Abstract
Recent studies of the nuclear envelope (NE) have emphasized its role in linking the nuclear and cytoplasmic compartments of mammalian cells. The inner face of the NE is bound to chromatin and this interaction is involved in regulating DNA replication and transcription. The outer face of the NE binds to different components of the cytoskeleton, and these interactions are involved in nuclear positioning. Many disease causing mutations in genes encoding NE proteins cause significant changes in nuclear architecture and cytoskeletal interactions with the NE. These mutations are also providing important new insights into nuclear-cytoplasmic interactions.
Introduction
During interphase of mammalian cells, the nuclear envelope (NE) establishes and maintains the overall shape, size and mechanical integrity of the nucleus. At the nuclear periphery, chromatin is anchored to the inner aspect of the NE, which provides a mechanism for the spatial control of DNA replication and transcription [1]. Recent studies have shown that the cytoskeletal systems are attached to the cytoplasmic face of the NE which appears to mediate interactions between the nucleus and the cytoplasm [2]. These interactions facilitate cellular processes including nuclear positioning and centrosome orientation during cell migration [3••].
Other insights into the functions of the NE have been derived from studies of disease mutations in genes encoding NE proteins, particularly the nuclear lamins. Some mutations frequently cause significant changes in nuclear shape, chromatin organization and gene expression [4], and they also modulate nuclear positioning and centrosome orientation [5•]. These changes reflect nuclear-cytoplasmic interactions.
This review focuses on the functions of the NE in mediating the molecular crosstalk between the nucleus and the cytoplasm.
The Nuclear Envelope Links the Nuclear and Cytoplasmic Compartments of Mammalian Cells
The NE is comprised of inner and outer nuclear membranes (INM and ONM), nuclear pore complexes (NPCs) and the nuclear lamina. Approximately 80 INM and ONM proteins and ~ 50 NPC proteins (nucleoporins) have been identified in mammalian cells [6•,7]. The major proteins of the lamina are the type V intermediate filament proteins, the A-type lamins (LA and LC) and the B-type lamins (LB1 and LB2). LA and LC are derived from a single gene (LMNA) by alternative splicing and are expressed only in differentiated cells. LB1 and LB2 are encoded by LMNB1 and LMNB2, respectively, and at least one of them is expressed in all cells throughout development [8]. Lamins within the lamina form filamentous structures [9,10] composed of separate but interacting A- and B-type lamin meshworks [11•]. The lamins also bind to other NE proteins, including some NPC and INM proteins (Fig. 1). These protein-protein interactions are critically important in regulating the proper assembly of the NE. For example, LB1 silencing induces changes in the LA/C meshworks creating LA/C rich microdomains devoid of LB1, LB2 and NPCs [11]. LA/C is also required for the proper localization of INM proteins such as emerin [12–14].
Figure 1.
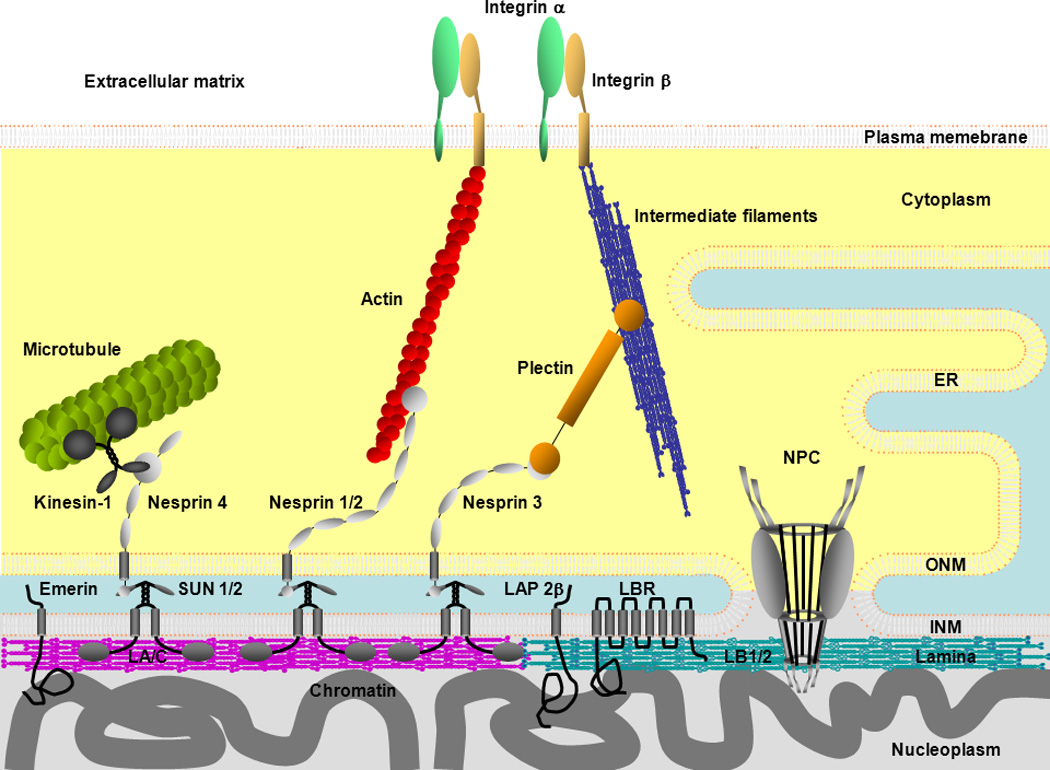
An overview of nuclear envelope (NE) connections with chromatin,and the cytoskeletal systems. The NE consists of the inner and outer nuclear membrane (INM, ONM), nuclear pore complexes (NPCs) and the lamina. The ONM is continuous with the endoplasmic reticulum (ER). NPCs cross the INM, ONM, the lamina and are associated with chromatin. A-type lamins (LA, LC) and B-type lamins (LB1, LB2) in the lamina bind to INM proteins such as emerin, lamina-associated polypeptide 2β (LAP2β), lamin B receptor (LBR) and SUN domain proteins (SUN1, SUN2) in the INM. All of the lamins and some of the INM proteins interact with chromatin. SUN1 and SUN2 bind to the KASH domain of nesprins in the luminal region between the INM and ONM to form the LINC complex. Nesprins in the ONM bind to cytoskeletal filaments such as actin, microtubules and intermediate filaments (IFs) directly or indirectly through plectin or kinesin. Actin and IFs are associated with the plasma membrane through integrin complexes.
