Abstract
Diabetics have an increased risk of developing cardiovascular disease, in part due to oxidative stress, resulting in endothelial nitric oxide synthase (eNOS) dysfunction. Studies have demonstrated that angiotensin-(1–7) [Ang-(1–7)] can activate eNOS activity. Because the bone marrow is a primary source of a number of progenitors important in physiological homeostasis and healing, the goal of this study was to evaluate the in vivo effects of Ang-(1–7) treatment on oxidative stress and the ensuing nitrative stress in diabetic bone marrow and its potential pathways. BKS.Cg-Dock7m +/+ Leprdb/J mice and their heterozygous controls were administered Ang-(1–7) alone or combined with A-779, losartan, PD123,319, nitro-l-arginine methyl ester, or icatibant sc for 14 d. The bone marrow was then collected to measure nitric oxide levels, eNOS phosphorylation, and expression of nitric oxide synthase, superoxide dismutase, and p22-phox. Nitric oxide levels in the bone marrow were significantly decreased in diabetic mice, and Ang-(1–7) treatment was able to significantly increase these measures (P < 0.01). This effect was blocked by the coadministration of PD123,319, A-779, nitro-l-arginine methyl ester, and icatibant. In addition, Ang-(1–7) treatment reversed the paradoxical increase in eNOS and neuronal nitric oxide synthase expression and decreased the phosphorylation of eNOS at Thr495 seen in diabetic mice. Ang-(1–7) also reversed diabetes-induced production of reactive oxygen species by decreasing p22-phox expression and increasing superoxide dismutase 3 expression, leading to a significant reduction in 3-nitrotyrosine formation in diabetic bone marrow (P < 0.05). Our findings demonstrate that Ang-(1–7) administration decreases diabetes-induced oxidative stress in the bone marrow and modifies pathways involved in eNOS dysfunction.
Studies show that oxidative stress is increased in diabetes, which can lead to long-term complications observed in diabetic patients (1). Oxidative stress may also be one pathological mechanism that leads to the development of diabetes (2, 3). Long-term diabetes-related complications, including hypertension and coronary artery disease, may stem from multiple factors including excessive production of reactive oxygen species (ROS) such as superoxide and a subsequent decrease in nitric oxide (NO) bioavailability upon its fast (diffusion controlled rates) reaction with superoxide to yield peroxynitrite. In addition to directly reducing NO levels, superoxide can also negatively alter the function of endothelial nitric oxide synthase (eNOS). Increases in ROS and reactive nitrogen species, such as peroxynitrite, can result in protein tyrosine nitration, ultimately modifying or impairing normal protein activity and function (4–6).
Angiotensin-(1–7) [Ang-(1–7)] is an endogenous seven-amino acid peptide of the renin-angiotensin system (RAS). The effects of Ang-(1–7) are believed to be mediated through Mas, a G protein-coupled receptor; however, there is speculation as to the role of other receptors, such as the fetal receptor angiotensin type 2 receptor (AT2) (7, 8). This is important because the expression of the AT2 receptor in adults is primarily up-regulated after injury and is also increased in an obese animal model (9).
Unlike angiotensin II (Ang II), the beneficial effects of Ang-(1–7) are due in part to antagonism of Ang II-induced vasoconstriction of the arteries as well as the stimulation of NO release from endothelial cells and cardiomyocytes resulting in vasodilation (10–17). Cross talk between the RAS and kinin-kallikrein system has been well documented (18, 19). After binding to the Mas receptor, Ang-(1–7) also potentiates the release of bradykinin, a ligand of the bradykinin B2 receptor that results in further vasodilatory effects (20–22).
Studies have also shown that Ang-(1–7) is effective in stimulating hematopoietic progenitor cell proliferation and increasing hematopoietic recovery after chemotherapy and irradiation injury in vivo (23, 24). In addition, Ang-(1–7) can activate eNOS and increase eNOS activity as well as increase the proliferation of bone marrow-derived progenitors (17, 25, 26). The bone marrow is a primary source of progenitors responsible for important biological processes such as vasculogenesis, angiogenesis, and hematopoiesis (28). Oxidative stress and damage, such as that seen in diabetes, can directly impact cell survival and function. Exposure of bone marrow cells to these insults could lead to many of the detrimental and irreversible complications of diabetes. The aim of the current study was to investigate multiple molecular markers of oxidative stress, including eNOS dysfunction and its resultant sequelae in diabetic bone marrow. In addition, we examined the effects of in vivo Ang-(1–7) administration on diabetes-induced oxidative stress in the bone marrow. Lastly, we analyzed the potential pathways through which Ang-(1–7) alters these processes by using pharmacological antagonists of angiotensin and bradykinin receptors as well as pharmacological inhibition of nitric oxide synthase (NOS).
