Abstract
BACKGROUND
A recent study showed that methylphenidate induces emergence from isoflurane general anesthesia. Isoflurane and propofol are general anesthetics that may have distinct molecular mechanisms of action. The objective of this study was to test the hypothesis that methylphenidate actively induces emergence from propofol general anesthesia.
METHODS
Using adult rats, the effect of methylphenidate on time to emergence after a single bolus of propofol was determined. The ability of methylphenidate to restore righting during a continuous target controlled infusion of propofol was also tested. In a separate group of rats, a target controlled infusion of propofol was established and spectral analysis was performed on electroencephalogram recordings taken before and after methylphenidate administration.
RESULTS
Methylphenidate decreased median time to emergence after a single dose of propofol from 735 seconds (95% CI: 598 to 897 seconds, n=6) to 448 seconds (95% CI: 371 to 495 seconds, n=6). The difference was statistically significant (p = 0.0051). During continuous propofol anesthesia with a median final target plasma concentration of 4.0 μg/ml (95%CI: 3.2 to 4.6, n=6), none of the rats exhibited purposeful movements after injection of normal saline. After methylphenidate, however, all 6 rats promptly exhibited arousal and had restoration of righting with a median time of 82 seconds (95% CI: 30 to 166 seconds). Spectral analysis of electroencephalogram data demonstrated a shift in peak power from delta (<4 Hz) to theta (4–8 Hz) and beta (12–30 Hz) after administration of methylphenidate, indicating arousal in 4/4 rats.
CONCLUSIONS
Methylphenidate decreases time to emergence after a single dose of propofol, and induces emergence during continuous propofol anesthesia in rats. Further study is warranted to test the hypothesis that methylphenidate induces emergence from propofol general anesthesia in humans.
INTRODUCTION
Anesthesiologists routinely reverse the actions of many drugs including opioids, benzodiazepines, muscle relaxants, and anticoagulants. However, emergence from general anesthesia is still treated as a passive process, dictated by the pharmacokinetics of anesthetic drug clearance. The classical approach of developing drugs that antagonize the actions of general anesthetics at the molecular level has not been feasible, due to the lack of clearly defined molecular targets through which general anesthetics induce loss of consciousness.
However, at the level of neural circuits and systems, there is growing evidence to suggest that arousal pathways play important roles in emergence from general anesthesia.1–10 We recently reported that methylphenidate, an inhibitor of dopamine and norepinephrine reuptake transporters, actively induces emergence from isoflurane general anesthesia in rats.11 Methylphenidate restored the righting reflex, induced electroencephalogram changes consistent with arousal, and increased respiratory drive. The behavioral and respiratory effects induced by methylphenidate were inhibited by droperidol, supporting the idea that methylphenidate induces arousal by activating a dopaminergic arousal pathway.
Propofol is a widely used intravenous general anesthetic thought to produce its effects by enhancing the function of specific γ-aminobutyric acid type A receptors that contain β3 subunits. Transgenic mice harboring a point mutation in the β3 subunit of the γ-aminobutyric acid type A receptor are resistant of the anesthetizing effects of propofol and etomidate but not volatile anesthetics,12,13 suggesting that the molecular mechanisms of action may be distinct for these two classes of general anesthetics. However, because methylphenidate likely induces emergence from isoflurane general anesthesia by activating monoaminergic arousal pathways rather than molecular level antagonism, we hypothesized that methylphenidate induces emergence from propofol anesthesia.
To test this hypothesis a rodent model was used to determine the effect of methylphenidate vs. normal saline (vehicle) on time to emergence after a bolus dose of propofol. We also investigated whether methylphenidate restores the righting reflex during a continuous, target controlled infusion of propofol. Finally, we performed spectral analysis on electroencephalogram recordings taken during continuous target controlled infusions of propofol to test whether methylphenidate induces neurophysiological evidence of arousal.
MATERIALS AND METHODS
Animal care and use
Animal studies were approved by the Subcommittee on Research Animal Care, Massachusetts General Hospital, Boston, Massachusetts, which serves as our Institutional Animal Care and Use Committee. Ten male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 375–508 grams were used for these studies. Six rats were used for the propofol bolus experiments and the continuous infusion experiments, in random order. At least 3 days of rest were provided between experiments. Four additional rats with implanted extradural electrodes were used for the electroencephalogram experiments. Animals were kept on a standard day-night cycle (lights on at 7:00 AM, and off at 7:00 PM), and all experiments were performed during the day.
