Abstract
Background.
Measurement of individual glomerular volumes (IGV) has allowed the identification of drivers of glomerular hypertrophy in subjects without overt renal pathology. This study aims to highlight the relevance of IGV measurements with possible clinical implications and determine how many profiles must be measured in order to achieve stable size distribution estimates.
Methods.
We re-analysed 2250 IGV estimates obtained using the disector/Cavalieri method in 41 African and 34 Caucasian Americans. Pooled IGV analysis of mean and variance was conducted. Monte-Carlo (Jackknife) simulations determined the effect of the number of sampled glomeruli on mean IGV. Lin’s concordance coefficient (RC), coefficient of variation (CV) and coefficient of error (CE) measured reliability.
Results.
IGV mean and variance increased with overweight and hypertensive status. Superficial glomeruli were significantly smaller than juxtamedullary glomeruli in all subjects (P < 0.01), by race (P < 0.05) and in obese individuals (P < 0.01). Subjects with multiple chronic kidney disease (CKD) comorbidities showed significant increases in IGV mean and variability. Overall, mean IGV was particularly reliable with nine or more sampled glomeruli (RC > 0.95, <5% difference in CV and CE). These observations were not affected by a reduced sample size and did not disrupt the inverse linear correlation between mean IGV and estimated total glomerular number.
Conclusions.
Multiple comorbidities for CKD are associated with increased IGV mean and variance within subjects, including overweight, obesity and hypertension. Zonal selection and the number of sampled glomeruli do not represent drawbacks for future longitudinal biopsy-based studies of glomerular size and distribution.
Keywords: glomerular heterogeneity, glomerular size, glomerular volume
Introduction
Chronic kidney disease (CKD) is a major health problem worldwide, with glomerular pathology accounting for >90% of CKD [1]. Glomerular enlargement is a common feature in several prevalent pathologies including hypertension [2, 3], diabetes mellitus [4, 5] and obesity [6]. Indeed, human mean glomerular volume (Vglom) is inversely correlated with age [7] and total nephron number (Nglom) [7, 8] and directly correlated with body surface area (BSA) [7, 8] and glomerulosclerosis [9, 10]. Several morphometric methods have been used to estimate Vglom [11, 12, 13]. Regardless of the method used, a single estimate of glomerular volume for an entire kidney is obtained. Because the structure and function of human glomeruli varies with their location in the cortex [14, 15], and human kidneys contain on average 900 000 nephrons with a reported 13-fold range (∼210 000 to 2.7 million nephrons) [16], a single estimate of glomerular volume for a kidney provides a limited insight of glomerular dimensions.
We have previously described a method for the unbiased estimation of individual glomerular volume (IGV) [18]. The method involves disector sampling of glomeruli and volume estimation using the Cavalieri principle [12, 19]. With this approach, associations between glomerular volume heterogeneity within single kidneys and relevant clinical features can be investigated [11, 20]. Reliable non-invasive methods for in vivo measurements of IGV and its distribution in humans are unavailable, although magnetic resonance imaging methods are available for ex vivo rat kidneys [21]. Thus, human studies currently depend on renal autopsy or biopsy samples for studying the course of glomerular hypertrophy. To date, few studies have used the unbiased disector/Cavalieri combination to measure IGV in renal biopsies [22, 23], mostly due a lack of (i) understanding as to which cortical zones should be sampled; (ii) the number of glomeruli needed to sample in order to get a reliable estimate and (iii) the financial burden and time-consuming nature of the process often favours other methods [11, 24]. Our previous autopsy-based studies of IGV have sampled 30 glomeruli, 10 from each cortical zone (superficial, middle and juxtamedullary). However, it is not known whether reliable IGV estimates can be obtained when only limited tissue is available, as is the case with renal biopsies.
This study is an in silico analysis of our IGV measurements of 75 American subjects, the largest database available. We present data that supports the relevance of IGV estimates through an analysis of accepted comorbidities for CKD. To provide guidelines for future biopsy studies, we explored IGV mean and variance in different cortical zones and determined how many glomeruli must be measured to generate reliable mean IGV estimates.
Materials and methods
Tissue
Tissue was obtained at coronial autopsies performed at the University of Mississippi Medical Center (Jackson, MS) between 1998 and 2005. Permission for autopsy was obtained from next-of-kin and ethical approval was provided by the respective boards of the University of Mississippi and Monash University. Right kidneys were perfusion-fixed with 10% buffered formalin, bisected and immersed in 10% formalin. After 10 days, both halves were cut into slices 4 mm thick and every fourth slice of both halves sampled for stereology. Selected slices were sent to Monash University for analysis.
Subjects
We reviewed data from 75 American males (41 African Americans and 34 Caucasians) who were included in previous publications [17, 25, 26] then stored in our Monash University Renal Autopsy Database. Clinical data were obtained from the forensic report, emergency department and hospital records. Variables such as body mass index (BMI) and BSA were calculated based on weight and height. Subjects were also categorized as hypertensive or normotensive based on a number of different criteria including confirmed blood pressure from medical records and histopathology [2, 27].
Other sub-groups were also defined: subjects were classified as obese with a BMI ≥30.0 kg/m2 and lean with a BMI ≤24.9 kg/m2. We further defined sub-groups based on accepted comorbidities for CKD: low-risk (age ≤30 years, BMI ≤29.0 kg/m2 and no evidence of hypertension) and high-risk (age ≥40 years, BMI ≥30.0 kg/m2 and the evidence of hypertension).
