Abstract
The hypothalamic suprachiasmatic nucleus (SCN) is the locus of a master clock that regulates circadian rhythms in physiology and behavior. Gonadectomy in male mice lengthens the period of circadian rhythms and increases the day-to-day variability of activity onset time. Both of these responses are rescued by the nonaromatizable androgen dihydrotestosterone. Androgen receptors (AR) are localized in SCN neurons that receive direct retinal input. To explore how androgens affect circadian clock function and its responsiveness to photic cues, we measured wheel-running behavior and SCN AR expression in intact, gonadectomized, and testosterone-replaced mice, held under various photic conditions. Gonadectomy lengthened circadian period in constant dim light but not in constant darkness. Increasing intensities of constant light parametrically increased circadian period, and this was potentiated at all intensities by gonadectomy. In contrast, gonadectomy did not alter light-induced pupil constriction, suggesting a nonretinal locus of hormone action. In hormone-replaced animals housed in constant darkness, T concentration was positively correlated with precision of activity onset and with SCN AR expression and negatively correlated with duration of activity. We infer the existence of two androgenic mechanisms: one modulates SCN responsiveness to light, and the second modulates SCN timekeeping and locomotor activity in a dose-dependent manner. Finally, the effects of androgens on period are a result of hormonal modulation of the SCN's response to photic input rather than to a change in the inherent period of oscillators in the absence of light.
Circadian rhythms are coordinated by a master clock located in the hypothalamic suprachiasmatic nucleus (SCN) (1, 2). The light-dark cycle is the principal environmental signal that synchronizes circadian rhythms (3). Additionally, endogenous endocrine cues such as gonadal hormone secretions can modulate the phase, period, and amplitude of these rhythms (4–7). The sites at which internal and external cues are integrated are as yet unspecified. A potential locus is the population of androgen receptor (AR)-expressing neurons in the SCN, described in mice, ferrets, and humans (5, 8, 9). In mice, AR-containing cells are restricted to the SCN region that receives photic cues from the retina, and AR occurs in about half of the SCN neurons that respond directly to an acute light pulse by expressing FOS (5). Behaviorally, gonadectomy (GDX) dramatically lengthens the free-running period and increases day-to-day variability in the start of the active phase (4, 5). These behavioral changes likely stem from changes in gene expression and morphology of the SCN. GDX reduces AR expression in the SCN, and testosterone propionate replacement restores expression (5). GDX alters synaptic connectivity, astrocyte morphology, and light-induced clock gene expression in the SCN (10). The nonaromatizable androgen dihydrotestosterone (DHT), rescues the gonadally intact phenotype, indicating that these effects are mediated by ARs rather than by estrogen receptors (5, 10).
Like GDX, tonic light exposure lengthens circadian period in mice, measured by locomotor activity rhythms. This is known as the Aschoff effect (11). According to this principle, which holds across a range of taxa, circadian period parametrically lengthens in nocturnal animals and shortens in diurnal animals with increasing intensity of constant light. The Aschoff effect mechanism is not known. The change in period nevertheless indicates an SCN site of action in mammals, because free-running period is a property of the brain clock and is not dependent on input from other brain regions. In proof, fetal SCN tissue implanted into the third ventricle restores circadian locomotor activity rhythms in arrhythmic SCN-lesioned animals, and if the free-running rhythm of the donor and host differ, the restored period is that of the donor rather than that of the host (12).
The foregoing evidence indicates that AR-containing SCN cells may integrate external photic and internal endocrine cues and may represent a common pathway by which both light and androgens modulate circadian period. In the present studies, we examined how a range of circulating concentrations of testosterone (T) alter clock function and photoresponsiveness. To this end, we tested 1) the dose-response relationship of T on circadian rhythms by measuring locomotor activity patterns in hormone-replaced mice, 2) the permissive effect of photic environment (constant darkness or constant dim light) on circadian responses to T, 3) whether androgens potentiate circadian photoresponsiveness, and 4) whether androgens alter retinal photosensitivity by measuring pupillary light reflex (PLR).
Materials and Methods
Animals and housing
Male C57BL/6 mice obtained from Charles River Laboratories (Wilmington, MA) were housed individually with food (LabDiet 5001; PMI Nutrition, Brentwood, MO) and water available ad libitum. Additional Calbindin D28k::GFP mice on a C57BL/6 background (13) were bred locally and used to measure pupil reflexes. Except as noted, mice were provided with a running wheel (11 cm diameter) in clear polycarbonate cages (32 × 14 × 13 cm) with pine shaving bedding. Reversibility of photic effects only (below) was tested in mice housed in translucent polypropylene cages (36 × 20 × 20 cm; 13-cm-diameter wheel) with corncob bedding. Environmental noise was masked by white noise (76 dB sound pressure level). Mice were acclimated in 12-h light, 12-h dark cycles, and then tested in constant darkness (DD) or constant light (LL) over a range of intensities. Light was provided by a strip of white light-emitting diodes (LED) or a rectangular array of red LED mounted on the ceiling of each shelf to evenly illuminate the cages (white: LL-RIBFLEX-W from LED Liquidators, Inc., Westlake Village, CA; red: HLMP-AD060-P0000, λpeak = 639 ± 9 nm, from Avago Technologies, San Jose, CA). The interbulb distance was 1.5 cm for white and 4–5 cm for red LED. Irradiance and photopic illuminance were measured 4 cm from the cage floor (IL1700; International Light Technologies, Peabody, MA). In DD, health checks and animal husbandry were performed with infrared goggles. A total of 132 mice were used in these experiments. All procedures were approved by the Institutional Animal Care and Use Committee of Columbia University.
Locomotor activity measurements
Wheel-running activity was monitored remotely by Vitalview (Minimitter, Bend, OR), with counts collected in 10-min bins, and visualized using double-plotted actograms. Daily onset of activity bouts were calculated by ClockLab (Actimetrics, Wilmette, IL), as previously described (14). In GDX mice with severely attenuated early night activity (5), onset was defined by the start of longer uninterrupted activity toward the end of the night. Onsets were used to calculate free-running period (slope of the best fit regression line through onsets of bouts at least 30 min long) and day-to-day variability in the time of activity onset around the regression line (sd of the onset residuals; lower values indicate a more precise clock). To validate the measures of free-running period, period was also calculated by χ2 periodogram analysis (ClockLab). In the T dose-response experiments, the correlation of periods as assessed by onset regression and by χ2 periodogram, across all groups and conditions before and after surgery, was high (r = 0.85; P < 0.001; n = 149). The results of ANOVA based on period derived from onset regression (shown below) and from periodograms (not shown) did not differ.