Materials and Methods
Animals
The National Institutes of Health Principles of Laboratory Animal Care were followed, and the Department of Animal Resources at the University of Southern California approved this study. Six- to 8-wk-old male BKS.Cg-Dock7m +/+ Leprdb/J mice and their heterozygous controls were purchased from Jackson Laboratories (Bar Harbor, ME). Mice homozygous for the diabetes spontaneous mutation Leprdb (BKS.Cg-Dock7m +/+ Leprdb/J), which is an obese model of type 2 diabetes due to truncation of the leptin receptor, were used in this study. The mice were quarantined for 1 wk before the initiation of the study, and all diabetic mice had verified plasma glucose levels greater than 500 mg/dl before the initiation of treatment. Food and water were available ad libitum, and all mice were kept on a 12-h light, 12-h dark cycle.
Chemical and reagents
Ang-(1–7) (prepared using good manufacturing practices) and d-Ala7-Ang I/II (1–7) (A-779), an antagonist of the Mas receptor, were purchased from Bachem (Torrance, CA). Losartan, an angiotensin type 1 receptor (AT1) antagonist, PD123,319, an AT2 receptor antagonist, and nitro-l-arginine methyl ester (L-NAME), an inhibitor of NOS, were purchased from Sigma-Aldrich (St. Louis, MO). Icatibant, an antagonist of bradykinin B2 receptors, was purchased from Tocris Bioscience (Ellisville, MO). CellROX* Deep Red reagent for oxidative stress detection was purchased from Invitrogen (Carlsbad, CA). The mouse antinitrotyrosine, clone 1A6, Alexa Fluor* 488-conjugated monoclonal antibody and IgG2bκ isotype control for flow cytometry was purchased from Millipore (Billerica, MA). Primers for quantitative RT-PCR were purchased from Integrated DNA Technologies (San Diego, CA), and antibodies used for Western blotting were purchased from Cell Signaling Technology (Danvers, MA).
Study design
BKS.Cg-Dock7m +/+ Leprdb/J mice and their heterozygous controls (n = 7/group) were administered saline (control), inhibitors alone (losartan, PD123,319, A-779 or L-NAME at 10 mg/kg·d or icatibant at 0.4 mg/kg·d), Ang-(1–7) alone (500 μg/kg·d), or Ang-(1–7) 500 μg/kg·d combined with an inhibitor at the aforementioned doses for 2 wk by sc injection. The mice were weighed three times weekly and the doses adjusted accordingly. The dose of Ang-(1–7) used in this study is based on unpublished data from our laboratory following multiple dose-response studies in mice, in which Ang-(1–7) 500 μg/kg·d administered as a once-daily sc dose was found to be the most effective dose in diabetic mice with no observable toxicities. Plasma glucose levels were measured at necropsy; however, there were no significant changes in any of the diabetic treatment groups (data not shown). In addition, there were no significant changes in any of the parameters investigated after treatment with inhibitors alone (data not shown). After the 14-d treatment period, the mice were euthanized, and the parameters were investigated as described below to determine the effect of diabetes and the Ang-(1–7) treatment with and without the coadministration of the various inhibitors.
Harvesting of bone marrow
The femurs from each mouse were collected and the bone marrow was harvested by flushing with PBS containing 2% fetal calf serum. After the collection of the bone marrow and aliquoting, red blood cells were lysed with a hypotonic solution and mixed with 0.04% trypan blue, and the number of nucleated cells was assessed using a hematocytometer under light microscopy.
Measurement of bone marrow nitrite levels
After permeabilization of aliquoted bone marrow cells with 0.1% Triton-X, NO levels were measured using the Griess reagent system (Promega, Madison, WI). Due to the instable and volatile nature of NO, this assay measures nitrite, one of the stable metabolites of NO. The assay was performed per the manufacturer protocol.