Estimation of total glomerular number (Nglom)
Total nephron (glomerular) number was estimated using the physical disector/fractionator combination as previously described [8]. This is a design-based stereological method that provides unbiased estimates. Macroscopically, kidney tissue was systematically sampled, embedded in glycolmethacrylate and exhaustively sectioned at 20 μm. Section pairs were used to count glomeruli at a unique point in a known fraction of the kidney. Total glomerular number was calculated using basic algebra.
Estimation of IGV
The technique used to estimate IGV has previously been described in detail [18]. Briefly, a tissue slice (∼10 × 10 × 1 mm) containing full thickness cortex and medulla was obtained from the mid-hilar region. After glycolmethacrylate embedding [28], blocks were exhaustively sectioned at 10 μm and stained with periodic acid-Schiff. Slides were projected onto a white surface using an Olympus BH-2 microscope at a magnification of ×320. Three evenly spaced cortical zones were defined: outer (superficial) located within four glomerular diameters of the capsule; inner (juxtamedullary) located within four glomerular diameters of the arcuate vessels and mid-cortical zone as intermediate between the outer and inner zones. Thirty glomeruli (10 per zone) in each kidney were sampled using disectors [29], and their volumes estimated using the Cavalieri method [30]. Each glomerulus used for volume estimation was exhaustively sectioned at a 10-μm. The glomerular profile tuft area of every second section was measured by point counting using an orthogonal test grid (area per point = 1 cm2 at a final magnification of ×320). IGV was represented by the glomerular tuft volume and was estimated using:
where ∑Pglom is the total number of grid points overlying the glomerular tuft, t is section thickness (10 μm) and SSF is the reciprocal of the section sampling fraction, in this Case 2 because every second section was analysed.
Statistical analysis
Statistical analyses were performed using Stata (version 8), and statistical significance was defined as a P < 0.05.
The general features of variables were described in mean, SD, minimum and maximum values. To assess distribution of IGV values within subjects, the Shapiro–Wilk W test was used and skewness defined when statistical significance was observed. Student’s t-test or analysis of variance tests were performed whenever applicable. For skewed variables, Mann–Whitney and Kruskal–Wallis tests were applied. For zonal analysis, the pooled mean IGV, SD, range (maximum value minus minimum value) and variance were estimated in order to assess differences between cortical zones.
A ‘gold standard’ IGV mean value was defined from the 30 sampled glomeruli per subject (10 glomeruli per zone). Then, Monte-Carlo simulation (Jackknife) was performed in order to simulate the effect of reducing the number of sampled glomeruli (from 29 to 3 sampled glomeruli per subject). This approach provided 27 new mean IGV values, which were compared with the gold standard mean IGV. This process was repeated three times in order to compare the variability generated by random selection. Our database contains IGV values for 2250 glomeruli from 75 well-matched subjects and therefore provides a greater power than which might be achievable in many settings. In order to simulate the effect of measuring fewer glomeruli in a smaller cohort size, a random sample of 10 subjects per race was selected and the effect of the Jackknife evaluated.
The coefficient of variation (CV, 100 × SD/mean IGV) and the coefficient of error (CE, 100 × SE/mean IGV) were calculated for each mean IGV generated by Jackknife simulation. Lin’s concordance coefficient (RC) [31] was used to assess precision, accuracy and the 95% limits of agreement of mean IGV values from the Jackknife simulation, again to determine whether reliable estimates of IGV size distribution could still be achieved whilst measuring fewer glomeruli. Finally, a regression analysis was conducted between Nglom and different mean IGV values. Nglom was selected because of its previously described inverse linear relationship with mean glomerular volume [8]. We tested whether this relationship was preserved when fewer glomeruli were measured.
Results
Demographics
General demographics of this cohort are provided in Table 1. Subjects were middle-aged men with a tendency towards overweight. Renal pathology findings (such as percentages of glomerulosclerosis, hyalinosis, corticofibrosis and vascular changes) were similar in the two racial groups with no overt renal disease (data not shown). The kidneys of African Americans contained 14% more nephrons than Caucasians, although this difference was not statistically significant.
Table 1.
Demographic and stereological data for male African and Caucasian Americans (N = 75)a
| African Americans (n = 41) |
Caucasian Americans (n = 34) |
||||
| Mean | SD | Mean | SD | P | |
| Age (years) | 39.85 | 10.96 | 44.59 | 14.33 | NS |
| Height (cm) | 179.85 | 7.84 | 177.21 | 8.83 | NS |
| Weight (kg) | 95.45 | 24.31 | 94.79 | 29.11 | NS |
| BSA (m2) | 2.19 | 0.32 | 2.15 | 0.38 | NS |
| BMI (kg/m2) | 29.32 | 6.31 | 29.98 | 8.46 | NS |
| Kidney weight (g) | 189.49 | 46.86 | 196.09 | 48.21 | NS |
| Hypertension (%) | 61 | NA | 47 | NA | NS |
| Glomerular number (Nglom) | 980 706 | 350 320 | 859 538 | 315 090 | NS |
NS, P > 0.05; NA, Non-Applicable.
Glomerular size and variability
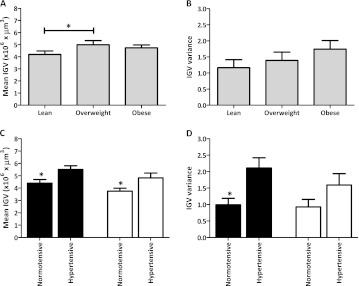
Mean IGV was larger with overweight (Figure 1A, P < 0.05) but did not increase any further in obesity (P > 0.05). However, as seen in Figure 1B, mean IGV variance progressively increased with BMI (F-test; P > 0.05). In both racial groups, hypertensives showed greater mean IGV (Figure 1C, P < 0.05). Hypertensive African Americans demonstrated ∼100% greater IGV variance than normotensives (Figure 1D, P < 0.05).
