Abstract
Allergy history has been consistently inversely associated with glioma risk. Two serologic markers, soluble CD23 (sCD23) and soluble CD14 (sCD14), are part of the innate and adaptive humoral immune systems and modulate allergic responses in opposite directions, with sCD23 enhancing and sCD14 blunting inflammatory responses. We measured sCD23 and sCD14 in serum from blood that was drawn at a single time point from 1079 glioma patients post diagnosis and 736 healthy controls. Glioma was strongly associated with high sCD14 (highest vs. lowest quartile OR = 3.94 (95% CI: 2.98-5.21) and low sCD23 (lowest vs. highest quartile OR=2.5 (95% CI: 1.89-3.23)). Results were consistent across glioma histologic types and grades, but were strongest for glioblastoma. While temozolomide treatment was not associated with either sCD14 or sCD23 levels among cases, those taking dexamethasone had somewhat lower sCD23 levels than those not taking dexamethasone. However, sCD23 was associated with case status regardless of dexamethasone treatment. These results augment the long observed association between allergies and glioma and support a role for the innate and adaptive humoral functions of the immune system, and in particular immunoregulatory proteins, in gliomagenesis.
Keywords: CD14, CD23, glioma, brain tumor, immune, tumor risk, epidemiology
Introduction
The etiology of adult glioma is currently unknown. Two recent genome wide association studies identified five susceptibility regions for glioma (1, 2). These are the only definitive risk factors for glioma apart from long established associations with rare inherited cancer syndromes and relatively high dose ionizing radiation exposures (3). Most epidemiological studies that addressed immune factors have reported that adults with glioma are 1.5 to 4 fold less likely than controls to report a variety of allergies (4-6), which ranks the absence of allergies among the most consistent risk factors for glioma reported to date. However, mechanisms accounting for this association have been difficult to establish. We hypothesized that allergy may reflect a state of balance of immune functions that could influence anti-tumor reactions. In one previous analysis, we found an inverse relationship between post-diagnosis serum IgE, an indicator of atopic immune response, and glioma (7). However, in a second study, the inverse association was only apparent in temozolomide treated patients and the analysis suggested that the lower levels of IgE in the patients could be related to temozolomide treatment (8). Although allergy-related IgE is an evidence of adaptive humoral immune response, allergy is a complex phenotype that involves both adaptive and innate branches of the immune system. Recent studies have shown clear interactions between the innate and adaptive branches of the immune system (9, 10). Here we focused on two candidate serologic markers of immune regulation that have been implicated in allergic immune responses; sCD23 (serum soluble CD23), the low affinity IgE receptor, is potentially stimulatory for atopic immunity whereas the innate immune marker, sCD14 (serum soluble CD14), may be inhibitory.
CD23 is an important mediator of the allergic response and can function to enhance antigen presentation of IgE antigen complexes (11). Soluble CD23 exhibits clear proinflammatory properties (12, 13), mediates macrophage (14, 15) or lymphocyte (16) activation, and has been shown to be concentrated in inflamed brain tissues and cerebrospinal fluid of encephalitis patients (17).
CD14 is an important component of the innate immune toll-like receptor (TLR) system and gram negative and positive bacterial pattern recognition (18, 19). The soluble form, sCD14 can bind to different B cell subsets and enhance IgG1 while suppressing IgE production (20), and inhibits the production of IL2 and IL4, thus contributing to reduced IgE isotype switching (21). An example that sCD14 plays a part in immune tolerance (22, 23) comes from a study of farmers' children; these children, exposed to high levels of endotoxin and other bacterial components as infants, have reduced risk of developing allergy and demonstrate relatively elevated sCD14 (24). Membrane bound CD14 is involved in Alzheimer's disease pathology (25) and sCD14 is found in cerebrospinal fluid where it may inhibit immune activation of glial cells and neurotoxicity (26). This demonstrates that CD14 is relevant to the central nervous system.
Given the inverse relationship of glioma with allergy history and IgE levels, the positive association of sCD23 with IgE, and the immune tolerance role of sCD14, we evaluated the hypotheses that glioma cases would have higher levels of sCD14 and lower levels of sCD23 than controls.
Materials and methods
Study participants
Population and clinic-based subject recruitment methods for the San Francisco Bay Area Adult Glioma study have been described in detail elsewhere (5, 7, 8). Briefly, all cases were adults age 20 or older with newly diagnosed histologically confirmed glioma (International Classification of Disease for Oncology, morphology codes 9380-9481). Population based cases from six Bay Area counties were ascertained using the Northern California Cancer Center's rapid case ascertainment system from May 1997 to August 1999 (Series 2) and from November 2001 to September 2005 (Series 3). Additionally, cases with same eligibility criteria diagnosed between 2002 and 2006 and seen at the UCSF Neuro-oncology Clinic were eligible to participate, regardless of place of residence; these are referred to as clinic-based cases. Pathological material was retrieved, when possible, for all resected brain cancers and reviewed and classified by one of two neuropathologists (Kenneth Aldape, MD Anderson, Houston, TX and Tarik Tihan, UCSF, San Francisco, CA). Blood and serum samples were usually collected at the time of interview. Controls aged 20 years or older from the same residential area as the population-based cases were identified using random digit dialing and were frequency matched to population-based cases on age, gender and ethnicity as previously described (8). Study methods were approved by the Committee on Human Research at the University of California, San Francisco, CA.