All of the lamins, as well as some nucleoporins and INM proteins, interact with chromatin and play a role in the regulation of transcription and DNA replication [1]. For example, some transcriptionally active genes are associated with nucleoporins at the nucleoplasmic face of NPCs [15], while silenced genes are tethered to the lamina [16–18]. However, these gene silencing effects associated with the lamina may be gene specific [19,20]. In addition, both the A- and B-type lamins and the lamina-associated polypeptide 2β (LAP2β) are involved in the initiation and elongation phases of DNA replication [21–23].
There is also evidence that some ONM proteins interact with specific proteins of the cytoskeletal systems (Fig. 1). These include the nesprins which span the ONM and components of the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex. The nesprin C-terminus located in the luminal region separating the ONM and INM contains a KASH (Klarsicht/ANC-1/Syne Homology) domain which binds to the SUN (Sad1p and UNC-84) domain proteins which span the INM [2]. At the cytoplasmic face of the ONM, the nesprins appear to bind directly to actin, associate with microtubules through interactions with dynein and kinesin, and interact with intermediate filaments via plectin (Fig. 1–2) [2,24]. At the nucleoplasmic face of the INM, there is evidence that SUN1 binds directly to LA [2]. It has also been shown that the LINC complex in association with LA is required for controlling nuclear positioning and centrosome reorientation during cell migration [3,5,25]. LB1 is also involved in anchoring the nucleus to the cytoskeleton through nesprin-1 and -2 [26].
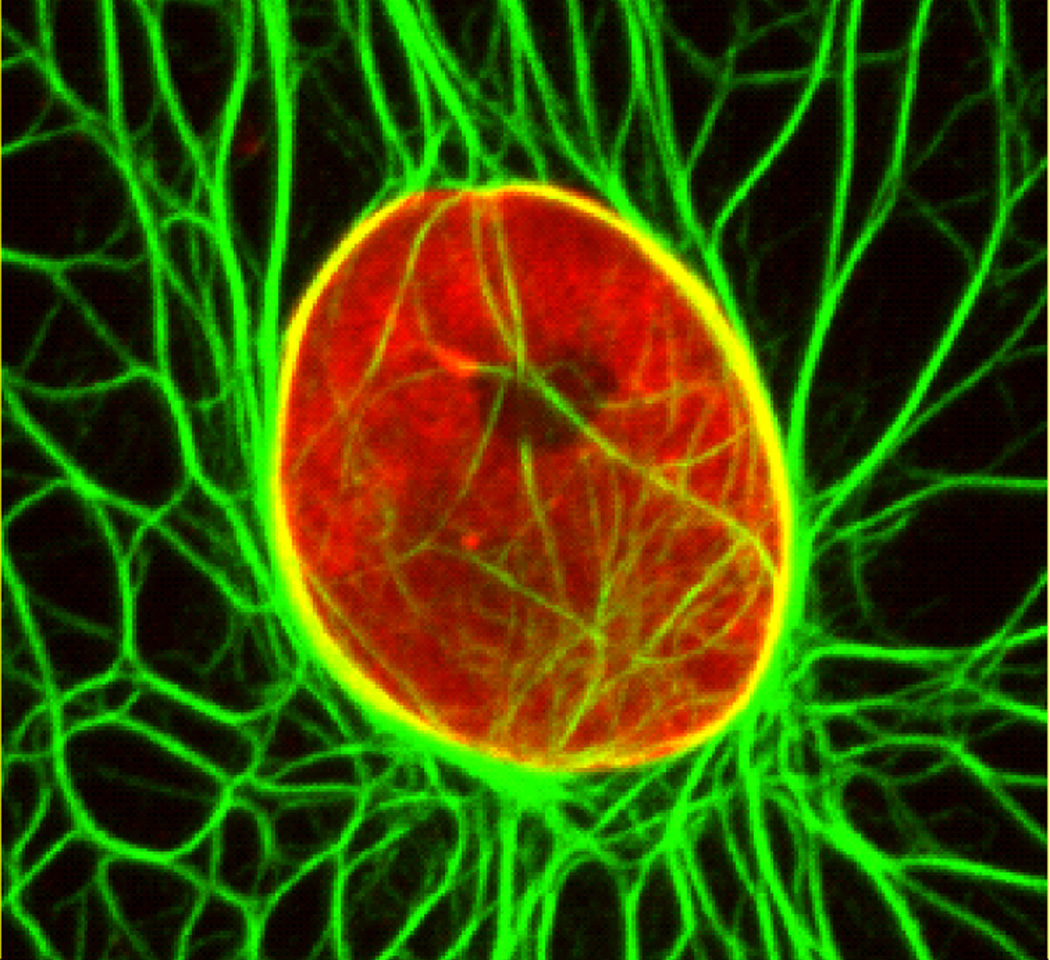
Figure 2.
Keratin-containing tonofibrils are distributed throughout the cytoplasm and surround, and are perhaps attached to the NE of a PtK2 rat kangaroo epithelial cell. This cell, expressing GFP-Keratin 18 (green), was fixed and immunostained with lamin A/C (red) antibody. Figure represents a projection of z-stack images obtained by confocal microscopy.