Preparation and delivery of drugs
Isoflurane, methylphenidate hydrochloride, and propofol were obtained from Henry Schein (Melville, NY), Sigma-Aldrich (St. Louis, MO), and APP pharmaceuticals (Shaumburg, IL) respectively. Sterile normal saline, methylphenidate, and propofol were always administered intravenously. Methylphenidate was weighed, dissolved in 0.5 ml of normal saline, and sterile filtered immediately prior to administration. The IV tubing (approximate volume 0.6 ml) was always flushed with 2 ml of normal saline after a bolus dose of propofol or methylphenidate was administered, to ensure complete delivery of drug. Investigators were not blinded (i.e. the identity of the administered drug was always known).
For continuous propofol infusions, STANPUMP* was run on an IBM Thinkpad T43 computer interfaced with a Physio 22 syringe pump (Harvard Apparatus, Holliston, MA). The pharmacokinetic file for propofol in rats was based on data published by Knibbe et al.14 and kindly provided by Dr. Steven L. Shafer, Columbia University, New York, NY. A constant plasma target concentration mode was selected and the drug was delivered through a dedicated lateral tail vein IV catheter.
Time to Emergence After Propofol Bolus
A single 24-gauge intravenous catheter was placed in a lateral tail vein during brief general anesthesia with isoflurane (2% to 3%) in oxygen, and then the animal was allowed to fully recover from the isoflurane general anesthetic in room air. At least 10 minutes after returning to a baseline level of activity, a bolus dose of propofol (8mg/kg IV) was administered. This dose was chosen because it is approximately twice the published EC50 dose for loss of righting.15 All rats lost the righting reflex within 30 seconds, and were gently placed in the supine position on a heating pad. Forty-five seconds after administration of propofol, methylphenidate (5 mg/kg IV) or normal saline (vehicle) was administered. Time to emergence was defined as the time from administration of propofol until restoration of the righting reflex (i.e. all four paws touching the floor). The same six rats were used on different days (at least 3 days apart) for both the normal saline group and the methylphenidate group. A crossover design was used (i.e. half of the animals received normal saline on the first day and methylphenidate on the second day, and the other half underwent the experiments in reverse order).
Administration of Methylphenidate During Continuous Propofol General Anesthesia
Rats were anesthetized in an induction chamber with 2% to 3% isoflurane in oxygen prior to the placement of two 24-gauge intravenous catheters, one on each lateral tail vein. One catheter was used as a dedicated line for the continuous target controlled infusion of propofol, and the other was used for bolus administration of methylphenidate or normal saline. The catheter used for bolus administration was kept open by using a second syringe pump that continuously infused normal saline at a rate of 2 ml/hr. After initiating the propofol infusion, isoflurane was discontinued and the rats were placed in the supine position on a heating pad in room air. A rectal temperature probe was used to maintain core body temperature between 36.5°C and 37.4°C.
To establish the minimum target plasma concentration of propofol sufficient to maintain loss of righting, the propofol infusion was initiated with a target plasma dose of 5.0 μg/ml. If the rat did not exhibit any purposeful movements, the propofol concentration was lowered by 0.5 μg/ml every 10 minutes. If the rat exhibited purposeful movements, the propofol concentration was increased until purposeful movements were no longer observed, and then lowered by 0.5 μg/ml every 10 minutes. The final target concentration was defined as 0.5 μg/ml above the highest dose at which purposeful movements were observed, and fixed for the remainder of the experiment. Pilot studies indicated that this protocol most reliably provided a steady state propofol general anesthetic with continuous loss of righting.
Establishing the final dose of propofol took a minimum of 45 minutes, during which time the rats inhaled room air and were not exposed to isoflurane. Fifteen minutes after arriving at the final target concentration for propofol, a bolus of normal saline was administered and the temperature probe was removed, to confirm that these mildly stimulating maneuvers were insufficient to induce an arousal response. At the final dose of propofol, no animal exhibited purposeful movement after bolus injection of normal saline or removal of the temperature probe. Five minutes after the normal saline bolus, methylphenidate (5 mg/kg IV) was administered. The experiment was terminated when the rat regained the righting reflex or when 30 minutes had elapsed after methylphenidate administration, whichever came first.
Electroencephalogram Electrode Placement, Recording, and Spectral Analysis
Extradural electroencephalogram electrodes were surgically implanted at least 7 days prior to recording. General anesthesia was induced and maintained with isoflurane. A microdrill (Patterson Dental Supply Inc., Wilmington, MA) was used to make four holes at the following stereotactic coordinates: A0L0, A6L3, A6L-3, and A10L2 relative to the lambda.16 An electrode with mounting screw and socket (Plastics One, Roanoke, VA) was screwed into each hole, and the sockets were inserted in a pedestal (Plastics One). The screws, sockets and pedestal were all permanently fixed with dental acrylic cement, and the animal underwent a minimum recovery period of 7 days.