Interview
Interview methods for subjects cases and controls were described previously (7). For population-based subjects, the full process lasted about 2 hours and used a structured questionnaire and show cards to facilitate recall. Allergy history data were collected in tabular form as described in detail in our earlier report (8). Extensive data were also collected about family and personal medical history including asthma and eczema, use of prescription and non-prescription medications, demographic factors, and lifestyle factors such as cigarette smoking and diet. Clinic-based patients were asked to answer a much shorter (about 30 minute) questionnaire, which contained key elements of the longer questionnaire. All participants who provided a blood sample were administered an additional questionnaire about current and recent medications and treatments.
Measurements of sCD14 and sCD23
A single serum sample was collected and tested for each study subject. The serological Luminex assays were developed using a standard sandwich capture format (27, 28). Briefly, monoclonal antibodies to human sCD14 (clone 55-3, BD Pharmingen, San Diego, CA) or sCD23 (clone 138633, R&D, Minneapolis, MN) were coupled to carboxylated Luminex microspheres by using a two-step carbodiimide reaction. Serum samples were diluted 1:100 in diluent for sCD14 and 1:10 for sCD23; serum diluent was a mixture of PBS, 10% (vol/vol) FBS and 2.5% (vol/vol) CBS-K (Millipore Corporation, Temecula, CA). The solution was then incubated at room temperature for one hour on a shaker. A standard curve was created by diluting known concentrations of recombinant human sCD14 (Cellsciences, Canton, MA) or sCD23 (R&D, Minneapolis, MN) using the standard serum diluent. The sCD14 and sCD23 standards or participant serum samples and coupled sCD14 or sCD23 microspheres were then incubated for 2 hours at room temperature on a shaker using a 96-well filter bottom plate (Bio-Rad Laboratories, Inc. Richmond, CA) and subsequently washed with the wash buffer (Laboratories, Inc. Richmond, CA). This step was followed by the addition of 25uL of 1:1000 diluted biotinylated anti-human sCD14 antibody (clone 3-C39, BD Pharmingen, San Diego, CA) or anti-human sCD23 antibody (clone BAF123, R&D, Minneapolis, MN) to each well and then incubating the mixture at room temperature for 30 minutes on a shaker. The serum solution was then washed and treated with 50uL of streptavidin-conjugated R-phycoerythrin 1:100 diluted stock (Bio-Rad Laboratories, Inc. Richmond, CA). After a 10 minute incubation and final wash the microspheres were resuspended in 125 uL of assay buffer (Bio-Rad Laboratories, Inc. Richmond, CA.). The amount of sCD14 or sCD23 bound to the microspheres by this antibody sandwich technique was determined by the median fluorescence intensity (MFI) of the reporter molecule, phycoerythrin (PE) using a Bio-plex 200 plate reader system. The MFI of the unknown serum sample was then converted into a picograms-per-milliliter value based on the known concentrations of the standard curve by using a five-parameter (5PL) regression formula. Each sample was run with a replicate and the intra-class correlation coefficient for sCD14 and sCD23 was 0.92 and 0.93, respectively. A single serum sample from a person without a brain tumor was repeated on most of the assay plates (39 out of 44) and the coefficient of variation between the assays was 11.5% for sCD14 and 18.3% for sCD23. We performed standard addition experiments that yielded recovery rates of 94% for sCD14 and 104% for sCD23.
IgE measurements
IgE levels were assessed using Pharmacia Diagnostics UniCAP fluorescent “sandwich” assay as described previously (29). IgE levels were determined using serum derived from the same blood draw as used for the current sCD14/sCD23 analysis. Total IgE was determined by measuring fluorescence against the standard curve with known quantity inputs. The intra-class correlation for replicate samples of IgE was 0.99.
Statistical methods
Statistical analyses were conducted using SAS v9 (SAS Institute, Cary NC). Odds ratios (ORs) for glioma cases versus controls were computed using unconditional logistic regression, adjusted for age, gender, ethnicity (white/nonwhite), education (college education yes/no) and smoking history (ever/never) because these characteristics were possible confounders of IgE levels and we thought they also might confound other serologic measures. Analyses were conducted for all gliomas and by histological subtype (using polytomous logistic regression) and treatment, e.g. temozolomide-treated versus not treated. Associations with continuous variables were determined using standard t-tests to compare means between groups. For the case/control comparisons, sCD14 and sCD23 values were categorized into quartiles based on the distribution among controls. In addition to analyses of the main effects of sCD23 and sCD14 on glioma, we determined whether the association between sCD23 and glioma was modified by level of sCD14 (or vice versa). To increase power for these statistical interaction analyses, sCD14 was dichotomized based on main effect results by combining the lowest three quartiles as the referent whereas sCD23 was dichotomized by combining the three highest quartiles as the referent group. sCD23 was ‘reverse’ coded to reflect the hypothesized inverse relationship between sCD23 and disease status and for ease of interpretation of the statistical interaction effects. Likelihood ratio tests were used to formally assess statistical interaction in nested adjusted unconditional logistic regression models with and without the cross-product term for the grouped sCD14 and sCD23 concentrations. Total IgE was analyzed both as a log-transformed continuous variable and for comparison with earlier studies, as a categorical variable with groups defined based on clinically relevant cutpoints (IgE > 100 kU/ L = “elevated”, 25–100 kU/L = “borderline” and < 25 kU/L = “normal”).
For any analyses involving allergy history, only population based subjects were included, because the shorter questionnaire used for the clinic patients yielded a much lower prevalence of ever having allergies and numbers of allergies could not be as thoroughly quantified as it could be from the long questionnaire.
Results
Study population
We selected 1079 cases and 737 controls with sufficient amounts of serum such that we would not deplete our biorepository. Serum analysis for sCD14 failed in one case and one control whereas, for sCD23, it failed in 15 cases and 10 controls. Therefore a total of 1078 cases and 736 controls were included in the CD14 analysis and 1064 cases and 736 controls in the sCD23 analysis. Of the 1079 cases included in this analysis, 671 completed a full questionnaire and 471 the abbreviated clinic questionnaire.