Activity onset and offset were defined from the 7-d activity profile (mean number of running wheel revolutions in each 10-min bin over the circadian cycle after correcting for period) as described previously (15). Duration of activity (α) was defined by offset minus onset time.
GDX and hormone replacement
GDX or sham surgery was performed under isoflurane anesthesia, with buprenorphine (0.5 mg/kg, sc) for analgesia. Testes were externalized via laparotomy and then removed after clamping the testicular artery. Shams were laparotomized, but the testes were left untouched. The incisions were closed with sterile suture and wound clips.
Several concentrations of circulating androgen were studied by replacing T (Steraloids, Inc., Newport, RI) in GDX mice (16). SILASTIC brand capsules (1.02 mm inner diameter, 2.16 mm outer diameter; Dow Corning Corp., Midland, MI) were packed with 100% T (high group), 25% T and 75% cholesterol (mid group), or 10% T and 90% cholesterol (low group), and sealed with SILASTIC adhesive (Dow Corning). Capsules were 5 mm long, with an additional 2 mm on each end for adhesive. The GDX and the sham-operated (intact) control groups received empty capsules. Capsules were washed in 70% ethanol, primed in 0.9% sterile saline at 37 C overnight, and then implanted sc between the scapulae under anesthesia after the GDX or sham surgery. The incision was closed with wound clips.
Concentration effects of T in DD
Mice were allowed to acclimate in running-wheel cages and then released into DD. After 2–3 wk in DD, mice were GDX and implanted with capsules to yield four experimental groups, differing by concentration of implanted T: high (n = 10), mid (n = 9), low (n = 8), and GDX (n = 8). Surgery was performed between circadian times 2 and 11 (with circadian time 12 defined as activity onset) to minimize light-induced phase shifts (17). Mice were then returned to DD. Locomotor activity measures were calculated for the 7 d before surgery and 10–16 d after surgery.
Concentration effects of T in dim light vs. DD
Mice were housed either in dim red light (0.2 lux) or in DD. After 2–3 wk, mice were divided into four experimental hormonal groups: intact, high T, mid T, and GDX (n = 7–10). Surgery was performed and activity measured as above.
Effects of photic intensity on circadian period and variability of activity onset
To test the Aschoff effect (period lengthening by LL) (11), mice were housed in DD and then either GDX (n = 13) or sham-operated (n = 11). Activity was then recorded during 2- to 4-wk exposures to LL in the following order: 400, 4, and 0.1 lux (white light). Period and onset variability were obtained from the last 7 d of each photic exposure.
Reversibility of tonic photic effects
The reversibility of light effects on circadian rhythms was tested in GDX mice (n = 11), whose period and activity onset variability were monitored in both dim red light (0.5 lux) and DD.
Androgenic effects on PLR
The PLR was tested in unanesthetized intact (n = 10) and GDX (n = 7) mice 6–10 h after lights on in a 12-h light, 12-h dark cycle as previously described (14, 18). After 1 h of dark adaptation, pupil size was recorded under infrared light with the camera in a fixed position relative to the eye [DCR-DVD610 (Sony, San Diego, CA) with a macro converter lens, DVS-WA45–30M (B&H, New York, NY]. Pupil size was measured using ImageJ just before and after 10 sec of light exposure from a single green LED placed 10 cm from the eye (λpeak = 520 nm; half-maximum spectral width = 35 nm; UniqueLEDs.com, Oklahoma City, OK).
Perfusion, androgen bioassay, and blood sampling
At the end of the experiments, mice were deeply anesthetized with an overdose of pentobarbital (200 mg/kg) and perfused transcardially with 50 ml saline followed by 50 ml cold fresh 4% paraformaldehyde in 0.1 m phosphate buffer (PB). Brains were removed, postfixed for 18–24 h, and cryoprotected in 20% sucrose in PB. In some mice, seminal vesicles were removed after perfusion and weighed wet to determine the state of steroid-dependent peripheral structures. For other mice, approximately 0.5 ml blood was drawn from the right ventricle, before perfusion, via a 21-gauge needle and transferred to heparinized (10 U) microcentrifuge tubes on ice. Blood was spun at 1800 × g at 4 C (Eppendorf 5417R, Hamburg, Germany) and the plasma stored at −80 C. Plasma T concentration was measured in duplicate by ELISA in two plates run simultaneously (no. 1880; Alpha Diagnostic International, Inc., San Antonio, TX). Pooled plasma from GDX mice yielded a nonspecific signal of 0.1 ng/ml. Parallelism was verified in serial dilutions of pooled plasma from mice with high T. Spike recovery was tested by spiking pooled plasma from GDX mice with kit standards and was 99 and 124% at predicted concentrations of 5 and 0.5 ng/ml, respectively. Based on multiple measures of pooled plasma from the high-T group (six samples per plate), the intra-assay coefficient of variation was 10.5%, consistent with the intra- and interassay coefficients of variation reported by the manufacturer of 10–12%.
Immunohistochemistry
Sections were cut at 40 μm in two series on a cryostat and collected in antifreeze [30% wt/vol sucrose, 1% wt/vol polyvinylpyrrolidone-40, 30% vol/vol ethylene glycol, in PB with 0.9% saline] and stored at −20 C until processing. For immunohistochemistry, sections were washed in PB and then in PB containing 0.1% Triton X-100 (0.1 PBT), blocked in 2% normal donkey serum in 0.3 PBT, and then incubated 72 h at 4 C with primary antibodies: rabbit polyclonal anti-AR (1:2000, sc-816; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and guinea pig anti-arginine vasopressin (AVP, 1:5000, T-5048; Peninsula Laboratories, Inc., San Carlos, CA). After the primary antibody step, sections were washed in 0.1 PBT and then incubated with the appropriate secondary antibodies in 0.3 PBT for 2 h at room temperature (Cy2- and Cy3-conjugated, made in donkey, 1:200; Jackson Immunoresearch Laboratories, Inc., West Grove, PA). Sections were washed once in 0.1 PBT and three times in PB, mounted on gelatin-subbed slides, dehydrated through alcohols, cleared in xylenes, and coverslipped with Krystalon (EMD Chemicals, Gibbstown, NJ).
