Abstract
The global prevalence of dementia is estimated to be as high as 24 million, and is predicted to double every 20 years through to 2040, leading to a costly burden of disease. Alzheimer disease (AD) is the leading cause of dementia and is characterized by a progressive decline in cognitive function, which typically begins with deterioration in memory. Before death, individuals with this disorder have usually become dependent on caregivers. The neuropathological hallmarks of the AD brain are diffuse and neuritic extracellular amyloid plaques—which are frequently surrounded by dystrophic neurites—and intracellular neurofibrillary tangles. These hallmark pathologies are often accompanied by the presence of reactive microgliosis and the loss of neurons, white matter and synapses. The etiological mechanisms underlying the neuropathological changes in AD remain unclear, but are probably affected by both environmental and genetic factors. Here, we provide an overview of the criteria used in the diagnosis of AD, highlighting how this disease is related to, but distinct from, normal aging. We also summarize current information relating to AD prevalence, incidence and risk factors, and review the biomarkers that may be used for risk assessment and in diagnosis.
Introduction
Dementia is characterized by deterioration in cognition, function and behavior, and places a considerable burden on society. In the US alone, Alzheimer disease (AD)—the most frequent cause of dementia—is associated with an estimated health-care cost of US$172 billion per year.1
The key pathological changes that are observed in AD brain tissue are increased levels of both the amyloid-β (Aβ) peptide, which is deposited extracellularly in diffuse and neuritic plaques, and hyperphosphory-lated tau (p-tau), a microtubule assembly protein that accumulates intracellularly as neurofibrillary tangles (nFts). In addition to these pathologies, widespread loss of neurons and synapses is observed. The mechanisms underlying the changes outlined above are unknown but, when present, these neuro pathological features confer a diagnosis of definite AD, according to current diagnostic criteria (see below).
In this article, we review the criteria used to diagnose AD, and summarize current knowledge regarding AD prevalence, incidence, and genetic and nongenetic risk factors. We also examine the value and limitations of biomarkers that may be used to determine AD risk and to aid diagnosis of this disease.
Diagnostic criteria
Over the past century, the classification of dementia subtypes has been revised repeatedly (Box 1). The key classification for the diagnosis of AD has been the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS–ADRDA; Box 2) criteria, which were established in 1984.1 These criteria link clinical patterns to neuropathology and use the labels possible, probable and definite AD in patient diagnosis.2 Despite the widespread use of this classification, several notable issues are associated with the extrapolation of clinicopathological findings for diagnosis (Box 3).
Box 1 | Historical perspective on the AD diagnosis.
1906–1950
During this period, individuals aged <65 years who developed dementia were diagnosed as having AD or presenile dementia when no other known causes of dementia were present. Individuals with dementia who had a strong history of vascular disease and exhibited executive symptoms were labeled as having multi-infarct dementia, independent of their age. Patients who developed dementia aged >65 years and for whom no other cause of this condition was known were diagnosed as having senile dementia. This form of dementia was understood to be vascular and prompted the use of vasodilators.
1950–1980
In this period, late-onset AD was recognized to be pathologically indistinguishable from the early-onset form of this disease. In addition, the vascular explanation for senile dementia was largely abandoned. Consequently, the age criterion for the label AD was lifted, the term senile dementia was abandoned, and the diagnosis of AD was assigned when all other possible causes of cognitive impairment were excluded. In addition, a key publication by Katman and colleagues noted the “malignancy” of AD.205
1980–present
The vascular component of late-onset AD has again been recognized. In 1984, the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association criteria were implemented. The key to these criteria is the linkage of clinical syndromal patterns to neuropathology after death through the use of possible, probable and definite labels for AD.2
Abbreviation: AD, Alzheimer disease.
Box 2 | The NINCDS–ADRDA criteria.
The NINCDS–ADRDA criteria categorize AD as definite, probable or possible.2 A diagnosis of definite AD requires supporting pathological evidence. Probable AD is the maximum level of certainty possible without pathological confirmation. This category requires a gradual onset and progressive decline in memory with involvement of at least one other cognitive domain that is established by clinical examination and confirmed by neuropsychological tests. The NINCDS–ADRDA criteria further require that the patient is fully conscious and does not have another condition that might explain their symptoms. Other supportive features of probable AD include evidence of progressive deterioration of language, praxis and visual recognition, with impaired activities of daily living or a positive family history of AD. Normal levels of routine cerebrospinal fluid measures, evidence of progressive cerebral atrophy on brain imaging and a normal or nonspecific pattern on EEG support probable AD diagnosis. Features that make the diagnosis of probable AD unlikely include an apoplectic onset, the presence of focal neurological findings (such as hemiparesis or sensory loss), and the presence of seizures or gait disturbances. Patients meet criteria for possible AD when the course of cognitive decline is atypical, focal neurological findings are evident, or disorders coexist that by themselves can explain the dementia, such as stroke or traumatic brain injury. The DSM-IV criteria for AD require a gradual onset and progressive impairment in memory function and at least one other cognitive domain that results in impairment of social and occupational function. Such cognitive impairment would not be explained by other psychiatric, neurological or systemic diseases. The NINCDS–ADRDA and DSM criteria are currently being revised.
Abbreviations: AD, Alzheimer disease; DSM, Diagnostic and Statistical Manual of Mental Disorders; NINCDS–ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association.
Box 3 | Issues relating to the diagnosis of AD.
Two notable issues surround the use of neuropathological studies to inform the diagnosis of AD. First, neuropathological studies are usually end-stage disease investigations and extrapolation of the findings of such studies to living patients in earlier stages of disease can be difficult. Second, neuropathological findings can differ between patients with similar clinical profiles before death. Thus, clinical diagnoses are not always in agreement with clincopathological diagnoses, which indicates that the relationship between clinical profiles and age is not stable. Estimates of AD prevalence and incidence will continue to vary until the key underlying pathologies of the disease have been identified, the relationship of specific pathology to clinical phenotype is understood, and the clinical diagnosis of AD is improved.
Abbreviation: AD, Alzheimer disease.
The original NINCDS–ADRDA criteria have received updates and undergone revisions, and have been incorporated into major international criteria, including the Diagnostic and Statistical Manual of Mental Disorders (DSM; Box 2) and the International Classification of Diseases. The NINCDS–ADRDA and DSM criteria are again under review, as the AD spectrum is now understood to be broader than was previously thought: pathological changes (such as cerebrovascular changes) other than the key AD pathologies (that is, amyloid plaques and NFTs) are now believed to precede or coexist with AD, and contribute to the cognitive and physical dysfunction observed in this disorder. The use of biomarkers may increase diagnostic specificity for AD and, consequently, will be considered in the updated criteria.
Since its instigation, the definition of an intermediate state between normal cognition and dementia, namely mild cognitive impairment (MCI), has been modified and investigated in many settings. In clinical settings, where cognitive impairment is most likely to be detected, MCI has proved a useful label to define people who are at risk of developing AD. In population settings, however, where cognitive impairment is less likely to be detected, this label has proved to be less valuable.
Prevalence and incidence
By 2005, 24.2 million people worldwide had dementia and 4.6 million new cases of this condition were arising every year (Table 1);3 ≈ 70% of these cases were attributed to AD. Among regional populations of individuals aged ≥60 years, those from North America and Western Europe exhibited the highest prevalence of dementia (6.4% and 5.4%, respectively), followed by those from Latin America (4.9%) and China and its western-Pacific neighbours (4.0%). Meanwhile, the annual regional dementia incidence rates (per 1,000 individuals in the population) were estimated to be 10.5 for North America, 8.8 for Western Europe, 9.2 for Latin America, and 8.0 for China and its western-Pacific neighbours.3 For all these populations, the incidence rate for dementia increased exponentially with age, with the most notable rise occurring through the seventh and eighth decades of life. The prevalence and incidence rates for AD also increase exponentially with age (Supplementary Figure 1 online).
Table 1.
Prevalence and incidence of dementia in developed and developing regions
| Region | Consensus dementia prevalence at age ≥60 years (%) |
Estimated annual incidence of dementia (per 1,000 individuals) |
People with dementia aged ≥60 years in 2001 (millions) |
Estimated increase in proportion of people with dementia from 2001 to 2040 (%) |
|---|---|---|---|---|
| Western Europe | 5.4 | 8.8 | 4.9 | 102 |
| Eastern Europe (regions with low adult mortality) | 3.8 | 7.7 | 1.0 | 169 |
| Eastern Europe (regions with high adult mortality) | 3.9 | 8.1 | 1.8 | 84 |
| North America | 6.4 | 10.5 | 3.4 | 172 |
| Latin America | 4.6 | 9.2 | 1.8 | 393 |
| North Africa and Middle Eastern Crescent | 3.6 | 7.6 | 1.0 | 385 |
| Developed western Pacific | 4.3 | 7.0 | 1.5 | 189 |
| China and developing western Pacific | 4.0 | 8.0 | 6.0 | 336 |
| Indonesia, Thailand and Sri Lanka | 2.7 | 5.9 | 0.6 | 325 |
| India and south Asia | 1.9 | 4.3 | 1.8 | 314 |
| Africa | 1.6 | 3.5 | 0.5 | 235 |
| Combined values | 3.9 | 7.5 | 24.3 | 234 |
Data taken from Ferri et al. (2005).3
The incidence rates of AD and dementia in people aged <75 years seem to be relatively similar across studies, but in the oldest age groups these rates vary (Supplementary Figure 1 online). Methodological issues partly account for the observed divergence; however, the estimates might also reflect geographical differences in age-dependent incidence owing to variation in survival and the prevalence of risk and protective factors.
Risk and protective factors
Various risk and protective factors have been linked to dementia and/or AD. Two types of analytical approaches have been used to identify such factors, namely observational studies (that is, case–control, cohort and cross-sectional studies) and experimental studies (that is, clinical trials). The strengths and weaknesses of such approaches are highlighted in Table 2.
Table 2.
Common types of epidemiological study design
| Study type | Methodological approach | Advantages | Disadvantages |
|---|---|---|---|
| Nonexperimental studies | |||
| Case–control study | Sampling conducted with respect to disease status Estimates the odds of having been exposed to a risk factor given the current case–control status |
Low costs in relation to efficiency | Disease and exposure status determined simultaneously, making the temporal sequence of events often difficult to establish |
| Cross-sectional study | Includes all individuals of a population regardless of exposure or disease status | Low costs in relation to efficiency | Disease and exposure status determined simultaneously, making the temporal sequence of events often difficult to establish Overrepresentation of cases with long duration and under-representation of cases with short duration of illness, leading to bias |
| Cohort study | Sampling conducted with respect to exposure status | Exposure status is ascertained before the occurrence of disease, allowing incidence rates of disease to be calculated in people with and without risk factors | Requires the follow-up of a large number of individuals until disease development and, hence, is costly Difficult to control confounding variables and maintain high follow-up rates Adverse outcomes may occur before the onset of the disease of interest, leading to survival bias |
| Experimental (randomized) studies | |||
| Clinical trial | Individuals are randomly assigned to intervention and comparison groups Aims to evaluate a cure or a preventive treatment (usually drugs) |
Minimizes bias and allows valid statistical testing, as factors that could potentially confound the examined association should occur with roughly equal frequencies in the intervention and comparison groups | Participants studied may not be representative of the general population |
Risk factors
Risk factors are antecedents of part of the disease pathway, and can be associated with the etiology or the outcome of a disease. Such factors may be used to assess disease risk but do not usually provide sufficient sensitivity and/or specificity to be employed as diagnostic markers.
