Abstract
Background: Many epidemiological studies have linked daily counts of hospital admissions to particulate matter (PM) with an aerodynamic diameter ≤ 10 μm (PM10) and ≤ 2.5 μm (PM2.5), but relatively few have investigated the relationship of hospital admissions with coarse PM (PMc; 2.5–10 μm aerodynamic diameter).
Objectives: We conducted this study to estimate the health effects of PMc on emergency hospital admissions for respiratory diseases in Hong Kong after controlling for PM2.5 and gaseous pollutants.
Methods: We conducted a time-series analysis of associations between daily emergency hospital admissions for respiratory diseases in Hong Kong from January 2000 to December 2005 and daily PM2.5 and PMc concentrations. We estimated PMc concentrations by subtracting PM2.5 from PM10 measurements. We used generalized additive models to examine the relationship between PMc (single- and multiday lagged exposures) and hospital admissions adjusted for time trends, weather conditions, influenza outbreaks, PM2.5, and gaseous pollutants (nitrogen dioxide, sulfur dioxide, and ozone).
Results: A 10.9-μg/m3 (interquartile range) increase in the 4-day moving average concentration of PMc was associated with a 1.94% (95% confidence interval: 1.24%, 2.64%) increase in emergency hospital admissions for respiratory diseases that was attenuated but still significant after controlling for PM2.5. Adjusting for gaseous pollutants and altering models assumptions had little influence on PMc effect estimates.
Conclusion: PMc was associated with emergency hospital admissions for respiratory diseases in Hong Kong independent of PM2.5 and gaseous pollutants. Further research is needed to evaluate health effects of different components of PMc.
Keywords: coarse particulate matter, emergency hospital admissions, fine particulate matter, generalized additive model, respiratory diseases, time-series study
Associations between particulate matter (PM) air pollution and cardiorespiratory hospital admissions have been reported by many epidemiological studies over the past two decades (Anderson et al. 2001; Atkinson 2004; Bell et al. 2004; Dominici et al. 2006; Ilabaca et al. 1999; Le Tertre et al. 2002; Lipsett et al. 1997; Morris 2001; Norris et al. 1999; Schwartz and Neas 2000; Slaughter et al. 2005). Most of the studies focused on PM with an aerodynamic diameter ≤ 10 μm (PM10) or ≤ 2.5 μm (PM2.5). Fewer studies have examined the potential health effects of the coarse fraction (PMc; 2.5–10 μm in aerodynamic diameter) and its relationship with cardiorespiratory hospital admissions (Brunekreef and Forsberg 2005; Chen et al. 2005; Halonen et al. 2009; Host et al. 2008; Lin et al. 2005; Peng et al. 2008; Tecer et al. 2008). In addition, excess relative risks (ERRs) estimated for daily respiratory admissions associated with PM2.5 and PMc have been quite inconsistent among these studies. A 2005 systematic review of studies on chronic obstructive pulmonary disease (COPD), asthma, and respiratory admissions noted that ERRs in response to short-term exposure to PMc were similar to or larger than corresponding estimates for PM2.5 and suggested that PMc might have adverse effects on the respiratory system (Brunekreef and Forsberg 2005). Several studies published after that review also reported significant positive associations between PMc and hospital admissions for respiratory diseases (Chen et al. 2005; Host et al. 2008; Lin et al. 2005; Tecer et al. 2008). On the other hand, the large National Mortality, Morbidity and Air Pollution Study conducted in 108 U.S. urban counties reported a large statistically significant ERR for PM2.5 but not for PMc (Peng et al. 2008).
Previous time-series studies on the health effects of air pollution in Hong Kong have focused on PM10 because of a lack of PM2.5 monitoring data (Ko et al. 2007; Lee et al. 2006; Wong CM et al. 2008a; Wong TW et al. 1999, 2002, 2006). In addition, the Air Quality Objectives (Environmental Protection Department 1987), the national air quality standards for Hong Kong, cover only PM10, although the Environmental Protection Department is considering PM2.5 regulation as well (Environmental Protection Department 2009). A standard specifically for PMc is not in place or under consideration, but additional studies could help support a PMc standard in the future. In the present study, we conducted a time-series analysis to estimate the health effects of PMc on emergency hospital admissions for total respiratory diseases, COPD, and asthma in Hong Kong after controlling for PM2.5 and gaseous pollutants.
