Abstract
Force generation in smooth muscle is driven by phosphorylation of myosin light chains (MYL), which is regulated by the equilibrium between the activities of myosin light chain kinase (MYLK) and myosin phosphatase (MYLP). MYLK is activated by Ca2+-calmodulin whereas MYLP is inhibited by phosphorylation of its myosin-binding subunit (MYPT1) by Ca2+-independent mechanisms, leading to generation of increased MYL phosphorylation and force for a given intracellular Ca2+ concentration, a phenomenon known as ‘calcium-sensitization’. The regulation of MYPT1 phosphorylation in human myometrium, which shows increasing phasic contractility at the onset of labour, has yet to be fully investigated. Here, we explore phosphorylation of MYPT1 at Thr696 and Thr853, alongside phosphorylation of MYL, in fresh human myometrial tissue and cultured myometrial cells. We report that pMYPT1 (Thr853) levels are dependent on the activity of Rho-associated kinase (ROCK), determined using the ROCK inhibitor g-H-1152 and siRNA-mediated knockdown of ROCK1/2, and are highly correlated to ppMYL (Thr18/Ser19) levels. Pharmacological inhibition of ROCK was associated with a decrease in oxytocin (OXT)-stimulated contractility of myometrial strips in vitro. Moreover, we have measured pMYPT1 and pMYL levels between and during spontaneous and OXT-stimulated phasic contractions by rapidly freezing contracting muscle, and demonstrate for the first time functional coupling between increases in pMYPT1 (Thr853), ppMYL (Thr18/Ser19) and phasic contractility that is ROCK-dependent. The combined approach of measuring contractility and phosphorylation has demonstrated that the phosphorylation of MYPT1 (Thr853) changes dynamically with each contraction and has elucidated a defined role for ROCK in regulating myometrial contractility through MYLP, providing new insights into uterine physiology which will stimulate further research into treatments for preterm labour.
Keywords: myosin light chains, myosin phosphatase, oxytocin, Rho-associated kinase, uterine contractility
Introduction
The pregnant uterus is a remarkable smooth muscle organ; after implantation of the embryo it grows and stretches to allow the development of the fetus and placenta, and is able to carry a considerable load for many weeks while remaining in a state of relative quiescence. However, the mechanisms involved in the transition from uterine quiescence to contractility at the onset of labour are not known and the endocrine or paracrine changes that promote parturition in women remain elusive. As a consequence, the prediction and management of complications such as preterm labour or uterine dystocia are poor, resulting in potentially severe complications for mother and baby.
Myometrium is a phasic smooth muscle and cycles of relaxation and contraction depend on the reversible equilibrium between unphosphorylated (MYL) and phosphorylated myosin light chain (P-MYL). As in other types of smooth muscle, in myometrium this equilibrium is driven by the opposing activities of myosin light chain kinase (MYLK) and myosin phosphatase (MYLP). MYLK is a calcium-dependent enzyme which responds rapidly to changes in intracellular calcium ([Ca2+]i) through interaction with Ca2+-calmodulin. On the other hand MYLP activity can be regulated by phosphorylation in a Ca2+-independent manner. Oxytocin (OXT) and other contractile agonists stimulate uterine contractility by binding to specific G protein-coupled receptors and promoting Ca2+ entry into the cells through activation of the Gq/phospholipase C signalling pathway (Lopez Bernal, 2003). Moreover, inhibition of MYLP potentiates the effect of MYLK, and the equilibrium requires less [Ca2+]i to shift towards P-MYL, a phenomenon called ‘Ca2+-sensitization’ (Somlyo and Somlyo, 2003).
Endogenous OXT is one of the most potent uterotonic agonists, and for many years it has been known that the sensitivity of the uterus to OXT increases dramatically in pregnancy (Caldeyro-Barcia and Theobald, 1968; Turnbull and Anderson, 1968), partly as a consequence of increased myometrial OXT receptor density (Fuchs et al., 1984; Bossmar et al., 1994). Moreover, synthetic OXT is commonly used for the induction and augmentation of labour, although many uncertainties remain about its mechanism of action. For instance, analysis of Ca2+-tension relationship in pregnant myometrial strips reveals a significant component of Ca2+-sensitization during OXT-induced contractions (McKillen et al., 1999; Woodcock et al., 2004); however, the pathways involved have not been fully elucidated.
The MYLP holoenzyme is composed of three subunits: a 38 kDa catalytic protein phosphatase subunit (PP1C); a large 110–130 kDa regulatory myosin-binding subunit (MYPT1, also known as PPP1R12A or MBP); and a small 20 kDa subunit (Hartshorne, 1998). The regulation of the MYPT1 in human myometrium is complex and requires detailed investigation. Experimental evidence shows that in smooth muscle the C-terminal end of MYPT1 is the target for several protein kinases, including Rho-associated kinase (ROCK) (Feng et al., 1999), integrin-linked kinase (ILK) (Muranyi et al., 2002) and zipper-interacting protein kinase (MacDonald et al., 2001; Borman et al., 2002). Phosphorylation of the C-terminal end of MYPT1 occurs simultaneously or independently at two different sites, namely Thr696 and Thr853, and it is proposed that phosphorylation of either or both sites blocks MYLP activity by a mechanism of auto-inhibition (Khromov et al., 2009). Phosphorylation by ROCK of one or both of these sites is Ca2+-independent and has been demonstrated in several cell types, including epithelial cells (Kawano et al., 1999), endothelial cells (Somlyo et al., 2003; Choi et al., 2009), platelets (Pandey et al., 2006), smooth muscle cells (Ito et al., 2003; Kitazawa et al., 2003; Patil and Bitar, 2006; Wang et al., 2006, 2009; Mizuno et al., 2008) and rat uterine strips (Tahara et al., 2002). It has therefore been proposed that phosphorylation of these sites may play a role in Ca2+-sensitization, however, little is known about their regulation and function in human myometrium. Inhibition of MYLP phosphorylation could explain the decrease in Ca2+-sensitization, and hence relaxation, evoked by ROCK inhibitors during OXT-induced myometrial contractility (Woodcock et al., 2004), however, other ROCK targets such as MYL itself (Amano et al., 1996; Katoh et al., 2001; Ueda et al., 2002), could be involved. MYLP can also be regulated through the 17 kDa protein kinase C (PKC)-activated phosphatase inhibitor (CPI-17), which is capable of inhibiting MYLP activity, thus potentiating P-MYL levels, when phosphorylated itself at Thr38 (Eto et al., 1995). Phosphorylation of Thr38 in response to agonist stimulation has been shown to be dependent on PKC, ROCK or ILK activities in vascular smooth muscle (Kitazawa et al., 2000; Pang et al., 2005; Huang et al., 2006). Up-regulation of CPI-17 mRNA and protein expression have been demonstrated during pregnancy, and these changes may explain the increased ability of PKC to mediate contraction of human myometrium during gestation (Ozaki et al., 2003).
We and others (Moore et al., 2000; Moran et al., 2002; Lartey et al., 2006, 2007) have found that extracts of pregnant human myometrium contain proteins of the Rho family of small GTPases and have demonstrated a pregnancy-related up-regulation of RND2 and RND3 in human myometrium (Lartey et al., 2006). RND proteins inhibit RhoA-mediated activation of ROCK by binding to a site which prevents RhoA–ROCK interaction. This increase in RND protein expression in rabbit myometrium is functionally coupled to a decrease in agonist- and GTP-induced Ca2+-sensitization during gestation (Cario-Toumaniantz et al., 2003). Moreover, the gestation-related up-regulation of RND2 and RND3 in human myometrium is associated with a loss of MYPT1 phosphorylation at Thr696 (a potential phosphorylation site of ROCK) in the pregnant samples (Lartey et al., 2006), suggesting an increase in the activity of MYLP in pregnancy. Furthermore, we have found that the level of active (GTP-bound) RhoA is elevated in pregnant compared with non-pregnant myometrium, and interestingly this increase is exacerbated in spontaneous preterm labour (Lartey et al., 2007). Additional Rho-effectors such as PKN1 and DIAPH1 are also up-regulated with pregnancy (Lartey et al., 2007). We and others have demonstrated no change in ROCK1 expression with pregnancy or labour (Riley et al., 2005; Lartey et al., 2007), while ROCK2 levels are decreased in pregnant versus non-pregnant myometrial tissue (Riley et al., 2005).
The purpose of this manuscript was to demonstrate phosphorylation of MYPT1 in pregnant human myometrial tissue and to clarify the involvement of ROCK under non- and OXT-stimulated conditions. Measurements of myometrial contractility and preservation of phosphorylation states during contraction and relaxation phases were used to elucidate that functional coupling exists between force, MYL (Thr18/Ser19) and MYPT1 (Thr853) phosphorylation that is ROCK-dependent. We have used ROCK inhibitors and have depleted ROCK1 and ROCK2 isoforms in human myometrial cells to demonstrate that Thr853 is the primary target for ROCK on MYPT1, and that both isoforms contribute to phosphorylation.
