235–238. doi: 10.1016/s0006-8993(99)02163-0. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left is doing? Trends Neurosci. 1999;22:122–127. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- Kool J, Hamdi M, Cornelissen-Steijger P, van der Eb AJ, Terleth C, van DH. Induction of ATF3 by ionizing radiation is mediated via a signaling pathway that includes ATM, Nibrin1, stress-induced MAPkinases and ATF-2. Oncogene. 2003;22:4235–4242. doi: 10.1038/sj.onc.1206611. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99:3246–3251. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leah JD, Herdegen T, Bravo R. Selective expression of Jun proteins following axotomy and axonal transport block in peripheral nerves in the rat: evidence for a role in the regeneration process. Brain Res. 1991;566:198–207. doi: 10.1016/0006-8993(91)91699-2. [DOI] [PubMed] [Google Scholar]
- Lee MK, Rebhun LI, Frankfurter A. Posttranslational modification of class III beta-tubulin. Proc Natl Acad Sci U S A. 1990;87:7195–7199. doi: 10.1073/pnas.87.18.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linda H, Piehl F, Dagerlind A, Verge VM, Arvidsson U, Cullheim S, Risling M, Ulfhake B, Hokfelt T. Expression of GAP-43 mRNA in the adult mammalian spinal cord under normal conditions and after different types of lesions, with special reference to motoneurons. Exp Brain Res. 1992;91:284–295. doi: 10.1007/BF00231661. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, O’Brien JA, Li M, Smith AG, Murphy LJ, Hunt SP. A Schwann cell mitogen accompanying regeneration of motor neurons. Nature. 1997;390:614–618. doi: 10.1038/37615. [DOI] [PubMed] [Google Scholar]
- Lorenzo LE, Ramien M, St LM, De KY, Ribeiro-da-Silva A. Postnatal changes in the Rexed lamination and markers of nociceptive afferents in the superficial dorsal horn of the rat. J Comp Neurol. 2008;508:592–604. doi: 10.1002/cne.21691. [DOI] [PubMed] [Google Scholar]
- Lu CR, Hwang SJ, Phend KD, Rustioni A, Valtschanoff JG. Primary afferent terminals that express presynaptic NR1 in rats are mainly from myelinated, mechanosensitive fibers. J Comp Neurol. 2003;460:191–202. doi: 10.1002/cne.10632. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Marklund N, Fulp CT, Shimizu S, Puri R, McMillan A, Strittmatter SM, McIntosh TK. Selective temporal and regional alterations of Nogo-A and small proline-rich repeat protein 1A (SPRR1A) but not Nogo-66 receptor (NgR) occur following traumatic brain injury in the rat. Exp Neurol. 2006;197:70–83. doi: 10.1016/j.expneurol.2005.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MR, Lieberman AR, Grenningloh G, Anderson PN. Transcriptional upregulation of SCG10 and CAP-23 is correlated with regeneration of the axons of peripheral and central neurons in vivo. Mol Cell Neurosci. 2002;20:595–615. doi: 10.1006/mcne.2002.1140. [DOI] [PubMed] [Google Scholar]
- Meiri KF, Pfenninger KH, Willard MB. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones. Proc Natl Acad Sci U S A. 1986;83:3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Kose A, Tsujino T, Tanaka C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J Comp Neurol. 1990;299:167–177. doi: 10.1002/cne.902990204. [DOI] [PubMed] [Google Scholar]
- Osborne SL, Latham CF, Wen PJ, Cavaignac S, Fanning J, Foran PG, Meunier FA. The Janus faces of botulinum neurotoxin: sensational medicine and deadly biological weapon. J Neurosci Res. 2007;85:1149–1158. doi: 10.1002/jnr.21171. [DOI] [PubMed] [Google Scholar]
- Petersen M, Eckert AS, Segond von BG, Heppelmann B, Klusch A, Kniffki KD. Plasticity in the expression of bradykinin binding sites in sensory neurons after mechanical nerve injury. Neuroscience. 1998;83:949–959. doi: 10.1016/s0306-4522(97)00465-x. [DOI] [PubMed] [Google Scholar]