Analysis
Images were captured with a Nikon Eclipse E800 epifluorescent microscope (Nikon, Tokyo, Japan) equipped with a cooled CCD camera (Retiga Exi; Q-Imaging, Surrey, Canada), using Q-capture Pro software (Q-Imaging). Sections were excited and emission filtered using filter cubes for Cy2 (FITC-HYQ, excitation 460–500 nm, emission 510–560 nm; Nikon) and Cy3 (Y-2E/C Texas Red, excitation 540–580 nm, emission 600–660 nm; Nikon). Relative OD of AR expression in the SCN core (defined by the absence of AVP expression) was measured in photomicrographs using ImageJ, and values are expressed relative to background measured in hypothalamic areas lateral to the SCN without AR expression. Grayscale images were inverted and adjusted for brightness and contrast using Photoshop (Adobe Systems, Mountain View, CA).
Statistics
Two-way ANOVA was used to test for the effects of endocrine status and light condition on behavior, physiology, and AR expression, with pre- and postsurgery values included as a repeated measure (pre/postoperative), and post hoc tests where appropriate. Effects of light intensity on behavior and pupil constriction were assessed by repeated-measures ANOVA followed by Tukey test (with hormonal treatment as a separate independent measure). The PLR dose response was fit by a four-parameter logistic function as described previously (18) (Prism, GraphPad Software, La Jolla, CA). Mean and se are reported throughout. Differences were considered significant at P < 0.05.
Results
Concentration effects of T in DD
In DD, GDX decreased the consolidation of activity (Fig. 1A). After GDX, activity onset became more variable, and mice were less active, especially between an initial onset bout and an accentuated offset bout. High T maintained normal behavior after GDX. Lower T doses led to an intermediate behavioral phenotype between intact- and GDX-typical activity. GDX with or without T replacement had no effect on free-running period (Fig. 1B, all effects, P > 0.05). In contrast, GDX increased the onset variability, decreased the amount of activity, and increased duration of activity, whereas T replacement rescued these traits in a graded manner [Fig. 1, C–E, repeated-measures ANOVA, onset variability: effects of group F(3,31) = 3.1, P < 0.05, pre/postoperative F(1,31) = 4.4, P < 0.05, interaction F(3,31) = 3.0, P < 0.05; amount of activity: group F(3,34) = 2.5, P = 0.08, pre/postoperative F(3,34) = 111, P < 0.001, interaction F(3,34) = 10, P < 0.001; activity duration: group F(3,31) = 0.8, P = 0.5, pre/postoperative F(3,31) = 53, P < 0.001, interaction F(3,31) = 3.9, P < 0.05]. For onset variability and activity duration, there was an ordered progression from low to high T concentration. In all cases, the presurgery phenotype was maintained in the high-T group.
Fig. 1.
Dose-response effects of T. A, Effect of T on running wheel activity in representative animals is demonstrated in double-plotted actograms (serial days are plotted on the y-axis, time of day for 48 h is plotted on the x-axis). The amount of running is represented by black shading. The day of GDX with T replacement or GDX alone is indicated by a circle. The T concentration and n/group are indicated at the top right of each panel. B–E, Quantification of surgery and group effects on parameters of wheel running (mean ± se), including period (B); onset variability (C), the sd of daily activity onset time residuals, where larger values indicate less day-to-day precision; amount of activity in counts/min (D); and duration of the active phase, alpha (E). Post-surgery values with different letters are significantly different (Fisher's protected least significant difference test, P < 0.05). Surg., Surgery.
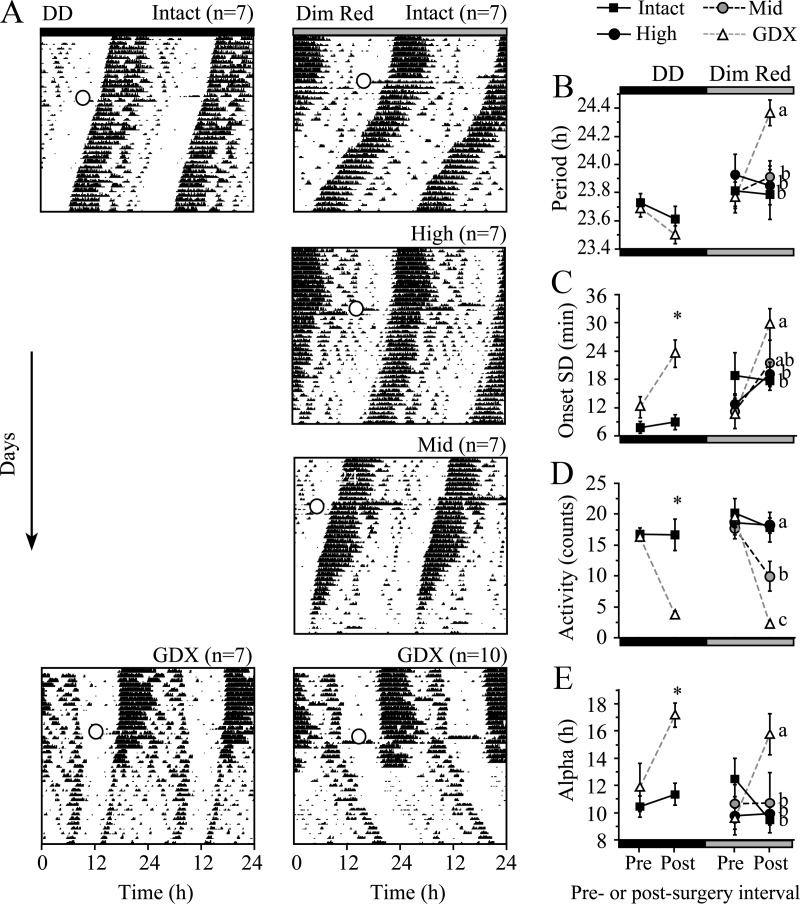
Concentration effects of T in dim light vs. DD
The lack of a GDX effect on free-running period in DD was surprising, given previous reports of GDX-induced period lengthening (4, 5). Unlike the experiment above, both previous reports employed constant dim red light for husbandry. Because light can lengthen period, and because AR is expressed in retinorecipient SCN neurons, we replicated the dose-response experiment with dim red light (0.2 lux) as an additional variable.
