Abstract
Although erythropoietin (Epo) is commonly used as a therapy for anemia, recent studies have suggested that Epo therapy is associated with adverse outcomes. A new study shows that polymeric IgA1 positively regulates erythropoiesis through binding to transferrin receptor 1 (Tfr1), suggesting new therapeutic routes for anemia (pages 1456–1465).
Low erythrocyte count, or anemia, is a major clinical problem that contributes to the morbidity and mortality associated with many diseases and that has a negative impact on quality of life. Anemia can be caused by direct defects in the erythropoietic machinery, as observed in congenital anemias such as thalassemia and sickle cell anemia, or by indirect causes, such as in the chronic anemia that can accompany inflammation.
For many years, recombinant human Epo has been used as a front-line therapy for anemia, as it stimulates erythropoiesis in people who cannot make Epo or in whom inflammation has inhibited erythropoiesis. Epo has improved quality of life and decreased the dependence of patients on blood transfusions, but recent studies have identified risks associated with Epo therapy. Several studies have associated Epo therapy, especially those regimens with high hemo globin target values, with increased risk of venous thromboembolic events1. Increased mortality has been observed in patients with cancer undergoing Epo therapy, and recent work has shown that Epo stimulation of Epo receptors (EpoRs) on breast cancer cells can antagonize the effects of chemotherapeutic agents2. Owing to these risks of Epo therapy, new ways of stimulating erythropoiesis are needed.
Decades of work have shown that erythropoietic capacity far exceeds what is necessary to maintain steady-state erythrocyte numbers3. A rational approach to identifying new targets for anemia therapy is to study the mechanisms that regulate elevated erythroid output at times of acute or chronic stress. For example, hypoxia has long been known to stimulate erythropoiesis. Mutations in hypoxia-inducible transcription factor 2α and in its negative regulator von Hippel-Lindau disease tumor suppressor lead to erythrocytosis4,5. So, logically, drugs that activate hypoxia-inducible transcription factor should augment erythropoiesis, as recently shown by Flygare et al.6.
Although the ‘rational’ approach is often success ful, sometimes an unexpected observation is made that could lead to the identification of a new therapeutic target. In this issue of Nature Medicine, Coulon et al.7 make such an observation and identify polymeric IgA1 (pIgA1) as a novel erythropoiesis-stimulating agent. IgA is one of the potential antibody iso-types generated by B cells during infection. In humans, only a small fraction of IgA is pIgA, which consists of a polymer of IgA molecules that are covalently attached by their J chains. Earlier work from the same group showed that pIgA1 binds transferrin receptor-1 (TfR1)8. Erythropoiesis requires iron, and TfR1 allows the transport of iron bound to transferrin into erythroid progenitors. However the new data of Coulon et al.7 show that, in addition to iron transport, TfR1 has another role in erythropoiesis.
Using both mouse and human erythroid progenitors, Coulon et al.7 show that pIgA1 improves the response to suboptimal Epo stimulation, which may be the relevant physiological state. Biochemically, pIgA1 stimulation results in increased activation of Akt and Erk signaling when erythroid cells are stimulated by low levels of Epo that normally only minimally activate these pathways. Akt activation can bypass the need for Jak2-Stat5 signaling in fetal liver erythroid progenitors9, which fits with the observation of these authors that pIgA1 does not affect the activation of the Jak2-Stat5 pathway7. This activation of Akt and Erk by pIgA1 seems to be a general response to TfR1 activation as iron-bound transferrin (Fe-Tf) and antibodies to TfR1 also augment the activation of these pathways in erythroid cells. Interestingly, the authors show that pIgA1 and Fe-Tf bind independently to TfR1 and that their effects on EpoR signaling are additive7. This observation suggests that both these ligands may upregulate erythropoiesis in response to anemic stress. Indeed, pIgA1 and Fe-Tf most likely act together, given that under physiological conditions most TfR1 will be bound by Fe-Tf.
The mechanism by which pIgA1 potentiates EpoR signaling is less clear. The cytoplasmic domain of TfR1 is small and contains no known signaling motifs. Although there are several potential phosphorylation sites in the cytoplasmic tail of TfR1, the authors show that TfR1 mediates its effect on EpoR signaling through Tyr20 (ref. 7), which is part of a motif that regulates receptor endocytosis. TfR2 also binds EpoR, but its function in EpoR signaling seems to be limited to facilitating transport of EpoR from the Golgi to the plasma membrane10. Further work will be needed to understand the role of this endocytosis motif in TfR1 signaling and how it couples to EpoR signaling in erythroid cells.