Cases and controls were comparable in their ethnicity, college graduate and household income distributions, and total years of education. Controls were older than cases and more likely to be female. Controls also were more likely to have smoked cigarettes compared with cases. (Table 1) Glioblastomas were the most common histologic subtype of brain tumor, followed by anaplastic astrocytomas, astrocytomas and oligodendrogliomas (Table 1).
Table 1. Age, Gender, Ethnicity, Education, Income, Smoking History and Histology of Participants with sCD14 or sCD23 Results, San Francisco Bay Area Adult Glioma Study (1997-2006).
| Glioma Cases (n=1079) | Controls (n=737) | |
|---|---|---|
| Mean Age ± SE | 50.6 ± 0.4 | 55.6 ± 0.6 |
| % White | 83 | 81 |
| % Male | 60 | 53 |
| % College Graduate | 51 | 52 |
| Mean Education (yrs) ± SE | 15.2 ± 0.1 | 15.4 ± 0.1 |
| Household Income (USD/yr) (%) | % | % |
| <=$29,999 | 17 | 20 |
| $30-49,999 | 17 | 17 |
| $50-69,999 | 14 | 16 |
| $70-99,999 | 17 | 18 |
| $100,000+ | 31 | 28 |
| Missing/Refused | 3 | 1 |
| Smoking History (%) | % | % |
| Never Smoked | 52 | 45 |
| Past Smoker | 36 | 42 |
| Current Smoker | 12 | 13 |
| Histology (%) | % | % |
| Glioblastoma | 57 | NA |
| Anaplastic Astrocytoma | 12 | NA |
| Astrocytoma | 8 | NA |
| Anaplastic Oligodendroglioma | 4 | NA |
| Oligodendroglioma | 8 | NA |
| Oligoastrocytoma | 3 | NA |
| Ependymoma | <1 | NA |
| Juvenile Pilocytic Astrocytoma | 2 | NA |
| Medulloblastoma | 1 | NA |
| Other/Unknown | 4 | NA |
| Astrocytoma NOS | <1 | NA |
sCD14, sCD23 levels and glioma case-control comparisons
The overall concentration of sCD14 was statistically significantly higher among glioma cases compared to controls (mean±SE: 10.3±0.13 μg/mL vs. 8.1±0.09 μg/mL respectively, p<0.01, Table 2). Glioma was strongly associated with high sCD14 (highest vs. lowest quartile OR = 3.94 (95% CI: 2.98-5.21)). Results were consistent for both GBM's and other glioma histologies (Table 2).
Table 2. Comparisons of sCD14 and sCD23 Levels in Glioma Cases and Controls, San Francisco Bay Area Adult Glioma Study (1997-2006).
| Cases | Controls | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Value | Case Category | n | Mean ± SE | n | Mean ± SE | t-test p-value** | |||
| sCD14 | All | 1078 | 10.3 ± 0.13 ug/mL | 736 | 8.1 ± 0.09 ug/mL | <0.01 | |||
| sCD23 | All | 1064 | 2.7 ± 0.08 ng/mL | 727 | 3.6 ± 0.13 ng/mL | <0.01 | |||
| Value | Category | Quartile | n | % | n | % | OR* (95% CI) | OR p-value* | Test for Trend |
| sCD14 | All | 1 | 174 | 16 | 184 | 25 | 1.00 | ||
| 2 | 130 | 12 | 184 | 25 | 0.79 (0.58-1.08) | 0.14 | |||
| 3 | 195 | 18 | 184 | 25 | 1.21 (0.90-1.63) | 0.21 | |||
| 4 | 579 | 54 | 184 | 25 | 3.94 (2.98-5.21) | <0.01 | P<0.01 | ||
| GBM | 1 | 94 | 15 | 184 | 25 | 1.00 | |||
| 2 | 66 | 11 | 184 | 25 | 0.71 (0.49-1.04) | 0.08 | |||
| 3 | 110 | 18 | 184 | 25 | 1.19 (0.84-1.69) | 0.33 | |||
| 4 | 347 | 56 | 184 | 25 | 4.02 (2.93-5.50) | <0.01 | P<0.01 | ||
| Non-GBM | 1 | 80 | 17 | 184 | 25 | 1.00 | |||
| 2 | 64 | 14 | 184 | 25 | 0.90 (0.60-1.36) | 0.61 | |||
| 3 | 85 | 18 | 184 | 25 | 1.21 (0.81-1.79) | 0.35 | |||
| 4 | 232 | 50 | 184 | 25 | 3.88 (2.73-5.53) | <0.01 | P<0.01 | ||
| sCD23 | All | 1 | 437 | 41 | 183 | 25 | 1.00 | ||
| 2 | 221 | 21 | 181 | 25 | 0.52 (0.40-0.68) | <0.01 | |||
| 3 | 232 | 22 | 182 | 25 | 0.53 (0.41-0.69) | <0.01 | |||
| 4 | 174 | 16 | 181 | 25 | 0.40 (0.31-0.53) | <0.01 | P<0.01 | ||
| GBM | 1 | 281 | 46 | 183 | 25 | 1.00 | |||
| 2 | 129 | 21 | 181 | 25 | 0.47 (0.35-0.63) | <0.01 | |||
| 3 | 117 | 19 | 182 | 25 | 0.41 (0.30-0.56) | <0.01 | |||
| 4 | 82 | 14 | 181 | 25 | 0.29 (0.21-0.41) | <0.01 | P<0.01 | ||
| Non-GBM | 1 | 156 | 34 | 183 | 25 | 1.00 | |||
| 2 | 92 | 20 | 181 | 25 | 0.62 (0.44-0.88) | <0.01 | |||
| 3 | 115 | 25 | 182 | 25 | 0.75 (0.54-1.05) | 0.09 | |||
| 4 | 92 | 20 | 181 | 25 | 0.62 (0.43-0.88) | <0.01 | P=0.02 | ||
Odds ratios were adjusted for age (continuous), race (white/non-white), gender, education (college vs no college), and smoking (ever vs never). ORs for the GBM and non-GBM groups were estimated using polytomous logistic regression models.