Measurement of bone marrow ROS levels
Isolated bone marrow cells were incubated with CellROX* Deep Red reagent at 5 μm and incubated for 30 min at 37 C per the manufacturer protocol. ROS levels were measured via flow cytometry and values reported as median fluorescent intensity.
Preparation of bone marrow cells for flow cytometry
Bone marrow cells were suspended at 106 cells/ml in DMEM containing 2% fetal calf serum, 1 mm HEPES, penicillin, and streptomycin. For antibody staining, bone marrow cells were suspended in Hanks' balanced salt solution containing 2% fetal calf serum, 1 mm HEPES, penicillin, and streptomycin at 108 cells/ml. An aliquot was first labeled with Alexa Fluor* 488 conjugated mouse antinitrotyrosine antibody added at 1 μg per 1 × 106 cells and then fixed with 4% paraformaldehyde. Flow cytometric analysis was performed on a LSR II flow cytometer using FACSDiva software (Becton Dickinson, Franklin Lakes, NJ) at the Flow Cytometry Core facility located at the University of Southern California School of Pharmacy.
Analysis of bone marrow mRNA expression
Total RNA was extracted from bone marrow cells using TRIzol (Invitrogen). For each sample, approximately 100 ng of RNA was reverse transcribed using Maxima reverse transcriptase (Fermentas, Glen Burnie, MD). Real-time PCR was conducted to examine expression of eNOS, neuronal NOS (nNOS), inducible NOS (iNOS), superoxide dismutase (SOD)-1, SOD2, SOD3, and p22-phox mRNA in bone marrow (for primer sequences, see Supplementary Material, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Amplification of the cDNA was performed using SYBR Green PCR master mix (Applied Biosystems by Life Technologies, Carlsbad, CA) using an ABI 7300. Expressions of eNOS, nNOS, iNOS, SOD1, SOD2, SOD3, and p22-phox mRNA were normalized against 18S mRNA and expressed as fold change compared with nondiabetic controls.
Analysis of bone marrow protein expression
Protein lysates isolated from bone marrow cells for Western blot analysis (30 μg) were resolved on SDS-PAGE gels and transferred to nitrocellulose membranes by electroblotting. The membranes were incubated with monoclonal rabbit antibodies against eNOS, phospho-eNOS (Ser1177 and Thr495), nNOS, SOD3, and p22-phox, followed by antirabbit horseradish peroxidase-conjugated antibody. An antibody against β-actin was used to normalize protein loading. SuperSignal West Pico enhanced chemiluminescence substrate was used to detect the bands (Thermo Scientific, Rockfield, IL). The resultant bands were quantified using densitometry using ImageJ, version 1.45l (National Institutes of Health, Bethesda, MD). The results were expressed as the ratio of target protein band to β-actin band intensity.
Statistical analysis
GraphPad Prism, version 5.0d for Mac OS X (GraphPad Software, San Diego, CA) was used to analyze the data. One-way ANOVA followed by a Tukey's test was used to compare data from more than two groups, and linear regression was used to determine the relationship between bone marrow nitrite levels and percentage of bone marrow tyrosine nitration. The level of statistical significance was set at 5%. Data are expressed as mean value ± sem.
Results
Ang-(1–7) effects on bone marrow ROS and nitrite levels
Bone marrow ROS levels were significantly increased in diabetic bone marrow, whereas administration of Ang-(1–7) for 14 d significantly reduced these ROS levels (Fig. 1A). Coadministration of Ang-(1–7) with losartan, PD123,319, A-779, L-NAME, or icatibant resulted in a significant increase in ROS in diabetic bone marrow compared with treatment with Ang-(1–7) alone.
Fig. 1.
