Abstract
Background. Paricalcitol and cinacalcet are common therapies for patients on haemodialysis with secondary hyperparathyroidism (SHPT). We conducted a multi-centre study in 12 countries to compare the safety and efficacy of paricalcitol and cinacalcet for the treatment of SHPT.
Methods. Patients aged ≥18 years with Stage 5 chronic kidney disease receiving maintenance haemodialysis and with intact parathyroid hormone (iPTH) 300–800 pg/mL, calcium 8.4–10.0 mg/dL (2.09–2.49 mmol/L) and phosphorus ≤6.5 mg/dL (2.09 mmol/L) were randomized within two strata defined by the mode of paricalcitol administration to treatment with paricalcitol- (intra-venous, US and Russian sites, IV stratum; oral, non-US and non-Russian sites, oral stratum) or cinacalcet-centred therapy. The primary endpoint is the proportion of patients in each treatment group who achieve a mean iPTH value of 150–300 pg/mL during Weeks 21–28 of treatment. Assuming efficacy response rates of 36 and 66% for cinacalcet and paricalcitol, respectively, and a 20% discontinuation rate, 124 subjects in each stratum were estimated to provide 81% power to detect a 30% absolute difference in the primary endpoint.
Results. Of 746 patients screened, 272 (mean age, 63 years; mean iPTH, 509 pg/mL) were randomized. Mean duration of haemodialysis at baseline was 3.7 years. Comorbidities included hypertension (90.4%), Type 2 diabetes (40.4%), congestive heart failure (17.3%), coronary artery disease (34.6%) and gastrointestinal disorders (75%).
Conclusions. The study participants are representative of a multinational cohort of patients on haemodialysis with elevated iPTH. The study results will provide valuable information on the best available treatment of SHPT in patients on haemodialysis.
Keywords: cinacalcet hydrochloride, haemodialysis, hyperparathyroidism, kidney disease, paricalcitol
Introduction
Secondary hyperparathyroidism (SHPT) is a major complication of chronic kidney disease (CKD) [1] resulting from impaired calcium and phosphate homeostasis and a lack of vitamin D receptor (VDR) activation secondary to active vitamin D deficiency [2, 3]. SHPT is characterized by increased parathyroid hormone (PTH) synthesis and secretion and progressive parathyroid gland hyperplasia [2]. Important pathogenic factors in SHPT include phosphate retention, hypocalcaemia, low levels of active vitamin D (1,25-dihydroxycholecalciferol, calcitriol) [3], high levels of fibroblast growth factor 23 (FGF23) [4], parathyroid resistance to inhibition by calcium, vitamin D [5] and FGF23 [6, 7] and skeletal resistance to PTH [3].
Severe SHPT is associated with a high risk of unfavourable clinical outcomes, particularly cardiovascular complications [1, 8] and high-turnover metabolic bone disease [3]. A strong association between vitamin D deficiency and cardiovascular disease is well established for the general population [9] and for patients on haemodialysis [10]. Other risk factors for mortality in patients on haemodialysis include hypocalcaemia, hyperphosphataemia [11] and high FGF23 [12]. Control of the levels of intact PTH (iPTH), calcium and phosphate is an important therapeutic goal in treating patients with SHPT on haemodialysis. For patients with Stage 5 CKD on haemodialysis, the 2003 guidelines of the Kidney Disease Outcomes Quality Initiative (KDOQI) of the National Kidney Foundation recommend specific targets for these parameters, including serum iPTH concentrations of 150–300 pg/mL [13]. In contrast, the 2009 Kidney Disease—Improving Global Outcomes (KDIGO) guidelines recommend maintaining PTH between two and nine times the upper limit of normal [14], which corresponds to a range of 130–600 pg/mL [15]. The KDIGO guidelines further recommend that a clinically relevant trend towards marked increase (or decrease) in iPTH levels should prompt initiation of or change in therapy to avoid progression to levels outside of this range.
A variety of treatments for SHPT are currently available including non-selective (calcitriol, alfacalcidol, doxercalciferol) and selective VDR activators (paricalcitol and maxacalcitol) and the calcimimetic cinacalcet. Paricalcitol [16, 17] is approved for the treatment of patients with SHPT with Stages 3–5 of CKD, whereas cinacalcet [18] is approved for the treatment of patients with SHPT on dialysis. A drawback of calcitriol and other non-selective VDR activators is that high doses may cause hypercalcaemia [19], which is associated with increased mortality in patients on haemodialysis [20]. The risk of hypercalcaemia associated with VDR activators can be reduced by using a selective VDR activator such as paricalcitol, which causes lesser hypercalcaemia due to smaller increases in intestinal calcium absorption than other VDR activators [21–23], or by using cinacalcet, which allosterically activates the calcium-sensing receptor to reduce PTH secretion [19]. Adding cinacalcet to conventional care or to low-dose VDR activator therapy in patients on haemodialysis has been shown to reduce the serum levels of calcium, phosphate, PTH [24–27] and FGF23 [28]. Randomized controlled studies also demonstrated that intra-venous and oral paricalcitol effectively reduce iPTH in patients with Stage 5 CKD without significantly increasing the risk of hypercalcaemia or hyperphosphatemia [29, 30]. Data from large observational studies suggest that paricalcitol compared with calcitriol is associated with a significantly lower risk of all-cause mortality [31] and that increasing doses of paricalcitol per unit of serum PTH may provide incremental survival benefits [32].
The purpose of this international, multi-centre, randomized controlled trial is to compare the safety and efficacy of paricalcitol and cinacalcet for the treatment of SHPT in patients on haemodialysis. The primary outcome (iPTH 150–300 pg/mL) is based on the 2003 KDOQI guidelines; the 2009 KDIGO guidelines for the management of CKD-associated mineral and bone disorder [14] were not yet available at the time this study was designed. In addition, despite the recent KDIGO guidelines, much clinical practice still employs KDOQI recommendations. Here, we provide details of the study design and of the demographic and baseline characteristics of the study population.
