Abstract
Hsp90s, molecular chaperones critically involved in many essential cellular processes, were the focus of a recent international conference held in Seeon, Germany. The scope of the conference ranged from structural and mechanistic insights all the way to medical applications.
Hsp90s (90-kDa heat-shock proteins) are molecular chaperones involved in both the stress response and the normal biosynthetic and homeostatic control mechanisms of the cell. They interact with a diverse clientele of proteins that include kinases, transcription factors and other structurally unrelated proteins. Hsp90s are not only able to stabilize these proteins and bring about their activation, but are also involved in directing them to proteasomal degradation. Consequently, Hsp90s have crucial roles in signal transduction, chromatin remodeling, cellular trafficking, epigenetic regulation, and development and morphological evolution.[Au: Correct as edited?] Johannes Buchner (Technische Universität München) and Didier Picard (University of Geneva) initiated this biannual meeting bringing together world-leading laboratories in Hsp90 research and providing a forum to discuss the latest findings. The present meeting, “The 4th International Conference on the Hsp90 Chaperone Machine”, was held in Seeon, Germany. The meeting focused on mechanistic, biochemical and structural aspects of in vivo functions of Hsp90, and on the development of new Hsp90 inhibitors and their current efficacy in ongoing clinical trials. [Au: Sentence correct as edited?]
Molecular mechanism of Hsp90 proteins
Hsp90 proteins are weak ATPases that consist of an N-terminal nucleotide binding domain (NBD), a middle domain (MD), which is involved in client binding, and a C-terminal dimerization domain. The reaction cycle of Hsp90 chaperones was first proposed on the basis of data from the interaction with steroid hormone receptors1. Steroid hormone receptors first interact with Hsp40 and Hsp70. With the aid of the cochaperone Hop (also known as Sti1)[Au: Ok?] the early Hsp70–Hsp90–Hop–client complex is formed. Hsp70 and Hop are replaced by p23 and one of the large peptidyl-prolyl-cis/trans isomerases (FKBP51, FKBP52 or Cyp40). ATP hydrolysis, accelerated by the cochaperone Aha1, leads to substrate dissociation.
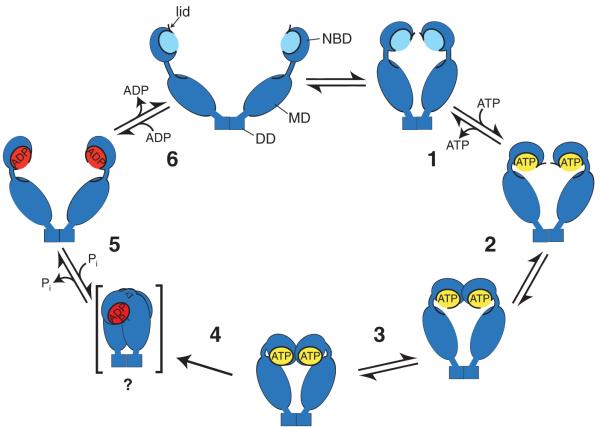
At the last Hsp90 conference in 2006, several crystal structures of full-length Hsp90 proteins were presented showing almost identical domain structures but substantially different domain orientations. One focus of this year’s conference was the conformational dynamics of Hsp90 and how these different conformations relate to the ATPase cycle. Several participants presented a careful analysis of the ATPase cycle of Hsp90 proteins, including the endoplasmic reticulum Hsp90, the mitochondrial Hsp90 Trap1 (Jochen Reinstein, Max Planck Institute, Heidelberg), yeast cytosolic Hsp82 (Johannes Buchner) and Escherichia coli HtpG (Matthias Mayer) (Fig. 1).[Au: Sentence correct as edited?] These data suggest that all Hsp90 proteins transit through the same number of intermediates in their ATPase cycle but have markedly different forward and backward conversion rates. The new analyses clarify several previously conflicting points; for instance, it is now clear that all Hsp90 proteins, including Grp94, hydrolyze ATP. The new data also explain the differences in ATPase rates for different Hsp90s.