Materials and Methods
Tissue collection
This study was approved by the North Somerset and South Bristol Research Ethics Committee and all women gave informed written consent. Myometrium was obtained from the upper border of the incision in women undergoing elective (not in labour) sections at term (37–41 weeks gestation). Indications for Caesarean sections included fetal malposition, previous section and maternal request. Women with signs of infection or who had received syntocinon for labour augmentation were excluded. The tissue was washed in ice-cold isotonic saline and transported to the laboratory where it was used to prepare tissue strips or freshly dispersed myometrial cells.
Materials
The following chemicals and reagents were obtained from the indicated sources: ON-TARGET plus smart pool siRNAs for human ROCK1 (gene Id: 6093), ROCK2 (gene Id: 9475), PKN1 (gene Id: 5585), PKN2 (gene Id: 5586); ON-TARGET plus non-targeting pool siRNA and Dharmafect 1 transfection reagent were purchased from Abgene (Epsom, UK). Collagenase type II, dispase, DMEM, fetal calf serum (FCS) and Alexa Fluor® 488 phalloidin were purchased from Invitrogen (Paisley, UK); elastase and DNAse were purchased from Sigma Aldrich (Poole, UK). ROCK inhibitor glycyl-H-1152-dihydrochloride (g-H-1152) and OXT were purchased from Merck (Darmstadt, Germany). PKC inhibitor bisindolylmaleimide I (BIS1, GF 109203X or Gö 6850) was purchased from Tocris Bioscience (Bristol, UK). Phospho-MYL (Thr18/Ser19), phospho-MYL (Ser19), phospho-MYPT1 (Thr853), phospho-MYPT1 (Thr696), MYPT1 and ROCK1 antibodies were purchased from Cell Signalling Technology (Danvers, MA, USA). MYL and ROCK2 antibodies were purchased from Abcam (Cambridge, UK). All other basic chemicals were supplied by Sigma Aldrich (Poole, UK) unless otherwise stated.
Measurement of myometrial contractility
Contractility measurements were carried out using a four chamber Myobath-II system from World Precision Instruments (WPI, Stevenage, UK). Strips of freshly isolated myometrial tissue were mounted using s-shaped hooks within a 10 ml chamber, and equilibrated in oxygenated Krebs solution (4.7 mM KCl, 1.25 mM MgSO4, 2.5 mM NaH2PO4, 1.25 mM CaCl2, 130 mM NaCl, 25 mM NaHCO3, 11.1 mM glucose and 10 mM Hepes pH 7.6) at 37°C for 45 min with three buffer changes. Hooks were connected to force transducers and data were recorded electronically using the Lab-Trax data acquisition system (WPI). Resting tension was set to 2 g and readjusted during the equilibrium phase, and spontaneous contractions were observed within ∼1 h of the final buffer change. Any strips that failed to contract during this time were excluded from further study. Agonists or inhibitors were added directly to the buffer chamber during experiments.
For g-H-1152 dose–response experiments (Figs 1 and 2), spontaneously contracting myometrial strips were treated with 10 nM OXT until consistent contractions were achieved for a period of 30 min. Cumulative additions of g-H-1152 (1×10−9 M, 1×10−8 M, 1 × 10−7 M, 1 × 10−6 M and 1 × 10−5 M) were added at 20 min intervals and dimethylsulphoxide (DMSO) control (maximum 0.1%) was added to a time-matched control strip present during every experiment. After the final g-H-1152 dose, muscle strips were quickly dismounted from the apparatus following a phasic contraction and rapidly frozen in liquid nitrogen for phosphorylation studies.
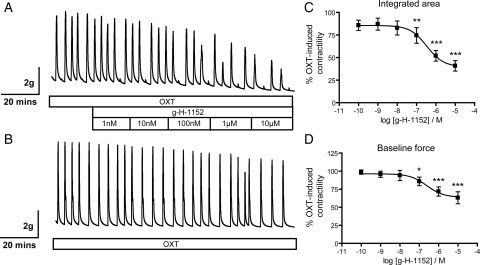
Figure 1.
Inhibition of ROCK with g-H-1152 causes dose-dependent decreases in phasic contractility and baseline force of freshly isolated human myometrium. (A) Cumulative doses of g-H-1152 were added at 20 min intervals to myometrial strips contracting in response to 10 nM OXT. (B) DMSO (maximum 0.1%) was added to time-matched control strips. (C) Integrated area under curve and (D) baseline force were calculated for each 20 min interval, g-H-1152 data were expressed as a percentage of the non-treated values and normalized to those obtained from the control strip. Data points represent mean ± SEM, n = 5; and were fitted to sigmoidal dose–response curves. Statistical significance was determined using repeated-measures ANOVA and Tukey's post hoc test; *P < 0.05, **P < 0.01 and ***P < 0.001 compared with non-treated values.
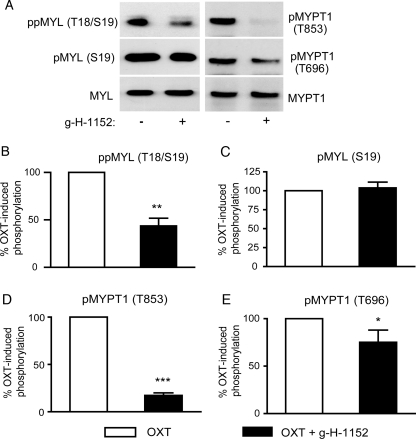
Figure 2.
Phosphorylation of MYL and MYPT1 is reduced in isolated myometrial strips treated with ROCK inhibitor g-H-1152. Myometrial strips treated with 10 nM OXT with and without cumulative additions of g-H-1152 to a maximum of 10 μM (as in Fig. 1), were snap frozen at the end of the experiment during a relaxed phase. (A) Solubilized proteins were subject to SDS–PAGE and immunoblotting for ppMYL (Thr18/Ser19), pMYL (Ser19), MYL, pMYPT1 (Thr853), pMYPT1 (Thr696) and MYPT1.(B–E) Signal from the phospho-specific antibody was normalized to that obtained from the associated total antibody, and expressed as a percentage of the OXT-treated value. Bars represent mean + SEM, and statistical analyses were performed using paired Student's t-tests, *P < 0.05, **P < 0.01, ***P < 0.001, n = 5.
Contractility data were analysed using Data-Trax software (WPI), from which integrated area under curve and minimum (baseline force) were calculated. For g-H-1152 dose–response experiments, data calculated for each dose during a 20 min window were expressed as a percentage of the OXT-induced contractility prior to g-H-1152 or DMSO additions. Data were further normalized using those from the control (DMSO treated) strip to take into account decreases in contractility over the experimental time course. Data were analysed using non-linear regression and fitted with sigmoidal dose–response curves using Prism v4.00 (GraphPad Software, La Jolla, CA, USA). Statistical analysis was carried out using repeated-measures analysis of variance (ANOVA) and Tukey's post hoc tests to compare each dose to the non-treated value.
For experiments comparing phosphorylation in relaxed versus contracting tissue, spontaneously contracting myometrial strips were stimulated with or without 10 nM OXT for 40 min, and for a further 40 min in the presence or in the absence of 1 μM g-H-1152 (Figs 3 and 4). Following these treatments, the strips were rapidly removed from the apparatus at the peak of (contracting), or immediately following (relaxed), a phasic contraction and snap-frozen in liquid nitrogen. The freezing process took an average of 5 s to complete, and the tissue was stored at −80°C until further use. The Myobath II system used in these studies had four chambers and only four conditions could be compared simultaneously. The experiments on spontaneously contracting tissue and OXT-stimulated tissue are therefore independent and are presented as such, each being carried out with tissue from seven different women.
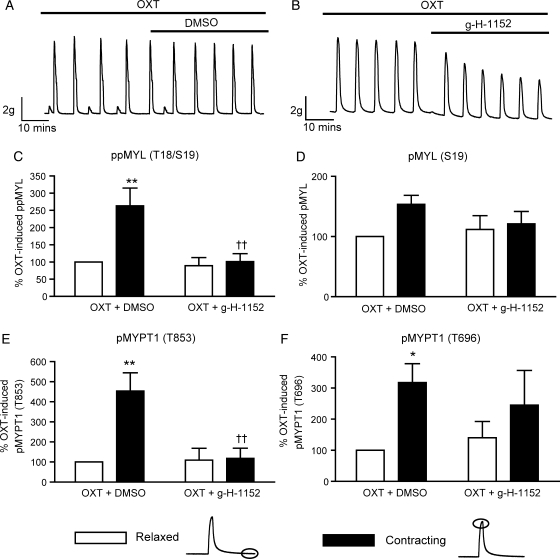
Figure 3.
