Abstract
BACKGROUND
People with schizophrenia and their biological relatives have deficits in executive control processes such as inhibition and working memory as evidenced by performance abnormalities on antisaccade (AS) and ocular motor delayed response (ODR) tasks.
METHODS
The present functional magnetic resonance imaging (fMRI) study was conducted to investigate brain activity associated with these putative indices of schizophrenia risk by (i) directly comparing neural functioning in 15 schizophrenia patients, 13 of their first-degree biological relatives (primarily siblings), and 14 healthy participants, and (ii) assessing executive function associated with volitional saccades by using a combination of AS and ODR tasks.
RESULTS
Behavioral data showed that patients and relatives both made more volitional saccade errors. Imaging data demonstrated that within the context of preserved activity in some neural regions in patients and relatives, there were two distinct patterns of disruptions in other regions. First, there were deficits observed only in the schizophrenia group (decreased activity in lateral FEF and SEF), suggesting a change associated with disease manifestation. Second, there were deficits observed in both patients and relatives (decreased activity in middle occipital gyrus, insula, cuneus, anterior cingulate, and BA10 in prefrontal cortex), indicating a potential association with disease risk.
CONCLUSIONS
Results indicate that decreased brain activation in regions involved in managing and evaluating early sensory and attention processing may be associated with poor volitional saccade control and risk for developing schizophrenia.
Keywords: antisaccades, saccades, working memory, inhibition, schizophrenia, delayed response tasks
Introduction
People with schizophrenia and their biological relatives have deficits in executive control processes such as inhibition and working memory (1–6), which can be successfully modeled using cognitively complex saccadic eye movement tasks. Saccades are fast redirections of gaze that exist in a hierarchy of increasingly complex behavior (7). At a basic level are refixation saccades to a target - on which schizophrenia participants have comparatively normal performance (8–10). At a more complex level are volitional saccades – on which schizophrenia participants’ performance is compromised. Volitional saccades require endogenous control, for example inhibiting an eye movement and/or maintaining internal representations of spatial locations as a target for a later eye movement (11–12).
Among a diverse range of volitional saccades tasks are two that are frequently used to assess such abilities in schizophrenia research: 1) antisaccade (AS; 13–14) and 2) ocular motor delayed response (ODR; 15–17) paradigms. These tasks both require visuo-spatial attention, inhibitory control, working memory and generation of a saccade to a specific spatial location in the absence of a visual target (13,14,16,18–22). For correct AS performance, participants must maintain the instruction to generate a saccade to the peripheral cue’s mirror image location, inhibit a reflexive saccade toward that cue upon presentation, and then program and generate a saccade to the cue's mirror image location. An error is defined as an initial glance toward the peripheral cue. For correct ODR performance, participants must maintain the instruction to generate a saccade to a remembered cue location, inhibit a response to that cue’s presentation and during a delay period, remember the spatial location of the cue, and finally, generate a saccade to the remembered cue location after the delay. An error is defined as an initial glance toward the peripheral cue or its location during the delay period.
Disrupted performance during both of these volitional saccade tasks is characteristic of schizophrenia participants and their biological relatives. Schizophrenia subjects (i) generate increased errors during AS tasks (9,13,23–29), (ii) generate increased anticipatory errors during ODR tasks (15,16,22,29–32) and (iii) demonstrate increased latencies and decreased gains of correct responses in both tasks (9,15–17,25,30,31,33). The similarities in characteristics of behavioral performance between AS and ODR tasks suggest that the two tasks may be indexing similar neural circuitry abnormalities among schizophrenia participants, although this possibility has yet to be evaluated. These data also suggest that use of both tasks within the same session may provide a constructive replication (i.e., probing organized neural circuitry with similar, but not identical, tasks). Use of both tasks, then, is expected to provide an inclusive view of the neural circuitry supporting volitional saccade tasks and maximize the probability that regions involved in volitional saccade tasks are observed and identified.
Volitional saccades are supported by both subcortical and cortical regions, and as might be inferred from the behavioral data, the neural regions identified as supporting AS and ODR performance are quite similar (34,35). The associated neural circuitry may be comprised of sub-circuits that support different aspects of saccadic performance. For instance, regions involved primarily in motor processes (i.e. saccade generation and triggering) include basal ganglia (BG), posterior parietal cortex (PPC), and lateral frontal eye fields (lFEF) (36–47). These regions may be fairly circumscribed, yet they collaborate with executive control regions necessary for selecting, inhibiting, sequencing and/or remembering correct responses, including anterior cingulate gyrus (ACG), medial frontal eye fields (mFEF), supplementary eye fields (SEF), and prefrontal cortex (PFC; 35,40,41,43–45,47–52). Many of these latter brain regions have been implicated in the neuropathology underlying cognitive control difficulties in schizophrenia (1,53–58).
The present study was conducted to further investigate neural correlates of executive control deficits by focusing on volitional saccade performance in schizophrenia by (i) directly comparing neural functioning in schizophrenia patients, their first-degree biological relatives (primarily siblings), and healthy participants, and (ii) making a more inclusive assessment of executive control by combining AS and ODR tasks into a single measure of “volitional control”. Behavioral performance on volitional saccade tasks has been proposed as a potential schizophrenia-related intermediate phenotype (28,59–61). The inclusion of relatives allows for a further evaluation of the extent to which intermediate phenotype status may extend to the brain activity patterns underlying the well-documented differences in behavioral performance.
The following hypotheses were evaluated: (i) schizophrenia participants and their relatives will show increased error rates on volitional saccade tasks relative to healthy participants; (ii) AS and ODR tasks will be highly similar in the neural circuitries supporting performance and in the activity patterns that differentiate schizophrenia and healthy participants1; (iii) relatives of schizophrenia participants will show disruptions in the neural circuitry mediating volitional saccade performance similar to that observed in the affected patients; (iv) these disruptions are particularly expected in regions mediating the executive component of volitional saccade performance such as PFC (38,40,41,48,62–65).
Methods and Materials
Participants
Fifteen participants diagnosed with DSM-IV schizophrenia (age: M=38 yrs, SD=11), 13 of their first-degree biological relatives (11 siblings, 1 parent, 1 offspring: age: M=40 yrs, SD=15), and 14 healthy participants (age: M=39 yrs, SD=12) were studied. Schizophrenia participants and their relatives were recruited from regional mental health centers and through newspaper advertisements. Schizophrenia participants were diagnosed using the Patient Edition of the Structured Clinical Interview for DSM-IV (66) and rated using Scales for the Assessment of Negative Symptoms (SANS), Scales for the Assessment of Positive Symptoms (SAPS) and Global Assessment Functioning (GAF). Eighty percent of schizophrenia participants were taking a single atypical antipsychotic medication2. One was taking two atypical antipsychotic medications3 and two were not medicated.
Relatives of schizophrenia participants were interviewed with the Non-Patient Edition of the Structured Clinical Interview for DSM-IV-TR (66) and screened using the Schizotypal Personality Questionnaire (SPQ; 67) and the Scales of Psychosis Proneness (68). Relatives did not score significantly higher than published norms (67, 68) on any of these measures. Eighty-five percent (N=11) of the relatives of schizophrenia participants had no DSM-IV Axis I disorder (two diagnosed with a nonpsychotic affective disorder). Healthy participants (matched by gender and age to the other groups) were recruited through newspaper advertisements and fliers posted throughout the community.
All participants were right-handed, free of serious physical health problems and absent of known neurological hard signs. Exclusion criteria included loss of consciousness for more than 30 minutes, history of severe head trauma, and current drug abuse. Participants were also screened for contraindications for fMR imaging. All participants provided informed consent as per UGA Institutional Review Board requirements and were paid for their time.
FMR Imaging
Brain imaging was performed at the Athens Orthopedic Clinic MRI Center using a GE Signa Horizon LX 1.5T MRI scanner (Milwaukee, WI). Immediately prior to entering the scanner, participants were given task instructions. During imaging, participants were provided with earplugs and positioned in a supine position. Their heads were stabilized with foam padding and head restraints. A dual mirror box was placed 16 cm above and in front of the participant's eyes designed to make stimuli visible to the participant, and the participant’s eyes visible to an eye-tracking camera. Eye movements were recorded using MRI compatible equipment (MeyeTrack LR, SensoMotoric Instruments, Inc., Berlin, Germany). The eye was illuminated via an infrared light source, and the eye image was relayed via a remote infrared camera with long-range optics. Eye movements were displayed on a computer monitor so performance could be monitored and recorded continuously (sampling rate = 60 Hz) for later analysis. An LCD Projector (NEC Viewtechnology, Ltd., Tokyo, Japan) displayed stimuli onto a rear projection screen standing 174 cm from participant's nasion. Stimulus presentation was controlled using Presentation software (Neurobehavioral Systems, Albany, CA).