Note: sCD14 Quartile cutoff values were: (1) Quartile 1=1.45-6.49ug/mL, (2) Quartile 2=6.50-7.65 ug/mL, (3) Quartile 3=7.66-9.26 ug/mL, and (4) Quartile 4=9.27-37.14 ug/mL.
sCD23 Quartile cutoff values were: (1) Quartile 1=0.03-1.57ng/mL, (2) Quartile 2=1.58-2.59 ng/mL, (3) Quartile 3=2.61-4.41 ng/mL, and (4) Quartile 4=4.43-38.72 ng/mL.
T-test comparing sCD14/sCD23 means between cases and controls.
SE=Standard error
In contrast, the mean serum sCD23 was lower for cases (2.7±0.08 ng/mL) compared to controls (3.6±0.13 ng/mL) (p<0.01). Glioma was strongly associated with low sCD23 (lowest vs. highest quartile OR=2.5 (95% CI: 1.89-3.23)). Although a similar pattern in ORs was observed for GBM and cases with other glioma histologies, the ORs were further from the null for those with GBM (Table 2).
Stratification of sCD14 and sCD23 levels by histological diagnosis show that, irrespective of subtype, levels are different for cases and controls, although differences were most pronounced for GBM (Supplementary Table 1). The median number of days between diagnosis and blood draw for cases was 89 days. Among cases, time between diagnosis and blood draw was weakly negatively correlated with sCD14 concentration (Spearman r2 = -0.08, p<0.01), whereas no correlation was observed with sCD23 concentration and time between diagnosis and blood draw (Supplementary Table 2). We also did not find any statistically significant association between smoking history and sCD14 or sCD23 levels.
Temozolomide treatment and sCD14 and sCD23 levels
Among the GBM cases diagnosed in 2001 or later, 406 of the 505 patients received temozolomide treatment. There was no significant difference of sCD14 levels in the temozolomide treated group compared to the non- temozolomide-treated group (p=0.6), with similar results in non-GBM patients (p=0.2). In stratified analyses there also was no evidence that history of temozolomide use altered the association of sCD14 and sCD23 with glioma (Supplementary Table 3). Glioma was associated with higher sCD14 quartiles in both groups (Supplementary Tables 3 and 4). Similarly, temozolomide was not associated with sCD23 levels in glioma patients (Supplementary Tables 3 and 4). Temozolomide treatment schedules are typically cyclical – 5 days on treatment followed by 23 days off. We assessed whether sCD14 or sCD32 levels were related to time since last dose of chemotherapy treatment among the 97 patients who were currently within a 28 day temozolomide cycle and found no correlation (data not shown).
Dexamethasone treatment and sCD14 and sCD23 levels
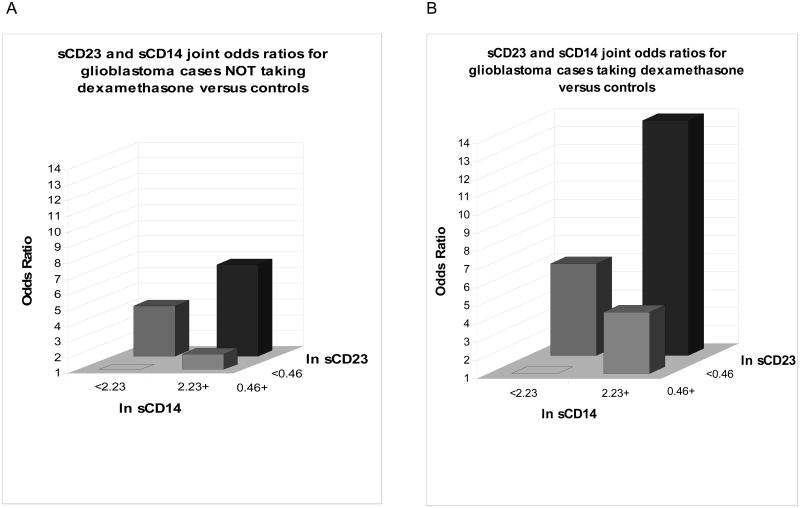
There was no difference in means or quartile distributions of sCD14 levels for GBM patients who reported taking dexamethasone at the time of blood draw versus those who did not (Supplementary Tables 5 and 6). In contrast, sCD23 levels in patients who reported dexamethasone use at time of blood draw were statistically significantly lower than in patients who did not (Supplementary Table 5). Although dexamethasone status didn't change the pattern of association between sCD23 and glioma (see Figure 1), the case-control OR was lower for patients reporting dexamethasone use versus those who were not using dexamethasone (Supplementary Table 6, dexamethosone OR= 0.25, 95% CI: 0.17-0.36 vs. non- dexamethasone: OR= 0.53, 95% CI: 0.39-0.73). Apart from dexamethasone, there were no significant associations of sCD14 or sCD23 with other medications (Supplementary Tables 7a and 7b).
Fig 1.