Materials and Methods
Data on particulate pollutants and meteorology variables. We obtained air pollution data for January 2000 through December 2005 from the Environmental Protection Department. There are a total of 11 general monitoring stations in Hong Kong. All of them monitored PM10 and gaseous pollutants [nitrogen dioxide (NO2) sulfur dioxide (SO2), and ozone (O3)] during the study period, but only three (Tsuen Wan, Tap Mun, and Tung Chung) collected simultaneous PM2.5 data. The Tap Mun and Tung Chung stations are located in remote areas of Hong Kong, whereas the Tsuen Wan station is located close to the geographic center of Hong Kong (Figure 1) and thus is likely to be more representative of Hong Kong’s air quality in general. In addition, the Tsuen Wan station is not in direct proximity to traffic, industrial sources, buildings, or residential sources of emissions from the burning of coal, waste, or oil. Therefore, instead of estimating average values for the three stations with simultaneous PM10 and PM2.5 data, we used data from the Tsuen Wan station only. We calculated 24-hr mean concentrations from nonmissing data if at least 18 of 24 hourly concentrations of PM10 or PM2.5 were available, and we did not impute data for the 195 days with missing PMc, which accounted for only 8.9% of the study period. We estimated PMc concentrations by subtracting daily mean PM2.5 from PM10. In contrast with studies that examined PMc using data collected every 3 or 6 days (Lin et al. 2005; Peng et al. 2008), we analyzed daily PMc data available during the study period. We also calculated daily 24-hr mean concentrations of NO2 and SO2 and 8-hr mean (1000 hours to 1800 hours) concentrations of O3 using data from the Tsuen Wan station and collected daily mean temperature and relative humidity data for the same period from the Hong Kong Observatory.
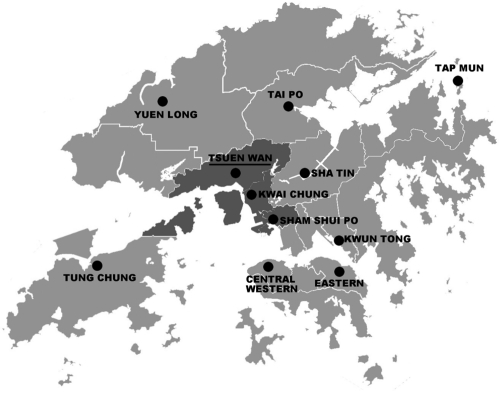
Figure 1.
Location of the Tsuen Wan air monitoring station, Tsuen Wan region (dark-gray area), and the other general air monitoring stations (black circles) in Hong Kong.
Data on hospital admissions. We collected citywide emergency hospital admissions (admissions through the accident and emergency services) for respiratory diseases in Hong Kong from January 2000 through December 2005. The hospitals included for compilation of hospital admissions were publicly funded hospitals that provide 24-hr accident and emergency services and 90% of hospital beds for Hong Kong residents (Wong et al. 1999). Patient data captured from the computerized medical record system included age, date of admission, source of admission, hospital, residential address, and principal diagnosis on discharge [International Statistical Classification of Diseases, 9th Revision (ICD-9); World Health Organization 1975)]. We chose hospital admissions through accident and emergency services for diseases of the respiratory system [ICD-9 codes 460–519, excluding influenza (487.0–487.8)] and for COPD (ICD-9 codes 491, 492, and 496) and asthma (ICD-9 code 493) specifically. We excluded influenza from respiratory diseases because a previous study demonstrated that influenza outbreaks may confound associations between PM and hospital admissions for respiratory diseases (Ren et al. 2006). We also compiled data on emergency hospital admissions for respiratory diseases among patients who are residents of the Tsuen Wan region (TW residents; residential addresses in the area around the Tsuen Wan air monitoring station, including Tsuen Wan, Kwai Tsing, and Sham Shui Po districts; Figure 1), to evaluate the potential influence of exposure misclassification.
Statistical methods. We used generalized additive modeling (GAM) with a log link and allowed Poisson autoregression and overdispersion to model the relationship between daily PMc concentrations and health outcomes (Hastie and Tibshirani 1990). All models were adjusted for the day of the week (DOW) and public holidays using categorical indicator variables (Schwartz et al. 1996), and for influenza outbreaks using a dichotomous variable to indicate weeks during which the number of influenza hospital admissions exceeded the 75th percentile for the year (Wong CM et al. 2002). In addition, we used penalized smoothing splines (Host et al. 2008; Kan et al. 2007) to adjust for seasonal patterns and long-term trends in daily morbidity, temperature, and relative humidity with degrees of freedom (df) selected a priori based on previous studies (Bell et al. 2008; Peng et al. 2008). Specifically, we used 7 df per year for time trend, 6 df for mean temperature on the current day (Temp0) and the moving average for the previous 3 days (Temp1–3), and 3 df for humidity (Humidity0) on the current day.
The resulting core model to estimate E(Yt), the expected daily emergency respiratory hospital admission count on day t, was specified as
log[E(Yt)] = α + s(t, df = 7/year) + s(Temp0, df = 6) + s(Temp1–3, df = 6) + s(Humidity0, df = 3) + β1 × DOW + β2 × Holiday + β3 × Influenza, [1]
where s(·) indicates a smoother based on penalized splines, and β are regression coefficients.