Fig. 1.
Mean IGV and variance in Caucasian and African Americans and associations with BMI (A, B) and hypertension (C, D). Black bars represent African Americans and white bars Caucasian Americans. BMI categories were defined as lean (BMI from 20.00 to 24.99 kg/m2), overweight (BMI from 25.00 to 29.99 kg/m2) and obese (BMI ≥30.00 kg/m2). *P < 0.05.
Zonal differences
Overall, African Americans presented larger glomeruli with a wider range and greater variance in all three cortical zones (see Table 2). Superficial glomeruli were significantly smaller than juxtamedullary glomeruli (all subjects, P < 0.01) and by racial group (P < 0.05). However, in lean, normotensive and low-risk subjects IGV mean and variance did not differ between the three zones. Interestingly, there was no association between zonal location and IGV mean or variance in hypertensive individuals nor did subjects at high-risk of kidney disease demonstrate location-specific changes in IGV (P > 0.05). In contrast, middle and juxtamedullary glomeruli from obese individuals, especially among obese Caucasians, showed larger and greater IGV variability (Table 2).
Table 2.
Zonal analysis comparing the mean and variance of IGVs for male African and Caucasian Americansa
| Number of glomeruli measured | Mean IGV (×106 μm3) | IGV variance (×106 μm3) | |||||
| Group | Zone | ||||||
| CA | AA | CA | AA | CA | AA | ||
| Race (n = 75) | S | 340 | 410 | 4.03 | 4.88b | 2.74 | 3.38b |
| M | 340 | 410 | 4.38 | 5.20b | 3.57 | 3.66 | |
| J | 340 | 410 | 4.28 | 5.16b | 2.61 | 4.15b | |
| Cardiovascular | NT | HTN | NT | HTN | NT | HTN | |
| AA (n = 41) | S | 160 | 250 | 4.23 | 5.29b | 2.60 | 3.46b |
| M | 160 | 250 | 4.56 | 5.61b | 2.30 | 4.11b | |
| J | 160 | 250 | 4.42 | 5.63b | 1.95 | 4.99b | |
| CA (n = 32) | S | 170 | 150 | 3.56 | 4.69b | 1.51 | 3.65b |
| M | 170 | 150 | 3.86 | 5.11b | 2.45 | 4.27b | |
| J | 170 | 150 | 4.00 | 4.70b | 1.95 | 3.22b | |
| Metabolic | Lean | Obese | Lean | Obese | Lean | Obese | |
| AA (n = 22) | S | 80 | 140 | 4.72 | 4.56 | 2.52 | 3.54b |
| M | 80 | 140 | 4.96 | 5.17 | 4.28b | 3.20 | |
| J | 80 | 140 | 4.56 | 5.21c | 3.84 | 3.56 | |
| CA (n = 26) | S | 110 | 150 | 3.85 | 4.10 | 1.85 | 2.62b |
| M | 110 | 150 | 3.83 | 4.81b | 1.17 | 3.90b | |
| J | 110 | 150 | 3.71 | 4.61b | 1.42 | 2.48b | |
| CKD comorbidities (n = 15) | Low | High | Low | High | Low | High | |
| S | 70 | 80 | 3.33 | 4.78b | 1.05 | 2.90b | |
| M | 70 | 80 | 3.24 | 5.41b | 0.68 | 2.22b | |
| J | 70 | 80 | 3.44 | 5.21b | 1.08 | 2.70b |
Group definitions: AA (African Americans), CA (Caucasian Americans), NT (mormotensive), HTN (hypertensive), lean (BMI ≤24.9 kg/m2), obese (BMI ≥30.0 kg/m2), low (age ≤30 years + BMI ≤29.9 kg/m2 + normotensive) and high (age ≥40.0 years + BMI ≥30.0 kg/m2 + hypertensive). Zones: S (superficial), M (middle) and J (juxtamedullary). Numbers indicted in bold type are statistically significant.
P < 0.001 between comparable groups and within the same zone.
P < 0.05 between comparable groups and within the same zone.
Notwithstanding the minimal effects of cortical zone, hypertensive individuals demonstrated IGVs that were significantly larger than normotensives (P < 0.001) and more heterogeneous (P < 0.001) in all three zones. A similar pattern was observed when comparing risk of kidney disease groups, where glomeruli from low-risk subjects were smaller and more homogeneous across all cortical zones than their high-risk counterparts (Table 2).
Optimizing technical efficiency
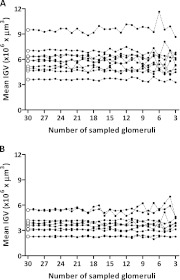
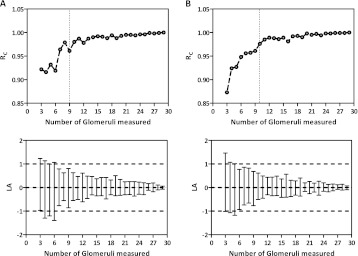
Following the Jackknife procedures to simulate the effects of sampling fewer than 30 glomeruli per subject, 27 new mean IGV values were generated for both racial groups (Table 3). Surprisingly, average IGV was barely altered by reducing the number of glomeruli measured, with little variation within and between groups. Mean IGV in African Americans (n = 41) was as stable as in Caucasian Americans (n = 34), even when the African Americans presented a larger mean IGV and more heterogeneous distribution. In African Americans, CV ranged from 29.02% (30 glomeruli measured per subject) to 29.33% (3 glomeruli measured) and CE from 4.53% (30 glomeruli measured) to 4.58% (3 glomeruli measured), whereas in Caucasian Americans, CV ranged from 32.21% (30 glomeruli measured) to 32.20% (3 glomeruli measured) and CE from 5.52% (30 glomeruli measured) to 5.52% (3 glomeruli measured). When 10 subjects were randomly sampled in each racial group, mean IGV for each subject showed little variation when more than nine glomeruli were measured per subject (Figure 2). Moreover, RC and the 95% limits of agreement were especially strong when more than nine glomeruli were measured per subject for each racial group (Figure 3).