A and B, Effect of Ang-(1–7) on bone marrow NO levels. Bone marrow ROS levels were significantly higher in diabetic mice compared with nondiabetic controls (P < 0.01). Treatment of diabetic mice with Ang-(1–7) resulted in a significant decrease in bone marrow ROS levels compared with saline-treated diabetic mice (P < 0.01). Coadministration of losartan, PD123,319, A-779, or L-NAME with Ang-(1–7) significantly increased bone marrow ROS levels compared with diabetic mice treated with Ang-(1–7) alone (P < 0.01). Bone marrow NO levels were significantly lower in diabetic mice compared with nondiabetic controls (P < 0.01). Treatment of diabetic mice with Ang-(1–7) for 14 d resulted in a significant increase in bone marrow NO levels compared with saline-treated diabetic mice (P < 0.01). Coadministration of PD123,319, A-779, or L-NAME with Ang-(1–7) significantly reduced bone marrow NO levels compared with diabetic mice treated with Ang-(1–7) alone (P < 0.05), whereas coadministration of Ang-(1–7) with either losartan or icatibant did not have a significant effect on bone marrow NO levels compared with Ang-(1–7) alone. **, P < 0.01; †, P < 0.05 compared with diabetic + Ang-(1–7) group; ††, P < 0.01 compared with diabetic + Ang-(1–7) group.
Due to the integral relationship between diabetes-induced oxidative stress, NO and the production of reactive nitrogen species leading to protein tyrosine nitration and posttranslational modifications, NO levels were measured in bone marrow cells isolated from both nondiabetic and diabetic mice. Bone marrow NO levels were significantly reduced in diabetic mice when compared with nondiabetic controls, whereas treatment of diabetic mice with Ang-(1–7) resulted in a significant increase in bone marrow NO levels (Fig. 1B). Administration of Ang-(1–7) to nondiabetic mice did not significantly affect bone marrow NO levels (data not shown). Administration of PD123,319, A-779, or L-NAME in combination with Ang-(1–7) in diabetic mice resulted in a significant decrease in bone marrow nitrite levels compared with diabetic mice treated with Ang-(1–7) alone. The combination of Ang-(1–7) with either losartan or icatibant resulted in a nonsignificant reduction in bone marrow nitrite levels.
Effects of Ang-(1–7) on NOS isoform expression and eNOS activation
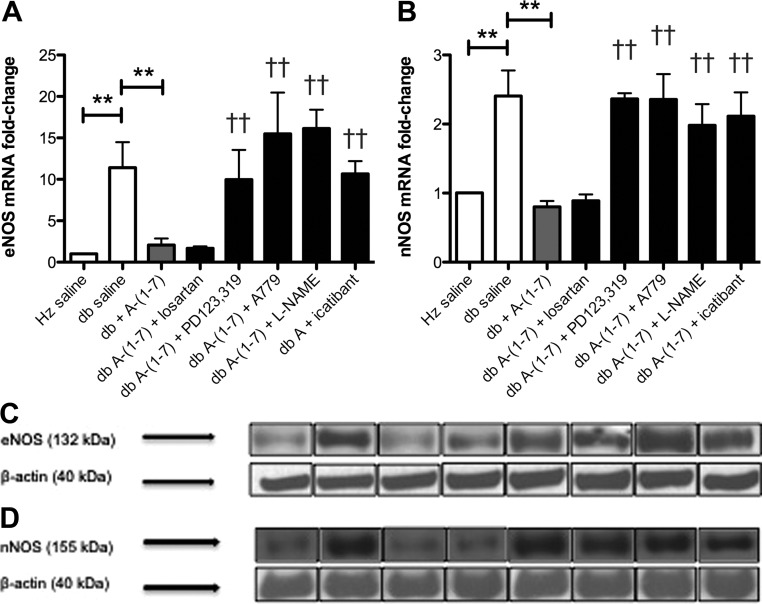
Because other investigators have observed a paradoxical increase in eNOS expression in endothelial cells despite decreased NO levels in type 2 diabetes (29, 30), bone marrow eNOS, nNOS, and iNOS mRNA and protein expression were assessed. Bone marrow eNOS and nNOS mRNA and protein expression were significantly increased in diabetic mice (Fig. 2, A–D), whereas there were no significant changes in bone marrow iNOS expression in any group (data not shown). Treatment of diabetic mice with Ang-(1–7) significantly decreased both eNOS and nNOS mRNA and protein expression, whereas the coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) resulted in a significant increase in eNOS and nNOS mRNA and protein expression compared with diabetic mice treated with Ang-(1–7) alone, whereas coadministration of losartan with Ang-(1–7) did not have a significant effect.
Fig. 2.