Unlike in DD, GDX dramatically lengthened free-running period by 36 min in constant dim light [Fig. 2, A and B, group F(3,27) = 1.2, P = 0.3; pre/postoperative F(1,27) = 7.6, P = 0.01; interaction F(3,27) = 9.0, P < 0.001]. Onset variability, amount of activity, and activity duration all varied with T concentration in dim light as they did in DD [Fig. 2, C–E, right side, onset variability: group F(3,27) = 0.7, P = 0.6, pre/postoperative F(1,27) = 20.3, P < 0.001, interaction F(3,27) = 5.3, P < 0.01; amount of activity: group F(3,30) = 8.6, P < 0.001, pre/postoperative F(1,30) = 46.2, P < 0.001, interaction F(3,30) = 15.0, P < 0.001; activity duration: group F(3,26) = 1.6, P = 0.2, pre/postoperative F(1,26) = 0.7, P = 0.4, interaction F(3,26) = 4.3, P < 0.01]. The results of intact and GDX mice in DD matched the previous run. As previously (compare Fig. 1 and DD portions of Fig. 2), GDX did not alter period [group F(1,12) =0.7, P = 0.4, pre/postoperative F(1,12) = 9, P < 0.05, interaction F(1,12) =0.5, P = 0.5], but did alter onset variability, amount of activity [Fig. 2, D and E, all effects F(1,12) > 6.4, P < 0.05], and activity duration [group F(1,12) = 9.2, P < 0.05, pre/postoperative F(1,12) = 8.2, P < 0.05, interaction F(1,12) = 4.1, P = 0.07].
Fig. 2.
Androgens change circadian responses to constant dim light. A, Actograms show wheel running activity in constant darkness (DD, left) or dim red light (right) in intact, GDX, and T-replaced animals. Open circle, Time of surgery. B–E, Quantification of surgery and group effects on parameters of wheel running (mean ± SE), including period (B), onset precision (C), amount of activity in counts/min (D), and activity duration, alpha (E). Different letters and * indicate significant between-group differences post-surgery (P < 0.05).
Effects of light intensity on circadian period and variability of activity onset
We measured LL-induced period lengthening (Aschoff effect) to test whether GDX lowers only the threshold to respond to light or whether it also potentiates circadian responses to light across a wide range of intensities. In DD, period decreased from before to after surgery with no difference between GDX and sham controls [hormone group, F(1,24) = 0.3, P = 0.6; pre/postoperative, F(1,24) = 12.1, P < 0.01; interaction, F(1,24) = 0.5, P = 0.5]. In contrast, during the same DD period, GDX increased onset variability relative to sham surgery [effect of pre/postoperative F(1,24) = 9.9, P < 0.01; hormone group F(1,24) = 4.2, P = 0.05; interaction F(1,24) = 5.0, P < 0.05]. In LL, both period and onset variability increased parametrically with increasing light intensity; the magnitude of these changes differed between intact and GDX mice [Fig. 3, period: group F(1,20) = 7.6, P < 0.05, light intensity F(3,60) = 147, P < 0.001, interaction F(3,60) = 3.7, P < 0.05; onset variability: group F(1,20) = 19.7, P < 0.001, light intensity F(3,60) = 16.4, P < 0.001, interaction F(3,60) = 1.9, P = 0.14].
Fig. 3.
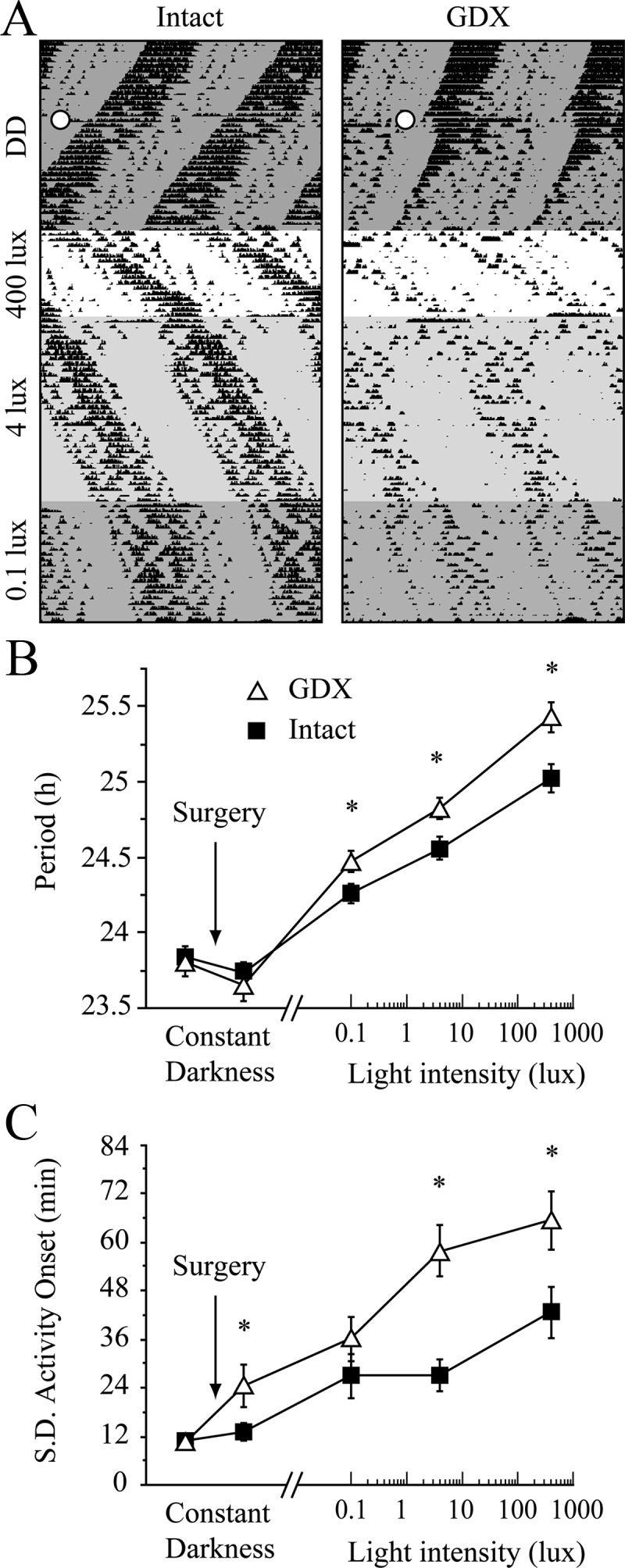
Effects of photic intensity on circadian period and onset variability. A, Representative actograms show the intensity-dependent lengthening of period in LL. Open circles indicate the time of sham surgery or GDX in DD. B and C, Quantification of the effects of endocrine status on circadian period (B) and the sd of activity onset (C) in DD and three intensities of LL. *, P < 0.05, Tukey test.