Figure 1 The ATPase cycle of Hsp90 proteins. Model of the ATPase cycle of Hsp90 proteins as revealed by kinetic analysis of ATP binding and/or conformational changes of yeast cytosolic Hsp82, human endoplasmic reticulum GRP94, human mitochondrial TRAP1 and E. coli HtpG. Hsp90 proteins proceed through two distinct intermediates before reaching the γ-phosphate cleavage–competent conformation. Forward and backward reaction rates are different for the individual Hsp90 proteins analyzed and may be subjected to regulation by cochaperones such as Aha1. A proposed compact post-hydrolysis state visible in EM seems to be transient and is not detectable by bulk measurements like SAXS or HX-MS.
David Agard (University of California, San Francisco) approached the question of different conformational states of Hsp90s by negative-stain EM and single-particle analysis, and by small-angle X-ray scattering (SAXS). Earlier, he had reported different nucleotide-dependent conformations for the E. coli homolog HtpG: an open V-shaped conformation in the absence of nucleotides; a more closed conformation with both N termini of the HtpG dimer in close proximity in the presence of AMPPNP; and a compact conformation in the presence of ADP2.[Au: Sentence correct as edited?] He now presented EM images of yeast and human Hsp90 proteins showing open and closed conformations in all nucleotide states, similar to the conformations observed in HtpG. He proposes that Hsp90 proteins are all in equilibrium between open and closed conformations and that nucleotides shift these equilibria3.
Johannes Buchner presented a highly sophisticated analysis of ATP-induced conformational changes in yeast Hsp82. Using fluorescence resonance energy transfer (FRET), he monitored the distances between the NBDs within the Hsp82 dimer and between the NBD and MD. The results supported an ATPase cycle with two intermediates before γ-phosphate cleavage, consistent with the kinetic analysis reported by Jochen Reinstein for TRAP1 (ref. 4).
Thorsten Hugel (Technische Universität München) in collaboration with the Buchner group used fluorescently labeled Hsp82 variants to analyze the changes in distance between protomers within the dimer at the single-molecule level. He directly observed the distance distribution and opening and closing events at individual molecules in the different nucleotide states. Surprisingly, opening and closing kinetics were more than a magnitude faster than expected from bulk steady-state kinetics.
Matthias Mayer reported on the analysis of the conformational dynamics of HtpG using amide hydrogen exchange in combination with MS (HX-MS). These experiments showed that ATP binding to HtpG and not hydrolysis induces a tightly folded conformation in the NBD and MD. Using pulse-labeling HX-MS, Mayer and colleagues were able to resolve the kinetics of the ATP-induced conformational transition within individual structural elements of NBD and MD. Surprisingly, not all parts of the domains showed the same transition rates, suggesting a stepwise mechanism whereby the conformational changes start at the nucleotide binding pocket and progress slowly toward the N terminus and the MD.
Taken together, it was gratifying to see a unifying mechanism for all Hsp90 proteins emerging. But what is the physiological meaning of the different conformations Hsp90 assumes through the ATPase cycle? First hints came from the Buchner laboratory. They found that Aha1 accelerated the cycle by bypassing one of the intermediates on the way to the γ-phosphate cleavage–competent state. Siligardi and co-workers had earlier come to a similar conclusion while analyzing Hsp82 mutant proteins and their ATPase rates in the presence of the cochaperones Aha1, Sti1 and p23 (ref. 5).[Au: Correct as edited?] Therefore, several energy barriers define the different conformational states. Cochaperones most likely affect the distribution of conformational states of Hsp90 by raising or lowering these barriers, probably in order to regulate Hsp90’s residence time in the different states competent for client binding.
Post-translational modifications add several layers of complexity to this multistep ATPase cycle of Hsp90 proteins.[Au: Sentence correct as edited?] Joanna Soroka from the Buchner laboratory and Mehdi Mollapour and Len Neckers (National Cancer Institute, US National Institutes of Health (NIH)) reported that yeast Hsp82 is modified at several sites by phosphorylation. The functional consequences of phosphorylation and whether there is a certain code for the activating and inhibiting influences on Hsp90’s activity are still largely unknown. Laura T. Donlin from the laboratory of Alexander Tarakhovsky (Rockefeller University) reported on lysine methylation in the C-terminal domain of Hsp90. Together with acetylation6 and S-nitrosylation7, Hsp90 is thus modified by four different functional groups. The influence of these modifications on the conformational dynamics, the ATPase cycle and interaction with cochaperones and clients is completely unexplored.