A–D, Ang-(1–7) effects on NOS isoform expression. Bone marrow eNOS mRNA expression (A) and protein levels (C) as well as nNOS mRNA expression (B) and protein expression (D) were significantly higher in diabetic mice compared with nondiabetic controls, respectively (P < 0.01). Diabetic mice administered Ang-(1–7) had significantly lower bone marrow eNOS and nNOS mRNA and protein expression compared with saline-treated diabetic mice after 14 d of treatment (P < 0.01). Coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) resulted in a significant increase in bone marrow eNOS and nNOS mRNA and protein expression in diabetic mice compared to treatment with Ang-(1–7) alone (P < 0.01). **, P < 0.01; ††, P < 0.01 compared to the db + Ang-(1–7) group.
To investigate the effect of diabetes and determine the impact of Ang-(1–7) on the activation of eNOS, we measured phosphorylation of eNOS at the primary activation (Ser1177) and inactivation sites (Thr495). Bone marrow eNOS protein isolated from diabetic mice exhibited significantly lower phosphorylation at Ser1177 and higher phosphorylation at Thr495 when compared with nondiabetic controls (Fig. 3, A and B). Ang-(1–7) treatment in diabetic mice resulted in a significant increase in eNOS phosphorylation at Ser1177 and a significant decrease in eNOS phosphorylation at Thr495. When PD123,319, A-779, L-NAME, or icatibant was coadministered with Ang-(1–7) in diabetic mice, the Ang-(1–7)-mediated effects on eNOS phosphorylation were blocked. No changes in eNOS phosphorylation were observed when Ang-(1–7) was coadministered with losartan.
Fig. 3.
A and B, Ang-(1–7) activates bone marrow eNOS. Phosphorylation of bone marrow eNOS at Ser1177 was significantly decreased in diabetic mice compared with nondiabetic controls (P < 0.01). Diabetic mice treated with Ang-(1–7) for 14 d had significantly increased phosphorylation of bone marrow eNOS at Ser1177 compared with saline-treated diabetic mice (P < 0.01). Coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) resulted in a significant reduction in phosphorylation of bone marrow eNOS at Ser1177 in diabetic mice compared with treatment with Ang-(1–7) alone (P < 0.01). Phosphorylation of bone marrow eNOS at Thr495 was significantly increased in diabetic mice compared with nondiabetic controls (P < 0.01). Diabetic mice treated with Ang-(1–7) for 14 d had significantly decreased phosphorylation of bone marrow eNOS at Thr495 compared with saline-treated diabetic mice (P < 0.01). Coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) resulted in a significant increase in phosphorylation of bone marrow eNOS at Thr495 in diabetic mice compared with treatment with Ang-(1–7) alone (P < 0.01). **, P < 0.01; ††, P < 0.01 compared to the db + Ang-(1–7) group.
Effect of Ang-(1–7) on SOD isoforms in the bone marrow
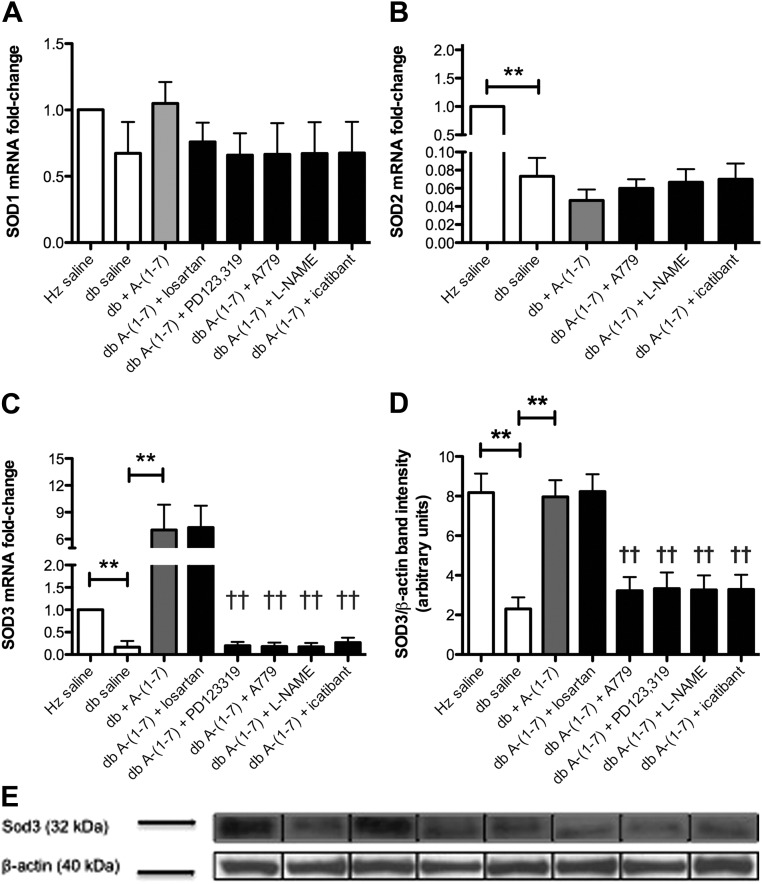