Reversibility of tonic photic effects
The effects of 0.5-lux dim red light on period and onset variability in GDX mice were reversible [Fig. 4, period: light effect F(3,30) = 23.9, P < 0.001; precision: light effect F(3,30) = 8.6, P < 0.001]. These data also demonstrate that although GDX increased onset variability in DD, light magnified this effect.
Fig. 4.
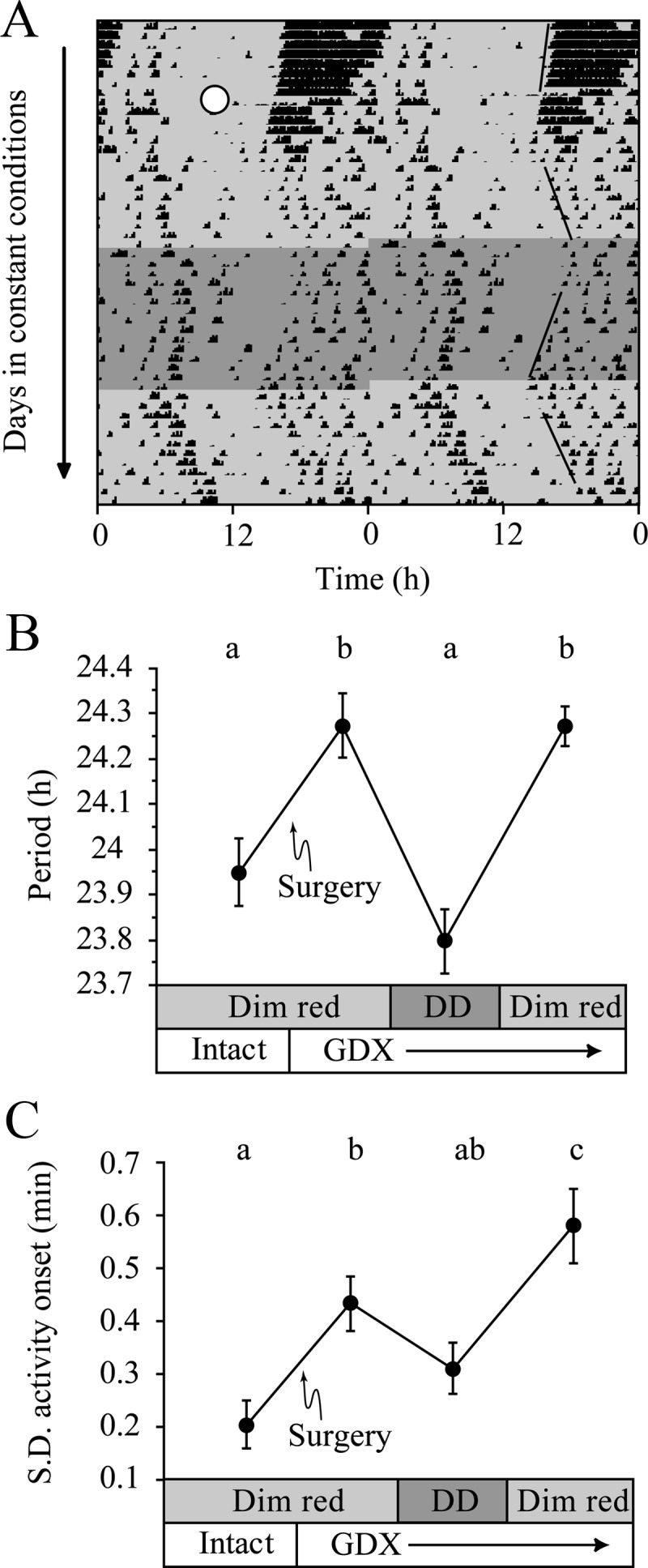
Tonic effects of LL are reversed in DD. A, Representative actogram of a GDX (white circle) mouse housed in dim red LL (light gray), then in DD (dark gray) for 2 wk, and then returned to dim red LL. Best-fit regression lines through onset are shown on the right. B, Period lengthens after GDX, and this is reversed by DD housing. Light and endocrine status are indicated on the x-axis. C, Variability of activity onset increases after GDX; variability is decreased in DD and increased in LL. Different letters indicate significant differences (Tukey test).
Androgen effects on PLR
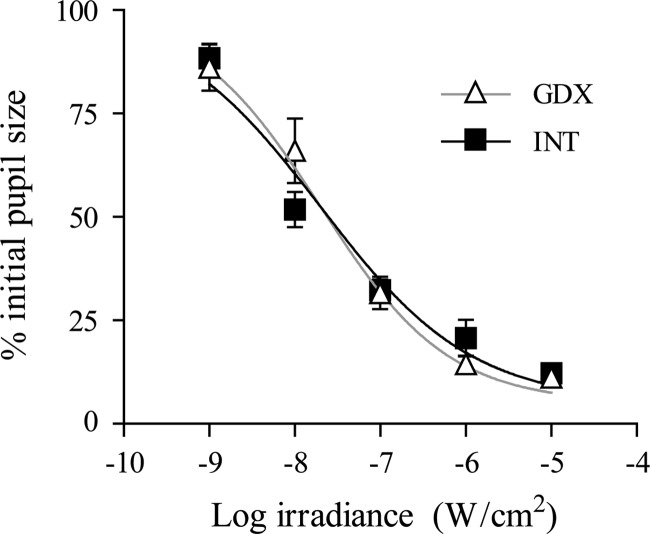
PLR was measured to determine whether the increased photoresponsiveness caused by GDX could be explained by a change at the level of the retina. Endocrine status had no effect on pupil constriction across a range of light intensities, including intensities at which GDX changed the Aschoff effect. These data indicate that GDX does not alter retinal photosensitivity as measured by PLR [Fig. 5, group F(1,11) = 0.23, P = 0.6; light intensity F(4,44) = 109, P < 0.001; interaction F(4,44) = 1.4, P = 0.2].