To minimize autocorrelation, which would bias the standard errors, we specified that the absolute values of the partial autocorrelation function for the model residuals had to be < 0.1 for the first 2 lag days (Wong et al. 2008b). When these criteria were not met, we added autoregressive terms for the outcome variable to Equation 1, resulting in the addition of three autoregressive terms (lag1, lag2, lag3) to model emergency hospital admissions for total respiratory diseases, two autoregressive terms (lag1 and lag2) for COPD, and one autoregressive term (lag1) for asthma.
We also estimated the linear effect of PMc according to different lag structures, including single-day lags [current day (lag0) up to 5 days before (lag5)] and multiday lags (moving averages for the current day and the previous 1, 2, or 3 days: lag01, lag02, and lag03, respectively). However, we focused on 4-day average PMc exposure (lag03) for two-pollutant models and sensitivity analyses (Chen et al. 2004). In addition, we estimated the effect of PMc on emergency respiratory hospitalizations after adjusting for exposures to gaseous pollutants (NO2, SO2, and O3). To justify the assumption of linearity between the logarithm of emergency hospital admissions and particle concentrations, we graphically examined concentration–response relationships derived using a smoothing function (Kan et al. 2007; Wong CM et al. 2002).
Sensitivity analysis. In addition to analyzing the entire range of particulate concentrations, we estimated effects after excluding days with extremely high or low PMc or PM2.5 concentrations (i.e., excluding days with the highest 1% and lowest 1% of values). We also examined the impact of degrees of freedom selection for time trend and weather conditions on PMc effect estimates. To address possible exposure misclassification resulting from the use of pollution data from a single monitoring station, we did a sensitivity analysis restricted to emergency respiratory hospital admissions among TW residents.
We conducted all analyses using the MGCV package in R (version 2.10.0; R Development Core Team, Vienna, Austria). We report results as the percent increase [ERR, with 95% confidence intervals (CIs)] in daily emergency respiratory hospital admissions for an interquartile range (IQR) increase in PM concentrations.
Results
From 1 January 2000 to 31 December 2005, we recorded a total of 710,247 hospital admissions for respiratory diseases in the study population. Of these, we included 518,864 hospital admissions through accident and emergency services (emergency hospital admissions) in our analyses. On average, there were 237 emergency hospital admissions per day for total respiratory diseases, 81 for COPD, and 20 for asthma (Table 1). The average number of daily emergency respiratory hospital admissions among TW residents was about 50 per day.
Table 1.
Summary statistics of daily emergency hospital admissions, air pollution concentrations, and weather conditions in Hong Kong, 2000–2005.
| No. of days | Percentile | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean ± SD | Minimum | 25th | 50th | 75th | Maximum | ||||
| Daily emergency hospital admissions | ||||||||||
| Total respiratory diseases | 2,192 | 236.7 ± 55.4 | 89 | 198 | 230 | 269 | 518 | |||
| COPD | 2,192 | 81.1 ± 20.3 | 22 | 68 | 80 | 95 | 165 | |||
| Asthma | 2,192 | 19.6 ± 8.0 | 1 | 14 | 19 | 25 | 61 | |||
| Respiratory diseases in TW residents | 2,192 | 50.0 ± 12.4 | 18 | 41 | 49 | 57 | 104 | |||
| Pollution concentration (μg/m3) | ||||||||||
| PM10 | 1,998 | 56.1 ± 27.8 | 13.5 | 34.9 | 49.2 | 72.5 | 231.5 | |||
| PM2.5 | 1,997 | 39.4 ± 20.7 | 8.9 | 23.8 | 34.8 | 50.1 | 179.8 | |||
| PMc | 1,997 | 16.6 ± 9.2 | 0.8 | 10.0 | 14.5 | 20.9 | 82.9 | |||
| NO2 | 1,995 | 64.4 ± 22.4 | 13.0 | 48.4 | 61.6 | 77.4 | 193.9 | |||
| SO2 | 1,998 | 22.9 ± 17.1 | 1.0 | 11.3 | 18.3 | 28.7 | 143.3 | |||
| O3 | 1,995 | 31.1 ± 24.3 | 1.0 | 13.2 | 24.2 | 42.8 | 171.7 | |||
| Meteorology measures | ||||||||||
| Temperature (°C) | 2,192 | 23.5 ± 5.0 | 8.2 | 19.6 | 24.9 | 27.8 | 31.8 | |||
| Relative humidity (%) | 2,192 | 78.2 ± 9.7 | 32 | 73 | 79 | 85 | 97 | |||
| Minimum is the lowest value, and maximum is the highest value in the full range. | ||||||||||
Daily mean concentrations of PM2.5 and PMc were 39.4 and 16.6 μg/m3, with IQRs of 26.3 and 10.9 μg/m3, respectively (Table 1). PM2.5 accounted for a substantial part of the mass concentration of PM10 in Hong Kong: the ratio of PM2.5 to PM10 ranged from 40% to 98%, with an average of 70%. Therefore, PMc accounted for about 30% of PM10 mass concentration. Daily mean concentrations of NO2, SO2, and O3 were 64.4, 22.9, and 31.1 μg/m3, respectively (Table 1). PM10 was strongly correlated with PM2.5 (correlation coefficient, r = 0.97) and with PMc (r = 0.84), and PM2.5 and PMc were moderately correlated (r = 0.68; Table 2). Correlation coefficients for PMc and gaseous pollutants were low to moderate (r = 0.56 for NO2, r = 0.27 for SO2, r = 0.37 for O3).