Table 3.
Mean IGV values generated by the Monte-Carlo Jackknife simulationa
| Number of sampled glomeruli per subject | African Americans (n = 41) |
Caucasian Americans (n = 34) |
||||||||||
| Mean (×106 μm3) | SD (×106 μm3) | Min (×106 μm3) | Max (×106 μm3) | CV (%) | CE (%) | Mean (×106 μm3) | SD (×106 μm3) | Min (×106 μm3) | Max (×106 μm3) | CV (%) | CE (%) | |
| 30 | 5.08 | 1.47 | 2.91 | 9.48 | 29.02 | 4.53 | 4.23 | 1.36 | 2.27 | 9.28 | 32.21 | 5.52 |
| 29 | 5.08 | 1.48 | 2.90 | 9.50 | 29.01 | 4.53 | 4.24 | 1.37 | 2.27 | 9.28 | 32.41 | 5.56 |
| 28 | 5.07 | 1.47 | 2.92 | 9.45 | 29.06 | 4.54 | 4.22 | 1.35 | 2.27 | 9.18 | 31.97 | 5.48 |
| 27 | 5.07 | 1.45 | 2.91 | 9.26 | 28.61 | 4.47 | 4.21 | 1.35 | 2.28 | 9.20 | 32.18 | 5.52 |
| 26 | 5.05 | 1.48 | 2.83 | 9.43 | 29.26 | 4.57 | 4.23 | 1.37 | 2.25 | 9.19 | 32.34 | 5.55 |
| 25 | 5.13 | 1.52 | 2.83 | 9.30 | 29.63 | 4.63 | 4.25 | 1.40 | 2.20 | 9.33 | 32.34 | 5.63 |
| 24 | 5.11 | 1.49 | 2.89 | 9.65 | 29.24 | 4.57 | 4.21 | 1.34 | 2.29 | 9.25 | 31.73 | 5.44 |
| 23 | 5.13 | 1.52 | 2.89 | 9.64 | 29.51 | 4.61 | 4.26 | 1.42 | 2.29 | 9.38 | 33.36 | 5.72 |
| 22 | 5.10 | 1.48 | 2.88 | 9.13 | 28.97 | 4.52 | 4.23 | 1.38 | 2.27 | 9.36 | 32.58 | 5.59 |
| 21 | 5.11 | 1.47 | 2.85 | 9.64 | 28.76 | 4.49 | 4.23 | 1.37 | 2.19 | 9.09 | 32.39 | 5.55 |
| 20 | 5.07 | 1.50 | 2.85 | 9.43 | 29.54 | 4.61 | 4.21 | 1.35 | 2.27 | 9.11 | 31.92 | 5.47 |
| 19 | 5.00 | 1.46 | 2.64 | 8.85 | 29.24 | 4.56 | 4.21 | 1.38 | 2.29 | 9.49 | 32.76 | 5.62 |
| 18 | 5.07 | 1.44 | 2.91 | 9.16 | 28.49 | 4.45 | 4.25 | 1.34 | 2.17 | 8.98 | 31.60 | 5.42 |
| 17 | 5.11 | 1.49 | 2.91 | 9.95 | 29.11 | 4.55 | 4.27 | 1.41 | 2.20 | 9.23 | 33.05 | 5.67 |
| 16 | 5.04 | 1.52 | 2.68 | 9.58 | 30.20 | 4.72 | 4.15 | 1.31 | 2.31 | 9.44 | 31.56 | 5.41 |
| 15 | 5.07 | 1.50 | 2.68 | 9.59 | 29.59 | 4.62 | 4.26 | 1.40 | 2.26 | 9.24 | 32.96 | 5.65 |
| 14 | 5.00 | 1.47 | 2.73 | 9.69 | 29.47 | 4.60 | 4.16 | 1.33 | 2.26 | 9.04 | 31.98 | 5.48 |
| 13 | 5.07 | 1.51 | 2.44 | 9.16 | 29.73 | 4.64 | 4.18 | 1.35 | 2.30 | 9.19 | 32.28 | 5.54 |
| 12 | 5.07 | 1.46 | 2.97 | 10.14 | 28.83 | 4.50 | 4.17 | 1.39 | 2.24 | 9.41 | 33.26 | 5.70 |
| 11 | 5.10 | 1.58 | 2.64 | 10.06 | 31.02 | 4.85 | 4.21 | 1.44 | 2.10 | 9.42 | 34.23 | 5.87 |
| 10 | 5.04 | 1.56 | 2.77 | 9.61 | 30.89 | 4.82 | 4.18 | 1.31 | 2.37 | 8.83 | 31.30 | 5.37 |
| 9 | 5.10 | 1.59 | 2.63 | 9.84 | 31.08 | 4.85 | 4.27 | 1.32 | 2.18 | 9.14 | 30.89 | 5.30 |
| 8 | 5.04 | 1.50 | 2.69 | 9.75 | 29.84 | 4.66 | 4.15 | 1.28 | 2.22 | 9.64 | 30.87 | 5.29 |
| 7 | 5.07 | 1.52 | 2.39 | 9.24 | 29.90 | 4.67 | 4.17 | 1.42 | 1.97 | 9.67 | 33.97 | 5.83 |
| 6 | 5.24 | 1.73 | 2.94 | 11.65 | 32.92 | 5.14 | 4.31 | 1.39 | 2.25 | 10.21 | 32.15 | 5.51 |
| 5 | 5.18 | 1.61 | 2.72 | 9.53 | 31.08 | 4.85 | 4.31 | 1.58 | 2.31 | 9.31 | 36.67 | 6.29 |
| 4 | 5.16 | 1.58 | 2.77 | 9.91 | 30.54 | 4.77 | 4.23 | 1.43 | 2.19 | 9.40 | 33.77 | 5.79 |
| 3 | 4.95 | 1.45 | 2.64 | 8.83 | 29.33 | 4.58 | 4.00 | 1.29 | 2.26 | 9.52 | 32.20 | 5.52 |
Number of sampled glomeruli per subject, number of glomeruli randomly sampled by the Monte-Carlo simulation; min, minimum volume (×106 μm3); max, maximum volume (×106 μm3); CV (%), coefficient of variation (100 × SD/mean); CE (%): coefficient of error (100 × SE/mean).