Fig. 5.
Androgenic effects on PLR. Intensity-response curves show the effects of 10 sec of 520 nm light on final pupil size in intact (INT) and GDX animals.
Concentration effects of T on SCN AR expression
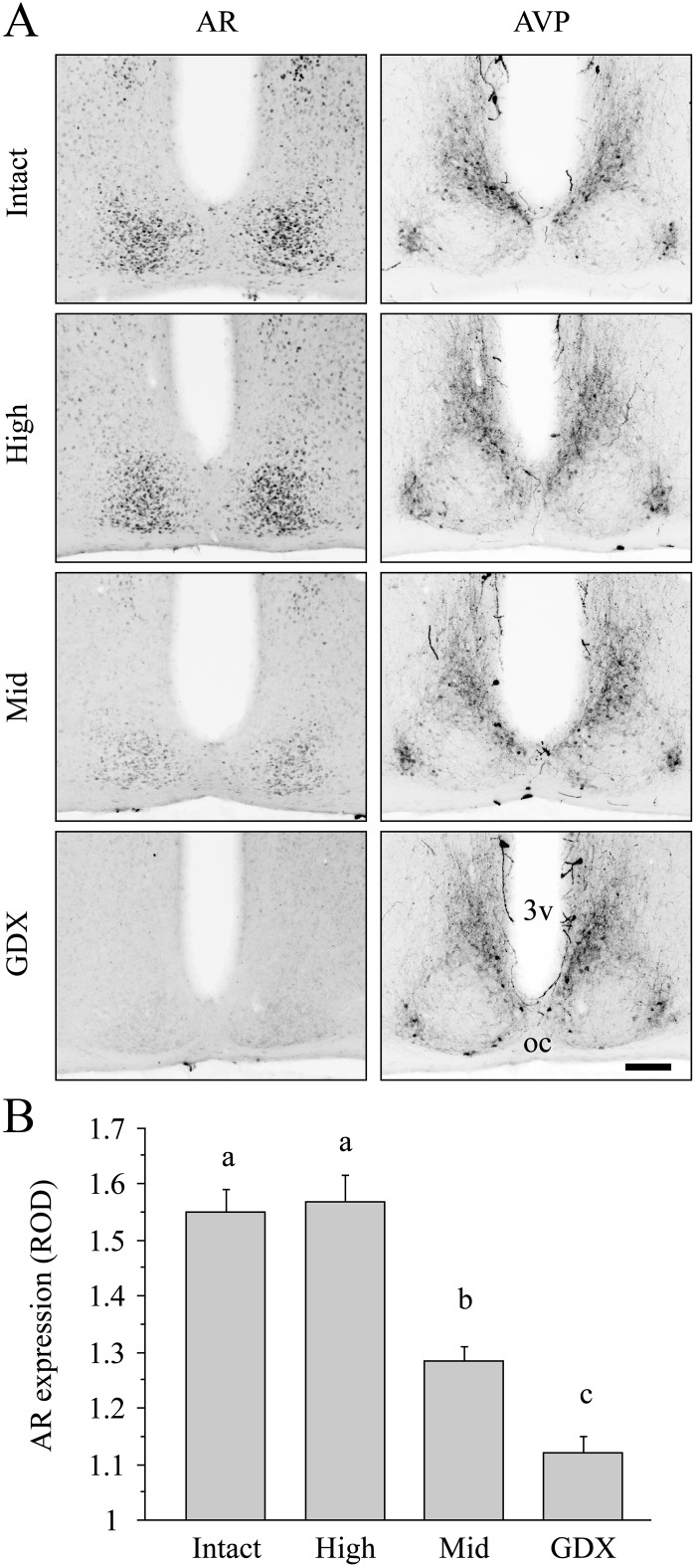
AR expression was restricted to the SCN core, as localized by the surrounding AVP immunoreactivity, and varied with T group (Fig. 6A). The expression level was normal in the high-T group and intermediate between intact and GDX expression levels in the mid-T group [Fig. 6B, F(3,19) = 34.7, P < 0.001].
Fig. 6.
Dose-dependent effects of T on SCN AR expression. A, Representative photomicrographs of the SCN, double labeled for AR and AVP immunoreactivity in intact, T-replaced, and GDX mice showing that decreased T concentration reduces SCN AR expression. Scale bar, 100 μm. 3v, Third ventricle; oc, optic chiasm. B, Quantification of AR expression in intact, T-replaced, and GDX mice. Different letters indicate significant differences (P < 0.05, Tukey test).
T concentration and seminal vesicle mass in hormone-replaced mice
The capsules established a range of subphysiological circulating T [Table 1, F(4,28) = 8.8, P < 0.001]. T replacement restored normal and intermediate seminal vesicle mass in the high- and mid-T groups, respectively, indicating restoration of physiologically relevant plasma T concentrations. Seminal vesicle mass differed between groups [Table 1, F(3,34) = 233, P < 0.001].
Table 1.
Capsules used in each group, resultant T concentration [T], and seminal vesicle mass
| Group | Capsule | Plasma [T] [ng/ml (n)] | Seminal vesicle mass [mg (n)] |
|---|---|---|---|
| Intact | Blank | 1.47 ± 0.30 (10)a | 260 ± 13 (11)a |
| High | 5 mm of 100% T | 0.80 ± 0.08 (8)b | 246 ± 9 (7)a |
| Mid | 5 mm of 25% T | 0.19 ± 0.04 (7)c | 60 ± 9 (6)b |
| Low | 5 mm of 10% T | 0.11 ± 0.02 (3)b,c | |
| GDX | Blank | 0.10 ± 0.01 (5)c | 17 ± 1 (14)c |
Within a column, values with different superscript letters differ (P < 0.05).
Discussion
The present study demonstrates that androgens alter the photoresponsiveness of the circadian clock, leading to a longer free-running period in GDX animals compared to intact or T-replaced animals. This effect on period was present in LL but absent in DD. Other consequences of GDX did not depend on the photic environment; mice with higher circulating T concentration had more precise clocks, and ran much more but for a shorter duration than counterparts with low T. We infer that the androgenic effect on photoresponsiveness is at the level of the SCN because GDX had no effect on PLR which is an SCN-independent measure of the non-image-forming visual system.