The Nuclear Envelopathies/Laminopathies Shed New Light on Nuclear-Cytoplasmic Interactions: Nuclear Shape, Chromatin Organization and Cytoskeleton-NE Interactions
Nuclear envelopathies/laminopathies are a large group of human diseases caused by mutations in genes encoding NE proteins such as the nucleoporins, INM and ONM proteins and the lamins (Table 1). Frequently cells from patients with these diseases exhibit abnormal nuclear shapes, alterations in chromatin organization and changes in the cytoskeletal systems [4,8]. For example, mutations in the gene encoding the INM protein emerin cause X-linked Emery-Dreifuss muscular dystrophy (XL-EDMD). Nuclei in XL-EDMD patients’ muscle cells are misshapen due to the formation of nuclear blebs or lobulations [41]. In contrast, nuclei in mature neutrophils are normally hyperlobulated [56]. Interestingly, mutations in another INM protein lamin B receptor (LBR) cause Pelger-Huët disease which inhibits hyperlobulation of nuclei and the proper maturation of neutrophils [44,57]. In the case of ONM proteins, there is a mutation resulting in a deletion of the KASH domain in nesprin-1, which causes arthrogryposis multiplex a disease resulting in abnormal joint contractures [45]. A model of cardiomyopathy has been described in mice expressing a nesprin-1 mutant missing the KASH domain, and the cardiocytes of these mice have abnormally elongated nuclei [46, 47•]. Furthermore, the knockout and silencing of nesprin-2 giant, another ONM protein which interacts with SUN domain proteins, induce nuclear blebbing in both mouse and human cells [58]. Since nesprins interact with cytoskeletal filaments such as actin and intermediate filaments, these findings suggest that the determination of nuclear shape is complex and to a great extent dependent on the interactions between the NE and the cytoskeletal systems.
Table 1.
Mutations in genes encoding NE proteins known to cause diseases collectively known as the nuclear envelopathies/laminopathies.
| Protein with mutations | Disease | Nuclear Shape | Reference |
|---|---|---|---|
| Lamins | |||
| Lamin A/C | Autosomal dominant and recessive Emery-Dreifuss muscular dystrophy (AD-EDMD, AR- EDMD) | Honeycomb lamina | [27] |
| Lamin A/C | Limb-girdle muscular dystrophy type 1B (LGMD1B) | Blebbed | [28,29] |
| Lamin A/C | Dilated cardiomyopathy with conduction defect disease (DCM-CD) | Blebbed | [27] |
| Lamin A/C | Familial partial lipodystrophy of the Dunnigan type (FPLD) | Honeycomb lamina, Blebbed | [27,30] |
| Lamin A/C | Lipoatrophy with diabetes, hepatic steatosis, hypertrophic cardiomyopathy, and leukomelanodermic papules (LDHCP) | Blebbed | [31] |
| Lamin A/C | Mandibuloacral dysplasia with type A lipodystrophy (MADA) | Honeycomb lamina, Blebbed | [32] |
| Lamin A/C | Charcot-Marie-Tooth disease type 2B1 (CMT2B1) | N/A | [33] |
| Lamin A/C | Hutchinson-Gilford progeria syndrome (HGPS) and atypical progeria syndrome | Blebbed, lobulated | [34••, 35,36] |
| Lamin A/C | Atypical Werner syndrome | blebbed | [37] |
| Lamin B1 (tandem gene duplication) | Autosomal dominant leukodystrophy (AD-LD) | Normal, distorted NE | [38] |
| Lamin B2 | Barraquer-Simons syndrome (BSS) | N/A | [39] |
| LNM proteins | |||
| Emerin | X-linked Emery-Dreifuss muscular dystrophy (XL-EDMD) | Honeycomb lamina, Blebbed, distorted NE | [40,41] |
| MAN1 | Buschke-Ollendorff syndrome (BOS), melorheostosis | N/A | [42] |
| Lamin B receptor (LBR) | Greenberg dysplasia | Hypolobulated | [43] |
| Lamin B receptor (LBR) | Pelger-Huet anomaly (PHA) | Ovoid (lobulated for normal nuclei) | [44] |
| ONM proteins and the associated protein | |||
| Nesprin-1 | Arthrogryposis multiplex congenita (AMC) | Normal in human, elongated in mice | [45–47] |
| Nesprin-1 | Dilated cardiomyopathy (DCM) | Normal | [47] |
| TorsinA | Torsion dystonia | Normal, distorted NE | [48] |
| Nucleoporins and the associated protein. | |||
| Nup155 | Atrial fibrillation and early sudden cardiac death | N/A | [49] |
| Nup62 | Autosomal recessive infantile bilateral striatal necrosis (IBSN) | N/A | [50] |
| Ran binding protein 2 (RanBP2) | Acute necrotizing encephalopathy (ANE) | N/A | [51] |
| ALADIN | Achalasia-Addisonianism-Alacrimia syndrome (AAA) | Normal | [52] |
| Others | |||
| Zinc metalloprotease STE24 homolog (Zmpste24) | HGPS, mandibuloacral dysplasia (MAD) and restrictive dermopathy (RD) | Blebbed | [53] |
| Zinc metalloprotease STE24 homolog (Zmpste24) | Autosomal recessive restrictive dermopathy (AR-RD) | Blebbed | [54] |
| Lamina-associated polypeptide 2α (LAP2α) | Dilated cardiomyopathy (DCM) | Normal | [55] |
Most information regarding the relationships between the NE, chromatin and the cytoskeletal systems comes from studies of the laminopathies, which represent the largest group of nuclear envelopathies. These are caused by hundreds of different mutations, mainly in human LMNA [59]. These mutations cause a remarkable number of different diseases including autosomal EDMD and Limb Girdle muscular dystrophy, dilated cardiomyopathy, lipodystrophy, Charcot-Marie Tooth disease, and premature aging diseases such as Hutchinson-Gilford Progeria Syndrome (HGPS, progeria) [59]. The structure of the lamina is altered by some mutations causing autosomal dominant EDMD (AD-EDMD). Nuclei of these patients’ cells have an enlarged lamin meshwork within the lamina known as honeycomb structures [27]. Other AD-EDMD mutations induce the formation of LA/C foci in the nucleoplasm [27] and block nuclear positioning and centrosome orientation [5•], suggesting that the interactions between the NE and the cytoskeletal systems are disrupted. The effects of these mutations on nuclear shape and chromatin organization vary depending on the locations of point mutations or deletions. For example, the HGPS mutation G608G (progerin/LAΔ50) located in the non-α-helical C-terminal domain of LA, causes an abnormal thickening of the lamina, nuclear blebbing, and abnormal distributions of B-type lamins and NPCs in skin fibroblasts (Fig. 3) [34••]. In addition, there is a dramatic loss of peripheral heterochromatin, accompanied by a decrease in histone methylation and acetylation of lysine residues in histones H3 and H4 and in gene expression [60, 61•, 62]. The atypical progeria mutation, E145K, located in the α-helical central rod domain of LA/C causes abnormal polymerization of LA/C, nuclear lobulations resulting in flower-shape nuclei, alterations in pericentric heterochromatin, abnormally clustered centromeres, and mislocalized telomeres [36]. Another atypical progeria mutation in LMNA, S143F, causes numerous blebs and lobulations of the NE as well as an enlarged lamin meshwork within the lamina [35]. Interestingly this phenotype appears to be rescued by the expression of nesprin-2 giant [35]. LA/C knockout mouse embrionic fibrobrasts (MEFs) and myocytes derived from LA/C knockout mice also display blebbed nuclei with displaced and fragmented heterochromatin [12,63].