Materials and methods
Study design
The primary objective of this 28-week, multi-centre, randomized open-label Phase 4 study (ClinicalTrials.gov identifier: NCT00977080) is to compare the efficacy of paricalcitol-centred therapy and that of cinacalcet-centred therapy in achieving the KDOQI goal for iPTH in subjects on haemodialysis with SHPT. The study design stipulates randomization and separate analysis within two strata defined by the mode of paricalcitol administration (intra-venous at US and Russian sites, IV stratum; oral at non-US and non-Russian sites, oral stratum). The primary efficacy analysis, conducted separately in each stratum, will compare the proportion of patients in each treatment group who achieve a mean iPTH value of 150–300 pg/mL during Weeks 21–28 of the treatment period. Secondary efficacy analyses will compare the proportions of patients who achieve at least a 30 or 50% reduction from baseline in iPTH and the proportions with a mean calcium <8.4 mg/dL (2.09 mmol/L) or >10.5 mg/dL (2.61 mmol/L) during Weeks 21–28. Safety will be evaluated through adverse events reporting and assessment of changes in complete chemistry and haematology measurements and vital signs. Additional laboratory tests will be conducted to assess changes in FGF23, bone-specific alkaline phosphatase (BSAP), alkaline phosphatase, phosphate, PTH-related peptide and 25-hydroxy vitamin D. Ibis testing, which combines polymerase chain reaction and mass spectrometry to detect DNA in biological samples, will be used to identify the presence of viral and microbial pathogens [33].
For each study site, the protocol was approved by an Institutional Review Board or Independent Ethics Committee. The study is being conducted in accordance with the protocol, International Conference on Harmonization guidelines, applicable regulations and guidelines governing clinical study conduct and the ethical principles established in the Declaration of Helsinki. All study participants provided written informed consent before undergoing any study-related procedures.
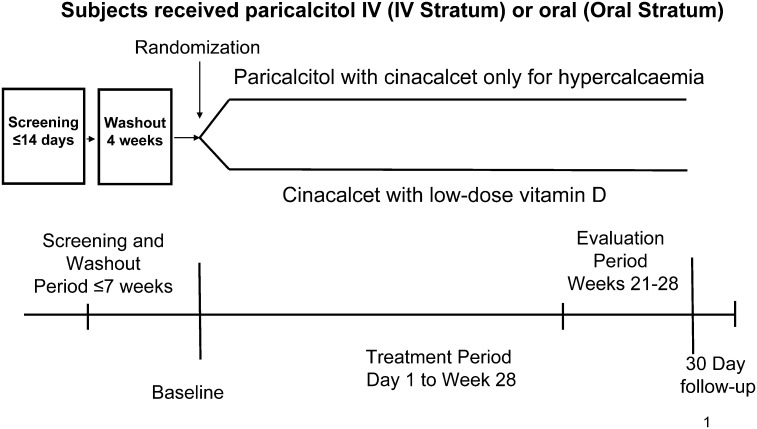
Enrolment has been completed. After an initial screening period, eligible participants underwent a 4-week washout period during which they discontinued prior VDR activator and cinacalcet therapy and after which they were re-evaluated for eligibility (Figure 1). A stratified randomization scheme was used. Random assignment at a ratio of 1:1 to paricalcitol or cinacalcet occurred within each of two strata defined by the mode of paricalcitol administration (oral or intra-venous). The randomization schedule was generated by the Clinical Statistics Department at Abbott Laboratories (Abbott Park, IL) before the start of the study. Treatment group assignment occurred at the Day 1 visit and was implemented through an interactive voice response system.
Fig. 1.
Study design. Paricalcitol arm: paricalcitol intra-venous (US and Russian sites) or oral (non-US and non-Russian sites) with cinacalcet for hypercalcaemia, if necessary. Cinacalcet arm: cinacalcet plus low-dose vitamin D.
Randomized patients receive one of two treatments for 28 weeks (Figure 1). Patients assigned to paricalcitol will receive intra-venous paricalcitol (US and Russian sites) or oral paricalcitol (non-US and non-Russian sites). The mean absolute bioavailability of paricalcitol capsules under low-fat diet conditions ranges from 72 to 86% in healthy subjects and in CKD Stage 5 patients on haemodialysis.