Fig. 2.
The effect of reduced glomerular sampling (through Jackknife simulation) on mean IGV values. Each dot represents one mean IGV value; dots united by a non-continuous line correspond to one subject. (A) (African Americans, n = 10 subjects) and (B) (Caucasian Americans; n = 10 subjects) represent the variation between mean IGV generated with 30 sampled glomeruli (open circles at the beginning of each horizontal line) and Jackknife generated values (black dots; from 29 to 3 sampled glomeruli per subject).
Fig. 3.
Lin’s concordance coefficient (RC) and its respective limits of agreement (LA, 95% CI) for different mean IGV values generated by the Jackknife in African Americans (Column A, n = 41) and Caucasian Americans (Column B, n = 34). The horizontal lines (----) illustrate 2 SD or ±1 × 106 μm3 and the vertical Lines (----) show the perfect agreement for RC.
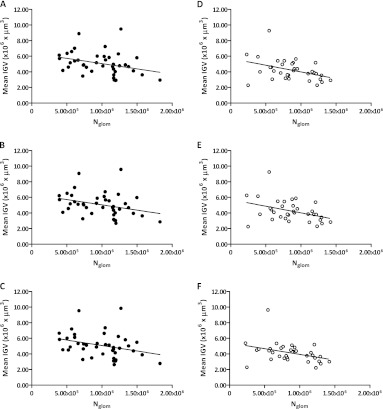
Figure 4 shows the linear relationship between mean IGV and Nglom. The general trend was preserved with all the different glomerular sample sizes (from 29 to 3 glomeruli measured per subject, data not shown) in both racial groups. The linear regression was statistically significant when eight or more glomeruli were measured from Caucasian Americans and nine or more glomeruli were measured from African Americans. Together, the results of the Lin’s concordance, Bland and Altman and linear regression analysis suggest that a stable estimate of mean IGV and IGV distribution are achieved where more than nine glomeruli are sampled per subject.
Fig. 4.
The effect of the Jackknife simulation on the relationship between mean individual glomerular volume (mean IGV) and total glomerular number (Nglom) in African and Caucasian American males. (A, B and C) (black circles) show African Americans (n = 41) and (D, E and F) (white circles) Caucasian Americans (n = 34). Mean IGV values were generated by the Jackknife simulation with different numbers of sampled glomeruli per subject; A and D (30 glomeruli), B and E (15 glomeruli), C (9 glomeruli) and F (8 glomeruli). C and F represent the minimum number of sampled glomeruli necessary to retain a statistically significant linear correlation between mean IGV and Nglom.
Discussion
This study reinforces the clinical relevance of IGV measurement, demonstrating that IGV mean and variance increase with established CKD comorbidities including age, obesity and hypertension. It also illustrates how reliable estimates of mean IGV can be obtained with at least nine sampled glomeruli.
This is the largest cohort study to show associations between obesity and hypertension and IGV. Hypertension and obesity are major contributors to the CKD epidemic, especially in populations with increased risk of kidney disease including Australian Aborigines [32], Pima Indians [33] and African Americans [34]. Of relevance to the present study, glomerular hypertrophy has been described in all these populations [32, 33, 35, 36]. Glomerular size and size variability are the two important markers of glomerular stress [16] and age [18, 37], BMI, hypertension [38] and Nglom [17, 26, 37] may act as glomerular stressors, increasing mean IGV and heterogeneity.
Glomerular morphology and function vary according to their location within the renal cortex [39]. Skov et al. [15] showed that in healthy primates, juxtamedullary glomeruli were up to four times larger than superficial glomeruli and our first autopsy study of IGV showed that superficial glomeruli were larger than juxtamedullary glomeruli in advanced age (>50 years) and high BSA (>2.11 m2) [18]. Our current analysis considered 2250 glomeruli from subjects with minimal comorbidities (lean, normotensive and low risk of kidney disease) and observed no zonal differences. Low-risk individuals presented smaller more homogeneous glomeruli in all three cortical zones compared with high-risk subjects. Moreover, middle and juxtamedullary glomeruli from high-risk subjects tended to be larger than superficial glomeruli (8–13%); a tendency that was not observed in the low-risk group. The data from the low-risk kidneys are in agreement with two human autopsy studies; neither Zimanyi et al. [26] nor McNamara et al. [37] found zonal differences in IGV, despite analysing 48 adult males (24 American and 24 Senegalese) and a total of 1440 glomeruli. Furthermore, Newbold et al. [40] found no differences between the size of juxtamedullary and superficial glomeruli in nosocomial human necropsies [7].