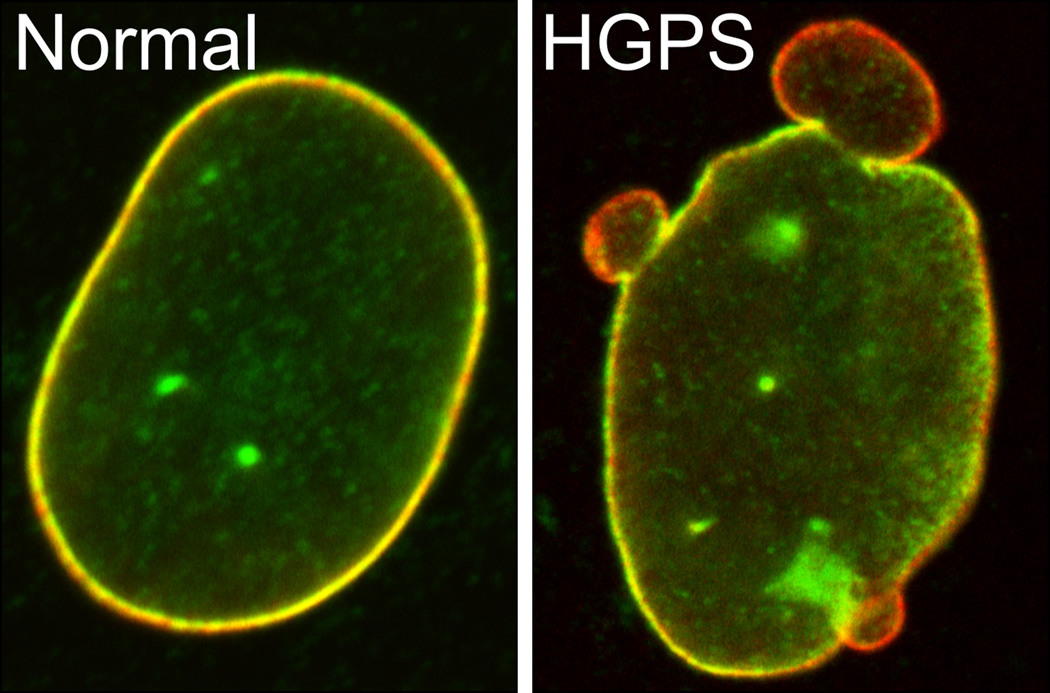
Figure 3.
The localization of lamins A/C and B1 in skin fibroblasts taken from a normal individual (left) and a patient with the HGPS mutation (G608G) in LMNA (right). These cells were fixed and immunostained with antibodies against lamin A/C (red) and lamin B1 (green). Note the separation of the A and B-type lamins in the blebbed regions.
Nuclear blebs can also be induced by silencing LB1 expression or by expression of a deletion mutation of LB1 in mice [11,64,65]. These nuclear blebs contain gene-rich euchromatin and the activated form of RNA polymerase II (pol II) but are transcriptionally defective, suggesting that pol II is stalled [11]. MEFs expressing a deletion mutant of LB1 contain nuclear blebs and exhibit changes in gene expression [66]. Further support for a role of NE proteins in transcription comes from the finding that they bind to transcriptional factors. For example, LA binds to MOK2, SREBP1 and c-Fos, and emerin binds to GCL and Lmo7 [67–71].
Taken together, this wide range of investigations provides a framework to describe in more detail the structural and functional linkages between the NE and chromatin on the one hand and with the NE and the cytoskeletal systems on the other (Fig. 1).
Mechanotransduction between the Nucleus and the Cytoplasm
Emerging evidence suggests the interesting possibility that nuclear shape may be regulated in part by the mechanical properties of the NE and the cytoskeletal systems attached to it. In support of this it has been found that mechanical stress exerted at the outer cell surface causes changes in nuclear shape possibly through the LINC complex [72•, 73••]. The intrinsic mechanical properties of the NE or the entire nucleus may also affect nuclear shape. For example, LA/C knockout MEFs have blebbed nuclei, which are “softer” relative to those of WT MEFs when assayed by mechanical strain [12, 74•]. In the case of HGPS, skin fibroblasts from the patients have “stiffer” nuclei with a thicker lamina compared to normal skin fibroblasts [34,75,76]. On the other hand, emerin knockout MEFs have normal nuclear mechanics but there are significant changes in nuclear shape [77], suggesting that changes in nuclear mechanics are not always coupled to changes in nuclear shape.