Effects of T replacement
A lower concentration of T was needed to restore a free-running period typical of an intact animal than was required to rescue normal onset variability, and amount and duration of activity. The lowest dose of T, in animals housed in constant dim light, fully rescued free-running period, despite low SCN AR expression. In contrast, T had dose-dependent effects on all other measures, irrespective of photic conditions. This is consistent with previous findings that T dose-response relationships differ among androgen-sensitive responses (19). The two dose-response dynamics suggest distinct AR-dependent mechanisms. Estrogen receptor-mediated signaling is not indicated, because implants of DHT restore all of these responses to normal with the exception of activity amount (5, 10). Furthermore, AR expression is robust, whereas estrogen receptor expression is sparse in the SCN (5, 20). Future work combining DHT and an AR antagonist such as flutamide will be necessary to rule out the possible role of other estrogen receptor-active androgen metabolites such as 3β-diol (21).
Effects of T on photoresponsiveness
Period, unlike the other circadian parameters measured, changed after GDX only in the presence of light. Indeed, GDX mice exhibit longer periods than their intact counterparts across a range of photic intensities. In the dimmest light tested, period lengthened in GDX but not in mice with circulating T. These data show that GDX lowers the threshold for the Aschoff effect and potentiates the effect in brighter light. Augmented photoresponsiveness in GDX mice may explain their larger phase shifts to light (10). One possible explanation is that GDX may increase N-methyl-D-aspartate receptor-mediated responses to glutamate secreted by retinal afferents (22). For instance, in hippocampal pyramidal neurons, GDX increases the response to N-methyl-D-aspartate, whereas DHT reverses this (23).
That GDX lengthened the period but did not increase PLR suggests, but does not prove, a direct effect on the SCN, independent of the retina. The retinal ganglion cells that innervate the olivary pretectal nucleus and mediate PLR do not completely overlap with those that innervate the SCN (24). Further work on androgenic effects on photoreceptor and retinal ganglion cell function is warranted given evidence for steroid receptors in the retina (25).
At a practical level, it is important that less than 0.5 lux red light lengthened circadian period in GDX mice. This intensity can impact the circadian system, even though it may not be bright enough to induce a detectable phase shift (26). These intensities are often used for animal husbandry, yet we found that they are sufficient to alter period in GDX animals and to entrain circadian rhythms of gonadally intact animals (18).
Effects of T on intra-SCN signaling
The SCN is structurally and functionally heterogeneous and can be conceptualized as a core of retinorecipient cells and a surrounding shell of circadian oscillators (27). Cells of the SCN core region play an important role in coordinating the rhythms of the shell oscillators. For example, in hamsters, small lesions of the core abolish circadian rhythms despite substantial survival of cells of the shell (28, 29). In vitro, oscillators in the shell region of the mouse SCN desynchronize when physically separated from the core (30). AR expression occurs predominantly in the retinorecipient SCN core (5). In this region, light pulses acutely induce FOS and PERIOD1 expression in gastrin-releasing peptide (GRP) neurons, more than 90% of which express AR (5, 13, 31, 32). GRP receptors occur in the SCN shell, and administration of GRP to the SCN either in vitro or in vivo causes phase shifts that mimic the response to light pulses (31, 33–35). Therefore, intra-SCN GRP signaling is a plausible androgen target, and this would be consistent with AR-GRP relationships described in the spinal cord and in prostate tumors (36, 37).
Additional evidence for an effect of GDX on intra-SCN signaling is derived from the observation that in DD, GDX increased activity duration without increasing period. These two properties are generally positively correlated (38). It has been proposed that activity duration is controlled by the strength of coupling between two putative oscillators that separately control the time of activity onset and activity offset (evening and morning oscillators) (39). Increased activity duration after GDX may indicate reduced coupling strength between these putative morning and evening oscillators.
Gonadal hormone effects on chronotype
In humans, AR is present in the SCN (9), and there is a correlation between plasma androgen levels and sleep onset in males. Sleep time gets progressively later from childhood to about 21 yr of age and then becomes progressively earlier (40), in parallel with changes in androgens (41, 42); directions, however, not predicted by the present results.
Conclusions
We show here that the effects of androgens on circadian timing are attributable to an interaction between hormonal status and circadian parametric responses to light. The effects of androgens on period are a result of hormonal modulation of photic input and are not due to a change in the inherent period of oscillators in the absence of light. Independent of light, androgens alter clock function as observed in the precision of the clock and the distribution of activity during the night (Fig. 4A) (5). These data support the emerging view that reproductive state interacts with the circadian clock (7, 43). This in turn has ramifications on endocrine control of growth, stress, and reproduction, aspects of which are coordinated in time by the circadian system (44, 45).
Acknowledgments
We thank Megan Rainbow, Pietro Muzzio, Mabel McLean, and Ben Meltzer for technical assistance and three anonymous reviewers for helpful comments on this manuscript.
Present address for M.P.B.: Division of Sleep Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts 02115.
Present address for I.N.K.: Department of Veterinary and Comparative Anatomy, Pharmacology and Physiology, Washington State University, Pullman, Washington 99163.
This work was supported by Grants NS37919 (to R.S.) and T32DK07328 (to M.P.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- AVP
- arginine vasopressin
- DD
- constant darkness
- DHT
- dihydrotestosterone
- GDX
- gonadectomy
- GRP
- gastrin-releasing peptide
- LED
- light-emitting diode
- LL
- constant light
- PB
- phosphate buffer
- PBT
- PB containing Triton X-100
- PLR
- pupillary light reflex
- SCN
- suprachiasmatic nucleus
- T
- testosterone.