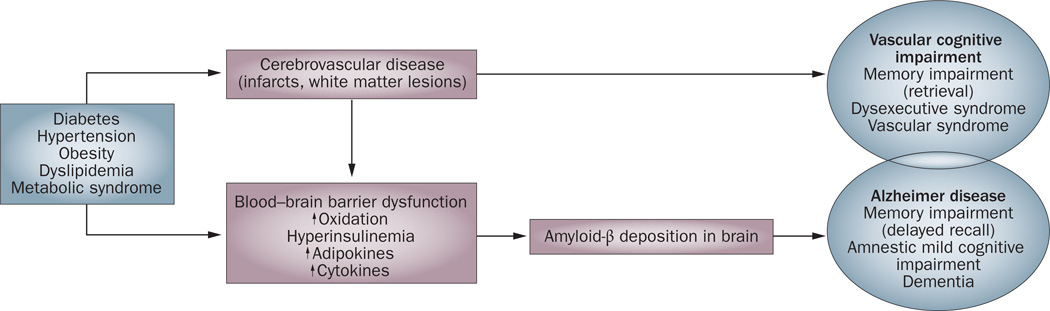
Various risk factors have been found to be associated with dementia and/or AD. Of note, many recognized vascular risk factors for ischemic heart disease and/or stroke are also risk factors for dementia. Diabetes, hyper tension, smoking and obesity have all been found to increase dementia risk. Nevertheless, while vascular risk factors and cerebrovascular disease clearly underlie vascular dementia, an etiological role for vascular changes in Aβ deposition and, hence, AD remains unclear. Figure 1 shows possible mechanisms linking vascular disease to dementia.
Figure 1.
Potential mechanisms linking vascular risk factors and cognitive impairment. At least two pathways exist that result in cognitive impairment and dementia: development of cerebrovascular disease may lead to vascular cognitive impairment syndromes, and deposition of amyloid-β may lead to other distinct amnestic clinical syndromes, including Alzheimer disease. In addition, these pathways may overlap and interact, resulting in mixed cognitive syndromes.
Cerebrovascular disease
Cerebrovascular changes such as hemorrhagic infarcts, small and large ischemic cortical infarcts, vasculopathies, and white matter changes all increase the risk of dementia. A meta-analysis incorporating data from 22 hospital-based and eight population-based cohorts4 found that 7.4% of patients with first-ever stroke developed poststroke dementia. Several mechanisms exist through which stroke could lead to cognitive impairment and AD. First, stroke may lead directly to damage of brain regions that are important in memory function, such as the thalamus and the thalamocortical projections. Second, stroke might increase Aβ deposition, which in turn can lead to cognitive decline. Third, the onset of stroke may induce inflammatory responses that impair cognitive function. Last, hypoperfusion can lead to overexpression of cyclin-dependent kinase 5 (CDK5), a serine–threonine kinase that is critical to synapse formation and synaptic plasticity and, hence, to learning and memory.5 Aberrant CDK5 activation is associated with neuronal apoptosis and death.6 This kinase may also be involved in the abnormal phosphorylation of tau, thereby contributing to the formation of NFTs,7 and might be a key protein linking NFT pathology to amyloid plaques.
Blood pressure
Inconsistencies exist between data from cross-sectional and longitudinal studies that have examined the effect of blood pressure on brain function. In part, these inconsistencies can be attributed to differences in study design; specifically, variation in the time between measurement of blood pressure and assessment of cognitive abilities, and in the age at which these parameters were measured. In contrast to the results of cross-sectional and longitudinal studies, data from observational studies exploring the association between elevated levels of blood pressure in midlife (40–60 years of age) and late-life cognitive impairment have proved to be relatively consistent across cohorts,8–11 although results relating to the association between late-life blood pressure levels and cognitive decline and dementia remain inconsistent.12–15
In middle age, elevated blood pressure increases the risk of cognitive impairment, dementia and AD. Hypertension may increase the risk of AD by decreasing the vascular integrity of the blood–brain barrier (BBB), resulting in protein extravasation into brain tissue.16 In turn, protein extravasation can lead to cell damage, a reduction in neuronal or synaptic function, apoptosis, and an increase in Aβ accumulation, resulting in cognitive impairment.17 With increasing age, the effect of elevated blood pressure on AD risk diminishes and may even become inverted, with an increase in blood pressure showing a protective effect. This observation might be explained by the fact that following the onset of AD, blood pressure begins to decrease, possibly as a result of vessel stiffening, weight loss and changes in the autonomic regulation of blood flow.
Seven randomized, placebo-controlled trials (RCTs) have evaluated the benefit of antihypertensive treatments in patients with cognitive impairment. Three studies showed that such agents had a beneficial effect. In the systeur trial, which included patients without dementia who were aged >60 years,18 the risk of dementia (diagnosed according to the revised DSM-III criteria) was reduced by 55% in the treatment group (nitren-dipine alone or in combination with enalapril maleate, hydrochlorothiazide or both add-on drugs) compared with the placebo group. In PROGRESS,19 which involved 6,105 individuals with prior stroke or transient ischemic attack, patients treated with perindopril (with or without indapamide) saw a 12% reduction in their risk of dementia (diagnosed according to DSM-IV criteria) in the absence of recurrent stroke, a 34% decrease in their risk of dementia with recurrent stroke, and a 54% drop in their risk of cognitive decline (decline of ≥3 points in the Mini Mental state examination [MMSE]) with recurrent stroke, in comparison with patients who received placebo. Among 81 community-screened, dementia-free individuals (aged >69 years, with no previous record of receiving antihypertensive treatment) who underwent captopril or bendrofluazid treatment in the HOPE trial,20 those in the most treatment-responsive quartile (blood pressure reduction ≥19 mmHg) had improved scores on the Anomalous sentences and Paired Associates Tests compared with those in the least treatment-responsive quartile (blood pressure reduction ≤5 mmHg). Nevertheless, other RCTs evaluating the benefit of antihypertensive treatment on cognitive decline (that is, the MRC study,21 SHEP,22 SCOPE23 and HYVET-COG24) have disclosed nonsignificant effects from such therapies, thereby failing to clarify the effect of blood-pressure-lowering medication on cognitive decline.
Type 2 diabetes
In observational studies, type 2 diabetes (T2D) has been found to nearly double the risk of AD.25–27 Various mechanisms have been proposed whereby diabetes might influence the development of AD. In cases of hyper-insulinemia accompanying diabetes, insulin may compete with Aβ for the insulin degrading enzyme (IDE), thereby hindering clearance of Aβ from the brain.28 Moreover, a histopathological study of hippocampal tissue from patients with AD and healthy controls showed relative reductions in IDE expression and IDE messenger RNA levels in AD brain tissue.29
Diabetes and impairment of glucose tolerance lead to the formation of advanced glycosylation end products (AGEs), and amyloid plaques and NFTs contain receptors for AGEs (RAGEs). Glycation of Aβ enhances its propensity to aggregate in vitro.30 In addition, RAGEs may facilitate the neuronal damage caused by Aβ,31 as the latter is a high-affinity ligand for cell-surface RAGEs.
Adipose tissue produces adipokines (such as adiponectin and leptin) and cytokines (including resistin, tumor necrosis factor [TNF] and interleukin [IL]-6), which are involved in metabolism and inflammation, respectively. Adipokine and cytokine levels increase with insulin resistance and hyperinsulinemia. Nevertheless, whether adipokines and cytokines are indicators of insulin resistance remains unclear, as is whether these signaling molecules are causally related to AD. Evidence suggests that leptin exerts beneficial effects on brain regions that are important for memory function, including the CA1 region of the hippocampus.32 Moreover, leptin receptor-deficient transgenic mice showed less synaptic plasticity and showed poorer performance on spatial memory tasks than did wild-type mice33 and, in the Framingham study, high circulating plasma leptin levels were associated with a decrease in AD risk and high cerebral brain volumes in cognitively normal adults, although these associations were restricted to non-obese people.34
A meta-analysis by Profenno et al., which included 10 longitudinal studies examining the relationship between T2D and AD,35 found that T2D increased the risk of AD by 54%. Nevertheless, studies examining the relationship between T2D and the neuropathological hallmarks of AD have proved inconsistent: while some studies showed that patients with diabetes had higher numbers of amyloid plaques or NFTs than healthy controls,36,37 other investigations found no association or an inverse relationship between such lesions and diabetes.37,38
Several RCTs have explored the effects of antidiabetic drugs on cognitive impairment. In a study by Reger and colleagues,39 intranasal administration of insulin altered cognitive performance in elderly patients with amnestic MCI or early AD in an apolipoprotein E (APOE) genotype-dependent fashion. In memory-impaired adults who did not harbor the APOE ε4 allele (a genetic risk factor for AD; see below), but not in APOE ε4-positive adults, insulin administration facilitated immediate recall at doses of 20 IU (P = 0.0006) and 40 IU (P = 0.0013) compared with placebo treatment. In a study of patients with AD or amnestic MCI by Watson and colleagues, which considered effect sizes (f2) of 0.02, 0.15 and 0.35 as small, medium and large effects, respectively, individuals who received 6 months of treatment with the peroxisome proliferator-activated receptor (PPAR)-γ agonist rosiglitazone (n = 20) showed moderate-to-large improvements in delayed recall (P = 0.0138; f2 = 0.31) and large improvements in selective attention (P = 0.0061; f2 = 0.38) compared with patients receving placebo (n = 10).40 A study of 511 patients with AD demonstrated that APOE ε4 noncarriers exhibited cognitive and functional improvement in response to rosiglitazone (mean changes in the AD Assessment Scale cognition subscale [ADAS-cog] for placebo and 8 mg rosiglita-zone were 1.5 and −2.0, respectively; P = 0.02).41 Finally, Sato et al. showed that daily treatment with 15–30 mg pioglitazone was associated with improvements in scores on the MMSE (23.1 ± 4.1 with pioglitazone versus 22.1 ± 3.5 with placebo) and on the ADAS-cog (142 ± 6.5 with pioglitazone versus 15.5 ± 5.9 with placebo) after 6 months (n = 21 in both treatment arms).42 Of note, PPARγ agonists have been shown to inhibit inflammatory gene expression, alter Aβ homeostasis and exhibit neuro protective effects.43
Body weight
Early studies examining body fat distribution and cognitive dysfunction showed that low BMI or being underweight seemed to be risk factors for dementia and age-related brain changes such as atrophy.44,45 Later prospective studies, however, have linked both low and high body weight to a heightened risk of AD and cognitive impairment, suggesting a U-shaped relationship between weight and cognitive performance.46–48 The association of body weight with the risk of AD seems to depend on the age at which body weight is measured. In addition, evidence exists for reverse causation in the years preceding dementia onset; that is, loss of body weight is caused by cognitive impairment during the prodromal phase of dementia.49 Of note, the relationship between body weight and cognitive impairment and dementia seems to be driven by central obesity.50 The aforementioned meta-analysis by Profenno et al. showed that obsesity (as assessed by BMI) increased the risk of AD by 59%.35
Plasma lipid levels
Conflicting data are available concerning the relationship between dyslipidemia and cognitive impairment or AD.51–56 Amyloid precursor protein (APP) can be broken down by enzymes, termed the secretases, via two routes, the nonamyloidogenic and amyloidogenic pathways. In the second pathway, APP is proteolytically cleaved by β-secretase and, subsequently, γ-secretase to gene rate Aβ, the most common isoforms of which comprise 40 (Aβ1–40) and 42 (Aβ1–42) amino acids, with the latter being the most fibrogenic of the two peptide species. Evidence exists that depletion of membrane cholesterol inhibits secretase cleavage of APP, thereby lowering Aβ1–40 and Aβ1–42 accumulation. Nevertheless, dyslipidemia increases the risk of vascular disease, which in turn is associated with a heightened risk of AD. In people at risk of cardiovascular and cerebrovascular disease, statins are the first-line treatments for reducing cholesterol levels. Three rCts—involving 748 participants aged 50–90 years—have explored the effect of statins on AD risk.58–56 Overall, these studies yielded insufficient evidence for a beneficial effect of statins on AD risk. The results of a large-scale trial of simvastatin to slow AD progression have yet to be published.61
Metabolic syndrome
Instead of exploring the effect of its subcomponents, several studies have assessed the relationship between metabolic syndrome as a whole and the risk of AD or cognitive decline. Most of these investigations demonstrated a positive association between the presence of this syndrome and cognitive dysfunction.62–64
Smoking
The relationship between smoking and cognitive decline remains uncertain. Case–control studies have largely suggested that smoking lowers the risk of AD,65–67 whereas prospective studies have shown that smoking increases this risk68–70 or has no effect on the probability of developing AD.71,72 A meta-analysis that examined the relationship between smoking and AD while accounting for tobacco-industry affiliation found that the combined results of 18 cross-sectional studies without industry affiliations yielded no association.73 By contrast, data from eight cross-sectional studies with tobacco-industry affiliations suggested that smoking protected against AD. Analysis of 14 cohort studies without tobacco-industry affiliations yielded a significant increase in the risk of AD in smokers.