First, a three-dimensional T1-weighted structural MRI scan for definition of anatomical structures within each brain [spoiled gradient-recall (SPGR) protocol: TE=2.8 msec, TR=10.8 msec, flip angle=20°, 2 NEX, matrix=256×256, field of view=24 (resulting in an in-plane resolution of 0.97×0.97), slice thickness of 1.5 mm, sagittal acquisition, 124 contiguous slices, scan time 5 min 41 sec]. Second, participants were reminded of task instructions, and two functional runs were conducted. For each, a series of T2*-weighted functional images were obtained [axial prescription, spoiled-gradient pulse sequence (SPGR) with a spiral readout pattern in k-space, (matrix=64×64, field of view=24 resulting in an in-plane resolution of 3.75×3.75, slice thickness=4 mm), TE=40 msec, TR=1912 msec with two interleaves resulting in an image acquisition time of 3.8 sec; flip angle=77°, 24 supratentorial contiguous slices; AS=81 TRs (scan time of 5 min 8 sec), ODR =97 TRs, (scan time of 6 min 9 sec)]. Brain coverage for functional scans was defined by placing the most superior scan plane tangent to the highest point of the somatosensory cortex. Each functional run began with two null repetitions (not included in analyses) to allow the magnetization to stabilize at steady state equilibrium.
Eye Movement Stimuli
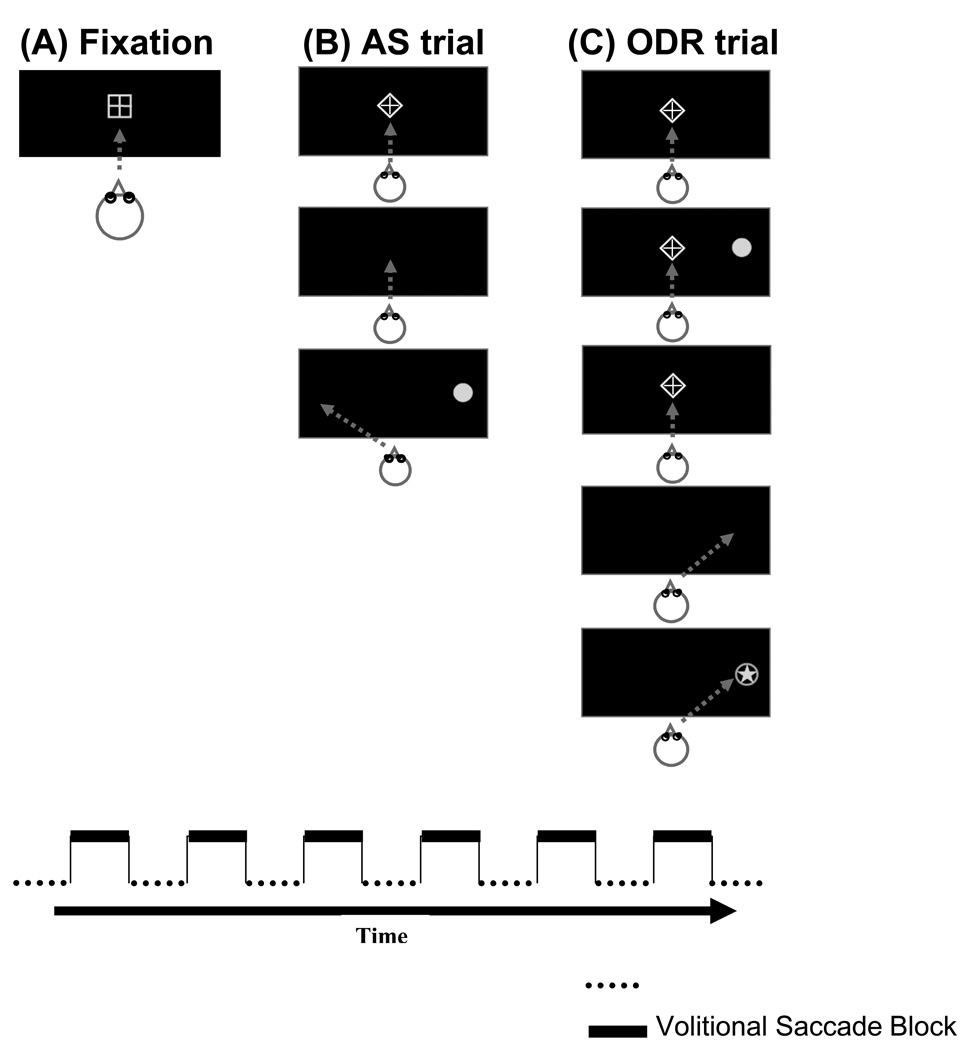
Participants performed two blocked runs, which alternated between blocks of fixation (see Figure 1A) and blocks of a single volitional saccade condition. A change in the geometric shape around the fixation cross signaled a task change: a bordering square signaled the baseline fixation condition and a bordering diamond signaled a volitional saccade condition. The volitional saccade blocks were either antisaccade trials (AS run; see Figure 1B), or ocular motor delayed response trials (ODR run; see Figure 1C). The order of runs was counterbalanced across subjects.
Figure 1. Antisaccade (AS) and Ocular Motor Delayed Response (ODR) Task Trials.
Stimuli presented during AS and ODR runs. During the AS run, participants were presented with 22.5-second blocks of fixation (1A) alternated with blocks of eight AS trials (1B). During the ODR run, participants were presented with 22.5-second blocks of fixation (1A) alternated with blocks of six ODR trials (1C). Gray arrows show correct eye position.
Fixation Block
Participants were instructed to fixate on a centrally presented cross bordered by a square for its duration (22.5 sec).
Antisaccade Block
Participants were instructed to keep their eyes on a centrally presented cross bordered by a diamond for its duration (1700 msec). The diamond was extinguished, and 200 msec later (gap), a 1° gray dot was presented 8° to the left or right of fixation in the horizontal plane (1250 msec). Participants were instructed to move their eyes as quickly and accurately as possible to the mirror image location of the cue (same amplitude, opposite side). Eight trials were presented per block (25.2 sec total).
ODR Block
Participants were instructed to keep their eyes on a centrally presented cross bordered by a diamond for its duration. After 1500 msec a 1° gray dot was presented (100 msec) at one of six pseudorandomly selected peripheral locations (+/− 4°,8°,or 12°). Participants were instructed to remember the location of the peripheral cue while keeping their eyes fixated on the central cross. After a delay period (2500 msec) the fixation cross was turned off, signaling the participant to move their eyes to the remembered location as quickly and accurately as possible. After 1300 msec of response time, a 1° gray star appeared in the correct location (500 msec) to reinforce the accuracy component of the task. Six trials were presented per block (35.4 sec total).
Data Analyses
Behavioral Analyses
Eye movements recorded in the scanner were analyzed (Matlab; The Mathworks, Natick, Massachusetts) for the following variables. First, the percentage of errors generated during AS and ODR trials were calculated ([number of trials with at least one error saccade/total number of useable trials]*100). For AS trials an error saccade was an initial glance towards (instead of away from) the cue. For ODR trials an error saccade was an initial glance toward the peripheral cue during its presentation or anytime during the remainder of the delay period. Second, the latencies of correct antisaccades and memory saccades were calculated (time in milliseconds between the cue presentation and the start of the saccade [>90 msec]). Third, the gain of correct antisaccades and memory saccades was determined ([initial saccade amplitude / cue amplitude]; 1.00 indicates perfect accuracy).
FMRI Analyses
Analyses were conducted with Analysis of Functional NeuroImages (AFNI; 69) software using methods similar to those previously published (34,48). Three-dimensional datasets were created from individual image files. For each run, all volumes were registered to the middle volume to correct for minor head movement over time. A full width, half-maximum (FWHM) Gaussian filter (4 mm) was applied to each dataset to account for individual variations in anatomy. For each voxel, the percent change in BOLD signal between the baseline (fixation) and volitional saccade (AS or ODR) blocks was calculated for each of the time points.