One of the most burning questions in the Hsp90 field concerns how Hsp90 interacts with client proteins and the location of the client binding site on the Hsp90 protein.[Au: Ok?] Franz Hagn from Horst Kessler’s laboratory (Technische Universität München) presented NMR data of the interaction of the MD of Hsp90 with the core domain of the tumor suppressor p53. Chemical shift perturbation experiments located the interaction surface within the MD of Hsp90 to one surface, which in the full-length Hsp90 dimer points outside, away from the central interdimer cleft.
Hsp90 interaction with cochaperones
Jill Johnson (University of Idaho, Moscow) presented a bioinformatics analysis of the distribution of Hsp90 cochaperones across 19 eukaryotic organisms. Although all cochaperones of Hsp90 are found in all major branches of the evolutionary tree of the eukarya, none of the cochaperones is universally present in all examined organisms. Hop was most frequently present, whereas Cdc37, an essential cochaperone in yeast8, was frequently missing from the genome. These data show that the cochaperone complement is organism- and therefore most likely client-specific, and they suggest that the Hsp90 system diverged in the different evolutionary branches according to different client needs.
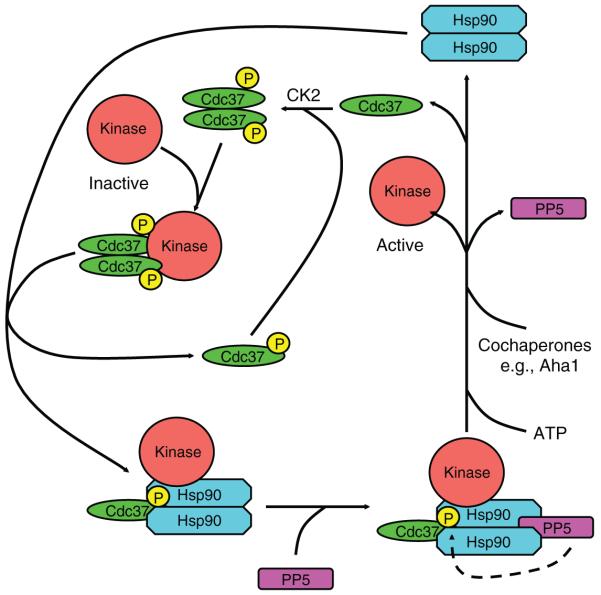
Cara Vaughan (Birkbeck College) presented exciting data suggesting that the Hsp90-dependant activation of kinases requires chaperone-targeted dephosphorylation of Cdc37 (ref. 9). The data reveal a cyclic mechanism in which PP5 (also known as Ppt1)[Au: Ok?] could dephosphorylate Cdc37 in a specific Hsp90-targeted[Au: Correct as edited?] manner in both yeast and human tumor cells. It seems that the phosphorylation of Cdc37 involves the constitutive action of CK2, and this is reversed by the regulated activity of PP5 (Fig. 2).
Figure 2.
A cycle of phosphorylation and dephosphorylation of Cdc37 is necessary for kinase-client loading onto Hsp90. Cdc37, phosphorylated by CK2, binds to kinases and forms a complex with Hsp90. The PP5 (also known as Ppt1) phosphatase binds to the C terminus of Hsp90 and dephosphorylates Cdc37, which subsequently dissociates from the Hsp90 complex. ATP binding and hydrolysis by Hsp90 and interaction with additional cochaperones lead to maturation and release of the kinase.[Au: e.g. instead of i.e. meant for Aha1?]