Smoking could affect the risk of AD via several mechanisms. Smoking may increase the generation of free radicals, leading to high oxidative stress, or affect the inflammatory immune system, leading to activation of phagocytes and further oxidative damage.74 In addition, smoking may promote cerebrovascular disease. Evidence also exists, however, that smoking can have a protective effect against AD. Nicotine has been suggested to induce an increase in the level of nicotinic acetyl choline receptors, thereby counterbalancing the loss of these receptors, and subsequent cholinergic deficits, observed in AD.75
Depressive symptoms
Depressive symptoms occur in 40–50% of patients with AD. Some longitudinal and case–control studies have found an increase in the risk of AD or MCI in individuals with a history of depression,76,77 but other studies have been unable to link AD with this mood disorder.78,79 The potential mechanisms underlying the possible association between these conditions might involve vascular pathways and effects of depression on the hippocampal formation or the hypothalamic–pituitary–adrenal axis.
Psychological stress
Evidence from rodent studies suggests that chronic psychological stress can alter brain morphology (such as hippocampal structure) and, as a result, exert a detrimental effect on brain function, including memory.80 Thus, chronic psychological stress might increase the risk of AD.
Traumatic brain injury
Retrospective studies81–83 suggested that individuals with a history of traumatic brain injury (TBI) had a higher risk of dementia than individuals with no history of such injury. Two meta-analyses84,85 demonstrated that among patients with TBI, the risk of dementia was higher in men than in women. Prospective studies of the relationship between TBI and AD have proved inconsistent, 86–88 but postmortem and experimental studies support a link between these conditions. Evidence also exists that after human brain injury, the extent of Aβ pathology89 and tau pathology increases in brain tissue, cerebrospinal fluid (CSF) Aβ levels are elevated, and APP is overproduced.90
Protective factors
Diet
Diets high in fish, fruit and vegetables are high in antioxidants and polyunsaturated fatty acids (PUFAS). In some observational population-based studies, people who had a high intake of vitamins E and C (both antioxidants) were less likely to show cognitive decline and had a lower AD risk than individuals with a low intake of these vitamins.91–93 By contrast, other large prospective studies of the effects of vitamins on AD risk found no such associations,94,95 and investigations examining the effect of dietary PUFAs on the risk of cognitive dysfunction proved inconclusive. Indeed, while several studies showed that the consumption of PUFAs led to reductions in the risks of dementia and AD,96–98 MCI99 and age-related cognitive decline,100 other studies found no association between dietary PUFAs and cognitive impairment.101 Scarmeas et al. reported that consumption of a Mediterranean-type diet (MeDi)—a diet characterized by a high intake of plant foods and fish (with olive oil as the primary source of monounsaturated fat), a moderate intake of wine and a low intake of red meat and poultry—reduced the incidence of AD102 and showed a trend towards reducing the risk of MCI.103 These effects were independent of levels of physical activity104 and vascular comorbidity.105 In a subsequent cohort study in France, MeDi was found not to alter performance on the Isaacs Set Test, the Benton Visual Retention Test or the Free and Cued Selective Reminding Test, but was associated with high MMSE scores,106 providing some support for the findings from the initial studies of this diet. In a meta-analysis of 15 prospective studies exploring the effect of alcohol on dementia risk,107 light to moderate alcohol consumption was associated with a reduction in the risk of AD and dementia.
Most RCTs examining the effects of antioxidant supplementation have found no association with cognitive performance.108–111 To date, prospective clinical trial data for dietary supplementation with omega-3 PUFAs have shown no overall effect on cognition in patients with MCI or AD, but have suggested that docosahexaenoic acid supplementation has a beneficial effect on cognitive function in people harboring the APOE ε4 allele and in the earliest stages of AD.112,113
Reactive oxygen species are clearly associated with neuronal damage in AD; however, whether the presence of these molecules reflects a primary or secondary event in the neurotoxic process remains unclear. Deposition of Aβ, which is an early event in AD, leads to a decrease in cerebral iron and copper concentrations, resulting in oxidative stress and neuronal damage.114 Evidence from in vitro studies indicates that vitamin E reduces the extent of Aβ-induced lipid peroxidation and cell death.115 In addition, carotenes and vitamin C protect against lipid peroxidation.116 Furthermore, vitamin C reduces the formation of nitrosamines and may affect catecholamine synthesis.117,118 Evidence also exists that antioxidant intake reduces AD risk through a reduction in the risk of cerebrovascular disease.119 Besides reducing oxidative stress, PUFAs have favorable effects on neuronal and vascular functions and inflammatory processes.120,121
Physical activity
Epidemiological and experimental data suggest that physical exercise may promote brain health. Conflicting results have, however, emerged from cross-sectional and longitudinal observational studies that examined the relationship between exercise levels and cognitive decline or dementia: while some studies indicated that physical activity has a beneficial effect on brain health, others showed no association between these variables.122–126
Physical activity could affect cognition via multiple mechanisms. An improvement in aerobic fitness increases cerebral blood flow, oxygen extraction and glucose utilization,122 and activates growth factors that promote structural brain changes, such as an increase in capillary density.128 In addition, rodent studies suggest that physical activity decreases the rate of amyloid plaque formation.129
RCTs exploring the effects of exercise on cognitive function in healthy elderly individuals have yielded conflicting results.130–133 A recent meta-analysis that included 11 RCTs involving cognitively healthy people aged >55 years suggested that undertaking of aerobic physical activities improves selective cognitive functions, including cognitive speed, as well as auditory and visual attention.134
Intellectual activity
Following initial reports that elderly people with higher levels of education had a lower incidence of dementia than individuals with no education, cognitive activity was suggested to decrease the risk of cognitive decline by increasing cognitive reserve. Several prospective studies subsequently found that both young135 and old136 people who engage in cognitively stimulating activities, such as learning, reading or playing games, were less likely to develop dementia than individuals who did not engage in these activities.
RCTs have shown a beneficial effect of intellectual interventions on cognitive function in elderly, dementia-free individuals.137 The benefits of cognitive training seem to be domain specific, however. Several trials found that while cognitive training can improve memory, reasoning and mental processing speed in older adults,137 cognitive training did not have an effect on all cognitive domains, and did not affect day-to-day functioning.138 In addition, one study found that among elderly individuals, those with memory impairment showed less improvement in cognition through memory training than those without such impairment.139 Consequently, in elderly people, the effect of cognitive training on the risk of dementia is unclear, but several trials are underway.
Genetic epidemiology
AD is usually classified according to its age of onset. The majority (>95%) of patients who develop this disease are aged >65 years (so-called late-onset AD), with 1–5% of AD cases exhibiting an earlier onset, typically in the late 40s or early 50s (so-called early-onset AD). Late-onset and early-onset AD are clinically indistinguishable; however, the latter is generally more severe than the former and is associated with a more rapid rate of progression. Moreover, the two forms of AD are associated with different patterns of genetic epidemiology.
Early-onset Alzheimer disease
Three genes have been firmly implicated in the pathophysiology of early-onset AD, namely APP itself, and the presenilin genes (PSEN1 and PSEN2), which encode proteins involved in APP breakdown and Aβ generation. AD-linked mutations in these three genes can be considered to be ‘diagnostic biomarkers’ of this disease: these mutations exhibit high penetrance (>85%), mostly show autosomal dominant inheritance, and lead with certainty to Aβ aggregation and early-onset disease.
APP mutations account for <0.1% of AD cases. Most dominantly inherited AD-linked missense mutations in APP affect the processing of the encoded protein, since the mutations are positioned in or near the Aβ-coding exons (APP exons 16 and 17).140 In addition to these dominant mutations, the APP mutation spectrum extends to two recessive mutations (which only cause disease in the homozygous state), as well as APP locus duplications, underscoring the importance of APP gene dosage in AD.140
At present, 182 different AD-related mutations from 401 families have been identified in PSEN1, while only 14 AD-linked mutations from 23 families have been detected in PSEN2.140 The majority of AD-linked PSEN1 and PSEN2 mutations are single-nucleotide substitutions, but small deletions and insertions have been described as well. The presenilins are functionally involved in the γ-secretase-mediated proteolytic cleavage of APP.141 Mutations in PSEN1 and PSEN2 impair this cleavage and cause an increase in the Aβ1–42:Aβ1–40 ratio. This rise might occur through either an increase in Aβ1–42 levels, as indicated in plasma and fibroblast media from PSEN-mutation carriers,142 or a decrease in Aβ1–40 levels, suggesting a loss-of-function rather than a gain-of-function mechanism.