Analysis of joint odds ratio (OR) of sCD23 and sCD14 in glioblastoma (GBM) patients taking or not taking dexamethasone. The patients were stratified into four groups by ln sCD14 and ln s CD23 values (ln sCD14 <2.23 or >2.23, ln sCD23 <0.46 or >0.46). A. In GBM patients who were not taking dexamethasone at the time of blood draw the OR in the group with high sCD14 and low sCD23 was more than 6.8-fold higher than the group with the low sCD14 and high sCD23 values. B. In GBM patients who were taking dexamethosone the OR in the high sCD14 and low sCD23 was more than 14-fold higher than those with the low sCD14 and high sCD23 values.
sCD14, sCD23, and IgE levels and allergy history
There were no statistically significant associations between sCD14 or sCD23 levels and IgE in controls (Supplementary Table 8). sCD23 levels in cases were lower among those with elevated IgE but not among controls. sCD14 concentration was not related to number of allergies, allergy type or age at first allergy in either cases or controls (Supplementary Table 9a). However, both cases and controls who reported any allergies had higher sCD23 levels than did those with no allergies, although the association was not statistically significant (p>0.05, Supplementary Table 9b).
Joint association of sCD14 and sCD23 with glioma
The joint associations of sCD14 and sCD23 by histopathological subtype and recent dexamethasone use are presented in Table 3. Results among all cases suggested that the association between sCD23 and GBM was modified by sCD14 level. Additional analyses conducted to assess the potential immune-related effect of dexamethasone on this relationship showed that the suggested statistical interaction between sCD14 and sCD23 in GBM's was limited to GBM patients who had recently used dexamethasone (Table 3 and Figure 1). Results also suggested statistical interaction between sCD23 and sCD14 among non-GBM patients. . Similar to GBM, it appears that dexamethasone use may explain the observed associations (data not shown). Small numbers of exposed patients prohibited our ability to adequately assess the effect of dexamethasone use on the relationship between sCD14 and sCD23 and rarer histological subtypes. The apparent interaction was thus simply an effect of dexamethasone on sCD23 and not sCD14 and therefore not a true interaction.
Table 3. Multiplicative Interaction Results, Combined sCD14 and sCD23 Levels (Dichotomous Groupings), Stratified by Histologic Type and Dexamethasone Use, San Francisco Bay Area Adult Glioma Study Participants (1997-2006).
| Number | |||||
|---|---|---|---|---|---|
| Case Grouping | Dichotomous Grouping** | Cases | Controls | OR* (95% CI) | Multiplicative p values |
| All Cases | Low sCD14 / High sCD23 | 276 | 416 | 1.00 | |
| Low sCD14 / Low sCD23 | 221 | 133 | 2.6 (2.0-3.4) | ||
| High sCD14 / High sCD23 | 344 | 126 | 4.7 (3.6-6.2) | ||
| High sCD14 / Low sCD23 | 222 | 51 | 7.2 (5.1-10.3) | 0.03 | |
| Cases Taking Dexamethasone | Low sCD14 / High sCD23 | 87 | 416 | 1.00 | |
| Low sCD14 / Low sCD23 | 113 | 133 | 4.1 (2.9-5.8) | ||
| High sCD14 / High sCD23 | 136 | 126 | 5.8 (4.1-8.1) | ||
| High sCD14 / Low sCD23 | 115 | 51 | 11.8 (7.8-17.8) | 0.01 | |
| Cases Not Taking Dexamethasone | Low sCD14 / High sCD23 | 189 | 416 | 1.00 | |
| Low sCD14 / Low sCD23 | 108 | 133 | 1.8 (1.3-2.5) | ||
| High sCD14 / High sCD23 | 208 | 126 | 4.3 (3.2-5.7) | ||
| High sCD14 / Low sCD23 | 107 | 51 | 5.0 (3.4-7.4) | 0.10 | |
| Glioblastoma Cases | Low sCD14 / High sCD23 | 134 | 416 | 1.00 | |
| Low sCD14 / Low sCD23 | 134 | 133 | 3.2 (2.3-4.3) | ||
| High sCD14 / High sCD23 | 189 | 126 | 5.1 (3.7-6.9) | ||
| High sCD14 / Low sCD23 | 151 | 51 | 9.8 (6.7-14.3) | 0.05 | |
| Non-Glioblastoma Cases | Low sCD14 / High sCD23 | 142 | 416 | 1.00 | |
| Low sCD14 / Low sCD23 | 86 | 133 | 1.9 (1.4-2.8) | ||
| High sCD14 / High sCD23 | 155 | 126 | 4.5 (3.3-6.3) | ||
| High sCD14 / Low sCD23 | 71 | 51 | 4.7 (3.1-7.3) | 0.04 | |
Odds ratios were estimated in logistsic regression models adjusted for age (continuous), gender, race (white/non-white), education (college vs no college), and smoking (ever vs never).
The stratified results were estimated using polytomous regression models.
Cutoff points for dichotomizing the concentration of sCD14 was 9.30 ug/mL and for sCD23 was 1.59 ng/mL.
Discussion
Immunological factors have long been hypothesized to play a role in glioma risk, and secretion of immunosuppressant molecules by high grade gliomas themselves is well documented (30). Despite this, there are few serum biomarkers to evaluate the etiological or prognostic immune aspects of this disease. Here we present the first analysis of the immune regulatory proteins sCD23 and sCD14 in glioma patients and controls showing that sCD14 levels were higher and sCD23 levels were lower in glioma cases compared with controls.