It has been proposed that the interactions between the plasma membrane and the cytoskeletal systems regulate gene expression in response to mechanical stress initiated at cell surfaces [78]. Such a mechanism for mechanotransduction is particularly interesting because mechanically-based signal propagation is faster than chemically based-diffusion [78]. Mechanotransduction appears to involve integrins in the plasma membrane interacting with cytoskeletal filaments such as actin and/or intermediate filaments. In turn these cytoskeletal components can transduce mechanical forces to the nucleus [79–81]. In support of this, endothelial cells subjected to shear stress exhibit rapid changes in gene expression and the organization of cytoskeletal intermediate filaments [82•,83]. There is also evidence that some NE proteins are involved in gene regulation in response to mechanical stress. For example, LA/C or emerin knockout MEFs subjected to mechanical strain are defective in expressing mechano-sensitive genes [74,77]. On the other hand, recent studies have shown that when the LINC complex is disrupted by the expression of a dominant negative KASH domain, the regulation of mechano-sensitive genes is normal in response to mechanical stress [73]. Although this result is not conclusive, it also suggests that interactions between the NE and the cytoskeletal systems may not be required for all aspects of mechanotransduction. One possibility to explain such findings is that other signaling pathways such as the propagation of chemical signals may act synergistically with mechanotransduction to facilitate interactions between the nucleus and the cytoplasm. Such synergistic interactions could provide compensatory mechanisms to explain the response of mechano-sensitive genes in the absence of the known complexes that link the nuclear lamina with the cytoskeleton. It should be noted, however, that this area of research is in its early phases and there are likely to be numerous linkages between the lamina, the NE and the cytoskeleton that have yet to be discovered.
Outlook
Knowledge is accumulating to show that during interphase the NE mediating the interactions between the nucleus and cytoplasm are involved in regulating nuclear shape, chromatin organization, gene expression and nuclear positioning.
Over the next few years we should see an explosion of interests in the structural synergy and molecular cross talk regulating the interactions between the nuclear and cytoplasmic compartments of mammalian cells. The NE is a critically important hub facilitating these interactions as it demarcates and provides a molecular interface between these two major cellular compartments. In addition to nuclear transport mechanisms for exchanging large molecules between the nucleus and the cytoplasm, the results to date provide compelling evidence for the presence of multiple chains of protein-protein interactions which pervade the entire cell. The following scenario can be pieced together from data derived from disparate sources. The outer surface of the plasma membrane/ECM connects with different but interacting cytoskeletal networks comprised of microtubules, intermediate filaments, microfilaments and their associated proteins. These cytoskeletal components are connected to the ONM via the LINC complex which forms transmembrane linkages to the lamina. In turn the lamina forms a complex interface between the INM and peripheral elements of interphase chromosomes. The latter are typically tethered to the lamina and the regulation of this tethering is involved in the regulation of gene expression. It is becoming more apparent that these interacting networks provide a structural framework for further defining mechanisms involved in the bidirectional propagation of signals and molecules between the nucleus and the cell surface. Sufficient components of the network are now in place to begin to speculate about what these structural pathways might be doing and how they might function. Most likely these pathways provide the cell with a complex of superhighways composed of interconnecting molecules capable of transmitting both mechanical and chemical signals from the external environment of cells to the nucleus, ultimately regulating gene expression. Of course the devil is in the detailed identification and analysis of the many molecules which undoubtedly are required for the functions of these proposed superhighways. Such knowledge is required to define functions and determining. In other words, it will be many years before we reach the same level of sophisticated structure/function relationships now in hand for some components of the NE, such as the NPCs as described.
Acknowledgements
We are supported by the National Cancer Institute, the Grus-Lipper Foundation and the Progeria Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
References and recommended reading Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Taddei A, Hediger F, Neumann FR, Gasser SM. The function of nuclear architecture: a genetic approach. Annu Rev Genet. 2004;38:305–345. doi: 10.1146/annurev.genet.37.110801.142705. [DOI] [PubMed] [Google Scholar]
- 2.Burke B, Roux KJ. Nuclei take a position: managing nuclear location. Dev Cell. 2009;17:587–597. doi: 10.1016/j.devcel.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 3. Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. Nuclear positioning and centrosome orientation require anchoring of the nucleus to actin cables through the LINC complex.
- 4.Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell. 2009;17:626–638. doi: 10.1016/j.devcel.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 5. Folker ES, Ostlund C, Luxton GW, Worman HJ, Gundersen GG. Lamin A variants that cause striated muscle disease are defective in anchoring transmembrane actin-associated nuclear lines for nuclear movement. Proc Natl Acad Sci U S A. 2011;108:131–136. doi: 10.1073/pnas.1000824108. Lack of lamin A expression or mutations in LMNA causing autosomal EDMD inhibits nuclear positioning and centrosome orientation through interactions with the LINC complex.
- 6. Schirmer EC, Florens L, Guan T, Yates JR, 3rd, Gerace L. Nuclear membrane proteins with potential disease links found by subtractive proteomics. Science. 2003;301:1380–1382. doi: 10.1126/science.1088176. Multidimensional protein identification technology (MudPIT) has identified 13 known and 67 novel putative nuclear envelope transmembrane proteins (NETs).
- 7.Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear Lamins: Major Factors in the Structural Organization and Function of the Nucleus and Chromatin. Genes and Development. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 10.Schermelleh L, Carlton PM, Haase S, Shao L, Winoto L, Kner P, Burke B, Cardoso MC, Agard DA, Gustafsson MG, et al. Subdiffraction multicolor imaging of the nuclear periphery with 3D structured illumination microscopy. Science. 2008;320:1332–1336. doi: 10.1126/science.1156947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimi T, Pfleghaar K, Kojima S, Pack C, Solovei I, Goldman AE, Adam SA, Shumaker DK, Kinjo M, Cremer T, et al. The A- and B-type Nuclear Lamin Networks: Microdomains Involved in Chromatin Organization and Transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. The lamina is comprised of A- and B-type lamin meshworks. Silencing LB1 expression induces nuclear blebs enriched in lamin A/C. These blebs contain gene rich euchromatin and activated RNA polymerase II but are transcriptionally defective.