For each subject, for each run, six factors were entered into a regression model including the baseline and experimental conditions, one linear drift factor, and three factors characterizing head motion in order to evaluate blood oxygenation level-dependent (BOLD) signal change associated with the experimental conditions. Anatomical and functional volumes were transformed into Talairach space (70) and resampled to 4×4×4 mm resolution.
To display volitional saccade-related BOLD signal change, data from all participants in all groups across runs were submitted to a one-sample t test on a voxel-by-voxel basis. To protect against false positives, a threshold/cluster method derived from Monte Carlo simulations4 was applied to the t map (71). The resulting averaged, clustered one-sample t map showed BOLD signal changes associated with volitional saccade performance, and results were consistent with previous fMRI studies (e.g. for a particularly large sample, see 34). This global activation map was then used to define regions of interest (ROIs), wherein a sphere (radius 8 mm) was placed at the center of mass of each cluster that showed significant volitional saccade-related signal change. For each ROI, mean intensity changes were calculated for each individual. Finally, for each ROI, a 3X2 ANOVA was used to evaluate effects of group (SZ, RL, HP) and task (AS, ODR).
Results
Behavioral Results
Behavioral data for AS and ODR task performance were available for 98% of participants. Data from one schizophrenia participant could not be collected due to insufficient contrast between the pupil and the iris. A group (SZ, RL, HP) by task (AS, ODR) analysis of variance on percent errors revealed no significant effect of task, F(1,38)=1.2, p=0.27, and error rates in the AS and ODR tasks were significantly correlated in all groups (SZ: r=0.68, p=0.01; RL: r=0.57, p=0.04; and HP: r=0.58, p=0.03). As such, performance data presented hereafter are combined across AS and ODR tasks and are presented as volitional saccade data.
Error Rate
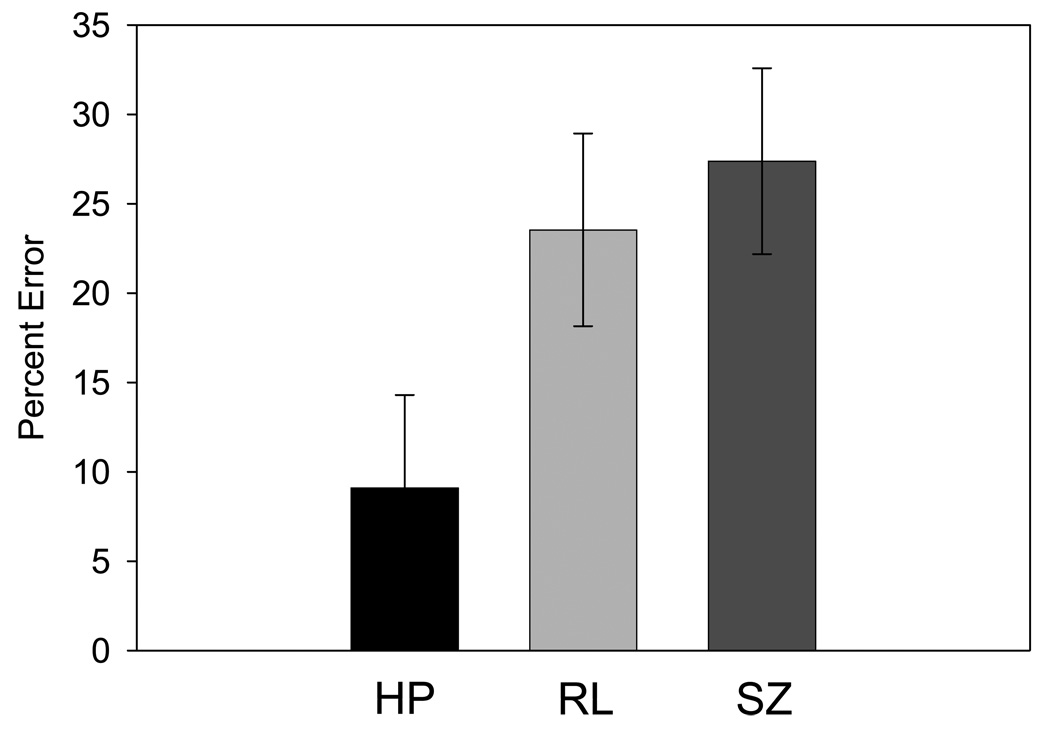
There was an overall significant difference in percent of volitional saccade errors generated between groups, F(2,38)=3.4, p=0.043. Post hoc Tukey tests revealed significantly higher error rates in the SZ group (M=27.4%, SE=5.2) than the HP group (M=9.1% error, SE=5.2, p=0.045). The error values for the RL group were intermediate (M=23.5%, SE=5.4) and differed significantly only from the HP group (p=0.045, see Figure 2).
Figure 2. Behavioral Results – Percent Error.
Bar graph showing mean percentage of errors (and standard error) generated during volitional saccade performance in the healthy participant group (HP), relative group (RL), and schizophrenia group (SZ).
Latency of Saccadic Responses
The latency of responses did not show a significant difference between groups during volitional saccade task performance, F(2,38)=0.725, p=0.49.
Gain of Saccadic Responses
There was a significant difference in the gain of the initial saccade, F(2,38)=5.194, p=0.01. Post hoc Tukey tests revealed that gain was significantly lower in the SZ group (M=0.78, SE=0.07) than in the HP group (M=1.06, SE=0.07; p=0.02) and RL group (M=1.05, SE=0.07; p=0.03)5.
FMRI Results
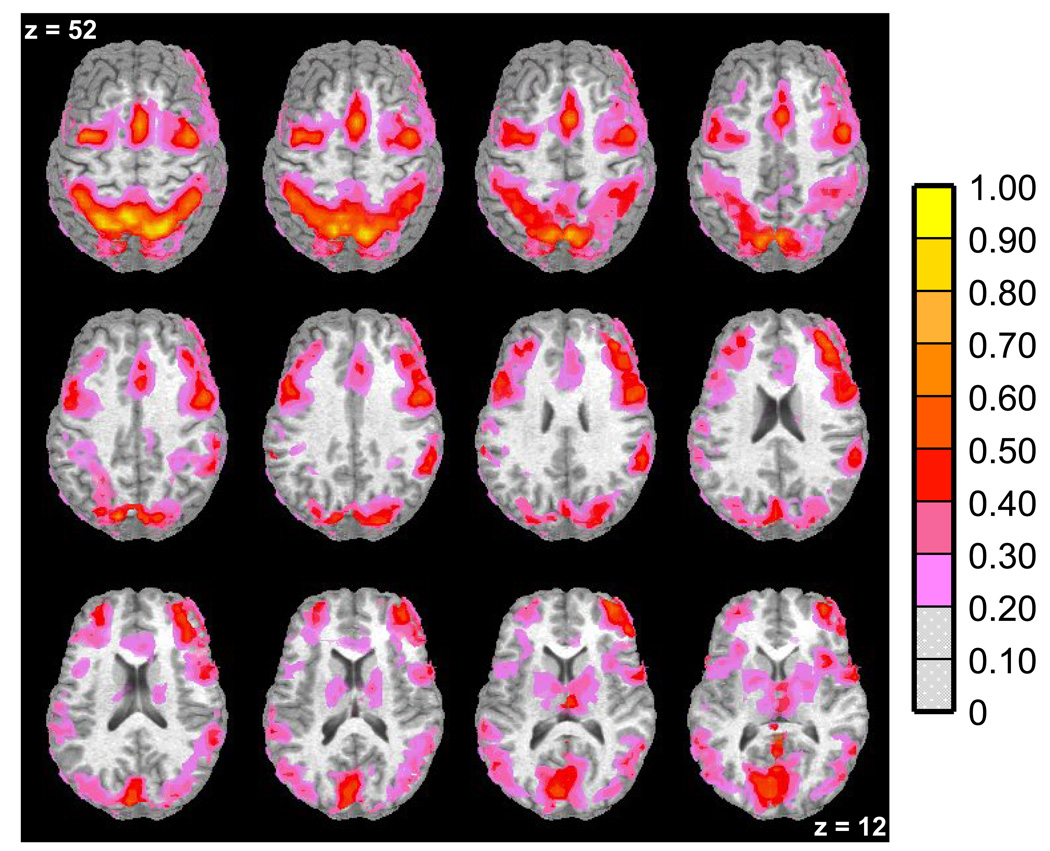
The clustered one-sample t map collapsed across groups and tasks revealed that the following bilateral regions showed significant BOLD signal activity during volitional saccade conditions compared to fixation conditions (see Table 1 and Figure 3). All groups showed increased signal in cortical and subcortical regions previously shown to support volitional saccade performance: striatum, insula, middle occipital gyrus (MOG), cuneus, anterior cingulate gyrus (ACG), superior parietal lobule (SPL), lateral frontal eye fields (lFEF), supplementary eye fields (SEF), medial frontal eye fields (mFEF), and bilateral BA9 and BA10 in dorsolateral prefrontal cortex (DLPFC) (e.g. 34,35,40,41,43,45,48). As such, these 11 bilateral regions comprised the ROIs for subsequent analyses.