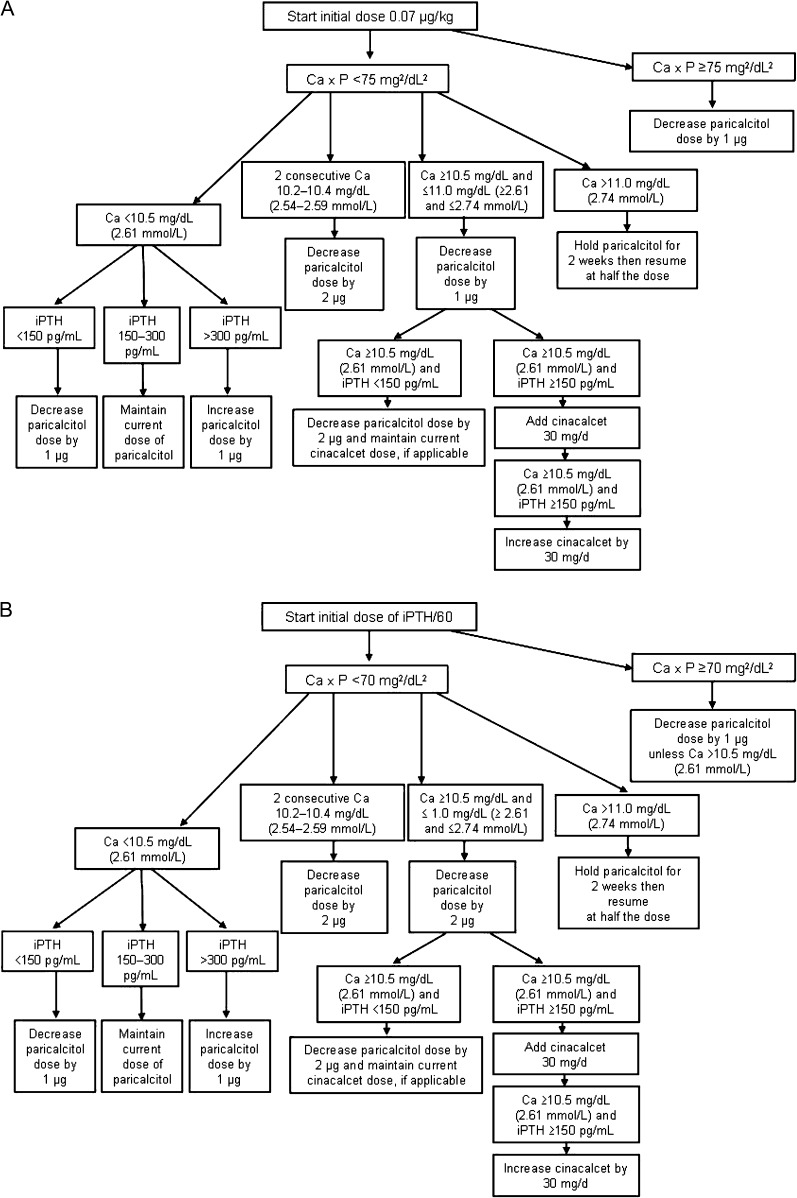
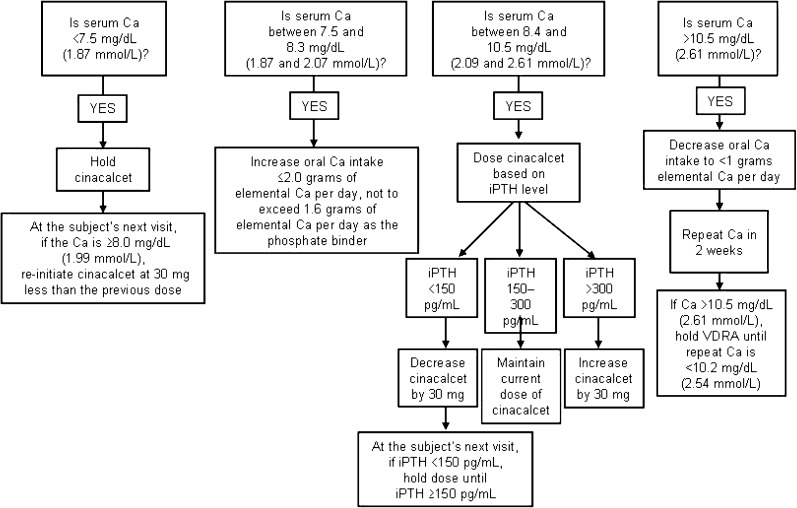
In the paricalcitol arm, cinacalcet will be administered as supplemental medication if serum calcium is ≥10.5 mg/dL (2.61 mmol/L) in samples of two consecutive blood tests. Doses of paricalcitol will be adjusted as shown in Figure 2. Patients assigned to the cinacalcet arm will receive cinacalcet and intra-venous doxercalciferol 1.0 μg three times weekly (US sites) or cinacalcet and oral alfacalcidol 0.25 μg/d (non-US sites). Planned dose adjustments for cinacalcet are shown in Figure 3. Dose adjustments will be based on chemistry results determined every 2 weeks until study completion during Week 28 or early termination.
Fig. 2.
Dose adjustments in the paricalcitol arm for sites inside (A) and outside (B) the USA and Russia. Ca, calcium; Ca × P, calcium–phosphorus product. (Adherence to the dosing algorithms is mandatory unless the investigator feels there is a risk to subject safety.)
Fig. 3.
Dose adjustments in the cinacalcet arm. Ca, calcium. (Adherence to the dosing algorithms is mandatory unless the investigator feels there is a risk to subject safety.)
Study population
Eligible patients were aged ≥18 years with Stage 5 CKD who had been receiving maintenance haemodialysis three times weekly for at least 3 months before screening and were to continue haemodialysis during the study. At screening, eligible patients had to have a serum iPTH 130–700 pg/mL, total alkaline phosphatase ≥40 U/L, calcium ≤10.0 mg/dL (2.49 mmol/L) and calcium–phosphorus product (Ca × P) ≤75 mg2/dL2 for US centres or ≤70 mg2/dL2 for non-US centres (based on recommendations in the prescribing information for the study drugs). Required pre-randomization values were iPTH 300–800 pg/mL, calcium 8.4–10.0 mg/dL (2.09–2.49 mmol/L) and phosphorus ≤6.5 mg/dL (2.09 mmol/L). Key exclusion criteria were allergic reaction or significant sensitivity to any study drug, an expected daily requirement of >2.0 g of oral elemental calcium, previous parathyroidectomy, chronic gastrointestinal disorders, clinically significant liver disease and use of known inhibitors or inducers of cytochrome P450 3A or of drugs metabolized by cytochrome P450 2D6 within 2 weeks before study drug administration.
Clinical trial simulations
Clinical trial simulations (Trial Simulator Version 2.2.1; Pharsight Corporation, Mountain View, CA) were performed to predict the efficacy of paricalcitol with regard to the primary endpoint. The results of the simulations justified the design of this comparative study and provided a basis for statistical power estimation.