To summarize, all three causal AD genes lend support to a common pathogenic AD pathway, with a pivotal role for Aβ. According to this amyloid hypothesis, neurodegenerative processes in AD are the consequence of an imbalance between aβ production and Aβ clearance, suggesting that other genes involved in these pathways might also be risk factors for this disease.
Late-onset Alzheimer disease
The genes involved in late-onset AD increase disease risk and are not inherited in a Mendelian fashion. First-degree relatives of patients with late-onset AD have twice the expected lifetime risk of this disease of people who do not have an AD-affected first-degree relative.143 In addition, AD occurs more frequently in monozygotic than in dizygotic co-twins,144 suggesting a substantial genetic contribution to this disorder. In the largest twin study of dementia, involving 11,884 participants in the swedish registry who were aged >65 years, 395 twin pairs were identified in which either one or both twins had AD.144 This study demonstrated a heritability of 58–79% for late-onset AD, depending on the model that was used in the data analysis.
Apolipoprotein E
APOE is the only established susceptibility gene for late-onset AD and maps to chromosome 19 in a cluster with the genes encoding translocase of outer mitochondrial membrane 40 (TOMM40), apolipoprotein C1 and apolipo-protein C2. APOE is a lipid-binding protein that is expressed in humans as one of three common isoforms, which are encoded by three different alleles, namely APOE ε2, APOE ε3 and APOE ε4. The presence of a single APOE ε4 allele is associated with a 2–3-fold increase in the risk of AD, while the presence of two copies of this allele is associated with a fivefold increase in the risk of this disease. Each inherited APOE ε4 allele lowers the age of AD onset by 6–7 years.145–147 Furthermore, the presence of this allele is associated with memory impairment, MCI, and progression from MCI to dementia.148 APOE ε4 has been suggested to account for as much as 20–30% of AD risk.
Despite the studies linking APOE ε4 with AD, the presence of this allele is neither necessary nor sufficient for disease: among participants in the Framingham study,149 55% of those who were homozygous for APOE ε4, 27% of those with one copy of this allele and 9% of those without an APOE ε4 allele developed AD by 85 years of age. Segregation analyses conducted in families of patients with AD support the presence of at least four to six additional major AD risk genes.150
Additional genetic risk variants
After APOE, the best-validated gene modulating late-onset AD risk is the sortilin-related receptor 1 (SORL1) gene, which is located on chromosome 11q23. SorL1 belongs to a group of five type I transmembrane receptors (the others being sortilin, SorCS1, SorCS2 and SorCS3) that are highly expressed in the CNS and are characterized by a luminal, extracellular vacuolar protein sorting 10 domain. From family-based and population-based studies that, together, included over 6,000 individuals from four ethnic groups, Rogaeva et al. identified two haplotypes in the 3' and 5' regions of SORL1 that are associated with late-onset AD risk.151 In addition, these researchers demonstrated that SorL1 promotes the translocation and retention of APP in subcellular compartments that exhibit low secretase activity, thereby reducing the extent of proteolytic breakdown into both amyloidogenic and nonamyloidogenic products.151 As a consequence, under-expression of SORL1 leads to overexpression of Aβ and an increased risk of AD. Several subsequent studies replicated these initial genetic association findings, and the results were further validated by a collaborative, unbiased meta-analysis of all published genetic data sets that included a total of 12,464 AD cases and 17,929 controls.152 In the past year, two studies demonstrated that, in addition, genetic variation in the SORL1 homolog SORCS1 influences AD risk, cognitive performance, APP processing and Aβ1–40 and Aβ1–42 levels through an effect on γ-secretase processing of APP,153,154 further emphazising the role of SorL1-related proteins in late-onset AD etiology.
Genome-wide association studies for AD using large numbers of cases and controls155–158 have revealed modest effect sizes for several genes on AD risk, with odds ratios in the range of 1.1–1.5, although most of these studies have only confirmed the association of APOE with this disease. One such study showed that variants of TOMM40—which is proximally located to and in linkage disequilibrium with APOE—were associated with AD risk, but whether these genetic associations are independent of the APOE locus remains unclear.159
Together, two genome-wide association studies identified variants in the clusterin gene (CLU), the phosphatidylinositol-binding clathrin assembly protein gene (PICALM) and complement receptor type 1 gene (CR1) as being associated with AD, but functional data confirming the roles in AD of the proteins encoded by these genes are still lacking.160,161 Clusterin is a lipoprotein that is expressed in mammalian tissues and is incorporated into amyloid plaques. This protein binds to soluble Aβ in CSF, forming complexes that can penetrate the BBB. Clusterin levels are positively correlated with the number of APOE ε4 alleles, suggesting a compensatory induction of CLU in the brains of AD patients with the APOE ε4 allele, who show low brain levels of APOE.162
CR1 encodes a protein that is likely to contribute to Aβ clearance from the brain,163 while PICALM protein is involved in clathrin-mediated endocytosis, allowing intracellular trafficking of proteins and lipids such as nutrients, growth factors and neurotransmitters.164 PICALM protein also has a role in the trafficking of vesicle-associated membrane protein 2, a soluble N-ethylmaleimide-sensitive factor attachment protein receptor that is involved in the fusion of synaptic vesicles to the presynaptic membrane in neurotransmitter release.
A third large genome-wide association study confirmed the associations of PICALM and CLU with AD165 and reported two additional loci as being associated with AD: rs744373, which is near the bridging integrator 1 gene (BIN1) on chromosome 2q14.3, and rs597668, which is located on chromosome 19q13.3.
Bin1 is a member of the Bar (Bin–amphiphysin–rvs) adaptor family, which has been implicated in caspase-independent apoptosis and membrane dynamics, including vesicle fusion and trafficking, neuronal membrane organization, and clathrin-mediated synaptic vesicle formation.166 Of note, the latter process is disrupted by Aβ.167 Changes in BIN1 expression have also been shown in aging mice and in transgenic mouse models of AD.168
The locus rs597668 is not in linkage disequilibrium with APOE, suggesting that the effect of this locus on AD risk is independent. Six genes are found in this region, of which at least two (genes encoding biogenesis of lysosomal organelles complex 1 subunit 3 and microtubule-associated protein–microtubule affinity-regulating kinase 4) are implicated in molecular pathways linked to AD or other brain disorders.169–171
The results of the published genome-wide association studies are informative, but the genetic associations need functional validation. Indeed, such studies alone cannot prove causality or assess the biological significance of an observed genetic association. Furthermore, while genome-wide association studies represent a method of screening the genome, limitations exist in their ability to detect true associations. Also, the results of such studies can be difficult to replicate if the actual effect is smaller than that observed in the initial study. Finally, the detection of associations with multiple rare variants at a single site (which are better detected by linkage studies) or with single rare variants (minor allele frequency <5%) may not be possible. These limitations have led researchers to focus on additional biological characteristics—including endophenotypes (Box 4) and epigenetic characteristics (Box 5)—to facilitate the identification of disease-causing mutations.
Box 4 | Endophenotypes and genetic epidemiology.
Endophenotypes (measurable intermediate phenotypes that are closer to the action of the gene than affection status) provide clues to the genetic underpinnings of a disease, and diagnoses can be deconstructed to increase the success of genetic analyses. Age at disease onset is a well-established endophenotype for Alzheimer disease (AD), and is a key indicator of genetic heterogeneity. Recognition of various age-at-onset profiles among families was critical in the initial identification of genetically distinct forms of AD. Age information, such as age-at-onset data for individuals affected by AD and censored age data for unaffected individuals, is also an important covariate used to modify the penetrance function, allowing determination of age-dependent penetrance of the disease as a function of disease genotype, while also increasing the power of genetic linkage studies and the accuracy with which disease genes can be localized. In addition to the use of age at onset as a covariate and as a stratifier in genome scans, this endophenotype has a genetic basis, with several contributing loci having been identified. Nevertheless, despite overwhelming evidence that age is an important variable to be considered in genetic risk factor research for AD, most genome scans for this disease have not incorporated age information. Other quantitative phenotypes that have been proven useful are pathological phenotypes, such as neuritic plaque and neurofibrillary tangle densities, structural brain changes on brain imaging such as white matter lesions, and cognitive function.
Box 5 | Epigenetic mechanisms.
Several epigenetic studies are underway using normal brain tissue to identify functional cis-acting regulatory polymorphisms that may be associated with brain disorders such as Alzheimer disease. Allele-specific DNA methylation—an epigenetic marker—is often strongly dependent on nearby single-nucleotide polymorphisms (SNPs) and haplotypes. This observation suggests that mapping allele-specific DNA methylation across the whole genome in human brain DNA could be useful for finding regulatory SNPs that act in this target tissue and are involved in specific brain disorders.
Biomarkers
The epidemiological research exploring environmental and genetic risk factors for late-onset AD has led to the identification of various biomarkers for this disease. In addition to being helpful tools for the determination of disease risk, biomarkers are invaluable in establishing a diagnosis. In contrast to the autosomal dominantly inherited mutations reviewed above, the additional biomarkers that have been identified for AD are not definite markers of disease but, rather, contribute to increasing the specificity of diagnosis. These additional biomarkers include various measurements from CSF, blood and neuroimaging. The reliability, specificity and sensitivity of these biomarkers are determined by epidemiological studies.
Cerebrospinal fluid biomarkers
The free transport of proteins between the brain and CSF provides a reflection of the cerebral metabolic processes occurring in the latter. CSF levels of Aβ1–42, total tau (t-tau) and p-tau are validated markers that may aid the accurate diagnosis of AD at an early stage of disease.172 CSF samples from patients diagnosed as having AD or MCI contain lower levels of Aβ1–42 and higher levels of t-tau or p-tau than do those from cognitively normal individuals.173 Furthermore, t-tau CSF levels increase with AD progression.174 A longitudinal study that aimed to clarify whether variation in the concentration pattern of different-length Aβ peptides increases diagnostic power suggested that the combined levels of Aβ1–37, Aβ1–38, Aβ1–39, Aβ1–40 and Aβ1–42 have a 91% sensitivity and a 64% specificity in predicting progression from MCI to AD.175
The relationship between concentrations of amyloid plaques and NFTs in the brain and levels of CSF biomarkers (that is, Aβ1–42, t-tau and p-tau) remains to be clarified. According to one hypothesis,176 accumulation of protofibrillar Aβ species in the brain may result in a reduction of soluble Aβ in the CSF and brain, and increased Aβ deposition in amyloid plaques. Some studies suggested a positive correlation between p-tau CSF levels and NFT concentration in people with AD;177 however, other studies were unable to validate these findings.178,179 In the interpretation of the data generated in these studies, the importance of various influencing factors on Aβ and tau levels in CSF needs to be recognized. Increasing age or carrying the APOE ε4 allele accelerates the deposition of Aβ1–42 in the brain and lowers CSF Aβ1–42 levels.180 Also, CSF APOE levels correlate positively with CSF levels of t-tau and 24S-hydroxycholesterol in patients with cognitive disorders.181 Supplementary Table 1 shows additional CSF biomarkers that have been reported for predicting the risk or aiding the diagnosis of AD. In a large-scale meta-analysis of 14 studies exploring biomarkers for pre clinical AD, the effect sizes of aβ1–42, t-tau and p-tau ranged from 0.91–1.11, suggesting that the sensitivity of these markers for this condition is modest.182
Plasma biomarkers
Owing to the properties of the BBB, plasma bio markers comprise only small or lipophilic proteins, and proteins carried by specific transporters that are able to cross this barrier. The exact mechanisms underlying the interaction between brain and plasma Aβ levels remain unclear; however, under physiological conditions, a steady-state level of brain Aβ exists that is maintained by a balance between the production and deposition of Aβ in the brain and the production of this peptide in the periphery by platelets. RAGEs transport plasma Aβ through the BBB into the brain, whereas the cell-surface low-density lipoprotein receptor related protein-1 transports this peptide from the brain into plasma. In healthy individuals, brain Aβ levels are, therefore, reflected by plasma Aβ concentrations. In patients with dementia, the relationship between brain Aβ levels and the concentration of Aβ in plasma is unclear, owing to deposition of Aβ in amyloid plaques.