On the day of recording, the potential difference between electrodes A0L0 and A6L3 (right somatosensory cortex) or between electrodes A0L0 and A6L-3 (left somatosensory cortex) was recorded based on which signal gave less motion artifact. The signal was referenced to A10L2 and recorded using a QP511 Quad AC Amplifier System (Grass Instruments, West Warwick, RI) and a USB- 6009 14-bit data acquisition board (National Instruments, Austin, TX). The sampling rate was 512 Hz, and no line filter was used. Data was filtered between 0.3 Hz and 50 Hz. A 10-minute baseline electroencephalogram was recorded during the awake state prior to the induction of general anesthesia.
In order to decrease motion artifacts associated with righting attempts, a higher dose of propofol was used for these studies. Using the same procedure described above in “Administration of Methylphenidate During Continuous Propofol General Anesthesia” (second paragraph), the final target concentration of propofol was established and maintained at 1.0 μg/ml above the highest dose at which purposeful movements were observed. After 15 minutes at the final dose of propofol, the electroencephalogram signal was recorded for an additional 10 minutes, and then normal saline was injected. None of the animals exhibited purposeful movement during or after administration of normal saline. Five minutes later, methylphenidate (5 mg/kg IV) was administered. Although we initially attempted to perform the electroencephalogram experiments at the same dose of propofol as the experiments described in the previous section, the animals moved too vigorously after the administration of methylphenidate. Therefore the higher dose of propofol was necessary to attenuate the methylphenidate-induced arousal response and reduce motion artifacts on the electroencephalogram.
Spectral analysis was performed using Matlab R2010b (Mathworks, Natick, MA) and Chronux software (Cold Spring Harbor, NY),17 as previously described.11 Briefly, mean power spectra were compared before and after methylphenidate administration using Kolmogorov-Smirnov tests.18 To determine the difference between two spectra, a two-sample Kolmogorov-Smirnov test19 was performed on the spectral power as a function of frequency computed from the 30 windows in the pre-methylphenidate and post-methylphenidate periods. We used a Bonferroni correction to adjust the significance level for multiple hypothesis-testing.
Statistical Analysis
Prism 4.03 (Graphpad Software, San Diego, CA) and Matlab R2010b (Mathworks, Natick, MA) were used for statistical analysis. Statistical significance was defined as a p value less than 0.05. The Mann-Whitney test was used to test the hypothesis that methylphenidate decreases time to emergence after a bolus dose of propofol.
To compare the effect of methylphenidate versus normal saline on return of righting during continuous propofol general anesthesia we use a Bayesian Monte Carlo procedure to compute Bayesian 95% (credibility) confidence intervals for the difference in the righting probabilities of the two groups. We also use the algorithm to compute the probability that the righting probability in one group is greater than the righting probability in the other group. Let pi denote the probability of righting for the animals in group i, where i = m is the methylphenidate group and i = s is the saline group. If there are n animals in each group, let ki be the number of animals in group i that right. Because each animal in a given group either has return of the righting reflex or does not, the outcome of the experiment for each animal is Bernoulli and the joint distribution or likelihood for the animals in group i is the binomial model20
| (1) |
To perform a Bayesian analysis we assume that the prior density for pi is the beta probability model20
| (2) |
for pi ∈ (0,1), parameters α > 0 and β > 0, and is the standard gamma function. It follows that the posterior density for group i is the gamma probability density20
| (3) |
For our analyses we take α = β = 1 in Eq. 2 so that the prior density for both groups is the uninformative, uniform probability density on the interval (0,1). Given the posterior densities for the methylphenidate and the saline groups, we compute the posterior density f (pm − ps | km, ks) by using the following Monte Carlo algorithm:
Draw pm from f (pm | km)
Draw ps from f (ps | ks)
Compute pm − ps
Do 1 to 3 10,000 times.