Diabetes causes a decreased expression of various SOD isoforms, resulting in a subsequent increase in oxidative stress (31). In this study, we measured SOD1 (Cu-Zn SOD), SOD2 (mitochondrial SOD), and SOD3 (extracellular SOD) mRNA expression, in which no significant changes in SOD1 expression was detected among the groups evaluated. In contrast, both SOD2 and SOD3 mRNA expression was significantly reduced in diabetic mice when compared with nondiabetic controls (Fig. 4, A–C). When diabetic mice were treated with Ang-(1–7), a significant increase in SOD3 expression was seen; however, changes in SOD2 mRNA expression were not statistically significant. These results were verified using Western blotting to determine protein expression (Fig. 4D). The ability of Ang-(1–7) to increase mRNA and protein expression of SOD3 was completely blocked when coadministered with PD123,319, A-779, L-NAME, or icatibant (P < 0.01) but not with losartan.
Fig. 4.
A–E, Effects of Ang-(1–7) on bone marrow SOD expression. There were no significant differences in bone marrow SOD1 mRNA expression between any groups (A). Both bone marrow SOD2 (B) and SOD3 (C) mRNA expression were significantly reduced in diabetic mice compared with nondiabetic controls (P < 0.01). Although treatment with Ang-(1–7) for 14 d did not have a significant effect on SOD2 mRNA expression, treatment with Ang-(1–7) significantly increased bone marrow SOD3 mRNA expression in diabetic mice. Coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) resulted in a significant decrease in bone marrow SOD3 mRNA expression in diabetic mice compared to treatment with Ang-(1–7) alone (P < 0.01). SOD3 protein expression (D and E) was significantly reduced in diabetic mice compared with nondiabetic controls (P < 0.01), and the administration of Ang-(1–7) to diabetic mice significantly increased bone marrow SOD3 protein expression (P < 0.01). Coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) significantly inhibited its effect in diabrtic mice (P < 0.01). **, P < 0.01; ††, P < 0.01 compared to the db + Ang-(1–7) group.
Ang-(1–7) treatment decreases bone marrow p22-phox expression in diabetes
Hyperglycemia resulting from diabetes induces the expression of nicotinamide adenine dinucleotide phosphate oxidase (NADPH) oxidase through protein kinase C activation, resulting in an increased production of superoxide (32, 33). This can initiate a cascade that ultimately leads to eNOS dysfunction. Bone marrow p22-phox (a subunit of the heterodimeric, membrane bound portion of NADPH oxidase) was significantly higher in diabetic mice when compared with nondiabetic controls. When treated with Ang-(1–7), the expression of p22-phox was reduced when compared with saline treated animals, in which the levels were similar to nondiabetic mice (Fig. 5A). Administration of Ang-(1–7) combined with pharmacological antagonists of the AT2, Mas, or B2 receptors using PD123,319, A-779, or icatibant was able to inhibit Ang-(1–7)-mediated activity. Similarly, coadministration with L-NAME, a NOS inhibitor, was able to inhibit Ang-(1–7) activity with regard to p22-phox mRNA expression. No antagonism was seen in diabetic mice given both Ang-(1–7) and losartan, an AT1 receptor antagonist. The protein expression of p22-phox was consistent with RNA expression (Fig. 5B).
Fig. 5.