The simulations used parameter estimates from a proprietary paricalcitol population exposure–clinical response model to identify a study design that would maximize safety and efficacy in meeting KDOQI targets for iPTH while remaining consistent with prescribing information recommendations for intra-venous (US and Russian sites) and oral (all other sites) paricalcitol. Each simulated clinical trial included 200 subjects on haemodialysis with SHPT who were randomized 1:1 to receive intra-venous or oral paricalcitol or placebo administered three times weekly. Overall, five simulated clinical trials were conducted, providing a total of 500 subjects per treatment arm. All enrolled subjects were assumed to be fully compliant with the assigned dosing and sampling schemes. The proportions of subjects predicted to achieve a mean iPTH value of 150–300 pg/mL and a mean calcium value of 8.4–10.5 mg/dL during Weeks 21–28 of treatment were summarized as the average of the five replicated clinical trials. The proportion of subjects experiencing two consecutive events of calcium ≥10.5 mg/dL also was predicted. These subjects would be eligible for addition of cinacalcet.
Determination of sample size
A point estimate of 25% for the proportion of cinacalcet patients who achieve the primary endpoint (an iPTH value of 150–300 pg/mL) was obtained from published results of the ACHIEVE study [26]. The upper limit of an exact 95% confidence interval for the 25% point estimate of cinacalcet subjects achieving the primary endpoint was 36%. Clinical trial simulation for paricalcitol-based therapy predicted an efficacy response rate ≥66% for the primary endpoint. Assuming efficacy response rates of 36 and 66% for the cinacalcet and paricalcitol groups, respectively, and allowing for a discontinuation rate of 20%, a sample size of 124 subjects per stratum was estimated to provide 81% power, with a Fisher’s exact test at a 0.050 two-sided significance level, to detect a 30% absolute difference between treatment groups in the proportion of patients who reach the primary efficacy endpoint.
Statistical and analytical methods
Data were summarized for all randomized subjects. Quantitative demographic and baseline laboratory variables were summarized with mean and SD. Rates and proportions were summarized with number of patients and percentage. Correlations between baseline FGF23 and baseline age, iPTH, calcium, phosphorus, alkaline phosphatase, BSAP, 25-hydroxy vitamin D and albumin were evaluated using Spearman rank correlation coefficient.
iPTH was measured by IMMULITE® chemiluminescent assay system (Siemens, Deerfield, IL) with a linear range of 3–2500 pg/mL. FGF23 was measured by an enzyme-linked immunosorbent assay for human intact FGF 23 (Immutopics, Inc., San Clemente, CA). Alkaline phosphatase concentrations were determined by enzymatic assay using p-nitrophenyl phosphate hydrolysis (Roche Diagnostics, Indianapolis, IN). BSAP was assayed after immune capture with selective high-affinity antibodies (Microvue™ BAP EIA kit; Quidel Corporation, San Diego, CA).
Patient-reported outcomes
To compare the effects of the two different treatments on kidney disease-related quality of life (KDQOL), the study uses the KDQOL instrument, which has been used extensively in the dialysis population [34–36]. The KDQOL questionnaire includes 36 items (RAND-36; Rand Corporation, Santa Monica, CA) identical to those included in the Short Form-36. Eight scales of self-reported health status (physical function, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) are scored 0–100, with higher scores indicate better function. Two normalized scores representing overall physical and mental functioning are calculated by using the dimensions related to physical and mental functioning, respectively. KDQOL also includes a symptom score for several kidney disease-specific symptoms. In addition to KDQOL, bone pain is assessed using a bone pain visual analogue scale of 0–100 (with higher scores indicating worse pain). Patients are asked to record their worst pain experienced in the past 4 weeks. Patients with missing data for a specific analysis were excluded from that analysis. Analyses were conducted using SAS Version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 746 patients screened, 272 were enrolled in the study (Table 1). Of 578 patients eligible before washout, 306 (53%) failed to meet eligibility criteria after washout. Most of those who failed to enter the treatment phase had high (21%) or low (41%) iPTH levels or a low calcium level (20%) (Table 1).
Table 1.
Screening/washout failures
| Screen failures, n (%) |
Washout failures, n (%) |
||
| Total entering screening | 746 (100) | Total entering washout | 578 (77) |
| Excluded after screening | 168 (23) | Excluded after washout | 306 (53) |
| Reason for failure | Reason for failure | ||
| iPTH > 700 pg/mL | 48 (29) | iPTH > 800 pg/mL | 65 (21) |
| iPTH < 130 pg/mL | 39 (23) | iPTH < 300 pg/mL | 124 (41) |
| Calcium > 10.0 mg/dL | 46 (27) | Calcium > 10 mg/dL | 22 (7) |
| Ca × P > 75 mg2/dL2 (US) or > 70 mg2/dL2 (non-US) | 19 (11) | Calcium < 8.4 mg/dL | 61 (20) |
| Serum alkaline phosphatase < 40 U/L | 21 (13) | Phosphorus > 6.5 mg/dL | 61 (20) |
Ca × P, calcium–phosphorus product.
Study participants had a median age of 63.5 years, and most participants were men (62.5%) (Table 2). Most participants were white (73.5%) or black (21.3%). Mean duration of dialysis was 3.7 years. Large majorities of patients had comorbidities, including hypertension (90%) and gastrointestinal disorders (75%), of which gastroesophageal reflux disease (18.4%) and nausea (17.6%) were the most common. Consequently, patients were receiving a variety of concomitant medications. Most patients (72.1%) received erythropoiesis-stimulating agents. Mean iPTH serum concentration was 509 pg/mL (Table 3). Participants showed substantial variations in vitamin D, FGF23 and BSAP levels (Table 3). A univariable analysis demonstrated highly significant correlations of FGF23 levels with those of iPTH, calcium, phosphorus and bone-specific phosphatase (Table 4). Scores for most patient-reported outcomes were similar for different racial groups; however, white compared with black patients had higher mean scores for work status, patient satisfaction and bone pain (visual analogue scale) and lower mean scores for effects and burden of kidney disease, cognitive function, quality of social interaction, sexual function, physical role limitations, pain, general health and emotional well-being. The overall mean scores for the majority of patient-reported outcomes (Table 5) were higher than those previously published for a global population with end-stage renal disease [37].