OXT-stimulated phasic contractions of freshly isolated myometrium are associated with ROCK-dependent increases in phosphorylation of MYL (Thr18/Ser19) and MYPT1 (Thr853). Spontaneously contracting myometrial strips were treated with 10 nM OXT for 40 min and for a further 40 min in the presence or in the absence of 1 μM g-H-1152 (A and B). Strips were subsequently snap-frozen at the peak of (contracting), or immediately following (relaxed), a phasic contraction. Solubilized proteins were subjected to SDS–PAGE and immunoblotting. Signals from phospho-specific antibodies were normalized to those obtained from their associated total antibodies, and data were expressed as a percentage of the control value (OXT, relaxed). (C) ppMYL (Thr18/Ser19) levels, (D) pMYL (Ser19) levels, (E) pMYPT1 (Thr853) levels, (F) pMYPT1 (Thr696) levels; bars represent mean + SEM, n = 7. Data were analysed using two-way ANOVA and Bonferroni post hoc tests to determine both the contribution of contraction and of g-H-1152 treatment. *P < 0.05, **P < 0.01 for the effect of contraction, ††P < 0.01 for the effect of g-H-1152 on contracting tissue.
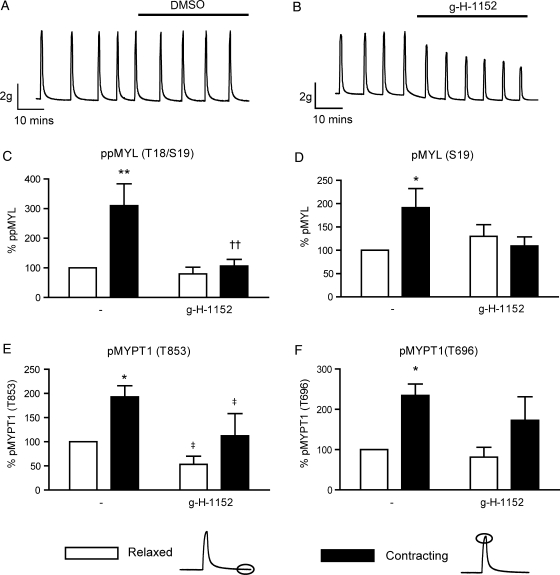
Figure 4.
Spontaneous phasic contractions of freshly isolated myometrium are associated with ROCK-dependent increases in phosphorylation of MYL (Thr18/Ser19) and MYPT1 (Thr853). Spontaneously contracting myometrial strips were treated with or without 1 μM g-H-1152 for 40 min (A and B). Strips were subsequently snap-frozen at the peak of (contracting), or immediately following (relaxed), a phasic contraction. Solubilized proteins were subjected to SDS–PAGE and immunoblotting. Signals from phospho-specific antibodies were normalized to those obtained from their associated total antibodies, and data were expressed as a percentage of the control value (OXT, relaxed). (C) ppMYL (Thr18/Ser19) levels, (D) pMYL (Ser19) levels, (E) pMYPT1 (Thr853) levels, (F) pMYPT1 (Thr696) levels; bars represent mean + SEM, n = 7. Data were analysed using two-way ANOVA (‡P < 0.05 for the overall effect of g-H-1152) and Bonferroni post hoc tests to determine both the contribution of contraction (*P < 0.05 and **P < 0.01) and of g-H-1152 treatment on contracting tissue (††P < 0.01).
Tissue homogenization
Frozen tissue strips were homogenized in buffer (25 mM Tris pH 6.8, 2% SDS, 10% glycerol, 50 mM β-glycerophosphate and 1 μg/ml each pepstatin, leupeptin and antipain) at ∼100 mg/ml using a Polytron homogenizer at 20°C. Lysates were immediately heated to 95°C for 5 min, and cleared by centrifugation at 16 000g for 10 min at 20°C. Protein concentration was determined using the BCA assay kit (Perbio Science, Cramlington, UK) before 12.5 mM dithiothreitol and 0.002% bromophenol blue were added prior to electrophoresis.
Myometrial cell culture
Myometrial tissue was digested in serum-free Dulbecco's Modified Eagle's medium (DMEM) containing 300 U/ml collagenase (type II), 0.3 U/ml dispase, 30 U/ml DNase I and 0.09 U/ml elastase at 37°C for 3 h with shaking. Liberated smooth muscle cells were then grown in adherent cell culture with DMEM containing 10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C. Experiments were carried out by plating cells of passage six or lower at 2.7 × 104/cm2 in 12- or 24-well plates and growing to confluency over 3 days. Cells were serum-starved for 4 h prior to experiments, and after treatment were washed with phosphate-buffered saline (PBS), lysed directly in boiling SDS–PAGE sample buffer (25 mM Tris pH 6.8, 10% glycerol, 2% SDS, 12.5 mM dithiothreitol and 0.002% bromophenol blue), and used for immunoblotting as described below.
Fluorescence staining of stress fibres
Cells were seeded on glass coverslips at 1.4 × 104/cm2 and grown overnight. Cells were starved for 4 h before being treated with 1 µM g-H-1152 or 2 µM BIS1 for 40 min. Cells were washed in PBS and fixed with 3.7% paraformaldehyde for 15 min at room temperature. After fixation, coverslips were washed 3 × 5 min with PBS and permeabilized with 0.1% Triton-X 100 in PBS for 15 min. Cells were then blocked with 1% bovine serum albumin (BSA) in PBS for 20 min. Filamentous actin was stained with Alexa Fluor 488® phalloidin (Invitrogen, Paisley, UK) at ∼165 nM with 1% BSA in PBS. Staining was performed for 20 min at room temperature protected from light. The coverslips were washed 3 × 5 min with PBS and subsequently mounted onto slides with Vectashield mounting medium containing DAPI (Vector Laboratories Ltd, Peterborough, UK) to stain nuclei, prevent rapid fading and allow short-term storage. Filamentous actin pools were visualized with an upright fluorescence microscope (Leica DMRB; Leica, Wetzlar, Germany) and images were captured with a Leica DFC340FX camera and processed using Leica Application Suite 3.3.1.
siRNA-mediated knockdown of ROCK, ROCK2, PKN1 and PKN2
Myometrial cells grown to 50% confluency were incubated in the presence of 25 nM siRNA (ROCK1, ROCK2, PKN1, PKN2 or non-targeting) and 3% (v/v) Dharmafect 1 reagent in DMEM/FCS for 72 h. Cells were lysed as above and protein knockdown verified by immunoblotting with ROCK1, ROCK2, PKN1 and PKN2 antibodies.
SDS–PAGE, immunoblotting and quantification of immuno bands
Cell or tissue lysates (15 or 50 μg protein, respectively) were resolved using 10% Bis-Tris gels (357 mM Bis-Tris pH 6.75, 10% acrylamide, 0.27% bis-acrylamide) and MOPS running buffer (250 mM MOPS, 250 mM Tris, 5 mM EDTA, 0.5% SDS and 5 mM sodium bisulfite). Proteins were transferred to polyvinylidene difluoride membrane (GE Healthcare, Buckinghamshire, UK) by wet transfer at 80 mA for 1 h in a buffer containing 25 mM Tris, 192 mM glycine, 10% methanol and 0.01% SDS. Membranes were blocked in 5% BSA in Tris-buffered saline plus 0.1% Tween-20 (TBS-T), before incubation with primary antibodies (1 μg/ml in 5% BSA/TBS-T) overnight at 4°C. After washing 6 × 5 min in TBS-T, membranes were incubated with a horseradish peroxidase-conjugated goat anti-rabbit antibody (Cell Signalling Technology, Danvers, MA, USA; 1:5000) in TBS-T for 1 hour at room temperature. Membranes were washed for a further 6 × 5 min and bands visualized using an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, UK). Membranes were probed initially with phospho-specific antibodies and re-probed with the equivalent total antibody after incubating for 3 h in stripping buffer (20 mM glycine and 1% SDS, pH 2). Bands were quantified by densitometry using Quantity One software (Bio-Rad, Hemel Hempstead, UK), and care was taken to avoid saturation of signals. Raw data were normalized using a control for each experiment, and the level of phosphorylation was always normalized using the level of equivalent total protein.
Results
ROCK inhibitor g-H-1152 causes dose-dependent reductions in OXT-stimulated phasic contractility of freshly isolated human myometrial strips
Glycyl-H-1152-dihydrochloride (g-H-1152) is a glycated isoquinoline derived from the ROCK inhibitor HA-1077 (Fasudil), and is a cell permeable, reversible and ATP-competitive inhibitor of ROCK1/2. It shows greater potency than both HA-1077 and the commonly used ROCK inhibitor Y27632 (IC50 for ROCK2 = 11.8 versus 158 and 220 nM, respectively) and shows 100-fold selectivity for ROCK over other protein kinases such as PKA and PKC (Ishizaki et al., 2000; Tamura et al., 2005). While others have shown previously that ROCK inhibitor Y27632 can reduce spontaneous and OXT-induced contractility of human myometrium in vitro (Kupittayanant et al., 2001; Moran et al., 2002; Woodcock et al., 2004), the effect of g-H-1152 on contractility has yet to be investigated. Representative contractility traces of g-H-1152-treated and time-matched DMSO-treated control strips are shown in Fig. 1A and B. When added to OXT-stimulated myometrial strips, g-H-1152 caused dose-dependent reductions in phasic contractility (determined by integrated area) and in baseline force, with statistically significant reductions seen from 100 nM g-H-1152 (Fig. 1C and D, P < 0.01 and P < 0.05, respectively). The net contractility remaining at the highest dose used, 10 µM H-1152, was 31.8 ± 6.3% for integrated area (P < 0.001) and 51.6 ± 6.1% for baseline force (P < 0.001).