First, we discuss sCD14 levels which are higher in glioma cases than controls. Since measurements of sCD14 are taken after glioma diagnosis, we need to consider whether sCD14 levels were affected by the brain tumor or treatment. In these data, there were no differences among glioma patients for sCD14 levels by medications, radiation, or extent of resection. Though it is not easy to track the details of temozolomide administration in this study, there was no evidence that temozolomide exposure altered the association of sCD14 or sCD23 with glioma risk. Temozolomide may induce lymphopenia and affect patients' levels of white blood cell subsets (31); however, IgE levels were not altered during temozolomide treatment schedule (8). Similarly, we found no relationship of sCD14 or sCD23 with timing of blood draw within the temozolomide treatment schedule. sCD14 levels were also not associated with a history of allergies in either cases or controls. Alternatively, high sCD14 may reflect an individual's high level of current bacterial antigen exposure or potentially a greater sensitivity to this exposure (32). We had no record of infections within our case population but note that self-reported use of antibiotics was not associated with sCD14. In addition, a modest increase in sCD14 with increasing age was observed in our controls but not in cases; age was included in all models assessing the association of sCD14 with glioma and hence was unlikely to have influenced case-control associations. It is possible that sCD14 levels reflect an individual's “set point” for the innate immune system's capacity to recognize foreign or pathologic molecular patterns independent of allergy, as has been suggested in other studies. Several studies have shown that sCD14 levels are modified in the first years of life by exposure to microbial antigens (24, 33), leading to an adaptive and developmentally normal immune system which may establish a permanent physiologic sCD14 set-point. Since this set-point may persist later in adult life, much as other immune factors do, it could represent a brain tumor susceptible phenotype or the effect of a tumor itself. These hypotheses will have to be tested in cohort studies. It has been suggested that sCD14 plays an immunomodulatory role in the normal central nervous system and appears to inhibit glial cell activation by interfering with LPS effects (26). In malignant brain tumors CD14 accumulates in GBM but not among the lower grade astrocytomas (34). This indicates that CD14 may modulate immunological reactions in these tumors, contributing to their grim prognosis (34). The increased levels of sCD14 in brain tumor patients could be generated either by shedding the glycophosphatidylinositol (GPI) anchor from previously membranous CD14, or by increased CD14 transcription without the GPI anchor attachment (35); our data were unable to distinguish the source of sCD14. It also is possible that brain tumors themselves increase monocyte/macrophage activity locally with concomitant expression of CD14 and sCD14.
Although no known immunologic mechanism links sCD14 with gliomagenesis, sCD14 has the capacity to negatively affect T lymphocyte activation and function by interacting directly with activated T cells. sCD14 inhibits T cell proliferation and the production of IL-2 and IFN-γ (36). In addition, sCD14 is known to suppress B cell development leading to reduced IgE production. An antitumor role for IgE has been proposed for solid tumors (37). Given that sCD14 has been shown to suppress both T cell and B cell functions, a chronic high level of sCD14 may predispose to a permissive immune reaction with respect to glioma formation and participate in the known immunosuppressive serological factors that promote T cell and immune anergy in the brain, including TGFβ, IL-13 decoy receptor (ILRα2), and PGE2. Although disease or treatment related factors might possibly affect sCD14 levels, neither temozolomide nor dexamethasone treatment appeared to influence sCD14 levels in this study. We did observe a weak negative correlation between the time since diagnosis and blood draw. This modest effect would in fact lead to an underestimation of sCD14 in some cases and a lower OR of association with glioma.
Second, we consider sCD23 levels in relation to glioma. We found that the concentration of serum sCD23 was lower in glioma cases compared to controls. CD23 plays a critical role during immune response including IgE-synthesis, B and T cell differentiation, and the secretion of inflammation mediators by various human cells (38). These mechanisms could be important in gliomagenesis. Our group has previously shown that lack of allergy in this patient population is a consistent risk factor for glioma. Although the relationship was not strong in our data, we did observe an association of high sCD23 with increased self-reported allergy history (Supplementary Table 9b). Thus, sCD23 may be a useful marker of upstream immune regulation in glioma research. Finally, we note that production of sCD23 is dependent on the proteolytic cleavage of membrane CD23 by the metalloproteinase ADAM10. Lower amounts or activity of ADAM10 in glioma patients could be responsible for the decreased levels of sCD23. There are no studies of ADAM10 in human blood samples although in glioma tissues the levels appear to be similar to that observed in normal brain (13, 14).
Consistent with other studies, sCD23 levels were lower in patients using dexamethasone than in those who were not using this glucocorticoid drug. Despite this association, sCD23 levels were lower in glioma cases than controls, both in patients who were and were not taking dexamethasone at or near time of blood draw (Figure 1). Thus, we believe our results provide support for the concept that sCD23 levels are a marker of glioma risk, and not merely a marker of glucocorticoid use.
Although we observed an interaction between sCD23 and sCD14 levels in serum, further analyses accounting for dexamethasone use indicate that the observed interaction was likely due to the underlying effects of dexamethasone use on immune function. This was suggested across all histological subtypes. However, we have limited power to adequately assess a dexamethasone effect in rare histological subtypes (specific non-GBM types). The number and activation of B cells is known to contribute to sCD23 levels in peripheral blood (39). If an interaction between sCD14 and sCD23 does exist, an explanation could be that high levels of sCD14 may suppress sCD23 by inhibiting B cell proliferation. However, this hypothesis requires more supporting evidence and data from glioma patients who have not yet been treated with dexamethasone.