Table 2.
Pearson correlation coefficients between PM concentrations, gaseous pollutants, and weather conditions.a
| Pollutants | PM10 | PM2.5 | PMc | NO2 | SO2 | O3 | Temperature |
|---|---|---|---|---|---|---|---|
| PM10 | 1.000 | ||||||
| PM2.5 | 0.969 | 1.000 | |||||
| PMc | 0.836 | 0.675 | 1.000 | ||||
| NO2 | 0.771 | 0.786 | 0.560 | 1.000 | |||
| SO2 | 0.432 | 0.461 | 0.267 | 0.493 | 1.000 | ||
| O3 | 0.475 | 0.472 | 0.370 | 0.303 | 0.022 | 1.000 | |
| Temperature | –0.304 | –0.285 | –0.278 | –0.298 | 0.163 | 0.054 | 1.000 |
| Relative humidity | –0.470 | –0.409 | –0.498 | –0.282 | –0.062 | –0.582 | 0.213 |
| aAll correlation coefficients except that between O3 and SO2 are statistically significant (p < 0.05). | |||||||
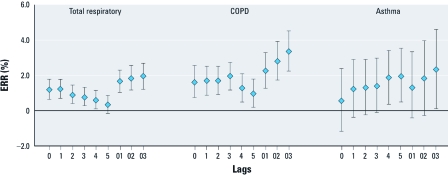
Regression results. PMc was significantly associated (p < 0.05) with total respiratory and COPD emergency hospital admissions at most of the lags examined in single-pollutant models, whereas associations with asthma hospitalization were positive but only statistically significant at lag4, lag5, and lag03 (Figure 2). An IQR increase in the 4-day moving average concentration of PMc (lag03) was associated with 1.94% (95% CI: 1.24%, 2.64%), 3.37% (2.26%, 4.49%), and 2.32% (0.14%, 4.55%) increases in emergency hospital admissions for total respiratory diseases, COPD, and asthma, respectively (Table 3). After adjusting for PM2.5 in two-pollutant models, estimated effects of PMc on respiratory and COPD hospital admissions were attenuated but remained statistically significant, with ERRs of 1.05% (95% CI: 0.19%, 1.91%) and 1.78% (0.41%, 3.16%), respectively. However, the effect estimate for PMc on asthma hospitalizations was close to the null after adjustment for PM2.5 (Table 3). Adjustment for gaseous pollutants had little influence on effect estimates for associations between PMc and total respiratory hospitalizations (Table 4).
Figure 2.
Percent increase (ERR with 95% CI) in emergency hospital admissions due to total respiratory diseases, COPD, and asthma for an IQR (10.9 μg/m3) increase in PMc concentrations with different lag days [single lags for the current day (lag0) to 5 days before the current day (lag5) and multiday lags for the current day plus 1 day before (lag01), 2 days before (lag02), or 3 days before (lag03)].
Table 3.
Estimated percent increase [ERR (95% CI)] in emergency hospital admissions associated with an IQR increase in PM concentrations, by disease.a
| Pollutant | Total respiratory | COPD | Asthma | |||
|---|---|---|---|---|---|---|
| Single-pollutant model | ||||||
| PMc | 1.94 (1.24, 2.64) | 3.37 (2.26, 4.49) | 2.32 (0.14, 4.55) | |||
| PM2.5 | 2.58 (1.73, 3.44) | 4.44 (3.11, 5.80) | 4.35 (1.66, 7.11) | |||
| Two-pollutant model | ||||||
| PMc | 1.05 (0.19, 1.91) | 1.78 (0.41, 3.16) | 0.27 (–2.42, 3.03) | |||
| PM2.5 | 1.81 (0.76, 2.87) | 3.13 (1.48, 4.81) | 4.14 (0.77, 7.63) | |||
| aThe effects of 4-day moving averages (current day to previous 3 days, lag03) of daily average PM concentrations were estimated in GAMs, adjusting for time trend, weather conditions, day of week, public holidays, and influenza outbreaks. IQRs: PMc, 10.9 μg/m3; PM2.5, 26.3 μg/m3. | ||||||
Table 4.