The histogram of the 10,000 pm − ps values is a Monte Carlo approximation to the posterior density f (pm − ps | km, ks). The lower and upper limits of the 95% Bayesian credibility (confidence) interval are 250th smallest value 9,750th smallest value in the Monte Carlo sample respectively. We compute the probability that pm − ps as , where ℓ is the number of times that pm > ps in the Monte Carlo sample. Unlike confidence intervals computed using frequentist methods, the Bayesian 95% confidence intervals can be interpreted as having probability 0.95 that the value of pm − ps lies between the lower and upper limits of the interval based on the data in the current sample.20 We used this Monte Carlo algorithm in our previous studies using methylphenidate to induce active emergence from isoflurane general anesthesia.11
RESULTS
Methylphenidate Decreases Time to Emergence After a Propofol Bolus
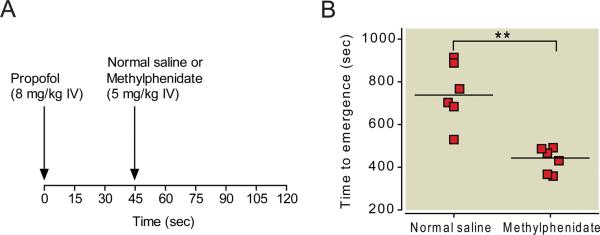
Figure 1A summarizes the protocol for this experiment. As shown in Figure 1B, the median time to emergence for animals that received normal saline was 735 seconds (95% CI: 598 to 897 seconds, n=6) versus 448 seconds (95% CI: 371 to 495 seconds, n=6) for animals that received methylphenidate. The median difference in time to emergence between these two groups was 282 seconds (95% CI: 166 to 458 seconds, Mann-Whitney test). This median difference was statistically significant (p = 0.0051).
Fig. 1.
Methylphenidate decreases time to emergence from propofol anesthesia. (A) Rats received a bolus of propofol (8 mg/kg IV), and 45 seconds later, received methylphenidate (5 mg/kg IV) or normal saline (vehicle). Time to emergence was defined as the time from administration of propofol to return of righting (i.e. all four paws touching the floor). (B) Scatter plot of time to emergence for rats that received normal saline vs. methylphenidate (5 mg/kg IV). The lines represent the medians. ** P<0.01.
Methylphenidate Restores the Righting Reflex During Continuous Propofol General Anesthesia
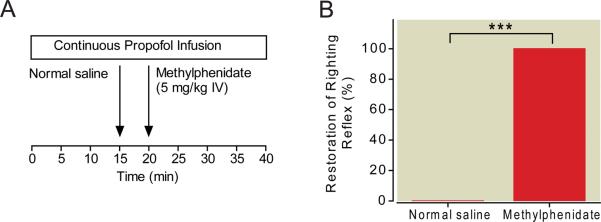
Figure 2A summarizes the protocol for this experiment. The final target concentration of propofol was maintained at a dose 0.5 μg/ml above the highest dose at which purposeful movements were observed, and fixed for the remainder of the experiment. The median final target plasma concentration of propofol was 4.0 (95% CI: 3.2 to 4.6, n=6). The results of the experiment are shown in Figure 2B. During continuous propofol anesthesia, none of the rats exhibited purposeful movements after intravenous injection of normal saline or removal of the temperature probe (n=6). However, after administration of methylphenidate (5 mg/kg IV), all of the rats promptly exhibited signs of arousal (e.g. lifting of the head, blinking of the eyes, twisting of the torso, kicking, clawing, and grooming) and had restoration of righting within 4 minutes, with a median time of 82 sec (95% CI: 30 to 166 sec, n=6). The Bayesian 95% CI for the difference in the propensities to have return of righting between rats in the methylphenidate group and those in the normal saline group was 0.373 to 0.968. The posterior probability was 0.9995.
Fig. 2.
Methylphenidate induces emergence during a continuous target controlled infusion of propofol. (A) The final target plasma concentration of propofol was established at 0.5 μg/ml above the highest dose at which purposeful movements were observed, and maintained for 15 minutes before normal saline (vehicle) was injected. Five minutes later, methylphenidate (5 mg/kg IV) was administered. The propofol infusion was continued at the same dose. (B) None of the rats exhibited an arousal response during the 5 minutes after normal saline administration (n=6). However, methylphenidate induced a profound arousal response and restored the righting reflex within 4 minutes in all rats (n=6), despite continuous propofol general anesthesia. ***posterior probability > 0.95.