A and B, Ang-(1–7) decreases bone marrow p22-phox expression. Bone marrow p22-phox mRNA expression was significantly higher in diabetic mice compared with nondiabetic controls (P < 0.01). Treatment of diabetic mice with Ang-(1–7) for 14 d resulted in a significant decrease in bone marrow p22-phox mRNA expression, comparable with levels measured in nondiabetic controls (P < 0.01). Coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) resulted in significantly higher p22-phox mRNA expression compared with treatment with Ang-(1–7) alone (P < 0.01). There was also a significant increase in bone marrow p22-phox protein expression diabetic mice compared with nondiabetic controls (P < 0.01). Administration of Ang-(1–7) for 14 d significantly decreased bone marrow p22-phox protein expression in diabetic mice, whereas the coadministration of PD123,319, A-779, L-NAME, or icatibant with Ang-(1–7) significantly blocked this effect compared with treatment with Ang-(1–7) alone (P < 0.01). **, P < 0.01; ††, P < 0.01 compared to the db + Ang-(1–7) group.
Ang-(1–7) treatment reduces bone marrow tyrosine nitration in diabetes
The increased production of superoxide and subsequent peroxynitrite formation after eNOS dysfunction in diabetes leads to protein tyrosine nitration, resulting in posttranslational modifications and alterations in protein function (4). Therefore, we measured nitrotyrosine in bone marrow cells isolated from nondiabetic and diabetic mice. The percentage of nitrated bone marrow cells in diabetic mice was significantly higher compared with nondiabetic controls (Fig. 6A). Administration of Ang-(1–7) to diabetic mice for 14 d resulted in a significant reduction in tyrosine nitration in the bone marrow. Coadministration of Ang-(1–7) with A-779, PD123,319, L-NAME, or icatibant blocked the Ang-(1–7)-mediated decreases of nitrotyrosine found in the bone marrow. However, coadministration of Ang-(1–7) with losartan did not block the effect seen after Ang-(1–7) administration alone.
Fig. 6.
A and B, Bone marrow tyrosine nitration and correlation with nitrite levels. Nucleated bone marrow cells from diabetic mice had a significantly higher percent tyrosine nitration compared with nondiabetic controls (P < 0.01). After treatment with Ang-(1–7) for 14 d, the percentage of cells nitrated in diabetic bone marrow was significantly decreased (P < 0.01). Coadministration of Ang-(1–7) with A-779, PD123,319, L-NAME, or icatibant resulted in a significant increase in bone marrow tyrosine nitration (P < 0.05), whereas coadministration of Ang-(1–7) with losartan did not result in a significant change compared with Ang-(1–7) treatment alone. The scatter plot of the relationship between bone marrow nitrite levels and percent tyrosine nitration in nondiabetic and diabetic mice shows a significant negative correlation (P < 0.01). **, P < 0.01; ††, P < 0.01 compared to the db + Ang-(1–7) group.
To demonstrate the potential role of eNOS dysfunction in diabetic bone marrow, NO levels measured in all groups were correlated with tyrosine nitration. There was a significant negative correlation between bone marrow NO levels and protein tyrosine nitration levels, in which lower bone marrow NO levels were associated with increased bone marrow protein tyrosine nitration (Fig. 6B). Diabetic mice treated with Ang-(1–7) for 14 d showed significantly increased bone marrow NO levels and decreased bone marrow protein tyrosine nitration, suggesting a potential reversal of the negative effects of eNOS dysfunction observed in diabetes, including increased superoxide formation, a subsequent decrease in NO levels, and increases in protein tyrosine nitration.
Discussion
Our study demonstrates the in vivo effects of Ang-(1–7) on markers of oxidative and nitrative stress in a murine model of type 2 diabetes. We showed that Ang-(1–7) administration decreased ROS levels in the bone marrow of db/db mice, coupled by a decrease in p22-phox expression and an increase in SOD3 expression. In addition, Ang-(1–7) increased NO levels. These changes were linked with increased eNOS phosphorylation at Ser1177 and reduced protein tyrosine nitration in the bone marrow of db/db mice. The effects of Ang-(1–7) were modulated by a Mas-, AT2-, and bradykinin B2 receptor-dependent mechanism.
Previous studies have shown that hyperglycemia can increase NADPH oxidase expression via protein kinase C activation (32, 33), in which increased NADPH oxidase expression results in an increased production of ROS. Elevated production of ROS through NADPH oxidase combined with a decrease in superoxide-removal systems, specifically SOD3, could further increase oxidative stress. The SOD family of enzymes is part of a critical defense mechanism for managing superoxide levels, in which down-regulation of this system can increase cytotoxic levels of ROS, ultimately leading to tissue damage and the long-term complications seen in diabetes.