Adjusted estimated percent increase [ERR (95% CI)] of emergency respiratory hospital admissions associated with an IQR increase in PM concentrations.a
| 1st–99th percentile PMc onlyc | In TW residents onlyd | Additionally adjusted for pollutantb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pollutant | NO2 | SO2 | O3 | |||||||
| Single-pollutant model | ||||||||||
| PMc | 2.37 (1.51, 3.24) | 2.66 (1.33, 4.02) | 1.58 (0.86, 2.30) | 1.96 (1.26, 2.67) | 1.85 (1.15, 2.56) | |||||
| PM2.5 | 2.55 (1.67, 3.43) | 3.02 (1.42, 4.65) | 1.98 (1.04, 2.94) | 2.74 (1.87, 3.63) | 2.43 (1.55, 3.32) | |||||
| Two-pollutant model | ||||||||||
| PMc | 1.32 (0.23, 2.42) | 1.78 (0.11, 3.47) | 1.07 (0.21, 1.94) | 1.02 (0.16, 1.89) | 1.08 (0.22, 1.95) | |||||
| PM2.5 | 1.70 (0.59, 2.82) | 1.72 (–0.26, 3.74) | 1.19 (0.05, 2.33) | 1.97 (0.89, 3.06) | 1.62 (0.53, 2.71) | |||||
| aThe effects of 4-day moving averages (current day to previous 3 days, lag03) of daily average PM concentrations were estimated in GAMs, adjusting for time trend, weather conditions, day of week, public holidays, and influenza outbreaks. IQRs: PMc, 10.9 μg/m3; PM2.5, 26.3 μg/m3. bAnalysis covered the entire range of PMc concentration and citywide respiratory admissions. cAnalysis restricted to 1st–99th percentiles (6.42–42.96 μg/m3) of PMc concentration. dAnalysis restricted to hospital admissions in TW residents. | ||||||||||
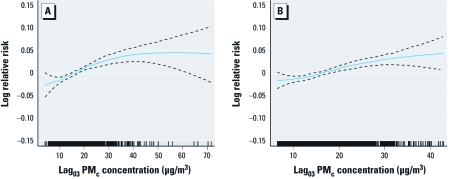
The concentration–response curve for PMc and emergency hospital admissions for total respiratory diseases tended to plateau at higher concentrations of PMc, but estimates were imprecise because of limited data in this range (Figure 3A). After we excluded the highest 1% and the lowest 1% extremes of PMc concentrations, the curve appeared essentially linear (Figure 3B). The estimated effect (slope) of PMc modeled as a linear variable increased slightly after excluding days with extreme concentrations, both before and after adjustment for PM2.5 (Table 4).
Figure 3.
Concentration–response curves between the logarithm of emergency respiratory hospital admission and PM concentration (df = 3). The density of the vertical bars on the x-axis shows the distribution of pollutant concentration. GAMs were used, adjusting for time trend, weather conditions, day of week, public holidays, and influenza outbreaks. (A) Analysis covering the entire range of PMc concentrations. (B) Restricted analysis excluding days with the lowest 1% and the highest 1% PMc concentrations.
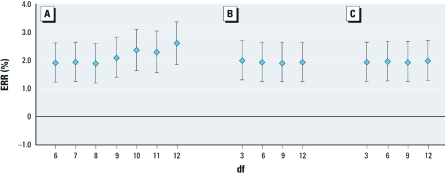
Varying the degrees of freedom for time trend (within the range of 6–12 per year) and weather conditions (mean temperature and humidity, within the range of 3–12) did not affect the regression results substantially (Figure 4), suggesting that effect estimates for PMc were relatively robust to changes in degrees of freedom for model covariates. ERR estimates based on data restricted to emergency respiratory hospitalizations among TW residents were less precise but slightly higher than corresponding estimates based on all observations.
Figure 4.
Sensitivity analyses for varying degrees of freedom for time trend and weather conditions on emergency respiratory hospital admissions based on an IQR increase of lag03 PMc concentrations: df = 6–12 per year for time trend (A), df = 3–12 for current day and previous 3 days’ mean temperature (B), and df = 3–12 for current day relative humidity (C).
Discussion
This study is one of the few to investigate the association between PMc and respiratory hospitalizations. We found significant positive associations between PMc concentrations and emergency hospital admissions for respiratory diseases in Hong Kong. To our knowledge, this study is the largest single-city study to date of the effects of PMc on emergency hospital admissions for respiratory diseases, including more than half a million admissions over 6 years. In contrast with studies based on PM data collected every third or sixth day (Lin et al. 2005; Peng et al. 2008), we evaluated daily data and were able to estimate effects of multiday average concentrations of PMc, which were larger in magnitude than estimated effects of single-day lags in most cases. We estimated statistically significant positive associations between PMc and emergency hospital admissions for total respiratory diseases and COPD for almost all lags examined. The estimated effect of PMc on asthma appeared to be strongest several days after exposure, consistent with a previous study (Lin et al. 2002).