The relaxing effect of g-H-1152 is associated with reductions in MYL and MYPT1 phosphorylation in freshly isolated myometrial tissue
In order to analyse tension–phosphorylation relationships, g-H-1152-treated myometrial strips (from Fig. 1) were snap-frozen after contractility had been measured, and phosphorylation of MYL and MYPT1 was analysed by immunoblotting. Phospho-specific antibodies were used to detect phosphorylation of MYPT1 at Thr696 or Thr853; and phosphorylation of MYL was detected using two separate antibodies: one for Ser19 and another for dual phosphorylation at both Thr18 and Ser19. Phosphorylation of Ser19 requires less MYLK activity and hence precedes phosphorylation at Thr18 (Ikebe and Hartshorne, 1985), but the latter increases myosin ATPase activity beyond that of Ser19 alone (Ikebe et al., 1988). A 56.4 ± 8.2% reduction in ppMYL (Thr18/Ser19) was measured in strips treated with 10 μM g-H-1152 when compared with non-treated strips (P < 0.01); however, there was no significant decrease in phosphorylation of MYL at the Ser19 site alone (Fig. 2B and C). This finding indicates that ROCK influences the phosphorylation of Thr18 to a greater extent than Ser19. To investigate the possibility that ROCK-mediated MYPT1 phosphorylation and subsequent MYLP inhibition contribute to MYL phosphorylation in myometrial tissue, phosphorylation of MYPT1 was monitored. Phosphorylation at Thr853, and to a lesser extent at Thr696, was significantly reduced by g-H-1152 (82.7 ± 2.6% reduction, P < 0.001 and 24.9 ± 12.8% reduction, P < 0.05, respectively; Fig. 2D and E). These data indicate that ROCK is the predominant kinase responsible for phosphorylating MYPT1 at Thr853, while other Thr696 kinases may exist in human myometrial tissue. Furthermore, as g-H-1152-treated strips showed reduced contractility, the data demonstrate a positive relationship between phosphorylation of MYL, MYPT1 and force in fresh myometrial tissue.
Phosphorylation of MYL and MYPT1 increases during OXT-stimulated phasic myometrial contractions in vitro
The tension–phosphorylation relationship was explored further by freezing myometrial tissue during the peak of a contraction or during a relaxation phase. These studies were carried out on strips stimulated with 10 nM OXT, and in the presence or absence of 1 µM g-H-1152. Contractility traces showing the relaxing effect of 1 µM g-H-1152 are shown in Fig. 3A and B. Table I describes the absolute tension recorded during relaxed and contracting phases of OXT-stimulated strips treated with either g-H-1152 or DMSO vehicle control, highlighting the function of the strips at the time of freezing. The phosphorylation of MYL and MYPT1 measured in these samples is quantified in Fig. 3C–F. OXT-induced phasic contractions were associated with significant increases in MYL (Thr18/Ser19), MYPT1 (Thr853) and MYPT (Thr696) phosphorylation (2.54 ± 0.61-fold, P < 0.01; 4.21 ± 1.23-fold, P < 0.01 and 2.98 ± 0.57-fold, P < 0.01, respectively, n = 7). There was evidence for contraction-induced increases in pMYL (Ser19) but the effect failed to reach statistical significance. Interestingly, the contraction-induced increases in ppMYL (Thr18/Ser19) and pMYPT (Thr853) were significantly attenuated in strips incubated with 1 µM g-H-1152 (P < 0.01, Fig. 3C and E), while the increases in pMYPT1 (Thr696) were not. This finding suggests a discrepancy between the affinity of ROCK for Thr853 and Thr696 of MYPT1 in human myometrium, and shows that dynamic changes in MYPT phosphorylation occur during contractions. Furthermore, the data indicate that phosphorylation at Thr18, resulting in dual phosphorylation of MYL, is more prominent during phasic contractions than phosphorylation of MYL at Ser19 alone.
Table I.
Tension of strips measured during the relaxed or contracting phases.
| Treatment | Tension (g) |
|||
|---|---|---|---|---|
| Pretreatment |
Post-treatment |
|||
| Relaxed | Contracting | Relaxed | Contracting | |
| OXT + DMSO | 1.47 ± 0.12 | 8.20 ± 1.08* | 1.82 ± 0.15 | 8.00 ± 1.0* |
| OXT + g-H-1152 | 1.91 ± 0.22 | 8.21 ± 1.05* | 1.22 ± 0.15 | 4.90 ± 0.79*† |
| DMSO | 1.53 ± 0.11 | 6.51 ± 0.71* | 1.47 ± 0.12 | 6.23 ± 0.75* |
| g-H-1152 | 1.51 ± 0.15 | 5.85 ± 0.40* | 1.11 ± 0.10 | 3.39 ± 0.37*† |
Results are means ± SEM, n = 7 for both spontaneous and OXT-treated experiments. *P < 0.001 and †P < 0.001 for the effect of contraction and treatment respectively, as determined by two-way ANOVA and Bonferroni post hoc tests.
Phosphorylation of MYL and MYPT1 also increases during spontaneous phasic myometrial contractions
The studies were extended to non-stimulated, spontaneously contracting strips. Contractility traces showing the relaxing effect of 1 µM g-H-1152 are shown in Fig. 4A and B. Table I describes the absolute tension recorded during relaxed and contracting phases of strips treated with either g-H-1152 or DMSO vehicle control. The phosphorylation of MYL and MYPT1 measured in these samples is quantified in Fig. 4C–F. Spontaneous phasic contractions were associated with significant increases in MYL (Thr18/Ser19), MYL (Ser19), MYPT1 (Thr853) and MYPT (Thr696) phosphorylation (3.10 ± 0.74-fold P < 0.01; 1.91 ± 0.41-fold, P < 0.05; 1.93 ± 0.23-fold, P < 0.05 and 2.35 ± 0.28-fold, P < 0.05, respectively, n = 7). The contraction-induced increases in ppMYL (Thr18/Ser19) were significantly attenuated in strips incubated with 1 µM g-H-1152 (P < 0.01, Fig. 3D), comparable to the effect in OXT-stimulated strips. The overall effect of g-H-1152 on pMYPT (Thr853) in these experiments was significant (two-way ANOVA; P < 0.05, Fig. 4E), but the inhibitory effect of g-H-1152 was smaller than in OXT-stimulated tissue. Comparing data from Figs 3 and 4, OXT caused a greater fold effect on contraction-induced MYPT (Thr696 and Thr853) phosphorylation than spontaneous contractions alone, while the effects on MYL phosphorylation were similar in OXT-stimulated and non-stimulated tissue. This observation was verified when spontaneous and OXT-stimulated contractions were studied simultaneously with the same tissue samples in a separate set of experiments (data not shown).
g-H-1152-induced reductions in MYL and MYPT1 phosphorylation in cultured myometrial cells
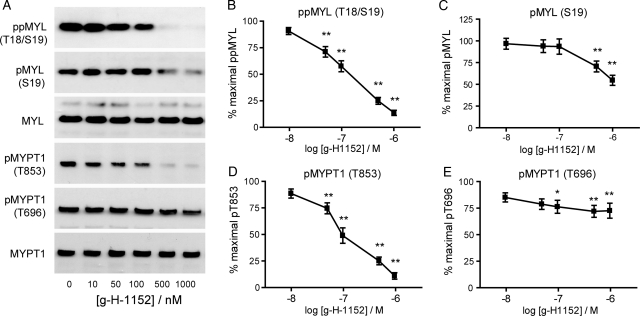
Studies from tissue were extended to cultured myometrial cells to investigate the involvement of ROCK in more detail. As shown in Fig. 5, g-H-1152 caused a dose-dependent decrease in ppMYL (Thr18/Ser19) with significant effects from 50 nM (P < 0.01) and a maximal 85.6 ± 3.5% reduction at 1 μM g-H-1152, P < 0.01 (Fig. 5B). The effects on MYL (Ser19) phosphorylation were less potent; phosphorylation was significantly decreased after incubation of cells with 500 nM g-H-1152 (P < 0.01), with a maximal reduction at 1 μM of 45.3 ± 5.9%, P < 0.01 (Fig. 5C). Figure 5D depicts a dose-dependent decrease in pMYPT1 (Thr853) with a significant reduction from 50 nM g-H-1152 (P < 0.01) and a maximal decrease of 86.5 ± 2.9% at 1 μM g-H-1152 (P < 0.01); however, ROCK inhibition caused a weaker reduction (maximum 24.2 ± 7.2%, P < 0.01) in phosphorylation at Thr696 (Fig. 5E).