Although we expected to see a relationship between IgE levels and sCD23 and sCD14, we did not observe a consistent dose-response association of sCD14 with IgE, and only a marginal inverse association of sCD23 with IgE (Supplementary Table 9). In addition there was no relationship between sCD14 and allergy, but we noticed that persons reporting allergies had higher levels of sCD23 than those reporting no allergy for both cases and controls. This is an interesting finding which implies that more allergies and higher sCD23 levels may be related since both have an inverse relationship with glioma. Another report on sCD14 in children with status asthmaticus showed that sCD14 levels were significantly higher during acute asthma attacks than at recovery, but there was no correlation between level of sCD14 or the change in sCD14 and the serum IgE concentration (40). It remains unclear whether allergies protect against tumors or whether immunosuppressive gliomas inhibit allergies (41). The original report on IgE and glioma also reported that non-IgE-related allergies were inversely related to glioma, suggesting that IgE per se may not be on the causal pathway driving the association but, rather, some other related immune factors may be responsible (6).
In summary, we believe the associations of sCD23 and sCD14 with glioma are very robust and unlikely to be due to chance because our study entailed a large sample size that included patients with various grades and histological types of glioma. Our epidemiological approach allowed us to investigate the potential role of factors such as age, gender, race/ethnicity, cigarette smoking and treatments on our results. We conclude that sCD14 and sCD23 measurements may provide information on how the balance of immune functions within an individual can play a role in glioma risk.
Supplementary Material
Acknowledgments
The work has been supported by US National Institutes of Health grants R01CA126831, R01ES06717, R01CA52689, UCSF Brain Tumor SPORE, P50CA097257 and R01CA109745. Authors also thank the Northern California Cancer Center for glioma patient case finding and the Pathology Departments of Alexian Hospital, Alta Bates Medical Center, Brookside, California Pacific Medical Center, DR Pinole, Eden Hospital, El Camino Hospital, Good Samaritan, Highland Hospital, John Muir, Kaiser Redwood City, Kaiser San Francisco, Kaiser Santa Teresa, Los Gatos Hospital, Los Medanos Hospital, Marin General, Merrithew, Mills Peninsula Hospital, Mt. Diablo Hospital, Mt. Zion Medical Center, Naval Hospital, O'Connor Hospital, Ralph K. Davies Medical Center, Saint Louise, San Francisco General, San Jose, San Leandro, San Mateo County, San Ramon Valley, Santa Clara Valley, Sequoia, Seton Medical Center, St. Francis, St. Lukes, St. Rose, Stanford, Summit, UC San Francisco, Valley Livermore, Veterans Palo Alto, Veterans SF and Washington Hospital for providing tumor specimens for review.
Work supported by NIH R01CA126831, R01ES06717, R01CA52689, UCSF Brain Tumor SPORE, P50CA097257, R01CA109745.
Abbreviations
- sCD14
serum soluble CD14
- sCD23
serum soluble CD23
- GBM
Glioblastoma multiforme
- CNS
Central nervous system
- SNP
Single nucleotide polymorphism
- OR
Odds Ratio
- Ig
Immunoglobulin
- UCSF
University of California San Francisco
References
- 1.Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41:905–8. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4:278–99. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartzbaum J, Jonsson F, Ahlbom A, et al. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. Int J Cancer. 2003;106:423–8. doi: 10.1002/ijc.11230. [DOI] [PubMed] [Google Scholar]
- 5.Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609–15. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 6.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99:1544–50. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 7.Wiemels JL, Wiencke JK, Patoka J, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64:8468–73. doi: 10.1158/0008-5472.CAN-04-1706. [DOI] [PubMed] [Google Scholar]
- 8.Wiemels JL, Wilson D, Patil C, et al. IgE, allergy, and risk of glioma: Update from the San Francisco Bay Area Adult Glioma Study in the Temozolomide era. Int J Cancer. 2009;125:680–7. doi: 10.1002/ijc.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomariz RP, Gutierrez-Canas I, Arranz A, et al. Peptides Targeting Toll-Like Receptor Signalling Pathways for Novel Immune Therapeutics. Curr Pharm Des. 2009 doi: 10.2174/138161210790963841. [DOI] [PubMed] [Google Scholar]
- 10.Sumida T. Mechanism of allergy--innate immunity and acquired immunity. Nippon Rinsho. 2009;67:2030–1. [PubMed] [Google Scholar]
- 11.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–37. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 12.Rambert J, Mamani-Matsuda M, Moynet D, et al. Molecular blocking of CD23 supports its role in the pathogenesis of arthritis. PLoS One. 2009;4:e4834. doi: 10.1371/journal.pone.0004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massa M, Pignatti P, Oliveri M, De Amici M, De Benedetti F, Martini A. Serum soluble CD23 levels and CD23 expression on peripheral blood mononuclear cells in juvenile chronic arthritis. Clin Exp Rheumatol. 1998;16:611–6. [PubMed] [Google Scholar]
- 14.Lecoanet-Henchoz S, Gauchat JF, Aubry JP, et al. CD23 regulates monocyte activation through a novel interaction with the adhesion molecules CD11b-CD18 and CD11c-CD18. Immunity. 1995;3:119–25. doi: 10.1016/1074-7613(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 15.Rezzonico R, Imbert V, Chicheportiche R, Dayer JM. Ligation of CD11b and CD11c beta(2) integrins by antibodies or soluble CD23 induces macrophage inflammatory protein 1alpha (MIP-1alpha) and MIP-1beta production in primary human monocytes through a pathway dependent on nuclear factor-kappaB. Blood. 2001;97:2932–40. doi: 10.1182/blood.v97.10.2932. [DOI] [PubMed] [Google Scholar]