Table 2.
Demographic and baseline characteristicsa
| Characteristicb | Total study population (N = 272) |
| Age, years | |
| Mean ± SD | 62.9 ± 12.9 |
| Median (range) | 63.5 (22.0–97.0) |
| Females | 102 (37.5) |
| Race | |
| White | 200 (73.5) |
| Black | 58 (21.3) |
| Asian | 6 (2.2) |
| Other | 8 (2.9) |
| Medical history | |
| Hypertension | 246 (90.4) |
| Diabetes mellitus Type 1 | 10 (3.7) |
| Diabetes mellitus Type 2 | 110 (40.4) |
| Congestive heart failure | 47 (17.3) |
| Coronary artery disease | 94 (34.6) |
| Cerebral vascular accident | 24 (8.8) |
| Gastrointestinal disorders | 204 (75.0) |
| Duration of dialysis, years, mean ± SD | 3.7 ± 3.4 |
| Blood pressure, mean ± SD | |
| Systolic, mmHg | 140.4 ± 23.2 |
| Diastolic, mmHg | 73.4 ± 13.6 |
| Concomitant medication history | |
| Phosphate binders | |
| Calcium containing | |
| Calcium acetate | 62 (22.8) |
| Calcium carbonate | 38 (14.0) |
| Non-calcium containing | |
| Lanthanum | 47 (17.3) |
| Sevelamer | 131 (48.2) |
| Anti-hypertensives | |
| ACE inhibitors | 65 (23.9) |
| Angiotensin II receptor blockers | 47 (17.3) |
| Beta blockers | 131 (48.2) |
| Calcium channel blockers | 97 (35.7) |
| Diuretics | 66 (24.3) |
| Anti-adrenergic, central | 27 (9.9) |
| Anti-adrenergic, peripheral | 21 (7.7) |
| Erythropoiesis-stimulating agents | 196 (72.1) |
| Neuropsychiatric | 88 (32.4) |
ACE, angiotensin-converting enzyme.
Values shown are n (%) unless otherwise specified.
Table 3.
Baseline laboratory values
| Total study population (N = 272) |
||
| Variable | Mean ± SD | Median (range) |
| 25-hydroxy vitamin D, ng/mL | 19.2 ± 10.8 | 16.5 (7.0–57.0) |
| Corrected calcium, mg/dL | 9.0 ± 0.6 | 9.0 (5.1–10.8) |
| Phosphorus, mg/dL | 4.8 ± 1.1 | 4.7 (1.3–9.3) |
| Serum iPTH, pg/mL | 509.0 ± 147.7 | 488.0 (130.0–794.0) |
| Fibroblast growth factor 23, pg/mL | 375.8 ± 551.2 | 146.3 (8.0–4418.8) |
| Alkaline phosphatase, IU/L | 110.0 ± 46.6 | 100.0 (34.0–368.0) |
| BSAP, U/L | 41.6 ± 24.5 | 35.9 (3.3–274.0) |
| Creatinine, mg/dL | 8.6 ± 2.6 | 8.5 (2.7–16.7) |
| Albumin, g/dL | 4.1 ± 0.3 | 4.1 (2.7–4.7) |
Table 4.
Correlation between FGF23 and continuous baseline variables
| Predictor variable | Spearman correlation coefficient | P-value |
| Age, years | −0.1479 | 0.024 |
| iPTH | 0.2161 | <0.001 |
| Calcium | 0.3431 | <0.001 |
| Phosphorous | 0.4944 | <0.001 |
| Alkaline phosphatase | −0.1339 | 0.041 |
| BSAP | −0.2074 | 0.001 |
| 25-hydroxy vitamin D | −0.0284 | 0.703 |
| Albumin | −0.0460 | 0.485 |
Table 5.