Figure 5.
ROCK inhibitor g-H-1152 causes dose-dependent decreases in MYL and MYPT1 phosphorylation in cultured myometrial cells. Resting myometrial cells were treated for 40 min with g-H-1152 at the concentrations shown; 0.1% DMSO was added to control wells. (A) Cell lysates were subjected to immunoblotting for total and phosphorylated MYL and MYPT1, representative blots are shown. Signal from the phospho-specific antibody was normalized to that obtained from the associated total antibody, and was expressed as a percentage of the non-treated value. (B) ppMYL (Thr18/Ser19) levels; (C) pMYL (Ser19) levels; (D) pMYPT1 (Thr853) levels and (E) pMYPT1 (Thr696) levels. Data points represent mean ± SEM, n = 11. Statistical significance was determined using one-way ANOVA and Dunnett's post hoc test; *P < 0.05 and **P < 0.01 compared with non-treated cells.
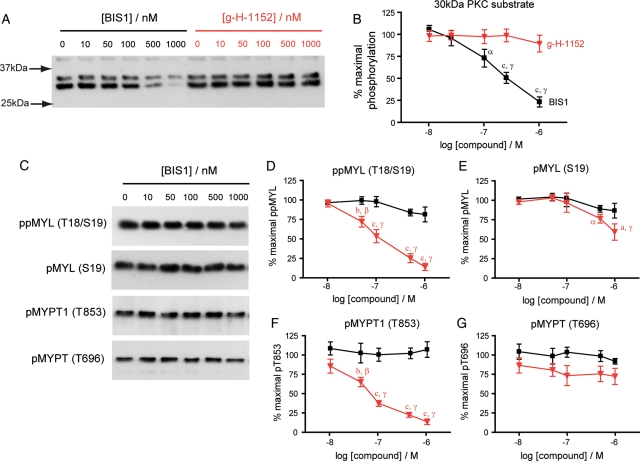
g-H-1152 does not attenuate PKC signalling in cultured myometrial cells, and PKC inhibition causes no significant decreases in MYL or MYPT1 phosphorylation
PKC is capable of phosphorylating CPI-17 at Thr38 leading to inhibition of MYLP (Eto et al., 1995; Kitazawa et al., 2000), therefore it could also contribute indirectly to increasing phosphorylated MYL levels in myometrial cells. During pregnancy there is increased sensitivity of human myometrial tissue to PKC inhibitors that decrease spontaneous and OXT-induced contractions (Ozaki et al., 2003; Yasuda et al., 2007). To examine the possibility that g-H-1152 may be having off target effects through interference of PKC signalling, we measured phosphorylation of a 30 kDa PKC protein substrate likely to be S6 ribosomal protein (Zhang et al., 2002). Dose–response studies with g-H-1152 and the PKC inhibitor bisindolylmaleimide I (BIS1 or GF109203X) were run in parallel, and while BIS1 clearly inhibited PKC activity from 100 nM (P < 0.05), g-H-1152 did not affect PKC-dependent phosphorylation up to 1 µM (Fig. 6A and B). Furthermore, BIS1 failed to inhibit phosphorylation of MYL (Ser19 or Thr18/Ser19) or MYPT1 (Thr853 or Thr696), whereas g-H-1152 had strong inhibitory effects, as expected (Fig. 6C–G). The clear discrepancies between the effects of g-H-1152 and BIS1 suggest that g-H-1152 is unlikely to be mediating its inhibitory effects on MYL or MYPT phosphorylation through altering PKC activity.
Figure 6.
g-H-1152 does not attenuate PKC signalling and inhibition of conventional PKC isoforms causes no significant decrease in MYL or MYPT1 phosphorylation. Resting myometrial cells were treated for 40 min with either bisindolylmaleimide I (BIS1) or g-H-1152 at the concentrations shown; 0.1% DMSO was added to control wells. Both compounds were used in tandem during each experiment. Cell lysates were subjected to immunoblotting using a PKC substrate antibody (A) and antibodies to detect total and phosphorylated MYL and MYPT1 (C), representative blots are shown. Signals from phospho-specific antibodies were normalized to those obtained from the associated total antibody, and were expressed as a percentage of the non-treated value. (B) 30 kDa PKC substrate phosphorylation; (D) ppMYL (Thr18/Ser19) levels; (E) pMYL (Ser19) levels; (F) pMYPT1 (Thr853) levels and (G) pMYPT1 (Thr696) levels. Data points represent mean ± SEM, n = 5, black: BIS1 and red: g-H-1152. Statistical significance was determined using two-way ANOVA and Bonferroni post hoc tests to compare both the effect of one compound against the other (a: P < 0.05, b: P < 0.01 and c: P < 0.001) and the effect of increasing dose (α: P < 0.05, β: P < 0.05 and γ: P < 0.001 compared with non-treated cells).
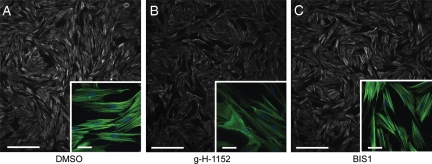
ROCK inhibition with g-H-1152 causes disassembly of stress fibres in cultured myometrial cells
Inhibition of ROCK with Y27632 has previously been shown to inhibit stress fibre formation in cultured myometrial cells (Gogarten et al., 2001), therefore the effect of g-H-1152 on stress fibres in our myometrial cultures was investigated. In resting cells, stress fibres can be seen to traverse the cells in parallel bundles as detected by F-actin staining (Fig. 7A). Treatment of cells with 1 µM g-H-1152 caused an overall reduction in F-actin staining and a noticeable decrease in individual stress fibres (Fig. 7B). In contrast, the PKC inhibitor BIS1 had no effect on F-actin staining or stress fibre morphology (Fig. 7C).
Figure 7.
ROCK inhibitor g-H-1152 causes disassembly of actin stress fibres in cultured myometrial cells. Representative examples of filamentous actin (F-actin) staining with Alexa Fluor 488® -phalloidin in resting myometrial cells; (A) in the absence of inhibitors or (B) in the presence of 1 µM ROCK inhibitor g-H-1152 or (C) 2 µM PKC inhibitor bisindolylmaleimide I (BIS1). Nuclear DNA was detected with DAPI (blue in insets). Wide-field images show decreases in overall F-actin staining (white) with ROCK inhibition, and insets highlight the parallel stress fibre bundles (green) in cells which decrease in the presence of ROCK inhibitor g-H-1152. Scale bars = 500 and 50 µm in insets.
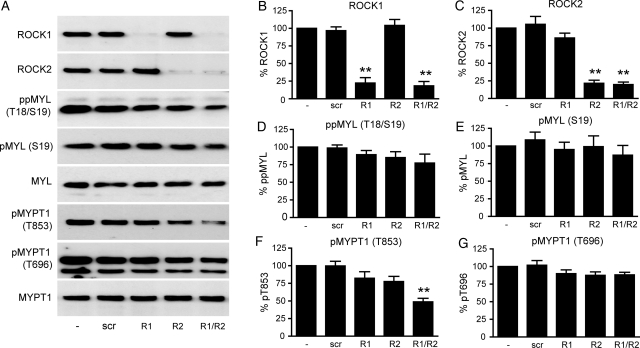
siRNA-mediated knockdown of both ROCK1 and ROCK2 results in a reduction in MYPT1 phosphorylation at Thr853 in cultured myometrial cells
siRNAs targeting both ROCK1 and ROCK2 were used individually and in combination to lower ROCK expression in cultured myometrial cells, and an 80% reduction in ROCK1/2 protein expression was achieved as demonstrated by immunoblotting (Fig. 8A–C). Knockdown of both ROCK isoforms was accompanied by a significant reduction in pMYPT1 (Thr853) (51.2 ± 5.0%, P < 0.01, n = 8) and modest decreases in ppMYL (Thr18/Ser19), pMYL (Ser19) and pMYPT1 (Thr696) that did not reach statistical significance (Fig. 8D, E and G). These data support the results obtained in fresh tissue and confirm that Thr853 is the primary site of ROCK-mediated MYPT1 phosphorylation, and strongly suggest that both ROCK isoforms are involved.