In familial AD142,183 and Down syndrome with APP triplication,184 total Aβ levels and Aβ1–42 levels in plasma are elevated. In sporadic AD, the usefulness of plasma Aβ as a risk biomarker remains controversial.185,186 Studies found associations between plasma Aβ1–40 levels and AD,186 reported no association between AD risk and plasma Aβ levels,187 showed that plasma Aβ1–42 levels are reduced in patients with AD compared with healthy controls,188 or demonstrated that levels of plasma Aβ1–42 decrease before the onset of AD.189 These contrasting findings result from variability in the timing of sample collection across studies, the use of different antibodies to detect Aβ, and a lack of validation of plasma Aβ as a risk biomarker for AD.
One study showed that in comparison with individuals who remained healthy during the follow-up period, participants who later went on to develop AD showed a significant increase in plasma Aβ1–42 levels but no rise in plasma Aβ1–40 levels after the initial examination.189 At the time of conversion to AD, however, levels of plasma Aβ1–42 and the Aβ1–42:Aβ1–40 ratio decreased significantly. These findings indicate that elevated plasma Aβ1–42 is an antecedent risk factor for AD, while decreasing levels of plasma Aβ1–42 or a decline in the Aβ1–42:Aβ1–40 ratio mark disease onset.
Several other molecules have been investigated as plasma biomarkers for AD risk, including homocysteine, various proteins linked to inflammation (for example, C-reactive protein, IL-1β, TNF, IL-6 and transforming growth factor β), and cholesterol. These molecules have, however, all yielded inconsistent data across studies.
Structural MRI
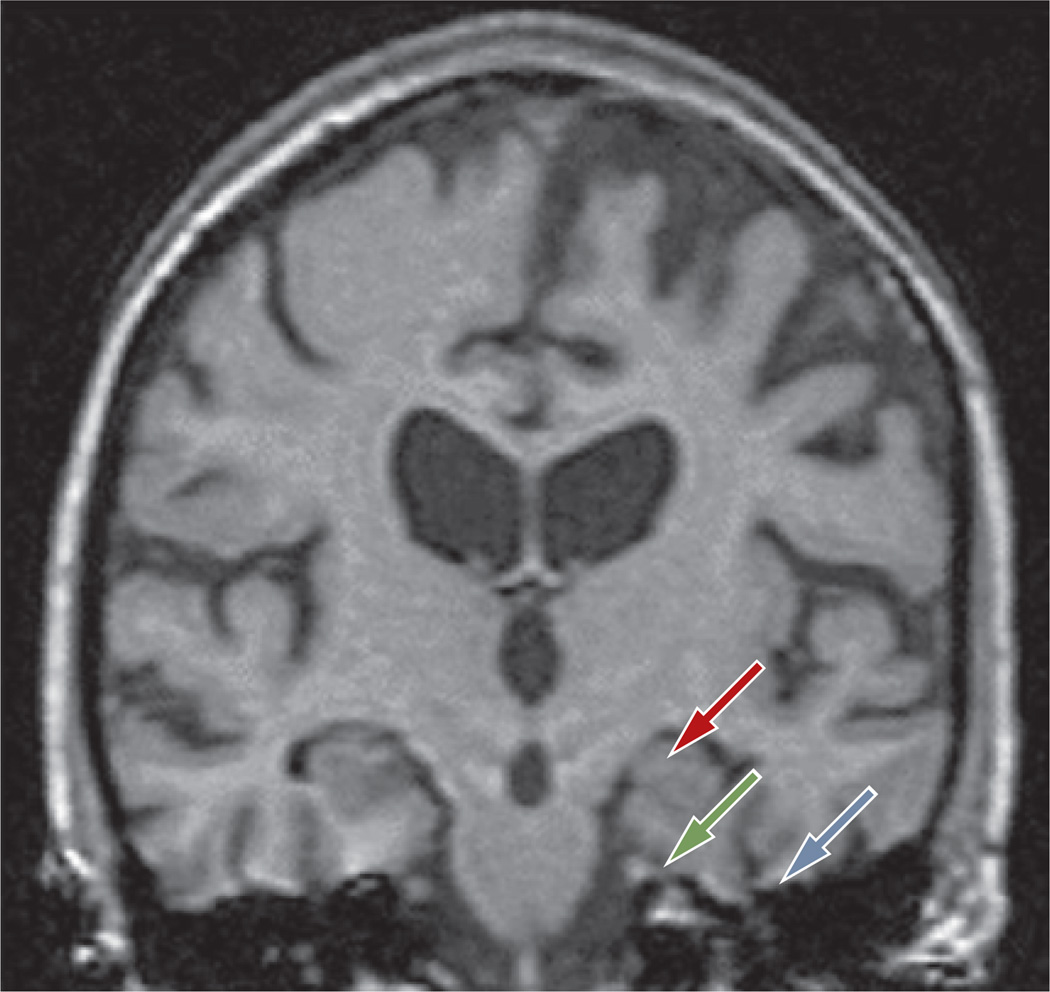
Late-onset AD is characterized by medial temporal lobe atrophy, particularly in the hippocampal formation and the amygdala (Figure 2). In early-onset AD, brain atrophy may be located more posteriorly than in late-onset AD, and may involve the posterior cortex,190 occipital lobes, precuneus, posterior cingulate191 and amygdala.192 Atrophy in the hippocampus and entorhinal cortex is associated with a decline in memory function, progression of memory impairment193 and an increased risk of AD.194 These changes are not specific to AD, however, and substantial anatomical overlap prevails between the types of atrophy observed in AD, various other neurodegenerative disorders and normal aging. Thus, such changes are not sufficient to establish a definitive diagnosis of AD. In addition, while non specific white matter changes appear frequently in healthy elderly individuals, such changes are also common in elderly people with cognitive decline, stroke or MCI. Nevertheless, several studies have suggested that certain structural MRI bio markers possess some degree of discriminative diagnostic power. Evidence exists that in AD, the corpus callosum (particularly the anterior area) exhibits atrophy. This change helps to distinguish AD from fronto temporal dementia, in which the posterior area of the corpus callosum shows greater atrophy than the anterior area of this brain structure.195 Evidence is also available that among patients with amnestic MCI, those who convert to AD show greater atrophy in the hippocampus and the inferior and middle temporal gyri than those who do not convert to AD.196
Figure 2.
T1-weighted MRI scan of a patient with a clinical diagnosis of late-onset AD. As is typical for late-onset AD, the MRI scan shows generalized brain atrophy and loss of gray matter affecting the hippocampus (red arrow), entorhinal cortex (green arrow) and perirhinal cortex (blue arrow). Abbreviation: AD, Alzheimer disease.
Functional MRI
Functional MRI (fMRI) can visualize neuronal activity either during rest or in association with a task that activates specific brain regions. The most common method is blood oxygen level-dependent (BOLD) fMRI, which measures alterations in blood flow on the basis of changes in deoxyhemoglobin. As the deoxyhemoglobin concentration depends on neuronal activity, BOLD reflects brain activity. This technique is widely used in research and in the diagnosis of various brain disorders because of its high sensitivity and easy implementation.
BOLD signals depend on several anatomical, physiological and imaging parameters, and can be interpreted qualitatively or semiquantitatively. As a result, inter-individual and intraindividual variability limits the use of such signals in the differential diagnosis of dementia-causing disorders. Nevertheless, fMRI can facilitate the characterization of functional abnormalities in specific diseases. People with AD exhibit reduced brain activity in parietal and hippocampal regions in comparison with healthy controls.197 In addition, some studies have found different neuronal activity patterns in healthy controls and patients with MCI.190 Recent advances in fMRI have allowed intrinsic functional networks in the human brain to be defined. The study of cognitive–behavioral function in the early stages of neurodegenerative disorders may allow the identification of the neuroanatomical networks affected by these diseases, and may assist in the differential diagnosis of the various disorders that underlie dementia.
PET and single-photon emission CT
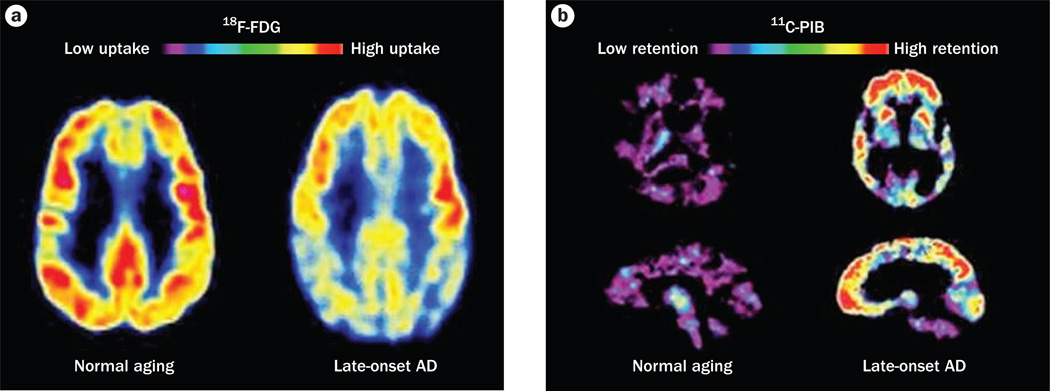
PET and single-photon emission CT (SPECT) have been widely explored as diagnostic tools for dementia, and both techniques have shown good diagnostic and prognostic capabilities. PET studies have mostly used the tracer 2-[18F]-fluoro-2-deoxy-d-glucose (18F-FDG), which provides a measure of cerebral glucose metabolism and, hence, indirectly demonstrates synaptic activity. In the early stages of AD, 18F-FDG-PET reveals a characteristic pattern of symmetric hypometabolism in the posterior cingulate and parietotemporal regions that spreads to the prefrontal cortices (Figure 3). These changes are distinct from the changes in cerebral glucose metabolism that are seen in healthy controls and cases of other forms of dementia, and the extent of hypometabolism inversely correlates with the degree of cognitive impairment.198 18F-FDG-Pet has a high sensitivity (94%) but a low specificity (73–78%) for the diagnosis of dementia.199 SPECT, which involves studying regional blood flow with Tc-hexamethylpropyleneamine oxime, has a similar specificity to 18F-FDG-PET for this condition.200
Figure 3.