- 12.Sullivan T, Escalante-Alcalde D, Bhatt H, Anver M, Bhat N, Nagashima K, Stewart CL, Burke B. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 1999;147:913–920. doi: 10.1083/jcb.147.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughan A, Alvarez-Reyes M, Bridger JM, Broers JL, Ramaekers FC, Wehnert M, Morris GE, Whitfield WGF, Hutchison CJ. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- 14.Ostlund C, Bonne G, Schwartz K, Worman HJ. Properties of lamin A mutants found in Emery-Dreifuss muscular dystrophy, cardiomyopathy and Dunnigan-type partial lipodystrophy. J Cell Sci. 2001;114:4435–4445. doi: 10.1242/jcs.114.24.4435. [DOI] [PubMed] [Google Scholar]
- 15.Brown CR, Kennedy CJ, Delmar VA, Forbes DJ, Silver PA. Global histone acetylation induces functional genomic reorganization at mammalian nuclear pore complexes. Genes Dev. 2008;22:627–639. doi: 10.1101/gad.1632708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guelen L, Pagie L, Brasset E, Meuleman W, Faza MB, Talhout W, Eussen BH, de Klein A, Wessels L, de Laat W, et al. Domain organization of human chromosomes revealed by mapping of nuclear lamina interactions. Nature. 2008;453:948–951. doi: 10.1038/nature06947. [DOI] [PubMed] [Google Scholar]
- 17.Kosak ST, Skok JA, Medina KL, Riblet R, Le Beau MM, Fisher AG, Singh H. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science. 2002;296:158–162. doi: 10.1126/science.1068768. [DOI] [PubMed] [Google Scholar]
- 18.Reddy KL, Zullo JM, Bertolino E, Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 19.Kumaran RI, Spector DL. A genetic locus targeted to the nuclear periphery in living cells maintains its transcriptional competence. J Cell Biol. 2008;180:51–65. doi: 10.1083/jcb.200706060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finlan LE, Sproul D, Thomson I, Boyle S, Kerr E, Perry P, Ylstra B, Chubb JR, Bickmore WA. Recruitment to the nuclear periphery can alter expression of genes in human cells. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000039. e1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moir RD, Spann TP, Herrmann H, Goldman RD. Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J Cell Biol. 2000;149:1179–1192. doi: 10.1083/jcb.149.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins S, Eikvar S, Furukawa K, Collas P. HA95 and LAP2 beta mediate a novel chromatin-nuclear envelope interaction implicated in initiation of DNA replication. J Cell Biol. 2003;160:177–188. doi: 10.1083/jcb.200210026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shumaker DK, Solimando L, Sengupta K, Shimi T, Adam SA, Grunwald A, Strelkov SV, Aebi U, Cardoso MC, Goldman RD. The highly conserved nuclear lamin Ig-fold binds to PCNA: its role in DNA replication. J Cell Biol. 2008;181:269–280. doi: 10.1083/jcb.200708155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20:1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji JY, Lee RT, Vergnes L, Fong LG, Stewart CL, Reue K, Young SG, Zhang Q, Shanahan CM, Lammerding J. Cell nuclei spin in the absence of lamin b1. J Biol Chem. 2007;282:20015–20026. doi: 10.1074/jbc.M611094200. [DOI] [PubMed] [Google Scholar]
- 27.Muchir A, Medioni J, Laluc M, Massart C, Arimura T, van der Kooi AJ, Desguerre I, Mayer M, Ferrer X, Briault S, et al. Nuclear envelope alterations in fibroblasts from patients with muscular dystrophy, cardiomyopathy, and partial lipodystrophy carrying lamin A/C gene mutations. Muscle Nerve. 2004;30:444–450. doi: 10.1002/mus.20122. [DOI] [PubMed] [Google Scholar]
- 28.Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- 29.Muchir A, van Engelen BG, Lammens M, Mislow JM, McNally E, Schwartz K, Bonne G. Nuclear envelope alterations in fibroblasts from LGMD1B patients carrying nonsense Y259X heterozygous or homozygous mutation in lamin A/C gene. Exp Cell Res. 2003;291:352–362. doi: 10.1016/j.yexcr.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Vigouroux C, Auclair M, Dubosclard E, Pouchelet M, Capeau J, Courvalin JC, Buendia B. Nuclear envelope disorganization in fibroblasts from lipodystrophic patients with heterozygous R482Q/W mutations in the lamin A/C gene. J Cell Sci. 2001;114:4459–4468. doi: 10.1242/jcs.114.24.4459. [DOI] [PubMed] [Google Scholar]
- 31.Caux F, Dubosclard E, Lascols O, Buendia B, Chazouilleres O, Cohen A, Courvalin JC, Laroche L, Capeau J, Vigouroux C, et al. A new clinical condition linked to a novel mutation in lamins A and C with generalized lipoatrophy, insulin-resistant diabetes, disseminated leukomelanodermic papules, liver steatosis, and cardiomyopathy. J Clin Endocrinol Metab. 2003;88:1006–1013. doi: 10.1210/jc.2002-021506. [DOI] [PubMed] [Google Scholar]
- 32.Novelli G, Muchir A, Sangiuolo F, Helbling-Leclerc A, D'Apice MR, Massart C, Capon F, Sbraccia P, Federici M, Lauro R, et al. Mandibuloacral dysplasia is caused by a mutation in LMNA-encoding lamin A/C. Am J Hum Genet. 2002;71:426–431. doi: 10.1086/341908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Sandre-Giovannoli A, Chaouch M, Kozlov S, Vallat JM, Tazir M, Kassouri N, Szepetowski P, Hammadouche T, Vandenberghe A, Stewart CL, et al. Homozygous defects in LMNA, encoding lamin A/C nuclear-envelope proteins, cause autosomal recessive axonal neuropathy in human (Charcot-Marie-Tooth disorder type 2) and mouse. Am J Hum Genet. 2002;70:726–736. doi: 10.1086/339274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, Gruenbaum Y, Khuon S, Mendez M, Varga R, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. A mutation in LMNA (G608G) causing Hutchinson-Gilford progeria syndrome (HGPS) induces an abnormal thickening of the lamina, loss of peripheral heterochromatin, nuclear blebbing, and abnormal distributions of B-type lamins and NPCs.