Tracy [41] suggested that glomerular size heterogeneity could be closely related to vascular changes, especially in hypertensive nephrosclerosis. Hoy et al. [42] showed that among subjects with hypertension, wall thickening of interlobular arteries was present at a younger age than the increase in glomerulosclerosis, suggesting that vascular changes preceded parenchymal loss. Moreover, in the scenario of hyperfiltration (such as obesity or any other type of parenchymal loss), renal blood flow would need to be re-distributed to preserve function and glomeruli closer to principal vessels (juxtamedullary) would possibly be favoured by their proximity to the blood supply. As described by Brenner [43], this hyperfiltration process would lead to progressive glomerular hypertrophy and finally glomerulosclerosis in the remaining glomeruli. Hoy et al. [38] proposed that small glomeruli may be more likely to sclerose and Samuel et al. [18] found that global glomerulosclerosis increased with age and was most severe in the superficial cortex. This observation is supported by our findings in high-risk subjects with smaller glomeruli in the superficial area. Whether this difference between superficial and juxtamedullary glomeruli reflects mostly vascular changes, or structural differences between glomeruli such as differences in podocyte number, is still to be defined.
We and others have estimated mean glomerular volume in renal biopsies [44, 45], but few studies have assessed variability in glomerular volumes within single biopsies with unbiased stereology [22, 47]. The method of Weibel and Gomez (1962) has been most commonly used to estimate glomerular volume in biopsies but requires knowledge of glomerular size distribution and shape [22, 44]. While the effect of size distribution has been previously addressed [48], the effect of assuming a uniform glomerular shape remains unknown and may be a source of significant bias.
We propose that measurements of IGV in renal biopsies would provide valuable insights in the diagnosis, prognosis and management of CKD [49]. However, several caveats must be mentioned. While biopsies are usually taken from the lower pole of the kidney, our estimates of IGV have been made in tissue from the mid-hilar region of the kidney. Lodrup et al. [50] report no differences in mean glomerular volume between mid-hilar region and renal poles in pigs but this has not been addressed in humans. Furthermore, biopsy samples have a preponderance of superficial glomeruli because of the risks of sampling in the juxtamedullary zone. Superficial glomeruli demonstrate changes in volume and volume distribution with disease but are stable under normal conditions, supporting their use for monitoring kidney health longitudinally.
The stereological dogma of ‘do more, less well’ may also be applicable to measurements of IGV [13, 51]. Although there are obvious advantages of designing studies in which as many glomeruli as possible are measured, our observation that a relatively modest number of glomeruli must be measured to return a reliable estimate of IGV mean and distribution suggests that longitudinal biopsy-based studies may be feasible. An average of 9–12 glomeruli [52] can be obtained from a percutaneous needle biopsy and the present study suggests that reliable estimates of mean IGV could be obtained with at least nine ‘complete’ or disector-sampled glomeruli per subject. Group analysis (mean IGV by racial group) and individual analysis (comparison of mean IGV values in 10 randomly sampled subjects per racial group) returned similar results, suggesting that the stability of IGV estimates is not a function of the large number of subjects available for this study.
Another relevant issue is the time-consuming nature of IGV estimation. MacLeod et al. [22] reported that five glomeruli per biopsy at a sectioning interval of 20 μm would provide satisfactory estimates of mean glomerular volume by the Cavalieri principle in normotensive type 1 diabetic patients. The present findings in subjects without overt renal pathology indicate that even three glomeruli per subject would barely alter the mean IGV, CV and CE compared with that observed from 30 glomeruli. However, valuable information about the spread or distribution of IGVs, the ability to perform correlations and statistical power would be lost with reductions below nine glomeruli per subject. Moreover, if the research question extends to an assessment of glomerular size distribution and variance within and between subjects, then the measurement of as many complete glomeruli as possible should be encouraged. Furthermore, new technologies such as the use of digital images and stereology-based software offer improvements in efficiency.
The present study has a number of limitations. Firstly, the IGV values utilized came from subjects carefully selected for various criteria in previous studies [18, 26]. Notwithstanding this, the matching criteria set previously were mainly based on age. Secondly, subjects in the three previous studies were selected for different end points, based on the extremes of the distributions for each variable of interest (age, Nglom, hypertension and BMI). Though this may appear to introduce a selection bias, we consider that our results based on subjects on the lower and upper end of their distributions would restrain our optimization process up to a sensible point. All glomeruli measured were contained in a mid-hilar slice of tissue. As such, any differences in the size or size distribution of glomeruli in the renal poles were not taken into account. Another important caveat would be the differences in the fixation process. Biopsy tissue is immersion-fixed, whereas we studied perfusion-fixed tissue. Mean glomerular capillary volume is lower in immersion versus perfusion fixed tissue [53]. Interestingly, Macleod et al. [22] have used the Cavalieri method to estimate glomerular volume in immersed-fixed human biopsy tissue, from diabetic individuals and showed a similar range of glomerular volumes to our present data. This outcome further strengthens the argument for estimating variability in glomerular volume in the study of renal disease.
In summary, this large cohort study of 2250 glomeruli from 75 individuals provides further evidence that the size distribution of glomeruli throughout the renal cortex is a relevant marker of risk factors for CKD including obesity, hypertension and low nephron number. Neither glomerular location within the renal cortex nor the number of sampled glomeruli per subject represent significant drawbacks for the application of IGV to tissue samples of limited size, including renal biopsies.