Positive associations between PMc and total emergency respiratory hospitalizations, especially COPD, remained after adjusting for PM2.5, but the estimated effect of PMc on emergency asthma hospitalizations was close to the null after adjusting for PM2.5. A few studies estimated effects of PMc on respiratory admissions after adjusting for PM2.5 (Burnett et al. 1999; Chen et al. 2004; Ito 2003; Peng et al. 2008), but only one (Burnett et al. 1999) reported statistically significant associations independent of PM2.5. However, unlike the daily measurements used in our study, daily levels of PMc and PM2.5 in that study were estimated from 6-day sampling and not directly measured. Two studies have reported positive associations between PMc and asthma hospitalization in children, but estimates were not adjusted for PM2.5 (Lin et al. 2002; Tecer et al. 2008). Estimated effects of PMc changed very little after we adjusted for possible confounding effects of gaseous pollutants (NO2, SO2, O3), and others have also reported positive associations between PMc and respiratory hospitalizations after adjusting for gaseous pollutants (Chen et al. 2004, 2005; Lin et al. 2002, 2005). The correlation coefficients between PMc and gases in these Canadian studies were low to moderate, consistent with our study (correlation coefficients ranging from 0.27 for PMc and SO2 to 0.56 for PMc and NO2).
Englert (2004) suggested that the relative sizes of effects attributed to fractions of PM10 depend on their relative mass percentages. Although PMc represented only about 30% of the PM10 mass concentration in our study, we estimated statistically significant ERRs for emergency respiratory hospital admissions in association with PMc, which supports a specific effect of this PM fraction.
The concentration–response relationship between PMc and emergency hospital admissions for total respiratory diseases was almost linear after excluding the highest 1% and the lowest 1% extreme concentrations of PMc, and the slope of the estimated association based on a linear model increased slightly. Our results were not substantially modified when we varied the degrees of freedom for smoothers of time and weather conditions. Analyses restricted to emergency hospitalizations among residents living near the monitoring station also were consistent with the overall results, which supports the use of PM data from a single central monitoring station in our main analyses.
Effects may vary for PMc from different sources and with different chemical compositions, and it has been proposed that differences in associations estimated for Hong Kong and U.S. populations (Peng et al. 2008) might be explained by differences in PMc composition. Further studies are needed to examine the health effects of the specific components in PMc.
Smaller particles offer a proportionally larger surface area resulting in potentially higher concentrations of adsorbed or condensed toxic air pollutants per unit mass. Hence, PM2.5 is frequently assumed to be a more relevant exposure indicator than are larger particles. However, the pathological mechanisms of particles on human health are not fully understood. Particle size may be associated with chemical, biological, and physical properties that contribute to specific pathological mechanisms. PMc originates mainly from the soil and abrasive mechanical processes and thus may carry biological materials such as bacteria, molds, or pollens that can produce adverse health effects in the respiratory system (Almeida et al. 2006). Our results lend support to possible adverse health effects of PMc exposure that are independent of PM2.5 and gaseous pollutants. Further study of seasonal differences in PMc composition and season-specific PMc effects may help clarify pathological mechanisms.
Some limitations of the present study should be noted. We estimated PMc concentrations by subtracting PM2.5 from PM10 measurements. A disadvantage of this method is that PMc exposure estimates are subjected to two sources of random error in measurement (standard error) rather than one, which may reduce the statistical power of detecting an association. Because we still observed significant associations between PMc and emergency respiratory hospital admissions in Hong Kong, these were likely true associations. As in other time-series studies, we used available outdoor monitoring data to represent the population exposure to ambient particles. Indoor air pollution and personal exposure data were not available. A simulation using data from a recent multipollutant (PM2.5, O3, and NO2) exposure assessment study conducted in Baltimore, Maryland (USA), suggested that for PM2.5, ambient concentrations available from local monitoring stations might be adequate surrogates for total personal exposures (Schwartz et al. 2007). On the other hand, PMc levels tend to be less spatially homogeneous than PM2.5 (Monn 2001), increasing the likelihood that personal exposure will be misclassified in monitor-based studies of ambient PMc.
In conclusion, we found evidence indicating that PMc may play an important role in emergency hospitalizations for respiratory diseases independent of PM2.5 and other gaseous pollutants. Our findings in Hong Kong add to the growing body of literature concerning adverse health effects of PMc. However, further studies are needed to elucidate toxicological differences related to effects of PMc with different compositions under different situations of time and place and to identify PMc component(s) posing the greatest health risk.