Methylphenidate Induces Changes in Electroencephalogram Spectral Content that Correlate with Increased Arousal
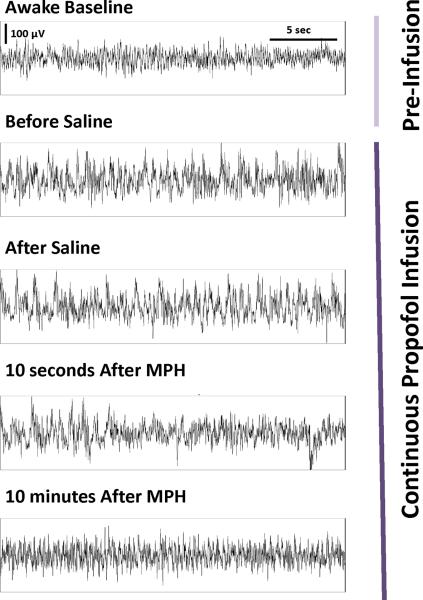
The electroencephalogram was recorded from four rats with pre-implanted extradural electrodes over the somatosensory cortex. In order to minimize motion artifacts, the final target concentration of propofol was maintained at a higher dose (1.0 μg/ml above the highest dose at which purposeful movements were observed), and fixed for the remainder of the experiment. Typical raw electroencephalogram traces recorded from a single rat are shown in Figure 3. In the awake state before the administration of any drugs, animals showed an active high-frequency, low-amplitude electroencephalogram pattern, which changed to a low-frequency, high-amplitude pattern during the target controlled infusion of propofol.
Fig. 3.
Methylphenidate induces electroencephalogram changes during a continuous target controlled infusion of propofol. Thirty-second epochs of electroencephalogram recordings from a single rat show the change from an active, theta-dominant pattern during the awake state to a delta-dominant pattern during the continuous target controlled infusion of propofol. The latter pattern was unchanged after the administration of normal saline, but after administration of methylphenidate (MPH, 5 mg/kg IV) there was a shift in the electroencephalogram back to an active theta-dominant pattern similar to that observed during the awake state. This pattern persisted for more than 10 minutes.
Although the electroencephalogram pattern did not change after injection of normal saline or removal of the temperature probe, administration of methylphenidate (5 mg/kg IV) induced a shift back to an active high-frequency, low-amplitude pattern similar to that observed during the awake state. This change persisted for more than 10 minutes despite the continuous target controlled infusion of propofol. After the administration of methylphenidate none of the rats had restoration of righting at this higher dose of propofol, but all four exhibited varying behavioral signs of arousal (e.g. opening of the eyes, lifting of the head, kicking, etc.).
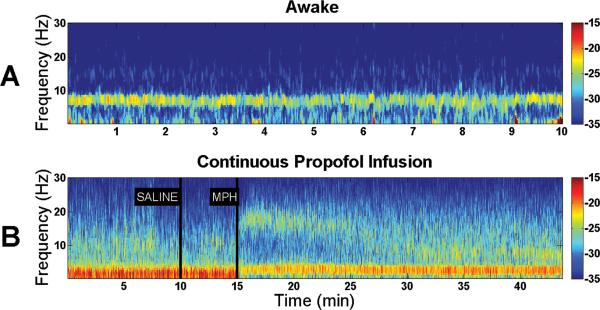
To assess changes in electroencephalogram power over time, spectrograms were computed from the continuous unfiltered electroencephalogram data recorded from each animal. Typical results from an individual rat are shown in Figure 4. During the awake state, electroencephalogram power was mainly in the theta frequency range (4–8 Hz). However, the target controlled infusion of propofol caused a large increase in delta power (<4 Hz). Although intravenous injection of normal saline produced no appreciable change in the power spectrum, administration of methylphenidate (5 mg/kg IV) produced a prompt reduction in delta power and increase in theta and beta power (12–30 Hz), providing evidence for a new arousal state distinct from the baseline awake state.
Fig. 4.
Spectral analysis of electroencephalogram data reveals a shift in power induced by methylphenidate during a continuous target controlled infusion of propofol. Warm colors (e.g. red) represent higher power at a given frequency, while cool colors (e.g. blue) represent lower power. (A) A representative spectrogram computed from a rat in the awake state shows predominance of theta power (4–8 Hz). (B) A representative spectrogram computed from a rat during propofol general anesthesia shows predominance of delta power (<4 Hz) before and after administration of normal saline. However, administration of methylphenidate (MPH, 5 mg/kg IV) promptly reduced delta power, and increased theta (4–8 Hz) and beta power (12–30 Hz), providing evidence for a new arousal state distinct from the baseline awake state.