- 16.Aubry JP. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358:3. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 17.Dugas N, Lacroix C, Kilchherr E, Delfraissy JF, Tardieu M. Role of CD23 in astrocytes inflammatory reaction during HIV-1 related encephalitis. Cytokine. 2001;15:96–107. doi: 10.1006/cyto.2001.0896. [DOI] [PubMed] [Google Scholar]
- 18.Schumann RR. Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: a short review. Res Immunol. 1992;143:11–5. doi: 10.1016/0923-2494(92)80074-u. [DOI] [PubMed] [Google Scholar]
- 19.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–4. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 20.Arias MA, Rey Nores JE, Vita N, et al. Cutting edge: human B cell function is regulated by interaction with soluble CD14: opposite effects on IgG1 and IgE production. J Immunol. 2000;164:3480–6. doi: 10.4049/jimmunol.164.7.3480. [DOI] [PubMed] [Google Scholar]
- 21.Vercelli D, Baldini M, Stern D, Lohman IC, Halonen M, Martinez F. CD14: a bridge between innate immunity and adaptive IgE responses. J Endotoxin Res. 2001;7:45–8. [PubMed] [Google Scholar]
- 22.Zdolsek HA, Jenmalm MC. Reduced levels of soluble CD14 in atopic children. Clin Exp Allergy. 2004;34:532–9. doi: 10.1111/j.1365-2222.2004.1921.x. [DOI] [PubMed] [Google Scholar]
- 23.Lundell AC, Andersson K, Josefsson E, Steinkasserer A, Rudin A. Soluble CD14 and CD83 from human neonatal antigen-presenting cells are inducible by commensal bacteria and suppress allergen-induced human neonatal Th2 differentiation. Infect Immun. 2007;75:4097–104. doi: 10.1128/IAI.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauener RP, Birchler T, Adamski J, et al. Expression of CD14 and Toll-like receptor 2 in farmers' and non-farmers' children. Lancet. 2002;360:465–6. doi: 10.1016/S0140-6736(02)09641-1. [DOI] [PubMed] [Google Scholar]
- 25.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci. 2009;29:11982–92. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin GN, Jeon H, Lee S, Lee HW, Cho JY, Suk K. Role of soluble CD14 in cerebrospinal fluid as a regulator of glial functions. J Neurosci Res. 2009;87:2578–90. doi: 10.1002/jnr.22081. [DOI] [PubMed] [Google Scholar]
- 27.Kellar KL, Kalwar RR, Dubois KA, Crouse D, Chafin WD, Kane BE. Multiplexed fluorescent bead-based immunoassays for quantitation of human cytokines in serum and culture supernatants. Cytometry. 2001;45:27–36. doi: 10.1002/1097-0320(20010901)45:1<27::aid-cyto1141>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 28.Martins TB, Pasi BM, Litwin CM, Hill HR. Heterophile antibody interference in a multiplexed fluorescent microsphere immunoassay for quantitation of cytokines in human serum. Clin Diagn Lab Immunol. 2004;11:325–9. doi: 10.1128/CDLI.11.2.325-329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeinbach SL, Barnes JH, Sullivan TJ, Williams PB. Precision and accuracy of commercial laboratories' ability to classify positive and/or negative allergen-specific IgE results. Ann Allergy Asthma Immunol. 2001;86:373–81. doi: 10.1016/S1081-1206(10)62481-7. [DOI] [PubMed] [Google Scholar]
- 30.Tada M, de Tribolet N. Immunobiology of malignant gliomas. J Clin Neurosci. 1996;3:102–13. doi: 10.1016/s0967-5868(96)90001-6. [DOI] [PubMed] [Google Scholar]
- 31.Heimberger AB, Sun W, Hussain SF, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wuthrich B, Kagi MK, Joller-Jemelka H. Soluble CD14 but not interleukin-6 is a new marker for clinical activity in atopic dermatitis. Arch Dermatol Res. 1992;284:339–42. doi: 10.1007/BF00372036. [DOI] [PubMed] [Google Scholar]
- 33.Riedler J, Braun-Fahrlander C, Eder W, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–33. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 34.Deininger MH, Meyermann R, Schluesener HJ. Expression and release of CD14 in astrocytic brain tumors. Acta Neuropathol. 2003;106:271–7. doi: 10.1007/s00401-003-0727-9. [DOI] [PubMed] [Google Scholar]
- 35.Labeta MO, Durieux JJ, Fernandez N, Herrmann R, Ferrara P. Release from a human monocyte-like cell line of two different soluble forms of the lipopolysaccharide receptor, CD14. Eur J Immunol. 1993;23:2144–51. doi: 10.1002/eji.1830230915. [DOI] [PubMed] [Google Scholar]
- 36.Rey Nores JE, Bensussan A, Vita N, et al. Soluble CD14 acts as a negative regulator of human T cell activation and function. Eur J Immunol. 1999;29:265–76. doi: 10.1002/(SICI)1521-4141(199901)29:01<265::AID-IMMU265>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Fu SL, Pierre J, Smith-Norowitz TA, et al. Immunoglobulin E antibodies from pancreatic cancer patients mediate antibody-dependent cell-mediated cytotoxicity against pancreatic cancer cells. Clin Exp Immunol. 2008;153:401–9. doi: 10.1111/j.1365-2249.2008.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lachaux A, Kaiserlian D. CD23: structures, functions and practical perspectives in allergy reactions. Pediatrie. 1993;48:305–12. [PubMed] [Google Scholar]
- 39.Bansal AS, Haeney MR, Cochrane S, et al. Serum soluble CD23 in patients with hypogammaglobulinaemia. Clin Exp Immunol. 1994;97:239–41. doi: 10.1111/j.1365-2249.1994.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garty BZ, Monselise Y, Nitzan M. Soluble CD14 in children with status asthmaticus. Isr Med Assoc J. 2000;2:104–7. [PubMed] [Google Scholar]
- 41.Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–68. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.