Acknowledgments
We thank the Hong Kong Environmental Protection Department for providing air pollution data, the Hong Kong Observatory for providing temperature and humidity data, and the Hospital Authority for providing hospital admissions data. We also thank W. Goggins for his advice on statistical modeling.
Footnotes
The authors declare they have no actual or potential competing financial interests.
References
- Almeida SM, Pio CA, Freitas MC, Reis MA, Trancoso MA. Approaching PM2.5 and PM2.5–10 source apportionment by mass balance analysis, principal component analysis and particle size distribution. Sci Total Environ. 2006;368:663–674. doi: 10.1016/j.scitotenv.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Anderson HR, Bremner SA, Atkinson RW, Harrison RM, Walters S. Particulate matter and daily mortality and hospital admissions in the West Midlands conurbation of the United Kingdom: associations with fine and coarse particles, black smoke and sulphate. Occup Environ Med. 2001;58:504–510. doi: 10.1136/oem.58.8.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RW; APHEA 2 Project. 2004Acute effects of air pollution on admissions: reanalysis of APHEA 2. Am J Respir Crit Care Med 1691257–1258. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Peng RD, Walker J, Samet JM, Zeger SL, et al. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 U.S. counties, 1999–2005. Am J Epidemiol. 2008;168:1301–1310. doi: 10.1093/aje/kwn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Samet JM, Dominici F. Time-series studies of particulate matter. Annu Rev Public Health. 2004;25:247–280. doi: 10.1146/annurev.publhealth.25.102802.124329. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J. 2005;26:309–318. doi: 10.1183/09031936.05.00001805. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Smith-Doiron M, Stieb D, Cakmak S, Brook JR. Effects of particulate and gaseous air pollution on cardiorespiratory hospitalizations. Arch Environ Health. 1999;54:130–139. doi: 10.1080/00039899909602248. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Q, Krewski D, Burnett RT, Shi Y, McGrail KM. The effect of coarse ambient particulate matter on first, second, and overall hospital admissions for respiratory disease among the elderly. Inhal Toxicol. 2005;17:649–655. doi: 10.1080/08958370500189420. [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Q, Krewski D, Shi Y, Burnett RT, McGrail K. Influence of relatively low level of particulate air pollution on hospitalization for COPD in elderly people. Inhal Toxicol. 2004;16:21–25. doi: 10.1080/08958370490258129. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert N. Fine particles and human health—a review of epidemiological studies. Toxicol Lett. 2004;149:235–242. doi: 10.1016/j.toxlet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Department. Hong Kong’s Air Quality Objectives. 1987. Available: http://www.epd-asg.gov.hk/english/backgd/hkaqo.html [accessed 30 June 2010]
- Environmental Protection Department. A Study to Review Hong Kong’s Air Quality Objectives. 2009. Available: http://www.epd.gov.hk/epd/english/environmentinhk/air/studyrpts/files/Final_Report_091013.pdf. [accessed 30 June 2010]
- Halonen JI, Lanki T, Yli-Tuomi T, Tiittanen P, Kulmala M, Pekkanen J. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology. 2009;20:143–153. doi: 10.1097/EDE.0b013e31818c7237. [DOI] [PubMed] [Google Scholar]
- Host S, Larrieu S, Pascal L, Blanchard M, Declercq C, Fabre P, et al. Short-term associations between fine and coarse particles and hospital admissions for cardiorespiratory diseases in six French cities. Occup Environ Med. 2008;65:544–551. doi: 10.1136/oem.2007.036194. [DOI] [PubMed] [Google Scholar]
- Ilabaca M, Olaeta I, Campos E, Villaire J, Tellez-Rojo MM, Romieu I. Association between levels of fine particulate and emergency visits for pneumonia and other respiratory illnesses among children in Santiago, Chile. J Air Waste Manag Assoc. 1999;49:154–163. doi: 10.1080/10473289.1999.10463879. [DOI] [PubMed] [Google Scholar]
- Ito K. In: Revised Analyses of the National Morbidity, Mortality, and Air Pollution Study, Part II: Health Effects Institute, Special Report. Revised Analyses of Time-Series Studies of Air Pollution and Health. Boston:Health Effects Institute, 143–156; 2003. Association of particulate matter components with daily mortality and morbidity in Detroit, Michigan. [Google Scholar]