Changes revealed by PET in the AD brain. a | 18F-FDG-PET patterns characteristic of metabolic activity in cognitively normal individuals and patients with late-onset AD. In comparison with people aging normally, individuals with late-onset AD show decreased bilateral glucose metabolism, particularly in the temporal and parietal regions. b | 11C-PIB PET images characteristic of elderly individuals without cognitive impairment and patients with late-onset AD. The high concentrations of 11C-PIB in the AD brain are suggestive of high amounts of amyloid deposits. Abbreviations: AD, Alzheimer disease; FDG, 2-fluoro-2-deoxy-d-glucose; PIB, Pittsburgh compound B.
A number of low-molecular-weight tracers have been developed for PET to assess Aβ deposits in vivo. The most frequently used tracer is Pittsburgh compound B (PIB). Compared with healthy controls, patients with AD show increased 11C-PIB retention in cortical regions targeted by Aβ deposits (Figure 3).201 Deposition of this peptide seems to reach a plateau by the early stages of AD.202 In MCI, PIB binding is bimodal, with ≈ 50% of patients showing an increase in 11C-PIB binding, resembling the 11C-PIB retention that is seen in AD, while the other ≈ 50% of patients exhibit low levels of 11C-PIB binding that are similar to the levels seen in controls.203 In MRI studies, 11C-PIB binding correlated positively with atrophy in the amygdala and hippocampus but not other cortical areas, suggesting that various brain areas have different susceptibilities to Aβ deposit-mediated toxicity, or that amyloid deposition is nonessential for neurodegeneration.203 New Pet tracers for amyloid deposits, such as 18F-FDDNP, are being developed. In studies comparing 11C-PIB and 18F-FDDNP, these tracers showed differences in regional binding and in the cognitive domains with which they seem to be associated, suggesting that these tracers measure related but different characteristics of AD.204
Conclusions
Substantial progress has been made over the past few decades in understanding AD. Neverthless, many researchers would argue that our knowledge of this disease is still profoundly imperfect, as demonstrated by the failure of all but symptomatic treatments for clinically diagnosed AD. We know that in people aged >85 years, dementia and cognitive impairment are common, reaching a combined prevalence >50% in the oldest old, and that the incidence of dementia continues to rise in the oldest age groups. Thus, screening is essential to identify cognitively normal individuals in midlife or old age who have a high risk of developing MCI and AD, so that interventions, when available, can be administered to stop the development of specific disease-related pathologies. In the oldest patients who develop AD, for whom this disease is closely associated with end-of-life issues, palliative therapies, rather than interventional treatments, are required to ensure the best possible quality of life and not necessarily an extension of lifespan.
Key points.
-
▪
The unprecedented level of aging occurring in developed nations will lead to an enormous burden of Alzheimer disease (AD)
-
▪
The primary pathological hallmarks in AD brain tissue—diffuse and neuritic extracellular amyloid plaques and intracellular neurofibrillary tangles—are well known, but the underlying etiologies of these pathologies remain unclear
-
▪
The diagnosis of AD in living patients is based on clinical examination—no definite diagnostic test is currently available—but may be supported by the use of clinical biomarkers
-
▪
AD heritability varies from 58–79% depending on age at onset; however, only a portion of the likely substantial genetic contribution to this disease has been determined
-
▪
Several nongenetic factors (including recognized vascular risk factors) have been associated with AD, but the underlying mechanisms linked to these factors are uncertain
Review criteria.
Alzheimer disease genetic studies were identified from searches of the AlzGene database, which was updated in May 2010. PubMed was searched for articles published in English between January 1990 and October 2010. Combinations of the following key words were used in the aforementioned searches: “dementia”, “Alzheimer’s disease”, “mild cognitive impairment”, “MCI”, “biomarker”, “risk”, “antecedent”, “gene”, “genetics”, “epigenetics”, “endophenotype”, “incidence”, “prevalence”, “criteria”, “diagnosis, “definition”, “history”, “pathology” and “autopsy”. The retrieved abstracts were read to identify studies addressing the topics included in this Review. We also performed a manual search of references cited in the published articles.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH and the National Institute on Aging (grants R37-AG15473 and P01-AG07232), as well as funding from The Blanchette Hooker Rockefeller Foundation and The Charles S. Robertson Gift from the Banbury Fund (R. Mayeux), and a Paul B. Beeson Career Development Award (K23AG034550) to C. Reitz.
Footnotes
Competing interests
R. Mayeux declares an association with the following organization: NIH. See the article online for full details of the relationship. The other authors, the journal Chief Editor H. Wood and the CME questions author L. Barclay declare no competing interests.
Laurie Barclay, freelance writer and reviewer, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author contributions
C. Reitz, C. Brayne and R. Mayeux researched the data for the article, provided substantial contributions to discussions of the content, and contributed equally to writing the article and to review and/or editing of the manuscript before submission.
supplementary information is linked to the online version of the paper at www.nature.com/nrneurol
Contributor Information
Christiane Reitz, Gertrude H. Sergievsky Center, Columbia University Medical Center, 630 West 168th Street, New York, NY 10032, USA.
Carol Brayne, Department of Public Health and Primary Care, Institute of Public Health, University Forvie Site, Robinson Way, Cambridge CB2 0SR, UK.
Richard Mayeux, Gertrude H. Sergievsky Center, Columbia University Medical Center, 630 West 168th Street, New York, NY 10032, USA.
References
- 1.Alzheimer’s Association. 2010 Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Ferri CP, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 5.Cheung ZH, Gong K, Ip NY. Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2. J. Neurosci. 2008;28:4872–4877. doi: 10.1523/JNEUROSCI.0689-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weishaupt JH, et al. Inhibition of CDK5 is protective in necrotic and apoptotic paradigms of neuronal cell death and prevents mitochondrial dysfunction. Mol. Cell. Neurosci. 2003;24:489–502. doi: 10.1016/s1044-7431(03)00221-5. [DOI] [PubMed] [Google Scholar]
- 7.Wen Y, et al. Cdk5 is involved in NFT-like tauopathy induced by transient cerebral ischemia in female rats. Biochim. Biophys. Acta. 2007;1772:473–483. doi: 10.1016/j.bbadis.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, et al. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 9.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu–Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 10.Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–2340. doi: 10.1161/01.str.29.11.2334. [DOI] [PubMed] [Google Scholar]
- 11.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 12.Glynn RJ, et al. Current and remote blood pressure and cognitive decline. JAMA. 1999;281:438–445. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- 13.Knopman D, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 14.Posner HB, et al. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–1181. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- 15.Skoog I, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 16.Kalaria RN. vascular basis for brain degeneration: faltering controls and risk factors for dementia. Nutr. Rev. 2010;68:S74–S87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deane R, Wu Z, Zlokovic Bv. RAGE (yin) versus LRP (yang) balance regulates Alzheimer amyloid β-peptide clearance through transport across the blood–brain barrier. Stroke. 2004;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 18.Forette F, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur.) study. Arch. Intern. Med. 2002;162:2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- 19.Tzourio C, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch. Intern. Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 20.Starr JM, Whalley LJ, Deary IJ. The effects of antihypertensive treatment on cognitive function: results from the HOPE study. J. Am. Geriatr. Soc. 1996;44:411–415. doi: 10.1111/j.1532-5415.1996.tb06412.x. [DOI] [PubMed] [Google Scholar]
- 21.Prince MJ, Bird AS, Blizard RA, Mann AH. Is the cognitive function of older patients affected by antihypertensive treatment? Results from 54 months of the Medical Research Council’s trial of hypertension in older adults. BMJ. 1996;312:801–805. doi: 10.1136/bmj.312.7034.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.[No authors listed] Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 23.Lithell H, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J. Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Peters R, et al. Association of depression with subsequent mortality, cardiovascular morbidity and incident dementia in people aged 80 and over and suffering from hypertension. Data from the Hypertension in the very Elderly Trial (HYvET) Age Ageing. 2010;39:439–445. doi: 10.1093/ageing/afq042. [DOI] [PubMed] [Google Scholar]
- 25.Leibson CL, et al. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann. NY. Acad. Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am. J. Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 27.Ott A, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 28.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr. Alzheimer Res. 2007;4:147–152. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 29.Cook DG, et al. Reduced hippocampal insulindegrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-ε4 allele. Am. J. Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamagishi S, Nakamura K, Inoue H, Kikuchi S, Takeuchi M. Serum or cerebrospinal fluid levels of glyceraldehyde-derived advanced glycation end products (AGEs) may be a promising biomarker for early detection of Alzheimer’s disease. Med. Hypotheses. 2005;64:1205–1207. doi: 10.1016/j.mehy.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Yan SD, et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 32.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XL, et al. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 34.Lieb W, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Profenno LA, Porsteinsson AP, Faraone Sv. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Janson J, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 37.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu–Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 38.Arvanitakis Z, et al. Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology. 2006;67:1960–1965. doi: 10.1212/01.wnl.0000247053.45483.4e. [DOI] [PubMed] [Google Scholar]
- 39.Reger MA, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol. Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Watson GS, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 41.Risner ME, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6:246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 42.Sato T, et al. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Q, Heneka M, Landreth GE. The role of peroxisome proliferator-activated receptorgamma (PPARγ) in Alzheimer’s disease: therapeutic implications. CNS Drugs. 2008;22:1–14. doi: 10.2165/00023210-200822010-00001. [DOI] [PubMed] [Google Scholar]
- 44.Grundman M, Corey-Bloom J, Jernigan T, Archibald S, Thal LJ. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46:1585–1591. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 45.White H, Pieper C, Schmader K. The association of weight change in Alzheimer’s disease with severity of disease and mortality: a longitudinal analysis. J. Am. Geriatr. Soc. 1998;46:1223–1227. doi: 10.1111/j.1532-5415.1998.tb04537.x. [DOI] [PubMed] [Google Scholar]
- 46.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 47.Razay G, vreugdenhil A. Obesity in middle age and future risk of dementia: midlife obesity increases risk of future dementia. BMJ. 2005;331:455. doi: 10.1136/bmj.331.7514.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart R, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu–Asia Aging Study. Arch. Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 49.Gustafson DR, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73:1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitmer RA, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 51.Muckle TJ, Roy JR. High-density lipoprotein cholesterol in differential diagnosis of senile dementia. Lancet. 1985;1:1191–1193. doi: 10.1016/s0140-6736(85)92866-1. [DOI] [PubMed] [Google Scholar]