Table 1.
Identification of anatomy, Brodmann area (BA), hemispheric location, and Talaraich coordinates for regions of interest (ROI) that showed blood-oxygen-level-dependent (BOLD) signal increase associated with volitional task performance.
| Anatomy of ROI | Left (L) /Right (R) | x | y | z |
|---|---|---|---|---|
| Normal activity in all groups | ||||
| Dorsolateral prefrontal cortex (DLPFC, BA 9) | L | −45 | 23 | 29 |
| R | 38 | 31 | 31 | |
| Medial frontal eye fields (MFEF, BA 6) | L | −27 | −3 | 41 |
| R | 26 | −1 | 46 | |
| Superior parietal lobule (SPL, BA 40) | L | −26 | −64 | 53 |
| R | 24 | −64 | 53 | |
| Striatum | L | −15 | −2 | 11 |
| R | 19 | −2 | 11 | |
| Reduced activity in schizophrenia group | ||||
| Lateral frontal eye fields (LFEF, BA 6) | L | −46 | −3 | 39 |
| R | 44 | −5 | 39 | |
| Supplementary eye fields (SEF, BA 6) | 3 | 15 | 40 | |
| Reduced activity in schizophrenia and relative group | ||||
| Dorsolateral prefrontal cortex (DLPFC, BA 10) | L | −27 | 54 | 14 |
| R | 34 | 53 | 10 | |
| Anterior cingulate gyrus (ACG, BA 32 & 24) | L | −10 | 43 | 8 |
| R | 15 | 36 | 14 | |
| Cuneus | L | −11 | −76 | 1 |
| R | 12 | −76 | 1 | |
| Insula | L | −39 | 20 | 1 |
| R | 42 | 13 | 1 | |
| Middle occipital gyrus (MOG, BA 18 & 19) | L | −43 | −74 | 8 |
| R | 28 | −82 | 21 | |
Figure 3. Functional Magnetic Resonance Imaging (fMRI) Results – Whole-Brain Analysis Results for all Groups.
Axial slices (top left z = 52 through bottom right z = 12, spacing = 8 mm) displaying regions with significant percent signal increase (indicated by the color scale) associated with volitional saccade performance in all groups. This one-sample t-map was used to determine regions of interest (ROIs). The background anatomical image is a structural image from one subject in neurological convention (left hemisphere on the left).
Between-Group Differences
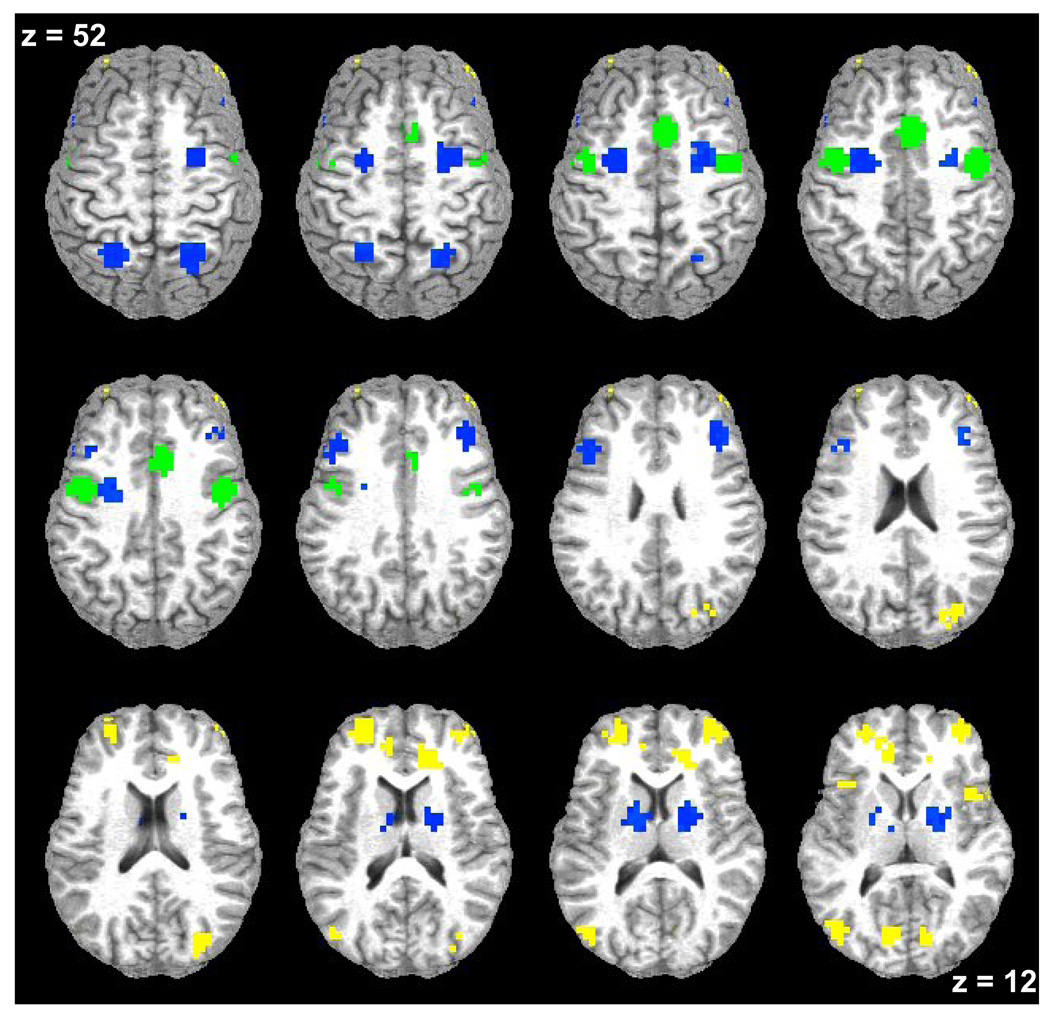
A group (SZ, RL, HP) by task (AS, ODR) ANOVA for each defined ROI revealed that there were no effects of task (all p-values from all ROIs were greater than p=0.16). The examination of group differences revealed three patterns of results (see Table 1 and Figure 4). First, groups did not differ on task-related signal increases in striatum, SPL, mFEF and in BA9 (see Figure 5). Second, only the SZ group showed significantly decreased task-related signal change when compared to the RL and HP groups in SEF and lFEF (see Figure 6). Third, both the SZ and RL groups showed significantly decreased task-related signal change when compared to the HP group in BA10, ACG, cuneus, insula and MOG (see Figure 7). In these five regions relatives tended to show intermediate task-related signal change.
Figure 4. Functional Magnetic Resonance Imaging (fMRI) Results – Differences between Groups.
Axial slices (top left z = 52 through bottom right z = 12, spacing = 8mm) displaying regions of interest with significant blood-oxygen-level-dependent signal increase associated with volitional task performance color coded by pattern of result. Regions in which all three groups showed task-related signal change are shown in blue. Regions in which the only the schizophrenia group is decreased are shown in green. Regions in which the schizophrenia and relative groups are decreased are shown in yellow. The background anatomical image is a structural image from one subject in neurological convention (left hemisphere on the left).