- Kan HD, London SJ, Chen GH, Zhang YH, Song GX, Zhao NQ, et al. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environ Int. 2007;33:376–384. doi: 10.1016/j.envint.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko FWS, Tam W, Wong TW, Chan DPS, Tung AH, Lai CKW, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax. 2007;62:780–785. doi: 10.1136/thx.2006.076166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Wong WHS, Lau YL. Association between air pollution and asthma admission among children in Hong Kong. Clin Exp Allergy. 2006;36:1138–1146. doi: 10.1111/j.1365-2222.2006.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Tertre A, Medina S, Samoli E, Forsberg B, Michelozzi P, Boumghar A, et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Community Health. 2002;56:773–779. doi: 10.1136/jech.56.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. The influence of ambient coarse particulate matter on asthma hospitalization in children: case-crossover and time-series analyses. Environ Health Perspect. 2002;110:575–581. doi: 10.1289/ehp.02110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Stieb DM, Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics. 2005;116:e235–e240. doi: 10.1542/peds.2004-2012. [DOI] [PubMed] [Google Scholar]
- Lipsett M, Hurley S, Ostro B. Air pollution and emergency room visits for asthma in Santa Clara County, California. Environ Health Perspect. 1997;105:216–222. doi: 10.1289/ehp.97105216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monn C. Exposure assessment of air pollutants: a review on spatial heterogeneity and indoor/outdoor/personal exposure to suspended particulate matter, nitrogen dioxide and ozone. Atmos Environ. 2001;35:1–32. [Google Scholar]
- Morris RD. Airborne particulates and hospital admissions for cardiovascular disease: a quantitative review of the evidence. Environ Health Perspect. 2001;109(suppl 4):495–500. doi: 10.1289/ehp.01109s4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. 1999An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect 107489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Chang HH, Bell ML, McDermott A, Zeger SL, Samet JM, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among medicare patients. JAMA. 2008;299:2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Williams GM, Tong S. Does particulate matter modify the association between temperature and cardiorespiratory diseases? Environ Health Perspect. 2006;114:1690–1696. doi: 10.1289/ehp.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Neas LM. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren. Epidemiology. 2000;11:6–10. doi: 10.1097/00001648-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Sarnat JA, Coull BA, Wilson WE. Effects of exposure measurement error on particle matter epidemiology: a simulation using data from a panel study in Baltimore, MD. J Expo Sci Environ Epidemiol. 2007;17(suppl 2):S2–S10. doi: 10.1038/sj.jes.7500619. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Spix C, Touloumi G, Bacharova L, Barumamdzadeh T, leTertre A, et al. Methodological issues in studies of air pollution and daily counts of deaths or hospital admissions. J Epidemiol Community Health. 1996;50(suppl 1):S3–S11. doi: 10.1136/jech.50.suppl_1.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter JC, Kim E, Sheppard L, Sullivan JH, Larson TV, Claiborn C. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J Expo Anal Environ Epidemiol. 2005;15:153–159. doi: 10.1038/sj.jea.7500382. [DOI] [PubMed] [Google Scholar]
- Tecer LH, Alagha O, Karaca F, Tuncel G, Eldes N. Particulate matter (PM2.5, PM10–2.5, and PM10) and children’s hospital admissions for asthma and respiratory diseases: a bidirectional case-crossover study. J Toxicol Environ Health A. 2008;71:512–520. doi: 10.1080/15287390801907459. [DOI] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. London: Chapman and Hall; 1990. Generalized Additive Models. [Google Scholar]
- Wong CM, Atkinson RW, Anderson HR, Hedley AJ, Ma S, Chau PYK, et al. A tale of two cities: effects of air pollution on hospital admissions in Hong Kong and London compared. Environ Health Perspect. 2002;110:67–77. doi: 10.1289/ehp.0211067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CM, Ou CQ, Chan KP, Chau YK, Thach TQ, Yang L, et al. The effects of air pollution on mortality in socially deprived urban areas in Hong Kong, China. Environ Health Perspect. 2008a;116:1189–1194. doi: 10.1289/ehp.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CM, Vichit-Vadakan N, Kan HD, Qian ZM. Public Health and Air Pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environ Health Perspect. 2008b;116:1195–1202. doi: 10.1289/ehp.11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TW, Lau TS, Yu TS, Neller A, Wong SL, Tam W, et al. Air pollution and hospital admissions for respiratory and cardiovascular diseases in Hong Kong. Occup Environ Med. 1999;56:679–683. doi: 10.1136/oem.56.10.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TW, Tam WS, Yu TS, Wong AH. Associations between daily mortalities from respiratory and cardiovascular diseases and air pollution in Hong Kong, China. Occup Environ Med. 2002;59:30–35. doi: 10.1136/oem.59.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TW, Tam W, Yu ITS, Wun YT, Wong AHS, et al. Association between air pollution and general practitioner visits for respiratory diseases in Hong Kong. Thorax. 2006;61:585–591. doi: 10.1136/thx.2005.051730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Geneva: World Health Organization; 1975. International Statistical Classification of Diseases, Injuries, and Causes of Death. 9th Revision. [Google Scholar]