Figure 8.
siRNA knockdown of ROCK1 and ROCK2 in cultured myometrial cells causes a significant decrease in MYPT1 phosphorylation at Thr853. Sub-confluent myometrial cells were transfected with 25 nM siRNA targeted for ROCK1 (R1), ROCK2 (R2), both (R1/R2) or non-targeting (scr) and grown for a further 72 h. (A) Cell lysates were subjected to immunoblotting for ROCK1, ROCK2, total and phosphorylated MYL and MYPT1, representative blots are shown. Signals from the phospho-specific antibody when used were normalized to those obtained from the associated total antibody, and data were expressed as a percentage of the non-transfected value. (B) ROCK1 levels; (C) ROCK2 levels; (D) ppMYL (Thr18/Ser19) levels; (E) pMYL (Ser19) levels; (F) pMYPT1 (Thr853) levels and (G) pMYPT1 (Thr696) levels. Bars represent mean + SEM, n = 8. Statistical significance was determined using one-way ANOVA and Dunnett's post hoc test; **P < 0.01 compared with non-transfected cells.
siRNA-mediated knockdown of Rho-effectors PKN1 or PKN2 does not affect MYL or MYPT phosphorylation in cultured myometrial cells
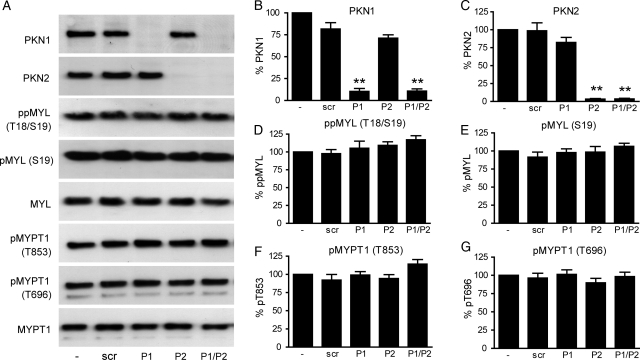
Data from experiments utilizing g-H-1152 have been supported by ROCK1/2 knockdown, and we have excluded the possibility that g-H-1152 interferes with PKC activity. PKN1 and PKN2 are members of the PKC superfamily and can be activated by Rho-family members. We therefore investigated the involvement of PKN1/2 in MYL and MYPT1 phosphorylation by siRNA-mediated knockdown. Expression of PKN1 and PKN2 proteins was reduced by ∼90 and 96%, respectively, both individually and in combination, as demonstrated by immunoblotting (P < 0.01, Fig. 9A–C). However, the loss of PKN proteins was not associated with any significant changes in MYL or MYPT1 phosphorylation (Fig. 9D–G).
Figure 9.
siRNA knockdown of PKN1 and PKN2 in cultured myometrial cells has no effect on MYL or MYPT1 phosphorylation. Sub-confluent myometrial cells were transfected with 25 nM siRNA targeted for PKN1 (P1), PKN2 (P2), both (P1/P2) or non-targeting (scr) and grown for a further 72 h. (A) Cell lysates were subjected to immunoblotting for PKN1, PKN2, total and phosphorylated MYL and MYPT1, representative blots are shown. Signals from the phospho-specific antibody when used were normalized to those obtained from the associated total antibody, and data were expressed as a percentage of the non-transfected value. (B) PKN1 levels; (C) PKN2 levels; (D) ppMYL (Thr18/Ser19) levels; (E) pMYL (Ser19) levels; (F) pMYPT1 (Thr853) levels and (G) pMYPT1 (Thr696) levels. Bars represent mean + SEM, n = 6. Statistical significance was determined using one-way ANOVA and Dunnett's post hoc test; **P < 0.01 compared with non-transfected cells.
Discussion
Inhibition of MYLP and the consequent decrease in MYL dephosphorylation have been proposed as primary mechanisms of Ca2+-sensitization in smooth muscle (Somlyo and Somlyo, 1994). OXT provokes Ca2+-sensitization in myometrium (Woodcock et al., 2004), however, phosphorylation of the sites on MYPT1 documented to cause auto-inhibition of the phosphatase (Thr696 and Thr853) has not been demonstrated previously in fresh human myometrial tissue. In the present study, we used phosho-specific antibodies to define the sites of MYPT1 phosphorylation in fresh tissue and cultured myometrial cells and have employed the inhibitor g-H-1152 to elucidate the role of ROCK. This study reveals that inhibition of ROCK with g-H-1152 reduces spontaneous and OXT-stimulated myometrial contractility and this reduction is associated with decreased phosphorylation of MYL and MYPT1 in fresh myometrial tissue. Moreover, we report for the first time a positive correlation between myometrial force and MYPT1 phosphorylation, and show that increased phosphorylation of MYL and MYPT1 (in particular on Thr18 and Thr853, respectively) during phasic contractions is ROCK-dependent. The fact that phosphorylation of MYPT1 (Thr853) changes so dynamically with each contraction is a very interesting finding that could only be detected with this type of experimental approach.
The effect of the ROCK inhibitor g-H-1152 on human myometrial contractility in vitro has not been tested previously. Here, it caused dose-dependent reductions in integrated area of phasic contractions and baseline force of OXT-stimulated myometrial strips. This supports other studies using an alternative ROCK inhibitor, Y27632, which reported decreases in amplitude and integrated area of OXT-induced contractions (Moran et al., 2002; Woodcock et al., 2004). However, other studies have suggested that inhibition of ROCK with Y27632 only affects force when the myometrium is stimulated tonically after depolarization with high K+, and has little effect on OXT-stimulated phasic contractions (Kupittayanant et al., 2001). It is important to note that we found significant decreases in integrated area (which is a robust overall assessment of contractions as it combines information about amplitude, rate and duration) when we used g-H-1152 at 100 nM, only 10-fold higher than the reported IC50 for ROCK2 (Tamura et al., 2005). At this concentration, g-H-1152 is unlikely to affect other kinases such as PKC or MYLK, and these data suggest that ROCK activity contributes to OXT-induced phasic contractility of myometrial tissue in vitro, and would support a role for ROCK during labour in vivo. Previous findings from our laboratory indicating an increase in Rho-GTP with spontaneous labour (Lartey et al., 2007) also support a role for augmented Rho-ROCK signalling during phasic myometrial contractions at the onset of labour.
Interestingly, when phosphorylation was examined in resting strips, ROCK inhibition with g-H-1152 was associated with decreases in MYL and MYPT1 phosphorylation, with pMYPT1 (Thr853) more affected than pMYPT1 (Thr696). Baseline force was concomitantly reduced by nearly 40% in these samples, thus we have demonstrated for the first time a positive correlation between force and MYPT1 phosphorylation in human myometrial tissue.
Exploration of the relationship between contractility and phosphorylation was extended when myometrial strips were frozen either during or between spontaneous and OXT-stimulated phasic contractions. This methodology has proven successful in a recent paper describing contraction-related increases in phosphorylation of high molecular weight caldesmon (h-CaD) and extracellular signal-regulated kinase 1/2 (ERK 1/2) (Paul et al., 2011). In our study, phosphorylation of MYL (Thr18/Ser19) increased during OXT-induced contractions in a ROCK-dependent manner, and the results with g-H-1152 suggest that ROCK activity is required for phosphorylation of MYL at Thr18 predominantly. Phosphorylation of MYPT1 (Thr853) followed a similar pattern, therefore providing evidence that MYL and MYPT1 phosphorylation are functionally coupled with phasic contractility of myometrial strips in vitro, and that ROCK activity is required. Phosphorylation of MYL and MYPT also increased during spontaneous contractions, and contraction-induced pMYL (Thr18/Ser19) showed significant ROCK dependency.
The ability of g-H-1152 to preferentially inhibit phosphorylation of MYPT1 at Thr853 over Thr696 in cultured myometrial cells indicates that basal ROCK activity is sufficient for maintaining MYPT1 phosphorylation at Thr853 under non-stimulated conditions. It appears that a combination of kinases including ROCK are responsible for phosphorylating MYPT1 at Thr696, and further work is required to elucidate the identity of these. The observation that significant decreases in MYPT1 (Thr853) and MYL (Thr18/Ser19) phosphorylation were observed at only 50 nM g-H-1152 reduces the likelihood that non-specific effects of the inhibitor were responsible. The possibility that g-H-1152 significantly affects PKC activity was ruled out by its inability to reduce phosphorylation of a PKC substrate. On the other hand, an inhibitor of conventional PKC isoforms (bisindolylmaleimide I) effectively decreased PKC substrate phosphorylation, while being unable to inhibit MYL or MYPT phosphorylation.