Ken Shirasu (RIKEN Plant Science Centre) reported NMR and mutagenesis experiments mapping the structural and functional interaction surfaces of the Sgt1–Hsp90 complex required for innate immunity in plants and animals10. Furthermore, Chrisostomos Prodromou (Institute of Cancer Research (ICR), London) presented results from a productive collaboration with Laurence H. Pearl (ICR) and Ken Shirasu, showing the three-dimensional X-ray structure of the CS domain[Au: Define CS.] of Sgt1 in complex with the N-terminal domain of Hsp90 (ref. 11). The structure confirmed that the CS domain is homologous to Sba1 (also known as p23). [Au: Ok?] Suprisingly, the interaction with the N-terminal domain of Hsp90 differs radically from p23. This confirmed the results from Ken Shirasu and revealed yet another binding site on the N terminus of Hsp90. The Hsp90–Sgt1 complex was shown to bind Skp1, suggesting roles in innate immunity, CBF3 kinetochore assembly and the formation and regulation SCF complexes.[Au: Sentence correct as edited?]
Further highlighting the important role of Hsp90 cofactors in modulating its activity, Edwin Sanchez (University of Toledo) and Marc Cox (Univeristy of Texas, El Paso) discussed the role of the prolyl isomerase Hsp90 cofactors FKBP51 and FKBP52. As cochaperones to Hsp90, the tetratricopeptide (TPR) proteins FKBP52, FKBP51, Cyp40 and PP5 are well-known regulators of steroid receptor (SR) action. Edwin Sanchez showed that these proteins have differential and opposing effects on SR transcriptional activity and on steroid-regulated physiology, ranging from progesterone receptor–and androgen receptor–mediated[Au: Correct?] control of reproduction to glucocorticoid receptor–mediated control of liver and muscle metabolism. Marc Cox presented the identification, characterization and targeting of a putative FKBP52 interaction surface on the androgen receptor hormone binding domain. Mutations within a specific surface of the androgen receptor hormone binding domain result in increased dependence on FKBP52 for normal function, and small molecules that bind this surface specifically inhibit FKBP52-enhanced receptor function. These small-molecule inhibitors will provide research tools for the study of FKBP52 regulation and may also represent attractive drug candidates for treatment of hormone-dependent diseases.
In vivo function of Hsp90
Didier Picard presented data on a gene trap mouse carrying a Hsp90α allele encoding a variant that is C-terminally truncated by 35 residues, greatly reducing the function of Hsp90.[Au: Correct as edited?] The homozygous mutant mice seemed morphologically normal; however, males but not females were sterile. Detailed analysis revealed clear defects in sperm formation in the testis, consistent with the observation that Hsp90α is expressed at high levels only in testis under nonstress conditions.
An important open question concerns the mechanism by which Hsp90 regulates the multiple cellular processes in which it is implicated. Two talks presented insight into this question. Using elegant biochemical reconstitution experiments, Brian Freeman (University of Illinois) explored the role of Hsp90 in telomere maintenance. Telomeres, the repetitive sequences present at the ends of eukaryotic chromosomes, must be maintained and extended by telomerase, an RNA-guided reverse transcriptase. Purified yeast telomerase is nonprocessive, but purified Hsp82 binds to telomerase and increases its nucleotide processivity by enhancing its affinity for both nucleotide and DNA. Freeman also described another function for Hsp90 in telomere-length regulation, namely, disrupting the Cdc13–telomere capping complex, which protects telomere DNA in vivo. This finding explains the previous paradoxical observations that overexpression of Hsp90 shortens telomere length. In a similar theme, Walid Houry (University of Toronto) identified the role of Hsp90 in the assembly of a four-protein complex containing the Rvb1 and Rvb2[Au: Ok?] hexameric AAA+ helicases, a small TPR domain protein termed Tah1 and another nuclear protein termed Pih1. The complex has been called the R2TP complex and has been shown to be required for the assembly of Box C/D small nucleolar ribonucleoproteins (snoRNPs) involved in pre–ribosomal RNA (rRNA) methylation and biogenesis12. Both Freeman and Houry highlight an emerging theme in Hsp90 function, namely, promoting, and regulating, assembly and disassembly of oligomeric complexes, a role consistent with results from recent genomic studies in yeast13.