- Piehl F, Arvidsson U, Johnson H, Cullheim S, Villar M, Dagerlind A, Terenius L, Hokfelt T, Ulfhake B. Calcitonin Gene-related Peptide (CGRP)-like Immunoreactivity and CGRP mRNA in Rat Spinal Cord Motoneurons after Different Types of Lesions. Eur J Neurosci. 1991;3:737–757. doi: 10.1111/j.1460-9568.1991.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Plunet W, Kwon BK, Tetzlaff W. Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J Neurosci Res. 2002;68:1–6. doi: 10.1002/jnr.10176. [DOI] [PubMed] [Google Scholar]
- Pradervand S, Yasukawa H, Muller OG, Kjekshus H, Nakamura T, St Amand TR, Yajima T, Matsumura K, Duplain H, Iwatate M, Woodard S, Pedrazzini T, Ross J, Firsov D, Rossier BC, Hoshijima M, Chien KR. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. EMBO J. 2004;23:4517–4525. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS, Bradbury EJ, Michael GJ, Lever IJ, McMahon SB. Glial cell line-derived neurotrophic factor increases calcitonin gene-related peptide immunoreactivity in sensory and motoneurons in vivo. Eur J Neurosci. 2003;18:2713–2721. doi: 10.1111/j.1460-9568.2003.03012.x. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J Neurosci. 2001;21:2651–2660. doi: 10.1523/JNEUROSCI.21-08-02651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkuhler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol. 2007;502:325–336. doi: 10.1002/cne.21311. [DOI] [PubMed] [Google Scholar]
- Saito N, Kose A, Ito A, Hosoda K, Mori M, Hirata M, Ogita K, Kikkawa U, Ono Y, Igarashi K. Immunocytochemical localization of beta II subspecies of protein kinase C in rat brain. Proc Natl Acad Sci U S A. 1989;86:3409–3413. doi: 10.1073/pnas.86.9.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin M, Zabihi S, Liu L, Wicher G, Kozlova EN. Metastasis-associated S100A4 (Mts1) protein is expressed in subpopulations of sensory and autonomic neurons and in Schwann cells of the adult rat. J Comp Neurol. 2004;473:233–243. doi: 10.1002/cne.20115. [DOI] [PubMed] [Google Scholar]
- Schreyer DJ, Skene JH. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J Neurobiol. 1993;24:959–970. doi: 10.1002/neu.480240709. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehab SA, Spike RC, Todd AJ. Do central terminals of intact myelinated primary afferents sprout into the superficial dorsal horn of rat spinal cord after injury to a neighboring peripheral nerve? J Comp Neurol. 2004;474:427–437. doi: 10.1002/cne.20147. [DOI] [PubMed] [Google Scholar]
- Shi TJ, Tandrup T, Bergman E, Xu ZQ, Ulfhake B, Hokfelt T. Effect of peripheral nerve injury on dorsal root ganglion neurons in the C57 BL/6J mouse: marked changes both in cell numbers and neuropeptide expression. Neuroscience. 2001;105:249–263. doi: 10.1016/s0306-4522(01)00148-8. [DOI] [PubMed] [Google Scholar]
- Shortland PJ, Baytug B, Krzyzanowska A, McMahon SB, Priestley JV, Averill S. ATF3 expression in L4 dorsal root ganglion neurons after L5 spinal nerve transection. Eur J Neurosci. 2006;23:365–373. doi: 10.1111/j.1460-9568.2005.04568.x. [DOI] [PubMed] [Google Scholar]
- Siegel SG, Patton B, English AW. Ciliary neurotrophic factor is required for motoneuron sprouting. Exp Neurol. 2000;166:205–212. doi: 10.1006/exnr.2000.7528. [DOI] [PubMed] [Google Scholar]
- Skene JH. Axonal growth-associated proteins. Annu Rev Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233:783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Skene JH, Willard M. Axonally transported proteins associated with axon growth in rabbit central and peripheral nervous systems. J Cell Biol. 1981;89:96–103. doi: 10.1083/jcb.89.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, Zhou FQ, Zhong J, Markus A. Signaling the pathway to regeneration. Neuron. 2002;35:13–16. doi: 10.1016/s0896-6273(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Spittaels K, Van den HC, Van DJ, Geerts H, Mercken M, Bruynseels K, Lasrado R, Vandezande K, Laenen I, Boon T, Van LJ, Vandenheede J, Moechars D, Loos R, Van LF. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J Biol Chem. 2000;275:41340–41349. doi: 10.1074/jbc.M006219200. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Nakagomi S, Kiryu-Seo S, Namikawa K, Imai Y, Ochi T, Tohyama M, Kiyama H. Expressed-sequence-tag approach to identify differentially expressed genes following peripheral nerve axotomy. Brain Res Mol Brain Res. 1999;64:34–40. doi: 10.1016/s0169-328x(98)00302-7. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Zwiers H, Lederis K, Cassar L, Bisby MA. Axonal transport and localization of B-50/GAP-43-like immunoreactivity in regenerating sciatic and facial nerves of the rat. J Neurosci. 1989;9:1303–1313. doi: 10.1523/JNEUROSCI.09-04-01303.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Kondo E, Fukuoka T, Yi D, Tsujino H, Sakagami M, Noguchi K. Differential regulation of P2X(3) mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- Van der Zee CE, Nielander HB, Vos JP, Lopes dS, Verhaagen J, Oestreicher AB, Schrama LH, Schotman P, Gispen WH. Expression of growth-associated protein B-50 (GAP43) in dorsal root ganglia and sciatic nerve during regenerative sprouting. J Neurosci. 1989;9:3505–3512. doi: 10.1523/JNEUROSCI.09-10-03505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Richardson PM, Wiesenfeld-Hallin Z, Hokfelt T. Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons. J Neurosci. 1995;15:2081–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Tetzlaff W, Richardson PM, Bisby MA. Correlation between GAP43 and nerve growth factor receptors in rat sensory neurons. J Neurosci. 1990;10:926–934. doi: 10.1523/JNEUROSCI.10-03-00926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig PJ, Subramony SH, Qin Z, McDaniel DO, Fratkin JD. Relationship between ataxin-1 nuclear inclusions and Purkinje cell specific proteins in SCA-1 transgenic mice. J Neurol Sci. 2000;174:100–110. doi: 10.1016/s0022-510x(00)00262-8. [DOI] [PubMed] [Google Scholar]
- Vogelaar CF, Hoekman MF, Gispen WH, Burbach JP. Homeobox gene expression in adult dorsal root ganglia during sciatic nerve regeneration: is regeneration a recapitulation of development? Eur J Pharmacol. 2003;480:233–250. doi: 10.1016/j.ejphar.2003.08.110. [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Wong LF, Yip PK, Battaglia A, Grist J, Corcoran J, Maden M, Azzouz M, Kingsman SM, Kingsman AJ, Mazarakis ND, McMahon SB. Retinoic acid receptor beta2 promotes functional regeneration of sensory axons in the spinal cord. Nat Neurosci. 2006;9:243–250. doi: 10.1038/nn1622. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Reynolds ML, Molander C, O’Brien C, Lindsay RM, Benowitz LI. The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury. Neuroscience. 1990;34:465–478. doi: 10.1016/0306-4522(90)90155-w. [DOI] [PubMed] [Google Scholar]
- Wujek JR, Lasek RJ. Correlation of axonal regeneration and slow component B in two branches of a single axon. J Neurosci. 1983;3:243–251. doi: 10.1523/JNEUROSCI.03-02-00243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin T, Sandhu G, Wolfgang CD, Burrier A, Webb RL, Rigel DF, Hai T, Whelan J. Tissue-specific pattern of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem. 1997;272:19943–19950. doi: 10.1074/jbc.272.32.19943. [DOI] [PubMed] [Google Scholar]
- Young BA, Girard BM, Parsons RL. Neurturin suppresses injury-induced neuronal activating transcription factor 3 expression in cultured guinea pig cardiac ganglia. J Comp Neurol. 2008;508:795–805. doi: 10.1002/cne.21711. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Roslan R, Lang D, Schachner M, Lieberman AR, Anderson PN. Expression of CHL1 and L1 by neurons and glia following sciatic nerve and dorsal root injury. Mol Cell Neurosci. 2000;16:71–86. doi: 10.1006/mcne.2000.0852. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA, McLachlan EM. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the rat dorsal root ganglia after peripheral nerve transection. J Neurosci. 1996;16:2901–2911. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.