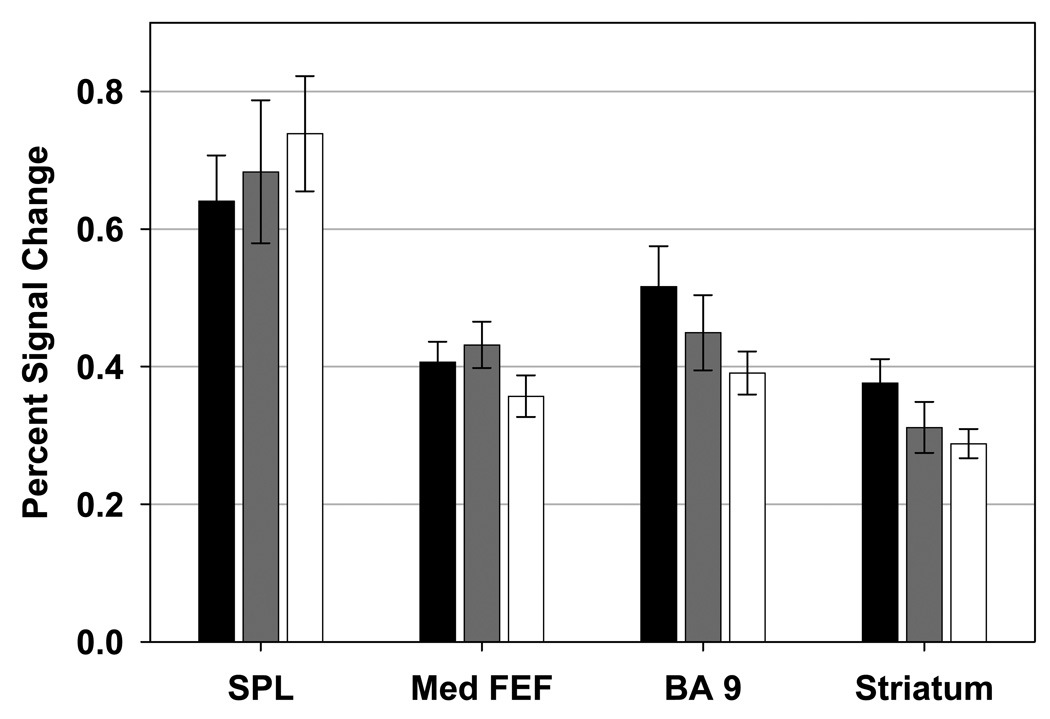
Figure 5. Regions of Interest with no Significant Differences between Groups.
Bar graphs showing mean percent signal change (and standard error) for the healthy participant (black), relative (gray), and schizophrenia (white) groups. Regions of interest depicted showed no significant differences in task-related signal change between groups. SPL: superior parietal lobule, Med FEF: medial frontal eye fields, BA 9: brodmann area 9.
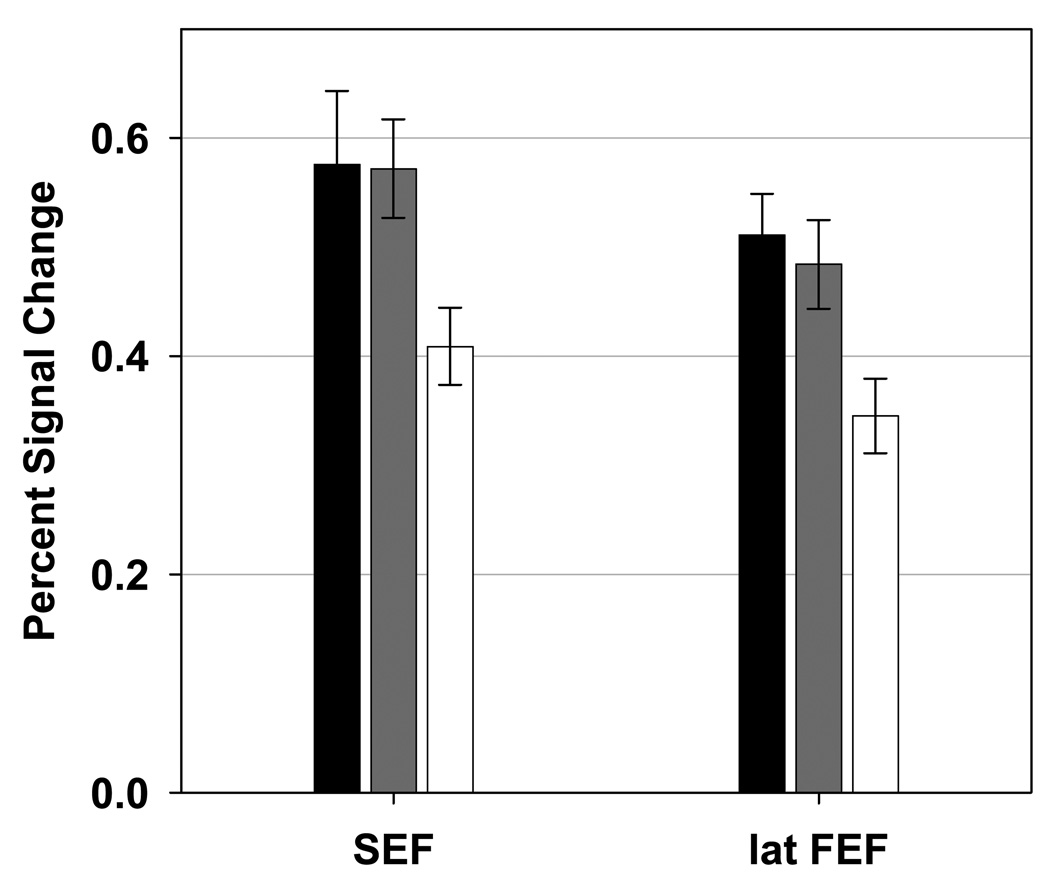
Figure 6. Regions of Interest in which the Schizophrenia Group Showed Decreased Activation.
Bar graphs showing mean percent signal change (and standard error) for the healthy participant (black), relative (gray), and schizophrenia (white) groups. Regions of interest depicted showed significantly lower task-related signal change was observed in the schizophrenia group than in the normal and relative groups. SEF: supplementary eye fields, lat FEF: lateral frontal eye fields.
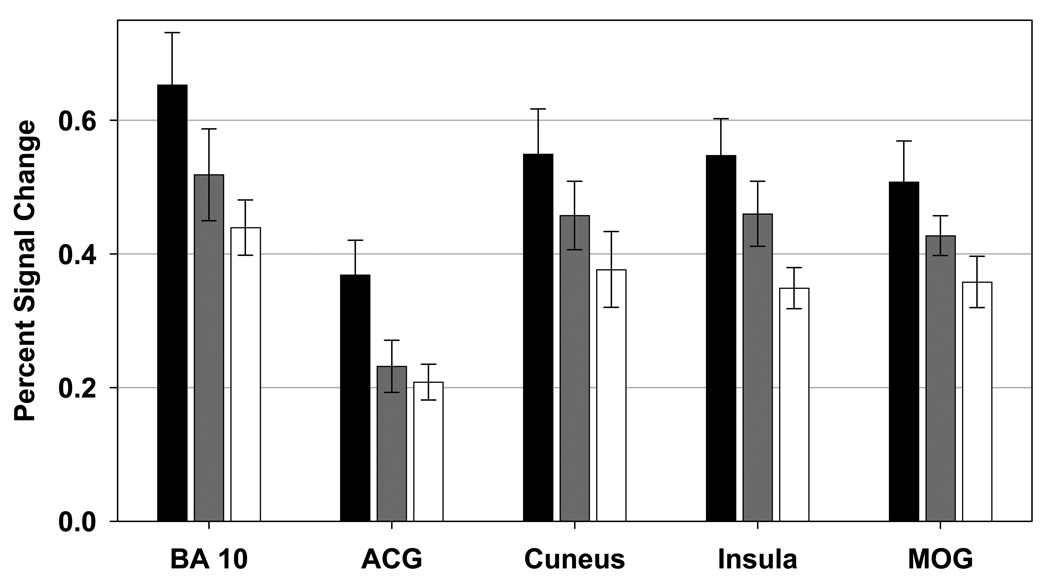
Figure 7. Regions of Interest in which both the Schizophrenia and Relative Groups Showed Decreased Activation.
Bar graphs showing mean percent signal change (and standard error) for the healthy participant (black), relative (gray), and schizophrenia (white) groups. Regions of interest depicted showed lower task-related signal change in the schizophrenia group than in the healthy participant group. Task-related signal change in the relative group did not differ from either the schizophrenia or normal groups. BA 10: brodmann area 10, ACG: anterior cingulate gyrus, MOG: middle occipital gyrus.
Discussion
The present study examined the neural substrates associated with executive control deficits in schizophrenia, which was assessed using two volitional saccade tasks that require visuo-spatial attention, inhibition, working memory and saccade generation to a location lacking a target. Behavior on both types of tasks is known to be impaired among people with schizophrenia and their first-degree biological relatives (20,23,72). Behavioral and fMRI data were acquired from participants with schizophrenia, their first-degree biological relatives (primarily siblings) and healthy participants recruited from the community. When neural activation patterns were compared across groups, differences were observed in particular regions of the saccade circuitry. This suggests that brain activity changes in some neural regions are more likely to be affected by disease-related factors (as indicated by decreased activity observed only in the schizophrenia participants) while activity in other regions are more closely associated with a risk for developing the illness (as indicated by decreased activity in both the patients and their relatives). These types of specific differentiations of disease-related and risk-related abnormalities in brain activations for a purported behavioral endophenotype have not been described previously.