Baseline patient-reported outcomes scores
| Scale, variable | Score, mean ± SD | n | Score by race, mean ± SD |
||
| White | Black | Other | |||
| KDQOL | |||||
| Symptoms/problems | 79.4 ± 14.5 | 256 | 79 ± 14.6 | 80.8 ± 12.1 | 80 ± 21 |
| Effects of kidney disease | 69.8 ± 21.2 | 256 | 68.1 ± 21.8 | 75.1 ± 15.1 | 73.5 ± 29 |
| Burden of kidney disease | 45.9 ± 27 | 255 | 43.4 ± 26.5 | 55 ± 26.7 | 45.2 ± 29.2 |
| Work status | 25.8 ± 32.7 | 250 | 27.2 ± 33.4 | 18.3 ± 28.1 | 35.7 ± 36.3 |
| Cognitive function | 77.4 ± 26.9 | 256 | 74.6 ± 28.8 | 87.2 ± 14.7 | 77.6 ± 28 |
| Quality of social interaction | 76.7 ± 25.6 | 257 | 73.6 ± 27.8 | 87 ± 13.8 | 79 ± 19.4 |
| Sexual function | 70.3 ± 32.8 | 116 | 67.7 ± 33 | 80 ± 29.8 | 66.1 ± 38.7 |
| Sleep | 64 ± 19.8 | 256 | 63.9 ± 21.1 | 63.6 ± 14.9 | 67.3 ± 17.6 |
| Social support | 77.3 ± 26.4 | 254 | 76 ± 27.5 | 82.7 ± 20.3 | 75 ± 29.8 |
| Dialysis staff encouragement | 81.4 ± 19.6 | 256 | 82.1 ± 20.2 | 80 ± 16.3 | 76.8 ± 23.4 |
| Overall health rating | 59.4 ± 19.4 | 253 | 58.6 ± 19.6 | 59.6 ± 17.7 | 70 ± 20.8 |
| Patient satisfaction rating | 75.6 ± 19 | 255 | 77.7 ± 18.3 | 70.1 ± 18.6 | 66.7 ± 23.6 |
| Physical composite score | 37.5 ± 10.7 | 253 | 37.1 ± 10.9 | 38.2 ± 9.6 | 39.6 ± 11.2 |
| Mental composite score | 51.7 ± 10.4 | 253 | 51.3 ± 10.6 | 53.5 ± 9.8 | 50.8 ± 10.2 |
| Physical functioning | 55.7 ± 28.6 | 257 | 55.7 ± 29.2 | 53.6 ± 26.6 | 63.9 ± 29.1 |
| Role limitations, physical | 51.1 ± 43.3 | 255 | 48.4 ± 42.8 | 55.5 ± 44 | 71.4 ± 43.7 |
| Pain | 67.6 ± 26.4 | 256 | 65.7 ± 27.5 | 75.8 ± 20.5 | 61.6 ± 26.3 |
| General health | 47.3 ± 21.1 | 257 | 46.4 ± 21.6 | 51.2 ± 19.7 | 45.4 ± 18.7 |
| Emotional well-being | 75.7 ± 20.1 | 256 | 74.4 ± 20.3 | 81.4 ± 17.7 | 72 ± 23.5 |
| Role limitations, emotional | 72.7 ± 39.8 | 255 | 71.3 ± 40.2 | 73.6 ± 40.5 | 88.1 ± 28.1 |
| Social function | 74.9 ± 25.3 | 257 | 75.2 ± 25.7 | 76.2 ± 23 | 65.2 ± 27.8 |
| Energy/fatigue | 53.1 ± 23.4 | 257 | 52.5 ± 23.9 | 54.5 ± 21.8 | 56.9 ± 22.5 |
| Bone pain visual analogue scale | 27.1 ± 27.9 | 248 | 29.1 ± 28.5 | 21.2 ± 26 | 20.3 ± 22.2 |
Discussion
The purpose of our study is to compare the efficacy of paricalcitol- versus cinacalcet-centred therapy in maintaining KDOQI targets for iPTH, calcium and phosphate in subjects on haemodialysis [13]. The doses selected for the study drugs are consistent with those indicated for dialysis patients with SHPT in the package inserts for paricalcitol injection [17], paricalcitol capsules [16] and cinacalcet tablets/capsules [18]. The doses of doxercalciferol and alfacalcidol are based on the dose equivalencies previously suggested for low-dose VDR activators in the ACHIEVE study [26].
Our clinical trial simulations predicted that a study duration of 28 weeks with a 4-week washout period will allow a majority of subjects in the paricalcitol arms to achieve a PTH value of 150–300 pg/mL and the KDOQI target for calcium. Approximately 85–95% of participants receiving paricalcitol are expected to complete the study without requiring cinacalcet supplementation for hypercalcaemia and >90% are predicted to maintain serum calcium of 8.5–10.5 mg/dL during the assessment phase.
The safety and efficacy of paricalcitol in the treatment of SHPT are well established, and observational studies have shown that VDR activators may be associated with improved survival [31, 38, 39]. Similarly, results of a large retrospective data analysis suggest that treatment of patients on haemodialysis with cinacalcet is associated with a significant reduction in all-cause and cardiovascular mortality [40]. This prospective study is the first that uses maximized and pre-specified doses in a direct comparison of the safety and efficacy of these two primary approaches to SHPT therapy in patients on haemodialysis. However, this study will not provide information regarding the long-term outcomes associated with each treatment.
The study has a number of secondary objectives. Of particular importance is the evaluation of changes in bone and mineral metabolic markers to gain a better understanding of the differential effects of paricalcitol- and cinacalcet-centred therapies on these markers. In a 3-year cohort study of 73 960 patients on haemodialysis, high serum concentrations of alkaline phosphatase, particularly those >120 U/L, were associated with increased risk of all-cause mortality [41]. Correlation analyses further suggest that elevated serum alkaline phosphatase is associated with low bone mineral density [42] and increased coronary artery calcification [43] in these patients. KDIGO has recommended monitoring BSAP in patients with CKD for the diagnostic evaluation of metabolic bone disease [14, 15]. Results of placebo-controlled Phase 3 studies have provided evidence that both paricalcitol and cinacalcet significantly reduce BSAP in patients with SHPT on dialysis [24, 30].
FGF23 is a key regulator of phosphate homeostasis that reaches extremely high circulating concentrations in patients with end-stage renal disease [4]. High levels of FGF23 were associated independently with increased risk of death in patients beginning haemodialysis [12] and with cardiovascular events in patients with pre-dialysis CKD [44]. VDR activators have been shown to increase FGF23 levels in vitro [45]. In addition, results from a recent prospective clinical study showed that treatment with calcitriol or doxercalciferol increased FGF23 by a factor of 4 in dialysis patients with SHPT [46]. In contrast, cinacalcet has been shown to reduce FGF23 in such patients, even if they receive low-dose VDR activators at the same time [28]. However, despite the increase in FGF23 associated with VDR activator therapy, results of epidemiological studies consistently suggested that such therapy provides survival benefits for patients on haemodialysis [47], at least some of which appear to be independent of effects on calcium, phosphate and iPTH [31]. Correlation analyses of baseline data from our study suggest that PTH, calcium, phosphorus and BSAP levels each are associated with FGF23 levels in subjects on haemodialysis. Although the results of this study may help to identify the FGF23 response to different therapeutic strategies for controlling SHPT, the significance of changes in FGF23 associated with various therapies remains to be determined. Other important secondary objectives include the comparison of patient-reported outcomes (e.g. pain and quality of life) and costs associated with each treatment regimen.