- 52.Kuo YM, et al. Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain Aβ 1–42 levels. Biochem. Biophys. Res. Commun. 1998;252:711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 53.Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J. Neurosci. Res. 2003;72:141–146. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- 54.Wieringa GE, et al. Apolipoprotein E genotypes and serum lipid levels in Alzheimer’s disease and multi-infarct dementia. Int. J. Geriatr. Psychiatry. 1997;12:359–362. [PubMed] [Google Scholar]
- 55.van Exel E, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann. Neurol. 2002;51:716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 56.Lesser G, et al. Elevated serum total and LDL cholesterol in very old patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2001;12:138–145. doi: 10.1159/000051248. [DOI] [PubMed] [Google Scholar]
- 57.Burns M, Duff K. Cholesterol in Alzheimer’s disease and tauopathy. Ann. NY. Acad. Sci. 2002;977:367–375. doi: 10.1111/j.1749-6632.2002.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 58.Jones RW, et al. The Atorvastatin/Donepezil in Alzheimer’s Disease Study (LEADe): design and baseline characteristics. Alzheimers Dement. 2008;4:145–153. doi: 10.1016/j.jalz.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Simons M, et al. Treatment with simvastatin in normocholesterolemic patients with Alzheimer’s disease: a 26-week randomized, placebo-controlled, double-blind trial. Ann. Neurol. 2002;52:346–350. doi: 10.1002/ana.10292. [DOI] [PubMed] [Google Scholar]
- 60.Sparks DL, et al. Circulating cholesterol levels, apolipoprotein E genotype and dementia severity influence the benefit of atorvastatin treatment in Alzheimer’s disease: results of the Alzheimer’s Disease Cholesterol-Lowering Treatment (ADCLT) trial. Acta Neurol. Scand. Suppl. 2006;185:3–7. doi: 10.1111/j.1600-0404.2006.00690.x. [DOI] [PubMed] [Google Scholar]
- 61.Sano M. Multi-center, randomized, double-blind, placebo-controlled trial of simvatatin to slow the progression of Alzheimer’s disease. Alzheimers Dement. 2008;4(Suppl. 2):T200. doi: 10.1016/j.trci.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raffaitin C, et al. Metabolic syndrome and risk for incident Alzheimer’s disease or vascular dementia: the Three-City Study. Diabetes Care. 2009;32:169–174. doi: 10.2337/dc08-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solfrizzi V, et al. Metabolic syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Ageing. J. Neurol. Neurosurg. Psychiatry. 2010;81:433–440. doi: 10.1136/jnnp.2009.181743. [DOI] [PubMed] [Google Scholar]
- 64.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch. Neurol. 2009;66:324–328. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tyas SL. Are tobacco and alcohol use related to Alzheimer’s disease? A critical assessment of the evidence and its implications. Addict. Biol. 1996;1:237–254. doi: 10.1080/1355621961000124856. [DOI] [PubMed] [Google Scholar]
- 66.Brenner DE, et al. Relationship between cigarette smoking and Alzheimer’s disease in a population-based case–control study. Neurology. 1993;43:293–300. doi: 10.1212/wnl.43.2.293. [DOI] [PubMed] [Google Scholar]
- 67.Ferini-Strambi L, Smirne S, Garancini P, Pinto P, Franceschi M. Clinical and epidemiological aspects of Alzheimer’s disease with presenile onset: a case control study. Neuroepidemiology. 1990;9:39–49. doi: 10.1159/000110750. [DOI] [PubMed] [Google Scholar]
- 68.Merchant C, et al. The influence of smoking on the risk of Alzheimer’s disease. Neurology. 1999;52:1408–1412. doi: 10.1212/wnl.52.7.1408. [DOI] [PubMed] [Google Scholar]
- 69.Launer LJ, et al. Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 70.Ott A, et al. Smoking and risk of dementia and Alzheimer’s disease in a population-based cohort study: the Rotterdam Study. Lancet. 1998;351:1840–1843. doi: 10.1016/s0140-6736(97)07541-7. [DOI] [PubMed] [Google Scholar]
- 71.Doll R, Peto R, Boreham J, Sutherland I. Smoking and dementia in male British doctors: prospective study. BMJ. 2000;320:1097–1102. doi: 10.1136/bmj.320.7242.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hebert LE, et al. Relation of smoking and alcohol consumption to incident Alzheimer’s disease. Am. J. Epidemiol. 1992;135:347–355. doi: 10.1093/oxfordjournals.aje.a116296. [DOI] [PubMed] [Google Scholar]
- 73.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s disease: an analysis controlling for tobacco industry affiliation. J. Alzheimers Dis. 2010;19:465–480. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Traber MG, van der vliet A, Reznick AZ, Cross CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin. Chest Med. 2000;21:173–187. doi: 10.1016/s0272-5231(05)70016-2. [DOI] [PubMed] [Google Scholar]
- 75.Kellar KJ, Wonnacott S. In: Nicotine Psychopharmacology: Molecular, Cellular, and Behavioral Aspects. Wonnacott S, Russell MA, Stolerman IP, editors. Oxford: Oxford University Press; 1990. pp. 341–373. [Google Scholar]
- 76.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust. NZ J. Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 77.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch. Gen. Psychiatry. 2006;63:273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 78.Becker JT, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am. J. Geriatr. Psychiatry. 2009;17:653–663. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Panza F, et al. Impact of depressive symptoms on the rate of progression to dementia in patients affected by mild cognitive impairment. The Italian Longitudinal Study on Aging. Int. J. Geriatr. Psychiatry. 2008;23:726–734. doi: 10.1002/gps.1967. [DOI] [PubMed] [Google Scholar]
- 80.Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol. Dis. 2006;22:453–462. doi: 10.1016/j.nbd.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Mayeux R, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon-4 in patients with Alzheimer’s disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 82.Rasmusson DX, Brandt J, Martin DB, Folstein MF. Head injury as a risk factor in Alzheimer’s disease. Brain Inj. 1995;9:213–219. doi: 10.3109/02699059509008194. [DOI] [PubMed] [Google Scholar]
- 83.Schofield PW, et al. Alzheimer’s disease after remote head injury: an incidence study. J. Neurol. Neurosurg. Psychiatry. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on, a partial replication. J. Neurol. Neurosurg. Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mortimer JA, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case–control studies. EURODEM Risk Factors Research Group. Int. J. Epidemiol. 1991;20(Suppl. 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 86.Guo Z, et al. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 87.Mehta KM, et al. Head trauma and risk of dementia and Alzheimer’s disease: the Rotterdam Study. Neurology. 1999;53:1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 88.Plassman BL, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 89.Hartman RE, et al. Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer’s disease. J. Neurosci. 2002;22:10083–10087. doi: 10.1523/JNEUROSCI.22-23-10083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franz G, et al. Amyloid β 1–42 and tau in cerebrospinal fluid after severe traumatic brain injury. Neurology. 2003;60:1457–1461. doi: 10.1212/01.wnl.0000063313.57292.00. [DOI] [PubMed] [Google Scholar]
- 91.Morris MC, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287:3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 92.Engelhart MJ, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 93.Masaki KH, et al. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54:1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- 94.Laurin D, Masaki KH, Foley DJ, White LR, Launer LJ. Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu–Asia Aging Study. Am. J. Epidemiol. 2004;159:959–967. doi: 10.1093/aje/kwh124. [DOI] [PubMed] [Google Scholar]
- 95.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch. Neurol. 2003;60:203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 96.Huang TL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE ε4. Neurology. 2005;65:1409–1414. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 97.Kalmijn S, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 98.Schaefer EJ, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch. Neurol. 2006;63:1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 99.Roberts RO, et al. Polyunsaturated fatty acids and reduced odds of MCI: the Mayo Clinic Study of Aging. J. Alzheimers Dis. 21:853–865. doi: 10.3233/JAD-2010-091597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Solfrizzi V, et al. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol. Aging. 2006;27:1694–1704. doi: 10.1016/j.neurobiolaging.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 101.Engelhart MJ, et al. Diet and risk of dementia: does fat matter?: The Rotterdam Study. Neurology. 2002;59:1915–1921. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 102.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scarmeas N, et al. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scarmeas N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302:627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch. Neurol. 2006;63:1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feart C, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am. J. Geriatr. Psychiatry. 2009;17:542–555. doi: 10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- 108.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch. Intern. Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 109.Yaffe K, Clemons TE, McBee WL, Lindblad AS. Impact of antioxidants, zinc, and copper on cognition in the elderly: a randomized, controlled trial. Neurology. 2004;63:1705–1707. doi: 10.1212/01.wnl.0000142969.19465.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petersen RC, et al. vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 111.Sano M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 112.Chiu CC, et al. The effects of omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1538–1544. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 113.Freund-Levi Y, et al. Effects of omega-3 fatty acids on inflammatory markers in cerebrospinal fluid and plasma in Alzheimer’s disease: the OmegAD study. Dement. Geriatr. Cogn. Disord. 2009;27:481–490. doi: 10.1159/000218081. [DOI] [PubMed] [Google Scholar]
- 114.Nagano S, et al. Peroxidase activity of cyclooxygenase-2 (COX-2) cross-links β-amyloid (Aβ) and generates Aβ–COX-2 hetero-oligomers that are increased in Alzheimer’s disease. J. Biol. Chem. 2004;279:14673–14678. doi: 10.1074/jbc.M313003200. [DOI] [PubMed] [Google Scholar]
- 115.Butterfield DA, Castegna A, Drake J, Scapagnini G, Calabrese V. vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr. Neurosci. 2002;5:229–239. doi: 10.1080/10284150290028954. [DOI] [PubMed] [Google Scholar]
- 116.Pitchumoni SS, Doraiswamy PM. Current status of antioxidant therapy for Alzheimer’s Disease. J. Am. Geriatr. Soc. 1998;46:1566–1572. doi: 10.1111/j.1532-5415.1998.tb01544.x. [DOI] [PubMed] [Google Scholar]
- 117.Weisburger JH. vitamin C and prevention of nitrosamine formation. Lancet. 1977;2:607. doi: 10.1016/s0140-6736(77)91451-9. [DOI] [PubMed] [Google Scholar]
- 118.Pardo B, Mena MA, Fahn S, Garcia de Yebenes J. Ascorbic acid protects against levodopa-induced neurotoxicity on a catecholamine-rich human neuroblastoma cell line. Mov. Disord. 1993;8:278–284. doi: 10.1002/mds.870080305. [DOI] [PubMed] [Google Scholar]
- 119.voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology. 2003;61:1273–1275. doi: 10.1212/01.wnl.0000090458.67821.a3. [DOI] [PubMed] [Google Scholar]
- 120.Calder PC. Polyunsaturated fatty acids, inflammation, and immunity. Lipids. 2001;36:1007–1024. doi: 10.1007/s11745-001-0812-7. [DOI] [PubMed] [Google Scholar]
- 121.Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol. Aging. 2002;23:843–853. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 122.Abbott RD, et al. Walking and dementia in physically capable elderly men. JAMA. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 123.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 124.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.verghese J, et al. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 126.Rovio S, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 127.Churchill JD, et al. Exercise, experience and the aging brain. Neurobiol. Aging. 2002;23:941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 128.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 129.Dishman RK, et al. Neurobiology of exercise. Obesity. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 130.Emery CF, Schein RL, Hauck ER, MacIntyre NR. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998;17:232–240. doi: 10.1037//0278-6133.17.3.232. [DOI] [PubMed] [Google Scholar]