Coulon et al.7 also modeled the pIgA1 and TfR1 system in vivo to extend their in vitro findings. They used a human α1 knock-in (α1KI) mouse model because the mouse Igα heavy chain does not contain the hinge region that interacts with TfR1. Compared with control mice, α1KI mice recovered significantly faster from anemia induced by the chemotherapy drug 5-fluorouracil or hypoxia and hemolytic anemia induced by either anti–red blood cell serum or phenylhydrazine. These effects are negated when the α1KI-encoding allele is crossed onto a J chain-negative background, which prevents the formation of pIgA. The authors also found that human pIgA1 injected into immunodeficient NOD-SCID mice leads to an expansion of erythroid progenitor cells. Furthermore, individuals with IgA deficiency have an increased serum Epo concentration, suggesting that compensatory erythropoiesis occurs in these individuals. These data all support a role for pIgA1 in augmenting erythropoiesis in response to anemic stress.
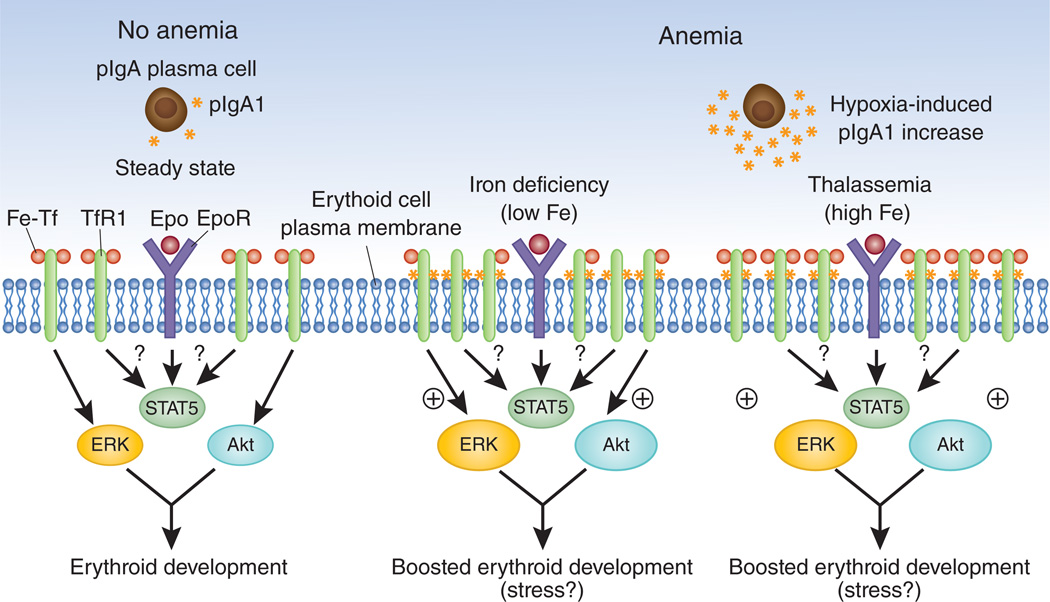
pIgA1 is produced by plasma cells, and on the basis of what is known about plasma cells, there is no reason to assume a connection between pIgA1 production and a response to anemia. However, Coulon et al.7 convincingly show that hypoxia increases pIgA1 production in the α1KI mice, and humans with chronic hypoxic conditions also have higher levels of pIgA1 in their serum compared to healthy volunteers. On the basis of these observations, the authors present a model where anemia leads to tissue hypoxia, which increases pIgA1 concentrations7. Fe-Tf and pIgA1 can stimulate TfR1 to boost erythroid output (Fig. 1). The role of pIgA1 becomes more important in iron deficiency anemia, in which transferrin saturation is low, limiting the ability of Fe-Tf to stimulate erythropoiesis. This model where activation of TfR1 by different ligands boosts erythropoiesis also explains why iron supplementation therapy reduces the requirement for Epo in the treatment of patients with anemia11 and why treatment with transferrin boosts erythropoiesis in thalassemic mice12,13.