The current data suggest that the control of both antisaccades and ocular motor delayed response tasks require similar neural circuitry. All groups showed increased signal in cortical and subcortical regions previously shown to support volitional saccade performance (e.g. 34,35,40,41,43,45,48). In the current study, no differences were observed between regions active during antisaccades and delayed response tasks, which may not be surprising given the similar processes involved in their performance. This is not to suggest that identical circuitry necessarily supports both types of task performance, but at the very least it is highly similar. Issues that may impact the ability to resolve differences between tasks in the current study include study design, the technical resolution of the equipment on which data were collected, and the analysis path which may have favored those areas active over both tasks as opposed to highlighting task-specific differences.
Consistent with previous literature, schizophrenia patients and their relatives showed evidence of disruptions in the circuitry supporting volitional saccade (43,48,62–65,73). Present results support three important conclusions regarding disruptions in volitional saccade control circuitry in schizophrenia: (i) they are not generalized across all involved brain regions, (ii) some aspects of observed abnormalities are related to manifestation of the disease, and (iii) other aspects of observed abnormalities may be related to the risk for developing the disease.
Disruptions in volitional saccade control circuitry in schizophrenia are not widespread because all three groups (patients, relatives, healthy subjects) showed comparable neural activation in cortical and subcortical regions, including BA9, mFEF, SPL and striatum (see Figure 5), which are known to support volitional saccade performance (34,52,74,75). These results suggest that the observed behavioral and neural abnormalities in schizophrenia participants are not associated with a “generalized deficit”, but are related to more specific disruption of the relevant neural circuitry.
Functional abnormalities in lFEF and SEF were observed only in the schizophrenia group (see Figure 6). Increased activity in these two frontal regions before response generation has been associated with the motor component of volitional saccade generation (lFEF; 49,76) and control of internally generated saccades (SEF; 7,77). Because the relative group did not show significant abnormalities in lFEF and SEF, specific aspects of the motor component of volitional saccade generation may be associated with the actual expression of the symptoms of the disease (78). This finding, however, may implicate several factors, including the disease process itself, active symptom subtypes, illness duration, and/or medication effects. The present project does not allow for an evaluation and determination of which of these factors are the most likely cause of the observed effects, but they would be important to consider in subsequent investigations.
Both the schizophrenia and relative groups showed decreased activity in MOG, insula, cuneus, ACG and BA10 in PFC (see Figure 7). Activity in MOG, insula and cuneus have been associated with early visuo-spatial processing, spatial attention and sensory awareness (44,79–81). Disruption of normal patterns of activity in these regions may be related to poor early sensory and attention processing after stimulus presentation. These regions also have reciprocal connections with prefrontal regions such as ACG and DLPFC (82–85). Perhaps top down control of early sensory areas are compromised during stimulus evaluation (86,87) and response preparation (88,89) among the schizophrenia groups. For instance, disruption of DLPFC and/or ACG-related top-down control signals may compromise evaluative processes involved in determining whether greater executive control is needed for proper task performance (e.g., 90,91) and/or disruption of DLPFC may impair appropriate saccade generation or inhibition (e.g., 84). Finally, it may be that regions showing lower activity in both the proband and relatives groups, may be the most effective indicators of disease risk (better intermediate phenotypes) because they manifest as a function of shared genes (not disease state).
There are at least two caveats applicable to this study. First, the samples sizes for the three groups are modest. Even given the sample sizes, however, the behavioral results essentially replicate previous findings of relative studies with larger sample sizes (Calkins et al., 2004), and imaging results show similar neural circuitry as that identified in other studies of antisaccade and ODR tasks in healthy subjects (34, 36). Also, by collapsing across two volitional saccade runs with similar behavioral requirements, we were able to increase the power to study the neural correlates of volitional saccades. Second, BOLD signal differences between groups might reflect differences in behavioral performance. For instance, more eye movements necessitated by errors and the subsequent corrections might be expected to result in more activity in saccadic circuitry. This would predict more activity in the schizophrenia or relative group, which is the opposite direction of what was actually observed.
In summary, the present study reports on the neural substrates supporting volitional saccade performance of different types (antisaccade and ocular motor delayed response tasks) among schizophrenia families. There were neural disruptions underlying behavioral manifestations of executive functioning deficits of two distinct types: (i) deficits observed only in the schizophrenia group, suggesting dysfunction associated with disease manifestation; and (ii) deficits observed in both patients and relatives, indicating changes associated with disease risk. Although, for the latter, both groups showed decreased activity in these regions, the amount of neural activity observed among the relatives was intermediate between the healthy and schizophrenia participants. This pattern mirrors well-documented differences in behavioral measurement of volitional saccade error rates in these groups (9,13,16,24,25,41). It appears, therefore, that decreased activation in regions involved in stimulus evaluation (ACG and DLPFC), and early sensory and attentional processing (MOG, insula and cuneus), may be associated with poor volitional saccade control and risk for developing schizophrenia.
These results also suggest that brain activity might serve effectively as an intermediate phenotype for schizophrenia. Putative endophenotypes (92) could include specific deficits in brain anatomy and/or function interposed between the predisposing genes and the overt clinical disease. Such measures are expected to provide more direct information about illness risk because they are closer in the etiological chain to the primary constitutional deviations resulting in disease (93,94,95). In practice, there are a number of other cases in which brain activity appears to be an effective intermediate phenotype (see e.g. 25,63,65,73,96). Importantly, the current study demonstrates that a similar level of behavioral performance (increased antisaccade and delayed response task errors of commission) does not guarantee that participants with schizophrenia and their relatives have the same deviations at the level of brain functioning. This suggests that identifying homogenous pathology-related mechanisms at the level of brain functioning among schizophrenia families could considerably enhance the usefulness of behavioral measures of volitional saccade control for a) understanding the neural underpinnings of schizophrenia, b) identifying heterogeneity at the level of specific brain functions between schizophrenia families, and c) defining refined and heritable phenotypes for use in genetic association and linkage studies.
Acknowledgements
This research was supported in part by grants from the National Institute of Mental Health (MH001852, MH57886). The authors would like to thank the subjects who participated in this study, Ms. Caroline Chapman for her assistance with recruitment and data collection, and Mr. Gary Washington for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Formulation of a hypothesis test in terms of the null often causes concern among investigators trained in the social sciences, although it is not a concern in other disciplines (97,98). The logical foundation for such an approach to theory testing, as opposed to the traditional use of “inferential statistics” that demand a statistically significant difference (p<.05), has been discussed at some length in numerous previous reports (99,100,101).
chlorpromazine [CPZ] equivalent dose mean=459 mg, SD=519; aripiprazole N=1, olanzapine N=4, quetiapine N=3, risperidone N=3, and ziprasidone N=1
(CPZ equivalent dose mean=825 mg; aripiprazole and risperidone)
Accounting for the 4 mm FWHM Gaussian filter and with a connectivity radius of 5.6 mm. Based on these simulations, the family-wise alpha of .05 was preserved with an a priori voxel-wise probability of .025 and three-dimensional clusters with a minimum volume of 1024 µL (16 or more voxels).
To further investigate the gain issue, all subjects were classified as “hypometric” if their average gains were below 1 or “hypermetric” if their average gains were above 1 (no subjects had a gain of 1.0). Hypometria was observed in 86% of the SZ group, 43% of the HP group, and 46% of the RL group.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Jazmin Camchong, University of Minnesota, Depts. of Psychology and Psychiatry.