Funding
This research was funded by grants from the National Institutes of Health (NIH 1 RO1 DK065970-01), the NIH Center of Excellence in Minority Health (5P20M000534-02), the National Health and Medical Research Council of Australia (NHMRC) and the American Hearth Association (Southeastern Affiliate). J.A.A. is a Monash Fellow and PhD scholarship funding for V.G.P. was provided by Monash Research Graduate School (MRGS) and Faculty of Medicine International Postgraduate Scholarship.
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Weiner DE. Public health consequences of chronic kidney disease. Clin Pharmacol Ther. 2009;86:566–569. doi: 10.1038/clpt.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughson MD, Douglas-Denton R, Bertram JF, et al. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 2006;69:671–678. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 3.Keller G, Zimmer G, Mall G, et al. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 4.Lemley KV. A basis for accelerated progression of diabetic nephropathy in Pima Indians. Kidney Int Suppl. 2003;83:S38–S42. doi: 10.1046/j.1523-1755.63.s83.9.x. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz A, Nyengaard JR, Bendtsen TF. Glomerular volume in type 2 (noninsulin-dependent) diabetes estimated by a direct and unbiased stereologic method. Lab Invest. 1990;62:108–113. [PubMed] [Google Scholar]
- 6.Chen HM, Li SJ, Chen HP, et al. Obesity-related glomerulopathy in China: a case series of 90 patients. Am J Kidney Dis. 2008;52:58–65. doi: 10.1053/j.ajkd.2008.02.303. [DOI] [PubMed] [Google Scholar]
- 7.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec. 1992;232:194–201. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 8.Douglas-Denton RN, McNamara BJ, Hoy WE, et al. Does nephron number matter in the development of kidney disease? Ethn Dis. 2006;16(2 Suppl 2):S2–S40-5. [PubMed] [Google Scholar]
- 9.Fogo A. Mechanisms and implications of heterogeneity of human glomerulosclerosis. Contrib Nephrol. 1996;118:6–11. doi: 10.1159/000425068. [DOI] [PubMed] [Google Scholar]
- 10.Fogo A, Hawkins EP, Berry PL, et al. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- 11.Lane PH, Steffes MW, Mauer SM. Estimation of glomerular volume: a comparison of four methods. Kidney Int. 1992;41:1085–1089. doi: 10.1038/ki.1992.165. [DOI] [PubMed] [Google Scholar]
- 12.Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–172. doi: 10.1016/s0074-7696(08)62497-3. [DOI] [PubMed] [Google Scholar]
- 13.Nyengaard JR. Stereologic methods and their application in kidney research. J Am Soc Nephrol. 1999;10:1100–1123. doi: 10.1681/ASN.V1051100. [DOI] [PubMed] [Google Scholar]
- 14.Bankir L, Bouby N, Trinh-Trang-Tan MM. Heterogeneity of nephron anatomy. Kidney Int Suppl. 1987;20:S25–S39. [PubMed] [Google Scholar]
- 15.Skov K, Nyengaard JR, Patwardan A, et al. Large juxtamedullary glomeruli and afferent arterioles in healthy primates. Kidney Int. 1999;55:1462–1469. doi: 10.1046/j.1523-1755.1999.00398.x. [DOI] [PubMed] [Google Scholar]
- 16.Puelles VG, Hoy WE, Hughson MD, et al. Glomerular number and size variability and risk for kidney disease. Curr Opin Nephrol Hypertens. 2011; 20: 7–15 doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- 17.Hughson M D, Hoy WE, Douglas-Denton R N, et al. Towards a definition of glomerulomegaly: clinical-pathological and methodological considerations. Nephrol Dial Transplant. 2011;26:2202–2208. doi: 10.1093/ndt/gfq688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuel T, Hoy WE, Douglas-Denton R, et al. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005;16:3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- 19.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 20.Pagtalunan ME, Drachman JA, Meyer TW. Methods for estimating the volume of individual glomeruli. Kidney Int. 2000;57:2644–2649. doi: 10.1046/j.1523-1755.2000.00125.x. [DOI] [PubMed] [Google Scholar]
- 21.Beeman SC, Zhang M, Gubhaju L, et al. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol. 2011;300(6):F1454–7. doi: 10.1152/ajprenal.00044.2011. [DOI] [PubMed] [Google Scholar]
- 22.Macleod JM, White KE, Tate H, et al. Measurement of glomerular volume in needle biopsy specimens. The ESPRIT Study Group (European Study of the Progression of Renal Disease in Type 1 Diabetes) Nephrol Dial Transplant. 2000;15:239–243. doi: 10.1093/ndt/15.2.239. [DOI] [PubMed] [Google Scholar]
- 23.Najafian B, Crosson JT, Kim Y, et al. Glomerulotubular junction abnormalities are associated with proteinuria in type 1 diabetes. J Am Soc Nephrol. 2006;17(4 Suppl 2):S53–S60. doi: 10.1681/ASN.2005121342. [DOI] [PubMed] [Google Scholar]
- 24.Basgen JM, Nicholas SB, Mauer M, et al. Comparison of methods for counting cells in the mouse glomerulus. Nephron Exp Nephrol. 2006;103:e139–e148. doi: 10.1159/000092905. [DOI] [PubMed] [Google Scholar]
- 25.S IM. The social welfare of children in Stockholm. Health Bull (Edinb) 1949;7:7. [PubMed] [Google Scholar]