Uwe Strähle (Universität Heidelberg) reported on the analysis of zebrafish variants that showed defects in motility. The Strähle group found that mutations in both Hsp90a and the cochaperone Unc45b cause loss of motility14. Unc45b and Hsp90a are expressed in skeletal and cardiac muscle and localize to the Z-band in mature fibrils15. During development, Hsp90a and Unc45b transiently colocalize with myosin in the A-band. Strähle showed that Hsp90a and Unc45b are essential for assembly and suggested a requirement of the two chaperones for maintenance of the myosin myofibrils.
Extracellular Hsp90, secreted via the unconventional exosome pathway by cancer cells, has an important role in cell motility and cancer metastasis. Data presented from Len Neckers (National Cancer Institute, NIH) showed that Hsp90 seems to lack a conventional signal for its secretion. However, a small hydrophobic sequence similar to one found in homeobox proteins such as Engrailed1 and Vsx1 was identified. Mutation of a residue in this motif abrogated Hsp90 secretion but also altered interactions with client proteins and cochaperones. Surprisingly, removal of the charged linker restored Hsp90 secretion as well as its ability to support yeast viability.
Wei Li (University of Southern California, Los Angeles) reported that HSP90α secretion by human keratinocytes and dermal fibroblasts in response to TGFα stimulation or hypoxia has a critical role in skin wound healing. Li reported that Hsp90α secreted via exosomes promotes migration of both epidermal and dermal cells from human skin, and identified Hsp90 as a ligand of the surface receptor LRP1 (also known as CD9;[Au: Ok?] refs. 16,17). This motility-promoting[Au: Ok?] activity resides within the charged linker and the MD region of Hsp90, and is consequently ATPase independent. Li showed[Au: Ok?] that the motility-promoting activity of Hsp90α could not be inhibited by TGFβ, a potent suppressor of dermal cell migration during skin wounds.
Two talks centered on the poorly understood function of the endoplasmic reticulum Hsp90 homolog Grp94, found only in higher eukaryotes but absent from yeast. The clients, cofactors and mechanistic cycle of this chaperone remain less well understood than those of the cytosolic counterpart. Yair Argon (University of Pennsylvania) showed through Grp94 knockout experiments in mice that Grp94 is essential. Argon’s research traced back the essential function to a crucial role of Grp94 in ATP-dependent folding of insulin-like growth factors, required for organ differentiation and development18. On the other hand, Ineke Braakman (Utrecht University), working with the low-density lipoprotein (LDL) receptor, showed that, even if this protein does not require Grp94 for folding, the Hsp90 homolog still contributes to its maturation and exit from the endoplasmic reticulum when the LDL receptor is expressed at high levels. Together, these observations suggest that Grp94 is essential for folding of some clients and may have a helping, nonstringent role in the folding of other proteins.
Whereas in eukaryotic organisms Hsp90 proteins are essential under all conditions, the E. coli homolog HtpG does not seem to have such an important role, despite its high sequence conservation. Now, Fumihiro Motojima (Tokyo Institute of Technology) reported on the first substrate for E. coli HtpG. He and his colleagues screened for substrate proteins by pulling down HtpG from cells incubated for 2 h at 45 °C. The ribosomal protein L2 copurified and the L2-HtpG interaction was verified in vitro. The physiological relevance of these findings, however, remains unclear.
Medical applications of Hsp90 research
The function of Hsp90 in protein maturation and assembly also emerges from Judith Frydman’s studies on the role of Hsp90 in folding and assembly of viral proteins. Viral replication requires high levels of viral protein expression, placing a heavy burden on the host chaperones that must fold them. For instance, Hsp90 is required for maturation and processing of the very large capsid precursor of picornavirus, a large virus family including rhino-, polio- and coxackie virus. Pharmacological inhibition of Hsp90 dramatically inhibits viral replication in vitro and in animal models of infection. Strikingly, the virus is unable to develop resistance to Hsp90 inhibitors, probably because mutations that reduce Hsp90 dependence also impair viral fitness. These observations, together with the favorable pharmacological profile of Hsp90 inhibitors in patients, suggest that Hsp90 inhibitors may also prove to be broad spectrum–effective antiviral drugs, in addition to their promising anticancer activity.