Recruitment for this study was characterized by a high degree of screening and washout failures, suggesting wide intra-subject fluctuations in iPTH and calcium levels. Significant intra-individual variance in iPTH and other markers of bone mineral metabolism has been observed previously in patients on haemodialysis [48]. After discontinuing treatment for SHPT, a large percentage (41%) of patients in our study did not experience the increase in iPTH necessary to meet the eligibility criterion for entering the treatment phase. The frequently observed decrease in calcium was most likely secondary to discontinuation of VDR activator therapy.
Participants are mostly older subjects with significant co-morbidities, particularly hypertension, diabetes and cardiovascular disease, who require a variety of concomitant medications, thus representing a cohort of real-life patients with CKD on haemodialysis. Participants include a large percentage of female and non-white patients, reflecting appropriate gender balance and ethnic diversity in this multinational study. Apart from the inclusion criteria, patients showed substantial variation in laboratory parameters, particularly vitamin D, FGF23 and BSAP. Therefore, the results of this study are likely to be applicable to a diverse population of subjects with SHPT on haemodialysis.
Conclusion
This ongoing study comparing the efficacy and safety of paricalcitol and cinacalcet in an international diverse study population will provide valuable information to determine the best available treatment for achieving biochemical control of SHPT in patients on haemodialysis. Planned analyses of bone and mineral metabolic markers such as FGF23, alkaline phosphatase, BSAP, calcium and phosphorus are expected to provide further information regarding the effects of the different treatments on these markers. In addition, the study will provide information for each treatment regarding its costs and effects on patient-reported outcomes.
Acknowledgments
The IMPACT study was funded by Abbott Laboratories, Inc. Writing and editorial assistance, funded by Abbott Laboratories, Inc., was provided by Roland Tacke, PhD, Marsha Hall and Colleen Hedge of Scientific Connexions, Newtown, PA. The sponsor was involved in the study design, collection and analysis of the data and in writing the report. Authors had access to the study data and the lead author attests for data accuracy. The lead author had the final decision to submit the publication. The study was overseen by an executive committee.
Transparency declaration: M.K. has received honoraria for speaking and advisory tasks from Abbott, Amgen, Fresenius Medical Care, Genzyme, Medice and Shire and has received research funding from Abbott and Amgen. K.J.M. has been a consultant for Abbott, Cytochroma, Kai and Shire, and a speaker for Abbott and Genzyme. M.C. has received honoraria from Abbott, Amgen, Shire, Genzyme and Roche. D.G. has received speaker fees from and has been a consultant for Abbott, Amgen, Novartis, Genzyme, Fresenius Medical Care and Shire. A.S. has been a consultant for Amgen, Genzyme and Abbott, has been a speaker for Amgen and Genzyme and has received research funding from Amgen. S.K., E.D., M.A., S.M. are employees of Abbott and may own Abbott stock or options. P.A. was an employee of Abbott and may own Abbott stock or options and is currently employed by Reata Pharmaceuticals.
Conflict of interest statement. None declared.
References
- 1.Joy MS, Karagiannis PC, Peyerl FW. Outcomes of secondary hyperparathyroidism in chronic kidney disease and the direct costs of treatment. J Manag Care Pharm. 2007;13:397–411. doi: 10.18553/jmcp.2007.13.5.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez M, Nemeth E, Martin D. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol Renal Physiol. 2005;288:F253–F264. doi: 10.1152/ajprenal.00302.2004. [DOI] [PubMed] [Google Scholar]
- 3.Martin KJ, Gonzalez EA. Metabolic bone disease in chronic kidney disease. J Am Soc Nephrol. 2007;18:875–885. doi: 10.1681/ASN.2006070771. [DOI] [PubMed] [Google Scholar]
- 4.Juppner H, Wolf M, Salusky IB. FGF-23: more than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25:2091–2097. doi: 10.1002/jbmr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsenfeld AJ, Rodriguez M, Aguilera-Tejero E. Dynamics of parathyroid hormone secretion in health and secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2007;2:1283–1305. doi: 10.2215/CJN.01520407. [DOI] [PubMed] [Google Scholar]
- 6.Komaba H, Goto S, Fujii H, et al. Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int. 2010;77:232–238. doi: 10.1038/ki.2009.414. [DOI] [PubMed] [Google Scholar]
- 7.Galitzer H, Ben-Dov IZ, Silver J, et al. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77:211–218. doi: 10.1038/ki.2009.464. [DOI] [PubMed] [Google Scholar]
- 8.Hagstrom E, Hellman P, Larsson TE, et al. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 9.Cozzolino M, Ketteler M, Zehnder D. The vitamin D system: a crosstalk between the heart and kidney. Eur J Heart Fail. 2010;12:1031–1041. doi: 10.1093/eurjhf/hfq112. [DOI] [PubMed] [Google Scholar]
- 10.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–592. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Kidney Foundation/KDOQI. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. New York, NY: National Kidney Foundation, Inc; 2003. http://www.kidney.org/professionals/KDOQI/guidelines_bone/index.htm (1 October 2010, date last accessed) [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009;76(Suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 15.Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the Diagnosis, evaluation, and treatment of CKD-mineral and bone disorder (CKD-MBD) Am J Kidney Dis. 2010;55:773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 16.Zemplar® (Paricaclitol) Capsules [Prescribing Information] North Chicago, IL: Abbott Laboratories; 2010. http://www.rxabbott.com/pdf/Zemplarcappi.pdf (1 October 2010, date last accessed) [Google Scholar]