- 35.Kandert S, Luke Y, Kleinhenz T, Neumann S, Lu W, Jaeger VM, Munck M, Wehnert M, Muller CR, Zhou Z, et al. Nesprin-2 giant safeguards nuclear envelope architecture in LMNA S143F progeria cells. Hum Mol Genet. 2007;16:2944–2959. doi: 10.1093/hmg/ddm255. [DOI] [PubMed] [Google Scholar]
- 36.Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, Erdos MR, Adam SA, Herrmann H, Medalia O, Collins FS, et al. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci U S A. 2009;106:20788–20793. doi: 10.1073/pnas.0911895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Lee L, Kudlow BA, Dos Santos HG, Sletvold O, Shafeghati Y, Botha EG, Garg A, Hanson NB, Martin GM, et al. LMNA mutations in atypical Werner's syndrome. Lancet. 2003;362:440–445. doi: 10.1016/S0140-6736(03)14069-X. [DOI] [PubMed] [Google Scholar]
- 38.Padiath QS, Saigoh K, Schiffmann R, Asahara H, Yamada T, Koeppen A, Hogan K, Ptacek LJ, Fu YH. Lamin B1 duplications cause autosomal dominant leukodystrophy. Nat Genet. 2006;38:1114–1123. doi: 10.1038/ng1872. [DOI] [PubMed] [Google Scholar]
- 39.Hegele RA, Cao H, Liu DM, Costain GA, Charlton-Menys V, Rodger NW, Durrington PN. Sequencing of the Reannotated LMNB2 Gene Reveals Novel Mutations in Patients with Acquired Partial Lipodystrophy. Am J Hum Genet. 2006;79:383–389. doi: 10.1086/505885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ognibene A, Sabatelli P, Petrini S, Squarzoni S, Riccio M, Santi S, Villanova M, Palmeri S, Merlini L, Maraldi NM. Nuclear changes in a case of X-linked Emery-Dreifuss muscular dystrophy. Muscle Nerve. 1999;22:864–869. doi: 10.1002/(sici)1097-4598(199907)22:7<864::aid-mus8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 41.Fidzianska A, Hausmanowa-Petrusewicz I. Architectural abnormalities in muscle nuclei. Ultrastructural differences between X-linked and autosomal dominant forms of EDMD. J Neurol Sci. 2003;210:47–51. doi: 10.1016/s0022-510x(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 42.Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PC, Costa T, Janssens K, Menten B, Van Roy N, Vermeulen SJ, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet. 2004;36:1213–1218. doi: 10.1038/ng1453. [DOI] [PubMed] [Google Scholar]
- 43.Waterham HR, Koster J, Mooyer P, Noort Gv G, Kelley RI, Wilcox WR, Wanders RJ, Hennekam RC, Oosterwijk JC. Autosomal recessive HEM/Greenberg skeletal dysplasia is caused by 3 beta-hydroxysterol delta 14-reductase deficiency due to mutations in the lamin B receptor gene. Am J Hum Genet. 2003;72:1013–1017. doi: 10.1086/373938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann K, Dreger CK, Olins AL, Olins DE, Shultz LD, Lucke B, Karl H, Kaps R, Muller D, Vaya A, et al. Mutations in the gene encoding the lamin B receptor produce an altered nuclear morphology in granulocytes (Pelger-Huet anomaly) Nat Genet. 2002;31:410–414. doi: 10.1038/ng925. [DOI] [PubMed] [Google Scholar]
- 45.Attali R, Warwar N, Israel A, Gurt I, McNally E, Puckelwartz M, Glick B, Nevo Y, Ben-Neriah Z, Melki J. Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Hum Mol Genet. 2009;18:3462–3469. doi: 10.1093/hmg/ddp290. [DOI] [PubMed] [Google Scholar]
- 46.Puckelwartz MJ, Kessler E, Zhang Y, Hodzic D, Randles KN, Morris G, Earley JU, Hadhazy M, Holaska JM, Mewborn SK, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–620. doi: 10.1093/hmg/ddn386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Puckelwartz MJ, Kessler EJ, Kim G, Dewitt MM, Zhang Y, Earley JU, Depreux FF, Holaska J, Mewborn SK, Pytel P, et al. Nesprin-1 mutations in human and murine cardiomyopathy. J Mol Cell Cardiol. 2010;48:600–608. doi: 10.1016/j.yjmcc.2009.11.006. Homozygous mutant mice expressing a nesprin-1 mutant missing the KASH domain develop cardiomyopathy with associated cardiac conduction system disease; cardiomyocytes of the mutant mice have abnormally elongated nuclei.