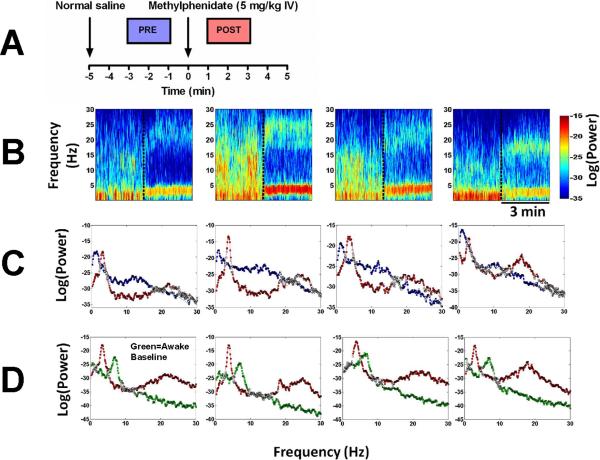
Figure 5 shows spectrograms and power spectra from four different animals during continuous target controlled infusions of propofol, with the results of the Kolmogorov-Smirnov test computed from two-minute time windows before and after methylphenidate administration. At a 0.05 significance level the two-sided Kolmogorov-Smirnov test with Bonferonni correction rejects the null hypothesis at all frequencies except those marked with white squares. Methylphenidate (5 mg/kg IV) decreased delta power and increased theta and beta power in all 4 animals. The difference in power before and after methylphenidate was statistically significant at most frequencies between 0–30 Hz.
Fig. 5.
Electroencephalogram power spectra and spectrograms computed for each of 4 animals reveal a consistent shift in peak power from delta to theta and beta after administration of methylphenidate during continuous propofol general anesthesia. (A) A schematic showing the two-minute windows used to compute power spectra before methylphenidate administration (blue, “PRE”), and after methylphenidate administration (red, “POST”). (B) Spectrograms computed from 4 different animals that received methylphenidate at the time point indicated by the vertical dashed line. (C) Power spectra computed from the same animals with results of the Kolmogorov-Smirnov test for the two-minute periods before (blue) and after (red) methylphenidate administration. At a 0.05 significance level (with Bonferonni correction) the Kolmogorov-Smirnov test rejects the null hypothesis at all frequencies except those marked with white squares. Statistically significant changes occurred at most frequencies between 0–30 Hz. (D) Power spectra computed from the same animals with results of the Kolmogorov-Smirnov test, comparing a two-minute period from the baseline awake state in the absence of any drugs (green) to the two-minute period after methylphenidate administration during continuous propofol general anesthesia (red). Statistically significant changes occurred at most frequencies between 0–30 Hz.
DISCUSSION
In this study we found that methylphenidate induces emergence from propofol general anesthesia in rats. Methylphenidate decreased time to emergence after a propofol bolus, and induced emergence during a continuous target controlled infusion of propofol. The effects of methylphenidate (5 mg/kg IV) were dependent on the dose of propofol. When the target plasma concentration of propofol was fixed at a dose 0.5 μg/ml above the highest dose at which purposeful movements were observed, methylphenidate restored the righting reflex. At a higher dose of propofol, methylphenidate induced electroencephalogram changes and varying behaviors consistent with arousal, but did not restore the righting reflex. The electroencephalogram pattern induced by methylphenidate during propofol general anesthesia was not identical to the pattern observed during the baseline awake state in the absence of drugs, suggesting that the two arousal states are distinct.
Although isoflurane general anesthesia was used for tail vein IV placement, the procedure typically took less than 5 minutes, and isoflurane was promptly discontinued after the IV was secured. For the bolus experiments, propofol was delivered at least 10 minutes after the animal recovered a baseline level of normal activity. For the continuous infusion experiments, establishing the final dose of propofol took a minimum of 45 minutes, during which time the rats inhaled room air and were not exposed to isoflurane. In both cases, residual isoflurane levels were likely minimal at the time methylphenidate was administered.
There has been a growing interest in the role of ascending arousal pathways in emergence from general anesthesia.2,7 The injection of nicotine in the central medial thalamus induces emergence from sevoflurane anesthesia in rats,4 and physostigmine, a centrally-acting cholinesterase inhibitor, restored consciousness in human volunteers during sevoflurane anesthesia10 as well as propofol anesthesia.5 These studies demonstrate that emergence from general anesthesia can be achieved by activating central cholinergic neurotransmission. It was recently reported that injection of the arousal-promoting neurotransmitters histamine or norepinephrine into the nucleus basalis magnocellularis induces behavioral and neurophysiological evidence of arousal during general anesthesia,21,22 providing evidence that monoaminergic arousal pathways are also important for emergence from general anesthesia.