- 131.Fabre C, Chamari K, Mucci P, Masse-Biron J, Prefaut C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sports Med. 2002;23:415–421. doi: 10.1055/s-2002-33735. [DOI] [PubMed] [Google Scholar]
- 132.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J. Appl. Physiol. 2006;101:1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 133.Lautenschlager NT, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300:1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 134.Angevaren M, Aufdemkampe G, verhaar HJ, Aleman A, vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database of Systematic Reviews. 2008;(Issue 3) doi: 10.1002/14651858.CD005381.pub3. Art. No.: CD005381. CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 135.Carlson MC, et al. Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement. 2008;4:324–331. doi: 10.1016/j.jalz.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J. Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 137.Acevedo A, Loewenstein DA. Nonpharmacological cognitive interventions in aging and dementia. J. Geriatr. Psychiatry Neurol. 2007;20:239–249. doi: 10.1177/0891988707308808. [DOI] [PubMed] [Google Scholar]
- 138.Ball K, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Unverzagt FW, et al. Effect of memory impairment on training outcomes in ACTIVE. J. Int. Neuropsychol. Soc. 2007;13:953–960. doi: 10.1017/S1355617707071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alzheimer Disease Mutation Database. Alzheimer Disease & Frontotemporal Dementia Mutation Database. 2010 [online], http://www.molgen.vib-ua.be/ADMutations/ [Google Scholar]
- 141.De Strooper B, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 142.Scheuner D, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat. Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 143.Green RC, et al. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA. 2002;287:329–336. doi: 10.1001/jama.287.3.329. [DOI] [PubMed] [Google Scholar]
- 144.Gatz M, et al. Role of genes and environments for explaining Alzheimer disease. Arch. Gen. Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 145.Corder EH, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 146.Kurz A, et al. Apolipoprotein E type 4 allele and Alzheimer’s disease: effect on age at onset and relative risk in different age groups. J. Neurol. 1996;243:452–456. doi: 10.1007/BF00900498. [DOI] [PubMed] [Google Scholar]
- 147.Poirier J, et al. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 148.Farlow MR, et al. Impact of APOE in mild cognitive impairment. Neurology. 2004;63:1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- 149.Myers RH, et al. Apolipoprotein E ε4 association with dementia in a population-based study: the Framingham study. Neurology. 1996;46:673–677. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- 150.Daw EW, et al. The number of trait loci in lateonset Alzheimer disease. Am. J. Hum. Genet. 2000;66:196–204. doi: 10.1086/302710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rogaeva E, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nature Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reitz C, et al. Meta-analysis of the association between variants in SORL1 and Alzheimer’s disease. Arch. Neurol. 2011;68:99–106. doi: 10.1001/archneurol.2010.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reitz C, et al. SORCS1 alters APP processing and variants may increase Alzheimer’s disease risk. Ann. Neurol. doi: 10.1002/ana.22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lane R, et al. Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-β metabolism: evidence for involvement of SorL1 and the retromer complex. J. Neurosci. 2010;30:13110–13115. doi: 10.1523/JNEUROSCI.3872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Beecham GW, et al. Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. Am. J. Hum. Genet. 2009;84:35–43. doi: 10.1016/j.ajhg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 157.Carrasquillo MM, et al. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer’s disease. Nat. Genet. 2009;41:192–198. doi: 10.1038/ng.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reiman EM, et al. GAB2 alleles modify Alzheimer’s risk in APOE ε4 carriers. Neuron. 2007;54:713–720. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Potkin SG, et al. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One. 2009;4:e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Harold D, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lambert JC, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 162.Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer disease. Brain Res. Mol. Brain Res. 1995;33:174–178. doi: 10.1016/0169-328x(95)00097-c. [DOI] [PubMed] [Google Scholar]
- 163.Wyss-Coray T, et al. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc. Natl Acad. Sci. USA. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baig S, et al. Distribution and expression of picalm in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2010;69:1071–1077. doi: 10.1097/NEN.0b013e3181f52e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Seshadri S, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wigge P, et al. Amphiphysin heterodimers: potential role in clathrin-mediated endocytosis. Mol. Biol. Cell. 1997;8:2003–2015. doi: 10.1091/mbc.8.10.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kelly BL, Ferreira A. Beta-amyloid disrupted synaptic vesicle endocytosis in cultured hippocampal neurons. Neuroscience. 2007;147:60–70. doi: 10.1016/j.neuroscience.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yang S, et al. Comparative proteomic analysis of brains of naturally aging mice. Neuroscience. 2008;154:1107–1120. doi: 10.1016/j.neuroscience.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 169.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 170.Starcevic M, Dell’Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J. Biol. Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- 171.Morris DW, et al. Dysbindin (DTNBP1) and the biogenesis of lysosome-related organelles complex 1 (BLOC-1): main and epistatic gene effects are potential contributors to schizophrenia susceptibility. Biol. Psychiatry. 2008;63:24–31. doi: 10.1016/j.biopsych.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 172.Hansson O, et al. Prediction of Alzheimer’s disease using the CSF Aβ42/Aβ40 ratio in patients with mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2007;23:316–320. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 173.Ewers M, et al. Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology. 2007;69:2205–2212. doi: 10.1212/01.wnl.0000286944.22262.ff. [DOI] [PubMed] [Google Scholar]
- 174.Andersson C, et al. Differential CSF biomarker levels in APOE-ε4-positive and -negative patients with memory impairment. Dement. Geriatr. Cogn. Disord. 2007;23:87–95. doi: 10.1159/000097354. [DOI] [PubMed] [Google Scholar]
- 175.Hoglund K, et al. Prediction of Alzheimer’s disease using a cerebrospinal fluid pattern of C-terminally truncated β-amyloid peptides. Neurodegener. Dis. 2008;5:268–276. doi: 10.1159/000119457. [DOI] [PubMed] [Google Scholar]
- 176.Fagan AM, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann. Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 177.Buerger K, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 178.Buerger K, et al. No correlation between CSF tau protein phosphorylated at threonine 181 with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2007;130:e82. doi: 10.1093/brain/awm140. [DOI] [PubMed] [Google Scholar]
- 179.Engelborghs S, et al. No association of CSF biomarkers with APOEε4, plaque and tangle burden in definite Alzheimer’s disease. Brain. 2007;130:2320–2326. doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- 180.Fukumoto H, et al. Age but not diagnosis is the main predictor of plasma amyloid β-protein levels. Arch. Neurol. 2003;60:958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- 181.Shafaati M, Solomon A, Kivipelto M, Bjorkhem I, Leoni V. Levels of ApoE in cerebrospinal fluid are correlated with Tau and 24S-hydroxycholesterol in patients with cognitive disorders. Neurosci. Lett. 2007;425:78–82. doi: 10.1016/j.neulet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 182.Schmand B, Huizenga HM, van Gool WA. Meta-analysis of CSF and MRI biomarkers for detecting preclinical Alzheimer’s disease. Psychol. Med. 2010;40:135–145. doi: 10.1017/S0033291709991516. [DOI] [PubMed] [Google Scholar]
- 183.Kosaka T, et al. The beta APP717 Alzheimer mutation increases the percentage of plasma amyloid-beta protein ending at A beta42(43) Neurology. 1997;48:741–745. doi: 10.1212/wnl.48.3.741. [DOI] [PubMed] [Google Scholar]
- 184.Schupf N, et al. Elevated plasma amyloid β-peptide 1–42 and onset of dementia in adults with Down syndrome. Neurosci. Lett. 2001;301:199–203. doi: 10.1016/s0304-3940(01)01657-3. [DOI] [PubMed] [Google Scholar]
- 185.Mayeux R, et al. Plasma Aβ40 and Aβ42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology. 2003;61:1185–1190. doi: 10.1212/01.wnl.0000091890.32140.8f. [DOI] [PubMed] [Google Scholar]
- 186.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MM. Plasma Aβ1–40 and Aβ1–42 and the risk of dementia: a prospective case–cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 187.Lopez OL, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70:1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lui JK, et al. Plasma amyloid-β as a biomarker in Alzheimer’s disease: the AIBL study of aging. J. Alzheimers Dis. 2010;20:1233–1242. doi: 10.3233/JAD-2010-090249. [DOI] [PubMed] [Google Scholar]
- 189.Schupf N, et al. Peripheral Aβ subspecies as risk biomarkers of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2008;105:14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Teipel SJ, et al. Relation of corpus callosum and hippocampal size to age in nondemented adults with Down’s syndrome. Am. J. Psychiatry. 2003;160:1870–1878. doi: 10.1176/appi.ajp.160.10.1870. [DOI] [PubMed] [Google Scholar]
- 191.Karas G, et al. Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology. 2007;49:967–976. doi: 10.1007/s00234-007-0269-2. [DOI] [PubMed] [Google Scholar]
- 192.Krasuski JS, et al. volumes of medial temporal lobe structures in patients with Alzheimer’s disease and mild cognitive impairment (and in healthy controls) Biol. Psychiatry. 1998;43:60–68. doi: 10.1016/s0006-3223(97)00013-9. [DOI] [PubMed] [Google Scholar]
- 193.Mungas D, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Apostolova LG, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch. Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 195.Likeman M, et al. visual assessment of atrophy on magnetic resonance imaging in the diagnosis of pathologically confirmed young-onset dementias. Arch. Neurol. 2005;62:1410–1415. doi: 10.1001/archneur.62.9.1410. [DOI] [PubMed] [Google Scholar]
- 196.Chetelat G, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27:934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 197.Rombouts SA, et al. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR. Am. J. Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- 198.Small GW, et al. Current and future uses of neuroimaging for cognitively impaired patients. Lancet Neurol. 2008;7:161–172. doi: 10.1016/S1474-4422(08)70019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Silverman DH, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 200.O’Brien JT. Role of imaging techniques in the diagnosis of dementia. Br. J. Radiol. 2007;80:S71–S77. doi: 10.1259/bjr/33117326. [DOI] [PubMed] [Google Scholar]
- 201.Klunk WE, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 202.Engler H, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 203.Frisoni GB et al. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–1511. doi: 10.1212/WNL.0b013e3181a2e896. [DOI] [PubMed] [Google Scholar]
- 204.Tolboom N, et al. Differential association of [11C]PIB and [18F]FDDNP binding with cognitive impairment. Neurology. 2009;73:2079–2085. doi: 10.1212/WNL.0b013e3181c679cc. [DOI] [PubMed] [Google Scholar]
- 205.Katzman R. Editorial: the prevalence and malignancy of Alzheimer disease. A major killer. Arch. Neurol. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.