Kara A. Dyckman, Massachusetts General Hospital, Department of Psychiatry
Benjamin P. Austin, University of Georgia, Department of Psychology, BioImaging Research Center
Brett A. Clementz, University of Georgia, Depts. of Psychology and Neuroscience, BioImaging Research Center
Jennifer E. McDowell, University of Georgia, Depts. of Psychology and Neuroscience, BioImaging Research Center, UGA Psychology Building, Athens, GA 30602, phone: (706) 542-3075, fax: (706) 542-3275, jemcd@uga.edu
References
- 1.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 2.Chan RC, Chen EY, Law CW. Specific executive dysfunction in patients with first episode medication-naive schizophrenia. Schizophr Res. 2006;82:51–64. doi: 10.1016/j.schres.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- 5.Kuha A, Tuulio-Henriksson A, Eerola M, Perala J, Suvisaari J, Partonen T, Lonnqvist J. Impaired executive performance in healthy siblings of schizophrenia patients in a population-based study. Schizophr Res. 2007;92:142–150. doi: 10.1016/j.schres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press; 2006. [Google Scholar]
- 8.Clementz BA, McDowell JE, Zisook S. Saccadic system functioning among schizophrenia patients and their first-degree biological relatives. J Abnorm Psychol. 1994;103:277–287. [PubMed] [Google Scholar]
- 9.Ettinger U, Picchioni M, Hall MH, Schulze K, Toulopoulou T, Landau S, et al. Antisaccade performance in monozygotic twins discordant for schizophrenia: the Maudsley twin study. Am J Psychiatry. 2006;163:543–545. doi: 10.1176/appi.ajp.163.3.543. [DOI] [PubMed] [Google Scholar]
- 10.Smyrnis N, Malogiannis IA, Evdokimidis I, Stefanis NC, Theleritis C, Vaidakis A, et al. Attentional facilitation of response is impaired for antisaccades but not for saccades in patients with schizophrenia: implications for cortical dysfunction. Exp Brain Res. 2004;159:47–54. doi: 10.1007/s00221-004-1931-0. [DOI] [PubMed] [Google Scholar]
- 11.Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 12.Hutton SB, Joyce EM, Barnes TR, Kennard C. Saccadic distractibility in first-episode schizophrenia. Neuropsychologia. 2002;40(10):1729–1736. doi: 10.1016/s0028-3932(01)00145-2. [DOI] [PubMed] [Google Scholar]
- 13.McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36:138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- 14.Reuter B, Kathmann N. Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychol (Amst) 2004;115:255–269. doi: 10.1016/j.actpsy.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Everling S, Krappmann P, Preuss S, Brand A, Flohr H. Hypometric primary saccades of schizophrenics in a delayed-response task. Exp Brain Res. 1996;111:289–295. doi: 10.1007/BF00227306. [DOI] [PubMed] [Google Scholar]
- 16.McDowell JE, Brenner CA, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Ocular motor delayed-response task performance among patients with schizophrenia and their biological relatives. Psychophysiology. 2001;38:153–156. [PubMed] [Google Scholar]
- 17.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 18.Broerse A, Crawford TJ, den Boer JA. Parsing cognition in schizophrenia using saccadic eye movements: a selective overview. Neuropsychologia. 2001;39:742–756. doi: 10.1016/s0028-3932(00)00155-x. [DOI] [PubMed] [Google Scholar]
- 19.Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutton SB, Huddy V, Barnes TR, Robbins TW, Crawford TJ, Kennard C, Joyce EM. The relationship between antisaccades, smooth pursuit, and executive dysfunction in firstepisode schizophrenia. Biol Psychiatry. 2004;56:553–559. doi: 10.1016/j.biopsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Nieman DH, Bour LJ, Linszen DH, Goede J, Koelman JH, Gersons BP, Ongerboer de Visser BW. Neuropsychological and clinical correlates of antisaccade task performance in schizophrenia. Neurology. 2000;54:866–871. doi: 10.1212/wnl.54.4.866. [DOI] [PubMed] [Google Scholar]
- 22.Ross RG, Harris JG, Olincy A, Radant A. Eye movement task measures inhibition and spatial working memory in adults with schizophrenia, ADHD, and a normal comparison group. Psychiatry Res. 2000;95:35–42. doi: 10.1016/s0165-1781(00)00153-0. [DOI] [PubMed] [Google Scholar]
- 23.Calkins ME, Iacono WG, Curtis CE. Smooth pursuit and antisaccade performance evidence trait stability in schizophrenia patients and their relatives. Int J Psychophysiol. 2003;49:139–146. doi: 10.1016/s0167-8760(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 24.Curtis CE, Calkins ME, Grove WM, Feil KJ, Iacono WG. Saccadic disinhibition in patients with acute and remitted schizophrenia and their first-degree biological relatives. Am J Psychiatry. 2001;158:100–106. doi: 10.1176/appi.ajp.158.1.100. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger U, Kumari V, Crawford TJ, Corr PJ, Das M, Zachariah E, et al. Smooth pursuit and antisaccade eye movements in siblings discordant for schizophrenia. J Psychiatr Res. 2004;38:177–184. doi: 10.1016/s0022-3956(03)00105-5. [DOI] [PubMed] [Google Scholar]
- 26.Karoumi B, Saoud M, d'Amato T, Rosenfeld F, Denise P, Gutknecht C, et al. Poor performance in smooth pursuit and antisaccadic eye-movement tasks in healthy siblings of patients with schizophrenia. Psychiatry Res. 2001;101:209–219. doi: 10.1016/s0165-1781(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 27.Katsanis J, Kortenkamp S, Iacono WG, Grove WM. Antisaccade performance in patients with schizophrenia and affective disorder. J Abnorm Psychol. 1997;106:468–472. doi: 10.1037//0021-843x.106.3.468. [DOI] [PubMed] [Google Scholar]
- 28.Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, et al. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr Res. 2007;89:320–329. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Ross RG, Harris JG, Olincy A, Radant A, Adler LE, Freedman R. Familial transmission of two independent saccadic abnormalities in schizophrenia. Schizophr Res. 1998;30:59–70. doi: 10.1016/s0920-9964(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 30.McDowell JE, Clementz BA. Ocular-motor delayed-response task performance among schizophrenia patients. Neuropsychobiology. 1996;34:67–71. doi: 10.1159/000119294. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- 32.Park S. Association of an oculomotor delayed response task and the Wisconsin Card Sort Test in schizophrenia. Int J Psychophysiol. 1997;27:147–151. doi: 10.1016/s0167-8760(97)00045-7. [DOI] [PubMed] [Google Scholar]
- 33.Hutton SB, Cuthbert I, Crawford TJ, Kennard C, Barnes TR, Joyce EM. Saccadic hypometria in drug-naive and drug-treated schizophrenic patients: a working memory deficit? Psychophysiology. 2001;38:125–132. [PubMed] [Google Scholar]
- 34.Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 35.Sweeney JA, Luna B, Keedy SK, McDowell JE, Clementz BA. fMRI studies of eye movement control: investigating the interaction of cognitive and sensorimotor brain systems. Neuroimage. 2007;36(Suppl 2):T54–T60. doi: 10.1016/j.neuroimage.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briand KA, Strallow D, Hening W, Poizner H, Sereno AB. Control of voluntary and reflexive saccades in Parkinson's disease. Exp Brain Res. 1999;129:38–48. doi: 10.1007/s002210050934. [DOI] [PubMed] [Google Scholar]
- 37.Boxer AL, Garbutt S, Rankin KP, Hellmuth J, Neuhaus J, Miller BL, Lisberger SG. Medial versus lateral frontal lobe contributions to voluntary saccade control as revealed by the study of patients with frontal lobe degeneration. J Neurosci. 2006;26:6354–6363. doi: 10.1523/JNEUROSCI.0549-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye movement control in Parkinson's disease. Neuropsychologia. 2005;43:784–796. doi: 10.1016/j.neuropsychologia.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95(6):3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- 40.DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89:1016–1023. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- 41.Ford KA, Goltz HC, Brown MR, Everling S. Neural processes associated with antisaccade task performance investigated with event-related FMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, et al. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 43.McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, Braff DL. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry. 2002;51:216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- 44.McDowell JE, Kissler JM, Berg P, Dyckman KA, Gao Y, Rockstroh B, Clementz BA. Electroencephalography/magnetoencephalography study of cortical activities preceding prosaccades and antisaccades. Neuroreport. 2005;16:663–668. doi: 10.1097/00001756-200505120-00002. [DOI] [PubMed] [Google Scholar]
- 45.Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol. 2004;17:17–25. doi: 10.1097/00019052-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Ploner CJ, Rivaud-Pechoux S, Gaymard BM, Agid Y, Pierrot-Deseilligny C. Errors of memory-guided saccades in humans with lesions of the frontal eye field and the dorsolateral prefrontal cortex. J Neurophysiol. 1999;82:1086–1090. doi: 10.1152/jn.1999.82.2.1086. [DOI] [PubMed] [Google Scholar]