- 26.Zimanyi MA, Hoy WE, Douglas-Denton RN, et al. Nephron number and individual glomerular volumes in male Caucasian and African American subjects. Nephrol Dial Transplant. 2009;24:2428–2433. doi: 10.1093/ndt/gfp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughson MD, Gobe GC, Hoy WE, et al. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis. 2008;52:18–28. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 28.Quester R, Knifka J, Schroder R. Optimization of glycol methacrylate embedding of large specimens in neurological research. Study of rat skull-brain specimens after implantation of polyester meshes. J Neurosci Methods. 2002;113:15–26. doi: 10.1016/s0165-0270(01)00469-1. [DOI] [PubMed] [Google Scholar]
- 29.Bertram JF, Soosaipillai MC, Ricardo SD, et al. Total numbers of glomeruli and individual glomerular cell types in the normal rat kidney. Cell Tissue Res. 1992;270:37–45. doi: 10.1007/BF00381877. [DOI] [PubMed] [Google Scholar]
- 30.Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 31.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 32.Hoy WE, Hughson MD, Singh GR, et al. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int. 2006;70:104–110. doi: 10.1038/sj.ki.5000397. [DOI] [PubMed] [Google Scholar]
- 33.Lemley KV. Diabetes and chronic kidney disease: lessons from the Pima Indians. Pediatr Nephrol. 2008;23:1933–1940. doi: 10.1007/s00467-008-0763-8. [DOI] [PubMed] [Google Scholar]
- 34.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2010;55:31–41. doi: 10.1053/j.ajkd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Young RJ, Hoy WE, Kincaid-Smith P, et al. Glomerular size and glomerulosclerosis in Australian aborigines. Am J Kidney Dis. 2000;36:481–489. doi: 10.1053/ajkd.2000.9788. [DOI] [PubMed] [Google Scholar]
- 36.Hughson M, Farris AB, 3rd, Douglas-Denton R, et al. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 37.McNamara BJ, Diouf B, Hughson MD, et al. Associations between age, body size and nephron number with individual glomerular volumes in urban West African males. Nephrol Dial Transplant. 2009;24:1500–1506. doi: 10.1093/ndt/gfn636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoy WE, Hughson MD, Zimanyi M, et al. Distribution of volumes of individual glomeruli in kidneys at autopsy: association with age, nephron number, birth weight and body mass index. Clin Nephrol. 2010;74:105–112. doi: 10.5414/cnp74s105. [DOI] [PubMed] [Google Scholar]
- 39.Trinh-Trang-Tan MM, Bouby N, Kriz W, et al. Functional adaptation of thick ascending limb and internephron heterogeneity to urine concentration. Kidney Int. 1987;31:549–555. doi: 10.1038/ki.1987.34. [DOI] [PubMed] [Google Scholar]
- 40.Newbold KM, Sandison A, Howie AJ. Comparison of size of juxtamedullary and outer cortical glomeruli in normal adult kidney. Virchows Arch A Pathol Anat Histopathol. 1992;420:127–129. doi: 10.1007/BF02358803. [DOI] [PubMed] [Google Scholar]
- 41.Tracy RE. The heterogeneity of vascular findings in the kidneys of patients with benign essential hypertension. Nephrol Dial Transplant. 1999;14:1634–1639. doi: 10.1093/ndt/14.7.1634. [DOI] [PubMed] [Google Scholar]
- 42.Hoy WE, Bertram JF, Denton RD, et al. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17:258–265. doi: 10.1097/MNH.0b013e3282f9b1a5. [DOI] [PubMed] [Google Scholar]
- 43.Brenner BM. Nephron adaptation to renal injury or ablation. Am J Physiol. 1985;249:F324–F337. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- 44.Najafian B, Basgen JM, Mauer M. Estimating mean glomerular volume using two arbitrary parallel sections. J Am Soc Nephrol. 2002;13:2697–2705. doi: 10.1097/01.asn.0000033381.53882.25. [DOI] [PubMed] [Google Scholar]
- 45.Najafian B, Kim Y, Crosson JT, et al. Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol. 2003;14:908–917. doi: 10.1097/01.asn.0000057854.32413.81. [DOI] [PubMed] [Google Scholar]
- 46.Weibel E R, Elias H. and International Society for Stereology. Quantitative methods in morphology. Quantitative Methoden in der Morphologie; proceedings. Berlin, NY: Springer-Verlag; 1967. vi, pp. 278. [Google Scholar]
- 47.Bertram JF, Young RJ, Seymour AE, et al. Glomerulomegaly in Australian Aborigines. Nephrology (Carlton) 1998;4:46–53. [Google Scholar]
- 48.Samuel T, Hoy WE, Douglas-Denton R, et al. Applicability of the glomerular size distribution coefficient in assessing human glomerular volume: the Weibel and Gomez method revisited. J Anat. 2007;210:578–582. doi: 10.1111/j.1469-7580.2007.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whittier WL, Korbet SM. Renal biopsy: update. Curr Opin Nephrol Hypertens. 2004;13:661–665. doi: 10.1097/00041552-200411000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Lodrup AB, Karstoft K, Dissing TH, et al. Kidney biopsies can be used for estimations of glomerular number and volume: a pig study. Virchows Arch. 2008;452:393–403. doi: 10.1007/s00428-007-0520-6. [DOI] [PubMed] [Google Scholar]
- 51.Bertram JF. Counting in the kidney. Kidney Int. 2001;59:792–796. doi: 10.1046/j.1523-1755.2001.059002792.x. [DOI] [PubMed] [Google Scholar]
- 52.Osterby R, Bangstad HJ, Rudberg S. Follow-up study of glomerular dimensions and cortical interstitium in microalbuminuric type 1 diabetic patients with or without antihypertensive treatment. Nephrol Dial Transplant. 2000;15:1609–1616. doi: 10.1093/ndt/15.10.1609. [DOI] [PubMed] [Google Scholar]
- 53.Miller PL, Meyer TW. Effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest. 1990;63:862–866. [PubMed] [Google Scholar]