We recently reported that methylphenidate induces emergence from isoflurane general anesthesia, an effect that is inhibited by droperidol.11 Methylphenidate acts by blocking dopamine and norepinephrine reuptake transporters,23 and it likely antagonizes the effects of isoflurane and propofol by activating a combination of dopaminergic, noradrenergic, and/or histaminergic arousal pathways.11 Transgenic mice harboring a point mutation in the β3 subunit of the γ-aminobutyric acid type A receptor are highly resistant to the anesthetizing effects of propofol but not volatile anesthetics,12,13 suggesting that propofol and isoflurane may produce general anesthesia by distinct molecular mechanisms. While the present study does not prove the mechanism of action of methylphenidate, the finding that methylphenidate induces emergence from both isoflurane and propofol general anesthesia further supports the hypothesis that methylphenidate acts by activating monoaminergic arousal pathways at the circuit level, rather than antagonizing general anesthetics at the molecular level.
In our previous study we used plethysmography to show that methylphenidate also increases respiratory drive in rats anesthetized with isoflurane.11 We concluded that the increase in minute ventilation induced by methylphenidate likely accelerated isoflurane elimination and contributed to the decrease in time to emergence. In this study we did not perform plethysmography experiments during propofol general anesthesia, because unlike inhaled anesthetics, the elimination of propofol does not involve the pulmonary system. However, there is considerable evidence that activating dopaminergic neurotransmission increases respiratory drive,24–26 so it is reasonable to predict that methylphenidate would also stimulate breathing during propofol general anesthesia. It is noteworthy that the animals in the present study inhaled room air (unlike the animals in our previous study that inhaled pure oxygen as the carrier gas for isoflurane), demonstrating that hyperoxia is not necessary for methylphenidate to induce emergence from general anesthesia.
To our knowledge, this is the first study to report the use of a STANPUMP-based target controlled infusion to maintain propofol general anesthesia in rodents. The rat pharmacokinetic profile was based on allometric extrapolations comparing adults, children and rats.14 Our results suggest that these kinetic parameters can be used to maintain a reliable steady-state general anesthetic with propofol. However, one weakness of this study is that we did not assay the propofol concentrations in blood over time to confirm that the target controlled infusion was providing a stable plasma concentration. Because we used long equilibration times and a careful titration protocol to arrive at the final dose of propofol, however, it is unlikely that large changes in the effect site concentration of propofol were occurring at the time of methylphenidate administration.
It has been reported that patients taking methylphenidate have an increased requirement for propofol,27,28 which agrees with the findings of this study. Further study is warranted to test the hypothesis that methylphenidate induces emergence from propofol general anesthesia in humans. Methylphenidate may be useful to restore consciousness in patients oversedated with propofol, and to induce emergence from propofol general anesthesia after prolonged infusions. Methylphenidate has a well-established safety profile in both children and adults for the treatment of Attention Deficit Hyperactivity Disorder.23 The results of the present study suggest that methylphenidate may be a clinically useful to induce emergence from propofol general anesthesia. The availability of such a drug may lead to improved patient safety and operating room efficiency.
What we already know about this topic
The psychostimulant methylphenidate was recently found to induce emergence from isoflurane anesthesia in rats, but its effects on anesthesia produced by other agents is unclear
What this article tells us that is new
Methyphenidate enhanced emergence from intravenous propofol in rats, which was accompanied by a shift in the electrocephalogram to higher frequencies
Further studies are required to determine whether methylphenidate might provide a useful agent to facilitate emergence from general anesthesia in humans
ACKNOWLEDGEMENT
The authors thank Steven L. Shafer, M.D., Professor of Anesthesiology, Columbia University, New York, NY, for kindly providing the propofol pharmacokinetics file for rats in STANPUMP.
DISCLOSURE OF FUNDING Supported by grants DP1-OD003646 and K08-GM094394 from the National Institutes of Health, Bethesda, Maryland, and the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
DISCLOSURE OF PATENT APPLICATION Jessica J. Chemali, Emery N. Brown, and Ken Solt submitted a patent application on September 1, 2011 for the use of intravenous methylphenidate to restore arousal during propofol general anesthesia.
PRIOR PRESENTATION OF WORK This work has been presented, in part, at annual meetings of the Society for Anesthesia and Sleep Medicine (Chicago, IL, October 14, 2011) and the Society for Neuroscience (Washington, DC, November 13, 2011).