- 47.Ploner CJ, Gaymard BM, Rivaud-Pechoux S, Pierrot-Deseilligny C. The prefrontal substrate of reflexive saccade inhibition in humans. Biol Psychiatry. 2005;57:1159–1165. doi: 10.1016/j.biopsych.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Camchong J, Dyckman KA, Chapman CE, Yanasak NE, McDowell JE. Basal ganglia-thalamocortical circuitry disruptions in schizophrenia during delayed response tasks. Biol Psychiatry. 2006;60:235–241. doi: 10.1016/j.biopsych.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Exp Brain Res. 1998;123:159–163. doi: 10.1007/s002210050557. [DOI] [PubMed] [Google Scholar]
- 50.Johnston K, Everling S. Neural activity in monkey prefrontal cortex is modulated by task context and behavioral instruction during delayed-match-to-sample and conditional prosaccade-antisaccade tasks. J Cogn Neurosci. 2006;18:749–765. doi: 10.1162/jocn.2006.18.5.749. [DOI] [PubMed] [Google Scholar]
- 51.Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003a;126:1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- 52.Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Rivaud-Pechoux S. Cortical control of ocular saccades in humans: a model for motricity. Prog Brain Res. 2003b;142:3–17. doi: 10.1016/S0079-6123(03)42003-7. [DOI] [PubMed] [Google Scholar]
- 53.Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 54.Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A. Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry. 2005;62(3):254–262. doi: 10.1001/archpsyc.62.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 56.Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53(1):25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- 57.Weinberger DR, Berman KF. Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1495–1503. doi: 10.1098/rstb.1996.0135. [DOI] [PubMed] [Google Scholar]
- 58.Weiss EM, Siedentopf C, Golaszewski S, Mottaghy FM, Hofer A, Kremser C, Felber S, Fleischhacker WW. Brain activation patterns during a selective attention test—a functional MRI study in healthy volunteers and unmedicated patients during an acute episode of schizophrenia. Psychiatry Res. 2007;154(1):31–40. doi: 10.1016/j.pscychresns.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 59.Calkins ME, Iacono WG. Eye movement dysfunction in schizophrenia: a heritable characteristic for enhancing phenotype definition. Am J Med Genet. 2000;97(1):72–76. doi: 10.1002/(sici)1096-8628(200021)97:1<72::aid-ajmg10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 60.Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, et al. The Consortium on the Genetics of Endophenotypes in Schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr Bull. 2007;33:33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 62.Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Res. 2006;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, Ramsey NF. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- 64.Raemaekers M, Ramsey NF, Vink M, van den Heuvel MP, Kahn RS. Brain activation during antisaccades in unaffected relatives of schizophrenic patients. Biol Psychiatry. 2006;59:530–535. doi: 10.1016/j.biopsych.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 65.Tu PC, Yang TH, Kuo WJ, Hsieh JC, Su TP. Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res. 2006;40:606–612. doi: 10.1016/j.jpsychires.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 66.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 21.0) New York: Biometrics Research Department, New York State Psychitric Institute; 1995. [Google Scholar]
- 67.Raine A, Benishay D. The SPQ-B: A brief screening instrument for schizotypal personality disorder. Journal of Personality Disorders. 1995;9:346–355. [Google Scholar]
- 68.Chapman LJ, Chapman JP. Scales for rating psychotic and psychotic-like experiences as continua. Schizophr Bull. 1980;6:477–489. [PubMed] [Google Scholar]
- 69.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 70.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: A-3-Dimentional Proportional System, An Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 71.Ward B. AlphaSim program documentation for AFNI. Milwaukee: Medical College of Wisconsin; 2000. Simultaneous Inference for fMRI Data. http://afni.nimh.nih.gov/pub/dist/doc/AlphaSim.pdf. [Google Scholar]
- 72.McDowell JE, Clementz BA. The effect of fixation condition manipulations on antisaccade performance in schizophrenia: studies of diagnostic specificity. Exp Brain Res. 1997;115:333–344. doi: 10.1007/pl00005702. [DOI] [PubMed] [Google Scholar]
- 73.Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1143–1149. doi: 10.1016/s0278-5846(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 74.Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Different roles of the frontal and parietal regions in memory-guided saccade: a PCA approach on time course of BOLD signal changes. Hum Brain Mapp. 2004;23:129–139. doi: 10.1002/hbm.20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- 76.Ettinger U, Ffytche DF, Kumari V, Kathmann N, Reuter B, Zelaya F, Williams SC. Decomposing the neural correlates of antisaccade eye movements using event-related fMRI. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- 77.Parton A, Nachev P, Hodgson TL, Mort D, Thomas D, Ordidge R, et al. Role of the human supplementary eye field in the control of saccadic eye movements. Neuropsychologia. 2007;45:997–1008. doi: 10.1016/j.neuropsychologia.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reuter B, Jäger M, Bottlender R, Kathmann N. Impaired action control in schizophrenia: the role of volitional saccade initiation. Neuropsychologia. 2007;45:1840–1848. doi: 10.1016/j.neuropsychologia.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 80.Hahn B, Ross TJ, Stein EA. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 2006;32:842–853. doi: 10.1016/j.neuroimage.2006.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manes F, Paradiso S, Springer JA, Lamberty G, Robinson RG. Neglect after right insular cortex infarction. Stroke. 1999;30:946–948. doi: 10.1161/01.str.30.5.946. [DOI] [PubMed] [Google Scholar]
- 82.Bullier J, Schall JD, Morel A. Functional streams in occipito-frontal connections in the monkey. Behav Brain Res. 1996;76(1–2):89–97. doi: 10.1016/0166-4328(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 83.Clavagnier S, Falchier A, Kennedy H. Long-distance feedback projections to area V1: implications for multisensory integration, spatial awareness, and visual consciousness. Cogn Affect Behav Neurosci. 2004;4(2):117–126. doi: 10.3758/cabn.4.2.117. [DOI] [PubMed] [Google Scholar]
- 84.Clementz BA, Brahmbhatt SB, McDowell JE, Brown R, Sweeney JA. When Does the Brain Inform the Eyes Whether and Where to Move? an EEG Study in Humans. Cereb Cortex. 2007;17:2634–2643. doi: 10.1093/cercor/bhl171. [DOI] [PubMed] [Google Scholar]
- 85.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 86.Kathmann N, Wagner M, Rendtorff N, Schöchlin C, Engel RR. Information processing during eye tracking as revealed by event-related potentials in schizophrenics, alcoholics, and healthy controls. Schizophr Res. 1995;16:145–156. doi: 10.1016/0920-9964(94)00066-h. [DOI] [PubMed] [Google Scholar]
- 87.Weiss KM, Chapman HA, Strauss ME, Gilmore GC. Visual information decoding deficits in schizophrenia. Psychiatry Res. 1992;44:203–216. doi: 10.1016/0165-1781(92)90024-w. [DOI] [PubMed] [Google Scholar]
- 88.Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- 89.Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, Manoach DS. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2007;131:971–986. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- 90.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- 91.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 92.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 93.Berrettini WH. Genetic bases for endophenotypes in psychiatric disorders. Dialogues Clin Neurosci. 2005;7(2):95–101. doi: 10.31887/DCNS.2005.7.2/wberrettini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;467:774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 95.Waldman ID. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1347–1356. doi: 10.1016/j.biopsych.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Schulze K, MacCabe JH, Rabe-Hesketh S, Crawford T, Marshall N, Zanelli J, et al. The relationship between eye movement and brain structural abnormalities in patients with schizophrenia and their unaffected relatives. J Psychiatr Res. 2006;40:589–598. doi: 10.1016/j.jpsychires.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Meehl PE. Theory-testing in psychology and physics: A methodological paradox. Phil Sci. 1967;34:103–115. [Google Scholar]
- 98.Meehl PE. Theoretical risks and tabular asterisks: Sir Karl, Sir Ronald, and the slow progress of soft psychology. J Consul Clin Psych. 1978;46:806–834. [Google Scholar]
- 99.Gigerenzer G. The superego, the ego and the id in statistical reasoning. In: Keren G, Lewis C, editors. A Handbook for Data Analysis in the Behavioral Sciences: Methodological Issues. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1993. pp. 311–339. [Google Scholar]
- 100.Lakatosh I. The Methodology of Scientific Research Programmes. Cambridge: Cambridge University Press; 1978. [Google Scholar]
- 101.Morrison DE, Henkel RE. The Significance Test Controversy: A Reader. Chicago: Aldine Publishing Company; 1970. [Google Scholar]