A simultaneous 80% knockdown of ROCK1 and ROCK2 isoforms was achieved in cultured human myometrial cells using siRNA and this was associated with a significant reduction in MYPT1 phosphorylation at Thr853. This supports the hypothesis that Thr853 is the preferred site for ROCK, and that both ROCK1 and ROCK2 isoforms are involved. The reductions in MYPT1 and MYL phosphorylation were less marked after ROCK knockdown in comparison to g-H-1152 treatment, and this discrepancy is likely due to the incomplete silencing of ROCK by siRNA methodology, with 20% ROCK1/2 protein remaining. However, we cannot rule out the possibility that g-H-1152 is inhibiting kinases other than ROCK, thereby exacerbating its effects over those of siRNA knockdown. PKN1 and PKN2 were proposed as candidates for such potential off-target effects of g-H-1152 for the following reasons: they are also Rho-activated kinases; PKN2 was partially inhibited by the non-glycyl H-1152 in an in vitro assay (Bain et al., 2007); and we have shown PKN1 is up-regulated in pregnancy (Lartey et al., 2007). However, successful siRNA-mediated knockdown of both PKN1 and PKN2 individually and in combination had no measurable effect, and suggests that these kinases are not involved in maintaining resting levels of MYPT or MYL phosphorylation in cultured myometrial cells. This result further supports the specificity of g-H-1152 for ROCK.
When Thr853 is phosphorylated, the catalytic activity of MYLP is inhibited by a process of auto-inhibition (Khromov et al., 2009) resulting in increased phosphorylation of MYL. Inhibition of phosphatase activity following phosphorylation of MYPT1 is likely to contribute to increased phosphorylation of MYL and phasic contractility as we have reported dynamic changes in pMYPT (Thr853) during contractions. Moreover, phosphorylated CPI-17 (PPP1R14A), a substrate for PKC, could also inhibit MYLP, but our data using the PKC inhibitor bisindolylmaleimide 1 suggest that PKC-mediated pathways do not play a role in maintaining phosphorylated MYL levels in myometrial cells. However, ROCK-dependent phosphorylation and activation of CPI-17 at Thr38 has been demonstrated in smooth muscle after agonist stimulation (Pang et al., 2005; Patil and Bitar, 2006). Thus, the regulation of CPI-17 by ROCK in the myometrium warrants further study.
While the contribution to myometrial contractility of ROCK-dependent MYLP inhibition is significant, its role is likely to be complementary to the substantial contribution of Ca2+-MYLK signalling (Wray et al., 2001). Inhibition of MYLK with wortmannin can completely ablate both spontaneous and OXT-induced contractions of human myometrium, highlighting the importance of Ca2+-MYLK activity (Longbottom et al., 2000), however, this finding does not exclude the possibility of ROCK-dependent Ca2+-sensitization in conjunction with Ca2+-MYLK signalling. The significant effect of g-H-1152 in the current study suggests that MYL phosphorylation is elevated during phasic contractions by both activation of MYLK and by ROCK-dependent mechanisms, including inhibition of phosphatase activity. However, without simultaneous measurements of [Ca2+]i and force we cannot be sure that g-H-1152 treatment is blocking Ca2+-sensitization. Uncoupling of [Ca2+]i and force has been demonstrated by others in response to OXT; there is evidence that the Ca2+-sensitizing effects of OXT are ROCK-dependent (Woodcock et al., 2004) and that they occur during the falling phase of the contraction (McKillen et al., 1999). Interestingly, the Ca2+-force relationship evoked by OXT is the same in myometrium taken before or after the onset of labour (McKillen et al., 1999).
Throughout our studies, inhibition of ROCK was less effective at decreasing pMYL (Ser19) than ppMYL (Thr18/Ser19), suggesting that phosphorylation of Thr18 shows greater ROCK dependency than phosphorylation of Ser19. If the action of ROCK is solely mediated via phosphatase inhibition then this discrepancy can be explained by concluding that MYLP acts preferentially on Thr18 of MYL. Alternatively, these data suggest that ROCK is capable of phosphorylating Thr18 of MYL directly during contractions, providing a ROCK-dependent but phosphatase-independent mechanism of force generation. In vitro phosphorylation of MYL by ROCK, primarily at Ser19, has been described (Amano et al., 1996; Ueda et al., 2002), and ROCK causes contraction of stress fibres isolated from human fibroblasts (Katoh et al., 2001). It is possible that ROCK-mediated phosphorylation of Thr18 occurs following Ca2+-dependent, MYLK-mediated phosphorylation of Ser19; however, the current data are limited and these possibilities require further investigation. Nevertheless, our data indicate that phosphorylation of MYL at Thr18 is more dynamically regulated during phasic contractions than phosphorylation of Ser19, and this supports an important role for MYL (Thr18) phosphorylation in human myometrium.
In summary, our findings provide strong evidence for ROCK-dependent phosphorylation of MYL (Thr18) and MYPT1 (Thr853) during spontaneous and OXT-stimulated phasic contractions of fresh human myometrial tissue in vitro. Pharmacological inhibition of ROCK is associated with decreases in OXT-stimulated myometrial contractility as determined by integrated area of phasic contractions and baseline force, strongly suggesting that ROCK-mediated inhibition of phosphatase activity makes an important contribution to the regulation of contractility in human myometrium. These data increase our understanding of the control of human myometrial activity and warrant further studies to investigate whether aberrations in ROCK signalling may contribute to increased contractility in preterm labour.
Authors' roles
C.A.H. involved in design, data aquisition and analysis and drafting of manuscript. K.J.H. and A.L.B. contributed in design, interpretation of data and revisions to manuscript.
Funding
This work was supported by the Wellcome Trust (083902/Z to A.L.B. and K.J.H.). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Conflict of interest
None declared
Acknowledgements
We are grateful for the help of research midwife Alison Kirby for obtaining informed consent from women at St Michael's hospital, Bristol, and collecting samples of myometrial tissue.
References
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. doi:10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. doi:10.1074/jbc.M201597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman MA, MacDonald JA, Muranyi A, Hartshorne DJ, Haystead TA. Smooth muscle myosin phosphatase-associated kinase induces Ca2+ sensitization via myosin phosphatase inhibition. J Biol Chem. 2002;277:23441–23446. doi: 10.1074/jbc.M201597200. doi:10.1074/jbc.M201597200. [DOI] [PubMed] [Google Scholar]
- Bossmar T, Akerlund M, Fantoni G, Szamatowicz J, Melin P, Maggi M. Receptors for and myometrial responses to oxytocin and vasopressin in preterm and term human pregnancy: effects of the oxytocin antagonist atosiban. Am J Obstet Gynecol. 1994;171:1634–1642. doi: 10.1016/0002-9378(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Caldeyro-Barcia R, Theobald GW. Sensitivity of the pregnant human myometrium to oxytocin. Am J Obstet Gynecol. 1968;102:1181. doi: 10.1016/0002-9378(68)90418-3. [DOI] [PubMed] [Google Scholar]
- Cario-Toumaniantz C, Reillaudoux G, Sauzeau V, Heutte F, Vaillant N, Finet M, Chardin P, Loirand G, Pacaud P. Modulation of RhoA–Rho kinase-mediated Ca2+ sensitization of rabbit myometrium during pregnancy—role of Rnd3. J Physiol. 2003;552:403–413. doi: 10.1113/jphysiol.2003.047738. doi:10.1113/jphysiol.2003.047738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SK, Ahn DS, Lee YH. Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc Res. 2009;82:324–332. doi: 10.1093/cvr/cvp054. doi:10.1093/cvr/cvp054. [DOI] [PubMed] [Google Scholar]
- Eto M, Ohmori T, Suzuki M, Furuya K, Morita F. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C.: Isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104–1107. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. doi:10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734–741. doi: 10.1016/0002-9378(84)90677-x. [DOI] [PubMed] [Google Scholar]
- Gogarten W, Emala CW, Lindeman KS, Hirshman CA. Oxytocin and lysophosphatidic acid induce stress fiber formation in human myometrial cells via a pathway involving Rho-kinase. Biol Reprod. 2001;65:401–406. doi: 10.1095/biolreprod65.2.401. doi:10.1095/biolreprod65.2.401. [DOI] [PubMed] [Google Scholar]
- Hartshorne DJ. Myosin phosphatase: subunits and interactions. Acta Physiol Scand. 1998;164:483–493. doi: 10.1046/j.1365-201X.1998.00447.x. doi:10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem J. 2006;396:193–200. doi: 10.1042/BJ20051772. doi:10.1042/BJ20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem. 1985;260:10027–10031. [PubMed] [Google Scholar]
- Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem. 1988;263:6432–6437. [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- Ito K, Shimomura E, Iwanaga T, Shiraishi M, Shindo K, Nakamura J, Nagumo H, Seto M, Sasaki Y, Takuwa Y. Essential role of rho kinase in the Ca2+ sensitization of prostaglandin F(2alpha)-induced contraction of rabbit aortae. J Physiol. 2003;546:823–836. doi: 10.1113/jphysiol.2002.030775. doi:10.1113/jphysiol.2002.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase-mediated contraction of isolated stress fibers. J Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. doi:10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. doi:10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khromov A, Choudhury N, Stevenson AS, Somlyo AV, Eto M. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J Biol Chem. 2009;284:21569–21579. doi: 10.1074/jbc.M109.019729. doi:10.1074/jbc.M109.019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. doi:10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol. 2003;546:879–889. doi: 10.1113/jphysiol.2002.029306. doi:10.1113/jphysiol.2002.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupittayanant S, Burdyga T, Wray S. The effects of inhibiting Rho-associated kinase with Y-27632 on force and intracellular calcium in human myometrium. Pflugers Arch. 2001;443:112–114. doi: 10.1007/s004240100668. doi:10.1007/s004240100668. [DOI] [PubMed] [Google Scholar]
- Lartey J, Lopez Bernal A. RHO protein regulation of contraction in the human uterus. Reproduction. 2009;138:407–424. doi: 10.1530/REP-09-0160. doi:10.1530/REP-09-0160. [DOI] [PubMed] [Google Scholar]
- Lartey J, Gampel A, Pawade J, Mellor H, Bernal AL. Expression of RND Proteins in Human Myometrium. Biol Reprod. 2006;75:452–461. doi: 10.1095/biolreprod.105.049130. doi:10.1095/biolreprod.105.049130. [DOI] [PubMed] [Google Scholar]
- Lartey J, Smith M, Pawade J, Strachan B, Mellor H, Bernal AL. Up-Regulation of myometrial RHO effector proteins (PKN1 and DIAPH1) and CPI-17 (PPP1R14A) phosphorylation in human pregnancy is associated with increased GTP-RHOA in spontaneous preterm labor. Biol Reprod. 2007;76:971–982. doi: 10.1095/biolreprod.106.058982. doi:10.1095/biolreprod.106.058982. [DOI] [PubMed] [Google Scholar]
- Longbottom ER, Luckas MJ, Kupittayanant S, Badrick E, Shmigol T, Wray S. The effects of inhibiting myosin light chain kinase on contraction and calcium signalling in human and rat myometrium. Pflugers Arch. 2000;440:315–321. doi: 10.1007/s004240000305. [DOI] [PubMed] [Google Scholar]
- Lopez Bernal A. Mechanisms of labour - biochemical aspects. BJOG. 2003;110(Suppl 20):39–45. doi: 10.1046/j.1471-0528.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- MacDonald JA, Borman MA, Muranyi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci USA. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. doi:10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillen K, Thornton S, Taylor CW. Oxytocin increases the [Ca2+]i sensitivity of human myometrium during the falling phase of phasic contractions. Am J Physiol Endocrinol Metab. 1999;276:E345–351. doi: 10.1152/ajpendo.1999.276.2.e345. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol. 2008;295:C358–364. doi: 10.1152/ajpcell.90645.2007. doi:10.1152/ajpcell.90645.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore F, Da Silva C, Wilde JI, Smarason A, Watson SP, Lopez Bernal A. Up-regulation of p21- and RhoA-activated protein kinases in human pregnant myometrium. Biochem Biophys Res Commun. 2000;269:322–326. doi: 10.1006/bbrc.2000.2290. doi:10.1006/bbrc.2000.2290. [DOI] [PubMed] [Google Scholar]
- Moran CJ, Friel AM, Smith TJ, Cairns M, Morrison JJ. Expression and modulation of Rho kinase in human pregnant myometrium. Mol Hum Reprod. 2002;8:196–200. doi: 10.1093/molehr/8.2.196. doi:10.1093/molehr/8.2.196. [DOI] [PubMed] [Google Scholar]
- Muranyi A, MacDonald JA, Deng JT, Wilson DP, Haystead TA, Walsh MP, Erdodi F, Kiss E, Wu Y, Hartshorne DJ. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem J. 2002;366:211–216. doi: 10.1042/BJ20020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Yasuda K, Kim YS, Egawa M, Kanzaki H, Nakazawa H, Hori M, Seto M, Karaki H. Possible role of the protein kinase C/CPI-17 pathway in the augmented contraction of human myometrium after gestation. Br J Pharmacol. 2003;140:1303–1312. doi: 10.1038/sj.bjp.0705552. doi:10.1038/sj.bjp.0705552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey D, Goyal P, Bamburg JR, Siess W. Regulation of LIM-kinase 1 and cofilin in thrombin-stimulated platelets. Blood. 2006;107:575–583. doi: 10.1182/blood-2004-11-4377. doi:10.1182/blood-2004-11-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang H, Guo Z, Su W, Xie Z, Eto M, Gong MC. RhoA-Rho kinase pathway mediates thrombin- and U-46619-induced phosphorylation of a myosin phosphatase inhibitor, CPI-17, in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2005;289:C352–360. doi: 10.1152/ajpcell.00111.2005. doi:10.1152/ajpcell.00111.2005. [DOI] [PubMed] [Google Scholar]
- Patil SB, Bitar KN. RhoA- and PKC-alpha-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G83–G95. doi: 10.1152/ajpgi.00178.2005. doi:10.1152/ajpgi.00178.2005. [DOI] [PubMed] [Google Scholar]
- Paul J, Maiti K, Read M, Hure A, Smith J, Chan EC, Smith R. Phasic Phosphorylation of Caldesmon and ERK 1/2 during Contractions in Human Myometrium. PLoS One. 2011;6:e21542. doi: 10.1371/journal.pone.0021542. doi:10.1371/journal.pone.0021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley M, Baker PN, Tribe RM, Taggart MJ. Expression of scaffolding, signalling and contractile-filament proteins in human myometria: effects of pregnancy and labour. J Cell Mol Med. 2005;9:122–134. doi: 10.1111/j.1582-4934.2005.tb00342.x. doi:10.1111/j.1582-4934.2005.tb00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. doi:10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- Somlyo AV, Phelps C, Dipierro C, Eto M, Read P, Barrett M, Gibson JJ, Burnitz MC, Myers C, Somlyo AP. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB J. 2003;17:223–234. doi: 10.1096/fj.02-0655com. doi:10.1096/fj.02-0655com. [DOI] [PubMed] [Google Scholar]
- Tahara M, Morishige K, Sawada K, Ikebuchi Y, Kawagishi R, Tasaka K, Murata Y. RhoA/Rho-kinase cascade is involved in oxytocin-induced rat uterine contraction. Endocrinology. 2002;143:920–929. doi: 10.1210/endo.143.3.8696. doi:10.1210/en.143.3.920. [DOI] [PubMed] [Google Scholar]
- Tamura M, Nakao H, Yoshizaki H, Shiratsuchi M, Shigyo H, Yamada H, Ozawa T, Totsuka J, Hidaka H. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta. 2005;1754:245–252. doi: 10.1016/j.bbapap.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Turnbull AC, Anderson ABM. Uterine contractility and oxytocin sensitivity during human pregnancy in relation to the onset of labour. BJOG: Int J Obstet Gynaecol. 1968;75:278–288. doi: 10.1111/j.1471-0528.1968.tb02078.x. doi:10.1111/j.1471-0528.1968.tb02078.x. [DOI] [PubMed] [Google Scholar]
- Ueda K, Murata-Hori M, Tatsuka M, Hosoya H. Rho-kinase contributes to diphosphorylation of myosin II regulatory light chain in nonmuscle cells. Oncogene. 2002;21:5852–5860. doi: 10.1038/sj.onc.1205747. doi:10.1038/sj.onc.1205747. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yoshioka K, Azam MA, Takuwa N, Sakurada S, Kayaba Y, Sugimoto N, Inoki I, Kimura T, Kuwaki T, et al. Class II phosphoinositide 3-kinase alpha-isoform regulates Rho, myosin phosphatase and contraction in vascular smooth muscle. Biochem J. 2006;394:581–592. doi: 10.1042/BJ20051471. doi:10.1042/BJ20051471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531–540. doi: 10.1161/CIRCRESAHA.108.188524. doi:10.1161/CIRCRESAHA.108.188524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock NA, Taylor CW, Thornton S. Effect of an oxytocin receptor antagonist and rho kinase inhibitor on the [Ca++]i sensitivity of human myometrium. Am J Obstet Gynecol. 2004;190:222–228. doi: 10.1016/s0002-9378(03)00925-6. doi:10.1016/S0002-9378(03)00925-6. [DOI] [PubMed] [Google Scholar]
- Wray S, Kupittayanant S, Shmygol A, Smith RD, Burdyga T. The physiological basis of uterine contractility: a short review. Exp Physiol. 2001;86:239–246. doi: 10.1113/eph8602114. doi:10.1113/eph8602114. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Nakamoto T, Yasuhara M, Okada H, Nakajima T, Kanzaki H, Hori M, Ozaki H. Role of protein kinase Cbeta in rhythmic contractions of human pregnant myometrium. Reproduction. 2007;133:797–806. doi: 10.1530/REP-06-0041. doi:10.1530/REP-06-0041. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zha X, Tan Y, Hornbeck PV, Mastrangelo AJ, Alessi DR, Polakiewicz RD, Comb MJ. Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs. J Biol Chem. 2002;277:39379–39387. doi: 10.1074/jbc.M206399200. doi:10.1074/jbc.M206399200. [DOI] [PubMed] [Google Scholar]