- 17.Zemplar (Paricalcitol Injection) Injection, Solution [Prescribing Information] North Chicago, IL: Abbott Laboratories; 2009. http://www.rxabbott.com/pdf/Zemplarivpi.pdf (1 October 2010, date last accessed) [Google Scholar]
- 18.Sensipar® (Cinacalcet) Tablets [Prescribing Information] Thousand Oaks, CA: Amgen Inc; 2010. http://pi.amgen.com/united_states/sensipar/sensipar_pi_hcp_english.pdf (1 October 2010, date last accessed) [Google Scholar]
- 19.Drueke TB, Ritz E. Treatment of secondary hyperparathyroidism in CKD patients with cinacalcet and/or vitamin D derivatives. Clin J Am Soc Nephrol. 2009;4:234–241. doi: 10.2215/CJN.04520908. [DOI] [PubMed] [Google Scholar]
- 20.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 21.Abdul Gafor AH, Saidin R, Loo CY, et al. Intravenous calcitriol versus paricalcitol in haemodialysis patients with severe secondary hyperparathyroidism. Nephrology (Carlton) 2009;14:488–492. doi: 10.1111/j.1440-1797.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 22.Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63:1483–1490. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 23.Lund RJ, Andress DL, Amdahl M, et al. Differential effects of paricalcitol and calcitriol on intestinal calcium absorption in hemodialysis patients. Am J Nephrol. 2010;31:165–170. doi: 10.1159/000266204. [DOI] [PubMed] [Google Scholar]
- 24.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350:1516–1525. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 25.Block GA, Zeig S, Sugihara J, et al. Combined therapy with cinacalcet and low doses of vitamin D sterols in patients with moderate to severe secondary hyperparathyroidism. Nephrol Dial Transplant. 2008;23:2311–2318. doi: 10.1093/ndt/gfn026. [DOI] [PubMed] [Google Scholar]
- 26.Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol. 2008;3:1718–1725. doi: 10.2215/CJN.01040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messa P, Macario F, Yaqoob M, et al. The OPTIMA study: assessing a new cinacalcet (Sensipar/Mimpara) treatment algorithm for secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2008;3:36–45. doi: 10.2215/CJN.03591006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wetmore JB, Liu S, Krebill R, et al. Effects of cinacalcet and concurrent low-dose vitamin D on FGF23 levels in ESRD. Clin J Am Soc Nephrol. 2010;5:110–116. doi: 10.2215/CJN.03630509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin KJ, Gonzalez EA, Gellens M, et al. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol. 1998;9:1427–1432. doi: 10.1681/ASN.V981427. [DOI] [PubMed] [Google Scholar]
- 30.Ross EA, Tian J, Abboud H, et al. Oral paricalcitol for the treatment of secondary hyperparathyroidism in patients on hemodialysis or peritoneal dialysis. Am J Nephrol. 2008;28:97–106. doi: 10.1159/000109398. [DOI] [PubMed] [Google Scholar]
- 31.Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–456. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 32.Shinaberger CS, Kopple JD, Kovesdy CP, et al. Ratio of paricalcitol dosage to serum parathyroid hormone level and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1769–1776. doi: 10.2215/CJN.01760408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampath R, Hall TA, Massire C, et al. Rapid identification of emerging infectious agents using PCR and electrospray ionization mass spectrometry. Ann N Y Acad Sci. 2007;1102:109–120. doi: 10.1196/annals.1408.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes AA, Bragg-Gresham JL, Goodkin DA, et al. Factors associated with health-related quality of life among hemodialysis patients in the DOPPS. Qual Life Res. 2007;16:545–557. doi: 10.1007/s11136-006-9143-7. [DOI] [PubMed] [Google Scholar]
- 35.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 36.Seica A, Segall L, Verzan C, et al. Factors affecting the quality of life of haemodialysis patients from Romania: a multicentric study. Nephrol Dial Transplant. 2009;24:626–629. doi: 10.1093/ndt/gfn506. [DOI] [PubMed] [Google Scholar]
- 37.Mapes DL, Bragg-Gresham JL, Bommer J, et al. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44(5 Suppl 2):S54–S60. doi: 10.1053/j.ajkd.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 39.Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 40.Block GA, Zaun D, Smits G, et al. Cinacalcet hydrochloride treatment significantly improves all-cause and cardiovascular survival in a large cohort of hemodialysis patients. Kidney Int. 2010;78:578–589. doi: 10.1038/ki.2010.167. [DOI] [PubMed] [Google Scholar]
- 41.Regidor DL, Kovesdy CP, Mehrotra R, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JC, Kovesdy CP, Duong U, et al. Association of serum alkaline phosphatase and bone mineral density in maintenance hemodialysis patients. Hemodial Int. 2010;14:182–192. doi: 10.1111/j.1542-4758.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shantouf R, Kovesdy CP, Kim Y, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seiler S, Reichart B, Roth D, et al. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol Dial Transplant. 2010;25:3983–3989. doi: 10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 45.Tang WJ, Wang LF, Xu XY, et al. Autocrine/paracrine action of vitamin D on FGF23 expression in cultured rat osteoblasts. Calcif Tissue Int. 2010;86:404–410. doi: 10.1007/s00223-010-9355-2. [DOI] [PubMed] [Google Scholar]
- 46.Wesseling-Perry K, Pereira RC, Sahney S, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79:112–119. doi: 10.1038/ki.2010.352. [DOI] [PubMed] [Google Scholar]
- 47.Kalantar-Zadeh K, Kovesdy CP. Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1529–1539. doi: 10.2215/CJN.02140309. [DOI] [PubMed] [Google Scholar]
- 48.Gardham C, Stevens PE, Delaney MP, et al. Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol. 2010;5:1261–1267. doi: 10.2215/CJN.09471209. [DOI] [PMC free article] [PubMed] [Google Scholar]