Leonard Petrucelli (Mayo Clinic) described the role of the Hsp90, which is intimately involved in tau clearance and refolding, in tauopathies, toxic aggregation diseases caused by accumulation of hyperphosphorylated tau. [Au: Correct as edited?] He presented data showing that the Hsp90-associated ubiquitin ligase CHIP promotes tau clearance, whereas Hsp90 together with folding-promoting cofactors Pin1 and p23 facilitate tau refolding and solubility. Interestingly, treatment with Hsp90 inhibitors in vitro or in animals enhances the CHIP-dependent degradation of tau and diminishes the pathological consequences of tau aggregation in an animal model.
William Balch (Scipps Research Institute) presented data on the cystic fibrosis transmembrane conductance regulator (CFTR) mutant CFTRΔF508, which is retained in the ER and rapidly degraded owing to a folding defect. Balch and colleagues searched for interaction partners of CFTR and found Hsp90 and Aha1. Overexpression of Aha1 led to degradation of wild-type and mutant CFTR; in contrast, small interfering RNA directed toward Aha1 increased traffic of CFTRΔF508 to the cell surface and restored functionality. Therefore, inhibitors that target specific Hsp90-cochaperone interactions may provide a new aspect for therapeutic intervention in diseases other than cancer.
Hsp90 as target for anti-tumor therapy[Au: Ok? Shortened to fit on one line.]
Paul Workman (Cancer Research UK Centre for Cancer Therapeutics, ICR) presented an update from the clinical trials being conducted with Hsp90 inhibitors. Cancer cells seem to be specifically sensitive to Hsp90 inhibitors, and one such compound, 17-AAG, seems to be useful in cases where Trastuzumed resistance has emerged in breast cancer patients.[Au: Sentence correct as edited?] A promising observation is that resistance to Hsp90 inhibitors does not seem to develop directly by changes in Hsp90 itself; rather, some patients show a loss of NQO1 activity, required for conversion of the quinone moiety of 17-AAG to the hydroquinone derivative, the more potent form of the inhibitor. However, cross-resistance to other quinone derivatives of 17-AAG was detected, but not against chemically unrelated inhibitors such as NVP-AUY922. Experiments reducing the expression of Hsc70 and Hsp70 simultaneously, although inhibiting Hsp90, showed a remarkable increase in toxicity in cancer cells, with apoptosis levels rising from a few percent to 35%.[Au: Correct as edited?] ‘Normal cells’, on the other hand, showed only a 2% increase in cell death relative to 17-AAG treatment alone.
Nicolas Winssinger (Institut de Science et d’Ingenierie Supramoleculaires Universite Louis Pasteur) reported the development of pochoxime A (NXD30001) and its analogs19. These inhibitors are based on the natural resorcylic acid lactones that include radicicol. These compounds seem to be useful in the treatment of breast and other forms of cancer, but with an improved dosing and therapeutic window compared to 17-AAG and possibly some other Hsp90 inhibitors.
An interesting alternative strategy was presented by Dario Altieri (University of Massachusetts Medical School). He showed data indicating that cancer cells, in contrast to normal cells, express the mitochondrial Hsp90 protein TRAP1 at high levels and that knockdown of TRAP1 results in spontaneous apoptosis. To specifically inhibit mitochondrial Hsp90, he synthesized several Geldanamycin derivatives that rapidly accumulate in the mitochondria, called Gamitrinibs (Geldanamycin mitochondrial matrix inhibitors). In isolated mitochondria, Gamitrinibs induced loss of membrane potential and release of cytochrome C. In vivo, Gamitrinibs had no effect on cytosolic Hsp90 client proteins but activated Caspases. Apoptosis was induced in cancer cells with high selectivity and not in comparable nontumor cells. Mice with tumor xenografts treated with Gamitrinibs showed regression of the tumor and no significant weight loss, which is indicative of a low general toxicity of these compounds. Clinical trials will show whether Gamitrinibs live up to their promises.