STANPUMP program. Available at: http://www.opentcl.org. Last accessed 4/26/2011.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Luo T, Leung LS. Involvement of tuberomamillary histaminergic neurons in isoflurane anesthesia. Anesthesiology. 2011;115:36–43. doi: 10.1097/ALN.0b013e3182207655. [DOI] [PubMed] [Google Scholar]
- 2.Franks NP. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 3.Hudetz AG, Wood JD, Kampine JP. Cholinergic reversal of isoflurane anesthesia in rats as measured by cross-approximate entropy of the electroencephalogram. Anesthesiology. 2003;99:1125–31. doi: 10.1097/00000542-200311000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–72. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 5.Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93:708–17. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–14. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–28. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zecharia AY, Nelson LE, Gent TC, Schumacher M, Jurd R, Rudolph U, Brickley SG, Maze M, Franks NP. The involvement of hypothalamic sleep pathways in general anesthesia: Testing the hypothesis using the GABAA receptor beta3N265M knock-in mouse. J Neurosci. 2009;29:2177–87. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plourde G, Chartrand D, Fiset P, Font S, Backman SB. Antagonism of sevoflurane anaesthesia by physostigmine: Effects on the auditory steady-state response and bispectral index. Br J Anaesth. 2003;91:583–6. doi: 10.1093/bja/aeg209. [DOI] [PubMed] [Google Scholar]
- 11.Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate Actively Induces Emergence from General Anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 13.Liao M, Sonner JM, Jurd R, Rudolph U, Borghese CM, Harris RA, Laster MJ, Eger EI., 2nd Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth Analg. 2005;101:412–8. doi: 10.1213/01.ANE.0000154196.86587.35. [DOI] [PubMed] [Google Scholar]
- 14.Knibbe CA, Zuideveld KP, Aarts LP, Kuks PF, Danhof M. Allometric relationships between the pharmacokinetics of propofol in rats, children and adults. Br J Clin Pharmacol. 2005;59:705–11. doi: 10.1111/j.1365-2125.2005.02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotten JF, Husain SS, Forman SA, Miller KW, Kelly EW, Nguyen HH, Raines DE. Methoxycarbonyl-etomidate: A novel rapidly metabolized and ultra-short acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111:240–9. doi: 10.1097/ALN.0b013e3181ae63d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vijn PC, Sneyd JR. I.v. anaesthesia and EEG burst suppression in rats: Bolus injections and closed-loop infusions. Br J Anaesth. 1998;81:415–21. doi: 10.1093/bja/81.3.415. [DOI] [PubMed] [Google Scholar]
- 17.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: A platform for analyzing neural signals. J Neurosci Methods. 192:146–51. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Box GEP, Jenkins GM, Reinsel GC. Time series analysis: Forecasting and control. 4th edition John Wiley; Hoboken, N.J.: 2008. [Google Scholar]
- 19.Sheskin DJ. Handbook of parametric and nonparametric statistical procedures. 2 edition Chapman Hall CRC; Boca Raton, FL: 2007. pp. 577–87. [Google Scholar]
- 20.DeGroot MH, Schervish MJ. Probability and statistics. 3rd edition Addison-Wesley; Boston: 2002. [Google Scholar]
- 21.Pillay S, Vizuete JA, McCallum JB, Hudetz AG. Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology. 2011;115:733–42. doi: 10.1097/ALN.0b013e31822c5ee1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology. 2009;111:725–33. doi: 10.1097/ALN.0b013e3181b061a0. [DOI] [PubMed] [Google Scholar]
- 23.Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: Insights on efficacy and safety. Neuropharmacology. 2009;57:608–18. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Lalley PM. Dopamine1 receptor agonists reverse opioid respiratory network depression, increase CO2 reactivity. Respir Physiol Neurobiol. 2004;139:247–62. doi: 10.1016/j.resp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Lalley PM. D1-dopamine receptor agonists prevent and reverse opiate depression of breathing but not antinociception in the cat. Am J Physiol Regul Integr Comp Physiol. 2005;289:R45–51. doi: 10.1152/ajpregu.00868.2004. [DOI] [PubMed] [Google Scholar]
- 26.Lalley PM. D1/D2-dopamine receptor agonist dihydrexidine stimulates inspiratory motor output and depresses medullary expiratory neurons. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1829–36. doi: 10.1152/ajpregu.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasuga T, Meno A, Honda M, Momoeda K, Nagase M, Hanaoka K. General anesthesia for two patients taking methylphenidate (Ritalin) Masui. 2008;57:748–51. [PubMed] [Google Scholar]
- 28.Ririe DG, Ririe KL, Sethna NF, Fox L. Unexpected interaction of methylphenidate (Ritalin) with anaesthetic agents. Paediatr Anaesth. 1997;7:69–72. doi: 10.1046/j.1460-9592.1997.d01-34.x. [DOI] [PubMed] [Google Scholar]