References
- 1. Dibner C, Schibler U, Albrecht U. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549 [DOI] [PubMed] [Google Scholar]
- 2. Welsh DK, Takahashi JS, Kay SA. 2010. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72:551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Golombek DA, Rosenstein RE. 2010. Physiology of circadian entrainment. Physiol Rev 90:1063–1102 [DOI] [PubMed] [Google Scholar]
- 4. Daan S, Damassa D, Pittendrigh CS, Smith ER. 1975. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci USA 72:3744–3747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karatsoreos IN, Wang A, Sasanian J, Silver R. 2007. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology 148:5487–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morin LP, Fitzgerald KM, Zucker I. 1977. Estradiol shortens the period of hamster circadian rhythms. Science 196:305–307 [DOI] [PubMed] [Google Scholar]
- 7. Abizaid A, Mezei G, Horvath TL. 2004. Estradiol enhances light-induced expression of transcription factors in the SCN. Brain Res 1010:35–44 [DOI] [PubMed] [Google Scholar]
- 8. Kashon ML, Arbogast JA, Sisk CL. 1996. Distribution and hormonal regulation of androgen receptor immunoreactivity in the forebrain of the male European ferret. J Comp Neurol 376:567–586 [DOI] [PubMed] [Google Scholar]
- 9. Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF. 2000. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 425:422–435 [DOI] [PubMed] [Google Scholar]
- 10. Karatsoreos IN, Butler MP, Lesauter J, Silver R. 2011. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology 152:1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aschoff J. 1960. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 25:11–28 [DOI] [PubMed] [Google Scholar]
- 12. Ralph MR, Foster RG, Davis FC, Menaker M. 1990. Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978 [DOI] [PubMed] [Google Scholar]
- 13. Karatsoreos IN, Yan L, LeSauter J, Silver R. 2004. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. J Neurosci 24:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Butler MP, LeSauter J, Sichel AN, Silver R. 2011. Targeted mutation of the calbindin D 28k gene selectively alters nonvisual photosensitivity. Eur J Neurosci 33:2299–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butler MP, Paul MJ, Turner KW, Park JH, Driscoll JR, Kriegsfeld LJ, Zucker I. 2008. Circadian rhythms of photorefractory Siberian hamsters remain responsive to melatonin. J Biol Rhythms 23:160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scordalakes EM, Rissman EF. 2003. Aggression in male mice lacking functional estrogen receptor α. Behav Neurosci 117:38–45 [PubMed] [Google Scholar]
- 17. Comas M, Beersma DG, Spoelstra K, Daan S. 2006. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms 21:362–372 [DOI] [PubMed] [Google Scholar]
- 18. Butler MP, Silver R. 2011. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. Proc R Soc B 278:745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. 2001. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:E1172–E1181 [DOI] [PubMed] [Google Scholar]
- 20. Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kalló I. 2008. Oestrogen receptor α and β immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol 20:1270–1277 [DOI] [PubMed] [Google Scholar]
- 21. Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. 2008. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5α-androstane-3β,17β-diol. Horm Behav 53:741–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. 1999. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci 19:5124–5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pouliot WA, Handa RJ, Beck SG. 1996. Androgen modulates N-methyl-d-aspartate-mediated depolarization in CA1 hippocampal pyramidal cells. Synapse 23:10–19 [DOI] [PubMed] [Google Scholar]
- 24. Chen SK, Badea TC, Hattar S. 2011. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature 476:92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA. 2000. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand 78:146–153 [DOI] [PubMed] [Google Scholar]
- 26. Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. 1984. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature 308:186–188 [DOI] [PubMed] [Google Scholar]
- 27. Butler MP, Silver R. 2009. Basis of robustness and resilience in the suprachiasmatic nucleus: individual neurons form nodes in circuits that cycle daily. J Biol Rhythms 24:340–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kriegsfeld LJ, LeSauter J, Silver R. 2004. Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus. J Neurosci 24:2449–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LeSauter J, Silver R. 1999. Localization of a suprachiasmatic nucleus subregion regulating locomotor rhythmicity. J Neurosci 19:5574–5585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. 2003. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302:1408–1412 [DOI] [PubMed] [Google Scholar]
- 31. Aida R, Moriya T, Araki M, Akiyama M, Wada K, Wada E, Shibata S. 2002. Gastrin-releasing peptide mediates photic entrainable signals to dorsal subsets of suprachiasmatic nucleus via induction of Period gene in mice. Mol Pharmacol 61:26–34 [DOI] [PubMed] [Google Scholar]
- 32. Earnest DJ, DiGiorgio S, Olschowka JA. 1993. Light induces expression of fos-related proteins within gastrin-releasing peptide neurons in the rat suprachiasmatic nucleus. Brain Res 627:205–209 [DOI] [PubMed] [Google Scholar]
- 33. Gamble KL, Allen GC, Zhou T, McMahon DG. 2007. Gastrin-releasing peptide mediates light-like resetting of the suprachiasmatic nucleus circadian pacemaker through cAMP response element-binding protein and Per1 activation. J Neurosci 27:12078–12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karatsoreos IN, Romeo RD, McEwen BS, Silver R. 2006. Diurnal regulation of the gastrin-releasing peptide receptor in the mouse circadian clock. Eur J Neurosci 23:1047–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piggins HD, Antle MC, Rusak B. 1995. Neuropeptides phase shift the mammalian circadian pacemaker. J Neurosci 15:5612–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakamoto H, Takanami K, Zuloaga DG, Matsuda K, Jordan CL, Breedlove SM, Kawata M. 2009. Androgen regulates the sexually dimorphic gastrin-releasing peptide system in the lumbar spinal cord that mediates male sexual function. Endocrinology 150:3672–3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schroeder RP, de Visser M, van Weerden WM, de Ridder CM, Reneman S, Melis M, Breeman WA, Krenning EP, de Jong M. 2010. Androgen-regulated gastrin-releasing peptide receptor expression in androgen-dependent human prostate tumor xenografts. Int J Cancer 126:2826–2834 [DOI] [PubMed] [Google Scholar]
- 38. Honma K, Honma S, Hiroshige T. 1985. Response curve, free-running period, and activity time in circadian locomotor rhythm of rats. Jpn J Physiol 35:643–658 [DOI] [PubMed] [Google Scholar]
- 39. Pittendrigh CS, Daan S. 1976. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol 106:333–355 [Google Scholar]
- 40. Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. 2004. A marker for the end of adolescence. Curr Biol 14:R1038–1039 [DOI] [PubMed] [Google Scholar]
- 41. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. 2001. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86:724–731 [DOI] [PubMed] [Google Scholar]
- 42. Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW. 2010. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem 56:1138–1147 [DOI] [PubMed] [Google Scholar]
- 43. Hagenauer MH, Lee TM. 2011. Time for testosterone: the suprachiasmatic nucleus gets sexy. Endocrinology 152:1727–1730 [DOI] [PubMed] [Google Scholar]
- 44. Butler MP, Kriegsfeld LJ, Silver R. 2009. Circadian regulation of endocrine functions. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, brain, and behavior. 2nd ed San Diego: Academic Press; 473–505 [Google Scholar]
- 45. Karatsoreos IN, Silver R. 2007. The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology 148:5640–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]