Figure 1.
pIgA1 and Fe-Tf bind TfR1 to stimulate Epo-dependent erythroblast proliferation and development. Coulon et al.7 present a new model of erythropoiesis, which might allow the development of new therapeutic approaches for anemia and other disorders associated with dyserythropoiesis. Under steady-state conditions (left), low concentrations of pIgA1 are produced by plasma cells, and most TfR1 is bound by Fe-Tf, with little stimulation of downstream ERK and Akt signaling pathways. Stress conditions such as hypoxia can lead to increased pIgA1 production, allowing erythroid development to be boosted via ERK and Akt signaling. The role of pIgA1 becomes more important in iron deficiency anemia, where Tf saturation is low, limiting the ability of Fe-Tf to stimulate erythropoiesis.
In addition to TfR1, IgA1 also binds CD89 (Fcα receptor). CD89 activation leads to reduced proinflammatory cytokine production and phagocytosis of erythrocytes, and previous work has shown that activation of CD89 by IgA1 is anti-inflammatory13. Proinflammatory cytokines such as interferon-γ and tumor necrosis factor-α inhibit erythropoiesis, so downregulating their expression may also help to improve erythropoietic output. Therefore, increasing the expression of (p)IgA1 during the response to anemia may improve erythropoiesis in two ways: first, by preventing phagocytic destruction of erythrocytes and the production of erythropoiesis-inhibiting cytokines through CD89, and, second, by boosting Epo-dependent erythropoiesis through TfR1. The potential roles of pIgA1-TfR1 and IgA1-CD89 in the response to anemia induced by chronic inflammation suggest that the IgA pathway could be a target for new anemia therapies, but further work is required to test this possibility.
Another issue concerns the target of pIgA1-TfR1 signaling. The α1KI mice showed a much greater stimulation of erythropoiesis in the spleen than in bone marrow7. In the mouse, spleen erythropoiesis is primarily erythropoiesis in response to stress, which relies on signals and progenitor cells that are distinct from those involved in bone marrow erythropoiesis14. Stress erythropoiesis is better understood in mice, in which hypoxia is known to induce bone morphogenetic protein 4–dependent expansion of stress erythroid progenitors. It will be of interest to test whether pIgA1 is part of the hypoxia-dependent stress erythropoiesis pathway or whether it upregulates both steady-state and stress erythropoiesis.
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Dicato M, Plawny L. Curr. Opin. Oncol. 2010;22:307–311. doi: 10.1097/CCO.0b013e32833aa9de. [DOI] [PubMed] [Google Scholar]
- 2.Groner B, Hynes NE. Cancer Cell. 2010;18:401–402. doi: 10.1016/j.ccr.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 3.Socolovsky M. Curr. Opin. Hematol. 2007;14:215–224. doi: 10.1097/MOH.0b013e3280de2bf1. [DOI] [PubMed] [Google Scholar]
- 4.Ang SO, et al. Nat. Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 5.Percy MJ, et al. N. Engl. J. Med. 2008;358:162–168. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flygare J, Rayon Estrada V, Shin C, Gupta S, Lodish HF. Blood. 2011;117:3435–3444. doi: 10.1182/blood-2010-07-295550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulon S, et al. Nat. Med. 2011;17:1456–1465. doi: 10.1038/nm.2462. [DOI] [PubMed] [Google Scholar]
- 8.Moura IC, et al. J. Exp. Med. 2001;194:417–425. doi: 10.1084/jem.194.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghaffari S, et al. Blood. 2006;107:1888–1891. doi: 10.1182/blood-2005-06-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forejtnikovà H, et al. Blood. 2010;116:5357–5367. doi: 10.1182/blood-2010-04-281360. [DOI] [PubMed] [Google Scholar]
- 11.Auerbach M, et al. J. Clin. Oncol. 2004;22:1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 12.Li H, et al. Nat. Med. 2010;16:177–182. doi: 10.1038/nm.2073. [DOI] [PubMed] [Google Scholar]
- 13.Pasquier B, et al. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Paulson RF, Shi L, Wu DC. Curr. Opin. Hematol. 2011;18:139–145. doi: 10.1097/MOH.0b013e32834521c8. [DOI] [PMC free article] [PubMed] [Google Scholar]