Closing remarks
In conclusion, the Hsp90 field has made both mechanistic and medical advances in the past 2 years. A consensus seems to have been reached regarding the mechanism by which Hsp90 hydrolyzes ATP. The rate-limiting step of the ATPase cycle seems to be a complex set of structural changes, including N-terminal dimerization and release of the catalytic loop, leading to the formation of a catalytic unit able to hydrolyze ATP5. Furthermore, all Hsp90s—the cytosolic yeast and human Hsp90s, the endoplasmic reticulum Grp94 and mitochondrial Trap1, as well as the bacterial HtpG—probably operate by the same mechanism4,20,21. Structurally, a better understanding of the client binding site on Hsp90 was achieved. It appears that the binding site for p53 overlaps with that previously described for Cdk4 on the MD of Hsp90 (ref. 22).
The effectiveness of treating various tumors with inhibitors of the Hsp90 N-terminal domain is being upheld. Most promising is the lack of Hsp90 mutations isolated that lead to inhibitor resistance. However, Peter Piper (University of Sheffield), pointed out that there are natural forms of resistance that he had isolated and that altering the yeast Hsp90 protein (L34I) conferred resistance to radicicol but not geldanamycin.[Au: We do not allow data to be listed as “in preparation”. Please provide first initial and last name of all authors up to 6 and cite as personal communication.] Hsp90 inhibitors and modulators also hold promise for the treatment of viral infections and metabolic diseases.
Contributor Information
Matthias P Mayer, Zentrum für Molekular Biologie der Universität Heidelberg, DKFZ-ZMBH-Alliance, Heidelberg, Germany.
Chrisostomos Prodromou, Section of Structural Biology at The Institute of Cancer Research, London, UK.
Judith Frydman, Department of Biology and BioX Program, James Clark Center, Stanford University, Stanford, California, USA.
References
- 1.Pratt WB, Toft DO. Exp. Biol. Med. (Maywood) 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 2.Shiau AK, Harris SF, Southworth DR, Agard DA. Cell. 2006;127:329–340. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Southworth DR, Agard DA. Mol. Cell. 2008;32:631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leskovar A, Wegele H, Werbeck ND, Buchner J, Reinstein J. J. Biol. Chem. 2008;283:11677–11688. doi: 10.1074/jbc.M709516200. [DOI] [PubMed] [Google Scholar]
- 5.Siligardi G, et al. J. Biol. Chem. 2004;279:51989–51998. doi: 10.1074/jbc.M410562200. [DOI] [PubMed] [Google Scholar]
- 6.Scroggins BT, et al. Mol. Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Ruiz A, et al. Proc. Natl. Acad. Sci. USA. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JL, Brown C. Cell Stress Chaperones. 2008;14:83–94. doi: 10.1007/s12192-008-0058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughan CK, et al. Mol. Cell. 2008;31:886–895. doi: 10.1016/j.molcel.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kadota Y, et al. EMBO Rep. 2008;9:1209–1215. doi: 10.1038/embor.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, et al. EMBO J. 2008;27:2789–2798. doi: 10.1038/emboj.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao R, et al. J. Cell Biol. 2008;180:563–578. doi: 10.1083/jcb.200709061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClellan AJ, et al. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins TA, et al. Development. 2008;135:1147–1156. doi: 10.1242/dev.018150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etard C, Roostalu U, Strahle U. J. Cell Biol. 2008;180:1163–1175. doi: 10.1083/jcb.200709128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CF, et al. Mol. Cell. Biol. 2008;28:3344–3358. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, et al. EMBO J. 2007;26:1221–1233. doi: 10.1038/sj.emboj.7601579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wanderling S, et al. Mol. Biol. Cell. 2007;18:3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barluenga S, et al. Angew. Chem. Int. Edn Engl. 2008;47:4432–4435. doi: 10.1002/anie.200800233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frey S, Leskovar A, Reinstein J, Buchner J. J. Biol. Chem. 2007;282:35612–35620. doi: 10.1074/jbc.M704647200. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan CK, Piper PW, Pearl LH, Prodromou C. FEBS J. 2008 Nov 20; doi: 10.1111/j.1742-4658.2008.06773.x. published online, doi:10.1111/j.1742-4658.2008.06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaughan CK, et al. Mol. Cell. 2006;23:697–707. doi: 10.1016/j.molcel.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]