- 48.Naismith TV, Heuser JE, Breakefield XO, Hanson PI. TorsinA in the nuclear envelope. Proc Natl Acad Sci U S A. 2004;101:7612–7617. doi: 10.1073/pnas.0308760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Chen S, Yoo S, Chakrabarti S, Zhang T, Ke T, Oberti C, Yong SL, Fang F, Li L, et al. Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell. 2008;135:1017–1027. doi: 10.1016/j.cell.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, Walsh CA, Magal N, Taub E, Drasinover V, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol. 2006;60:214–222. doi: 10.1002/ana.20902. [DOI] [PubMed] [Google Scholar]
- 51.Neilson DE, Adams MD, Orr CM, Schelling DK, Eiben RM, Kerr DS, Anderson J, Bassuk AG, Bye AM, Childs AM, et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet. 2009;84:44–51. doi: 10.1016/j.ajhg.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cronshaw JM, Matunis MJ. The nuclear pore complex protein ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci U S A. 2003;100:5823–5827. doi: 10.1073/pnas.1031047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shackleton S, Smallwood DT, Clayton P, Wilson LC, Agarwal AK, Garg A, Trembath RC. Compound heterozygous ZMPSTE24 mutations reduce prelamin A processing and result in a severe progeroid phenotype. J Med Genet. 2005;42:e36. doi: 10.1136/jmg.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navarro CL, De Sandre-Giovannoli A, Bernard R, Boccaccio I, Boyer A, Genevieve D, Hadj-Rabia S, Gaudy-Marqueste C, Smitt HS, Vabres P, et al. Lamin A and ZMPSTE24 (FACE-1) defects cause nuclear disorganization and identify restrictive dermopathy as a lethal neonatal laminopathy. Hum Mol Genet. 2004;13:2493–2503. doi: 10.1093/hmg/ddh265. [DOI] [PubMed] [Google Scholar]
- 55.Taylor MR, Slavov D, Gajewski A, Vlcek S, Ku L, Fain PR, Carniel E, Di Lenarda A, Sinagra G, Boucek MM, et al. Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy. Hum Mutat. 2005;26:566–574. doi: 10.1002/humu.20250. [DOI] [PubMed] [Google Scholar]
- 56.Olins AL, Olins DE. Cytoskeletal influences on nuclear shape in granulocytic HL-60 cells. BMC Cell Biol. 2004;5:30. doi: 10.1186/1471-2121-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olins AL, Ernst A, Zwerger M, Herrmann H, Olins DE. An in vitro model for Pelger-Huet anomaly: Stable knockdown of lamin B receptor in HL-60 cells. Nucleus. 2010;1:506–512. doi: 10.4161/nucl.1.6.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luke Y, Zaim H, Karakesisoglou I, Jaeger VM, Sellin L, Lu W, Schneider M, Neumann S, Beijer A, Munck M, et al. Nesprin-2 Giant (NUANCE) maintains nuclear envelope architecture and composition in skin. J Cell Sci. 2008;121:1887–1898. doi: 10.1242/jcs.019075. [DOI] [PubMed] [Google Scholar]
- 59.Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000760. a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, Erdos MR, Eriksson M, Goldman AE, Khuon S, Collins FS, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc Natl Acad Sci U S A. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. Cell nuclei from old individuals acquire defects similar to those of HGPS patient cells, including a decrease in trimethylation of histone H3 on lysine 9 and an increase in phosphorylation of H2AX.
- 62.Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol. 2008;10:452–459. doi: 10.1038/ncb1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nikolova V, Leimena C, McMahon AC, Tan JC, Chandar S, Jogia D, Kesteven SH, Michalicek J, Otway R, Verheyen F, et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J Clin Invest. 2004;113:357–369. doi: 10.1172/JCI19448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci U S A. 2004;101:10428–10433. doi: 10.1073/pnas.0401424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coffinier C, Jung HJ, Nobumori C, Chang S, Tu Y, Barnes RH, 2nd, Yoshinaga Y, de Jong PJ, Vergnes L, Reue K, et al. Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol Biol Cell. 2011 doi: 10.1091/mbc.E11-06-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malhas A, Lee CF, Sanders R, Saunders NJ, Vaux DJ. Defects in lamin B1 expression or processing affect interphase chromosome position and gene expression. J Cell Biol. 2007;176:593–603. doi: 10.1083/jcb.200607054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dreuillet C, Tillit J, Kress M, Ernoult-Lange M. In vivo and in vitro interaction between human transcription factor MOK2 and nuclear lamin A/C. Nucleic Acids Res. 2002;30:4634–4642. doi: 10.1093/nar/gkf587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lloyd DJ, Trembath RC, Shackleton S. A novel interaction between lamin A and SREBP1: implications for partial lipodystrophy and other laminopathies. Hum Mol Genet. 2002;11:769–777. doi: 10.1093/hmg/11.7.769. [DOI] [PubMed] [Google Scholar]
- 69.Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O'Connor JE, Burke B, Andres V. A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev. 2006;20:307–320. doi: 10.1101/gad.349506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holaska JM, Lee KK, Kowalski AK, Wilson KL. Transcriptional repressor germ cell-less (GCL) and barrier-to- autointegration factor (BAF) compete for binding to emerin in vitro. J Biol Chem. 2002;18:18. doi: 10.1074/jbc.M208811200. [DOI] [PubMed] [Google Scholar]
- 71.Holaska JM, Rais-Bahrami S, Wilson KL. Lmo7 is an emerin-binding protein that regulates the transcription of emerin and many other muscle-relevant genes. Hum Mol Genet. 2006;15:3459–3472. doi: 10.1093/hmg/ddl423. [DOI] [PubMed] [Google Scholar]
- 72. Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. By micromanipulating bound microbeads or micropipettes attached to integrins at cell surface it is demonstrated that cytoskeletal filaments are connected to the nucleus and transduct force from the cell surface to the nucleus.
- 73. Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J Biol Chem. 2011;286:26743–26753. doi: 10.1074/jbc.M111.233700. Experiments of microneedle manipulation and mechanical strain demonstrate that the LINC complex mediates mechanical force transmission between the nucleus and cytoskeletal filaments but is not involved in mechanically induced activation of mechanosensitive genes.
- 74. Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 2004;113:370–378. doi: 10.1172/JCI19670. Lamin A/C is involved in maintaining nuclear shape, mechanics and regulating mechanotransduction.
- 75.Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci U S A. 2006;103:10271–10276. doi: 10.1073/pnas.0601058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell. 2008;7:383–393. doi: 10.1111/j.1474-9726.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lammerding J, Hsiao J, Schulze PC, Kozlov S, Stewart CL, Lee RT. Abnormal nuclear shape and impaired mechanotransduction in emerin-deficient cells. J Cell Biol. 2005;170:781–791. doi: 10.1083/jcb.200502148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 79.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 80.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci U S A. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhattacharya R, Gonzalez AM, Debiase PJ, Trejo HE, Goldman RD, Flitney FW, Jones JC. Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. J Cell Sci. 2009;122:1390–1400. doi: 10.1242/jcs.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Davies PF, Shi C, Depaola N, Helmke BP, Polacek DC. Hemodynamics and the focal origin of atherosclerosis: a spatial approach to endothelial structure, gene expression, and function. Ann N Y Acad Sci. 2001;947:7–16. discussion 16–17. Transcription profiles of the intercellular communication protein connexin 43 are more heterogeneous in individual endothelial cells isolated from disturbed flow than cells isolated from undisturbed flow.
- 83.Helmke BP, Rosen AB, Davies PF. Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys J. 2003;84:2691–2699. doi: 10.1016/S0006-3495(03)75074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]