Similar to studies examining the effects of diabetes in other tissues, we observed a decrease in NO levels and an increase in eNOS and nNOS mRNA and protein expression in the bone marrow, which is often a direct result of NOS dysfunction (6, 34). The end result is a decrease in the production of NO and an increase in the production of superoxide anions via eNOS due to the dissociation of the heme ferrous-dioxygen complex in the oxygenase domain of eNOS. Phosphorylation of eNOS was also altered in diabetic bone marrow, in which an increase in phosphorylation at Thr495 and a decrease in phosphorylation at Ser1177 was observed. Phosphorylation of eNOS at Ser1177 results in activation, whereas Thr495 phosphorylation inactivates eNOS.
Lastly, increased oxidative stress in diabetes, specifically an increased production of superoxide anions, can result in the formation of peroxynitrite, an oxidant and nitrating agent. Peroxynitrite can cause protein tyrosine nitration, a posttranslational modification that can ultimately result in altered protein structure and function, and may also be the cause of many of the long-term complications of diabetes (4, 35, 36). Indeed, we observed an increase in protein tyrosine nitration in the bone marrow of db/db mice. In addition to the commonly referred to circulating or systemic RAS, tissue-specific RAS also exist in the body, including in the pancreas, kidneys, heart, skin, and bone marrow (37). Both in vitro and in vivo studies have shown an important role for the RAS in the bone marrow microenvironment, especially in hematopoiesis. For example, Ang-(1–7) itself has been demonstrated to stimulate hematopoietic progenitor cells after chemotherapy in patients diagnosed with cancer (24, 38). The bone marrow plays a vital role in the generation of progenitor cells responsible for multiple functions including wound healing, neovascularization, and immune function, all of which are compromised in diabetes. Evidence points to the role of oxidative stress in cellular damage, which may result in many of the long-term complications of diabetes such as cardiovascular and immune dysfunction.
Ang-(1–7) has been shown to bind to the G protein-coupled receptor Mas, which results in an increased production of both NO and bradykinin. However, there is evidence for the involvement of the AT2 receptor. In agreement with our present results, a recent paper showed that in spontaneously hypertensive rats, Ang-(1–7) administration up-regulated phosphorylated eNOS (Ser1177)/eNOS protein expression in cardiac tissues, an effect that was blocked by bradykinin B2 receptor and AT2 receptor antagonism (26). Because AT2 receptors are up-regulated after injury (e.g. damage from increased oxidative stress), there may be a role for AT2 receptors coupled with Mas receptors in diabetes. This role could potentially involve heterodimerization of the two receptors (39). In addition, other Ang-(1–7) signaling pathways that could potentially explain our results have been suggested in previous studies. This includes the counterregulation of Ang II signaling by Ang-(1–7), showing specifically that the activation of NADPH oxidase by Ang II is attenuated by Ang-(1–7) treatment in vitro (40). Studies have also shown that Ang-(1–7), through binding to the Mas receptor, activates Akt-dependent pathways including the stimulation of Akt phosphorylation via Akt kinase (41, 42). These pathways ultimately lead to eNOS activation and increases in NO production through mechanisms similar to those seen in our study, specifically phosphorylation of eNOS at Ser1177 and dephosphorylation of eNOS at Thr495 (41).
An interesting result was seen when measuring ROS in the bone marrow of db/db mice, in which blockade of the AT1 receptor using losartan inhibited the effects of Ang-(1–7). This was the only parameter measured that was affected by Ang-(1–7) that was blocked by losartan in this study. There are a few potential explanations for this effect. First, RAS receptor expression may be altered in diabetic bone marrow, which could change the dynamics of AT1 receptor blockade or Mas and AT2 receptor activation. Although we did not investigate bone marrow RAS expression in the current study, other groups have demonstrated that AT1 receptor expression can be altered by various factors. Specifically, decreased circulating Ang II levels have been shown to up-regulate AT1 receptor expression (43). Losartan administration itself has also been shown to decrease myocardial AT1 receptor expression (44). In addition, other non-RAS factors can alter AT1 receptor expression, including the cytokines IL-1β, IL-1, and TNF-α, which are increased in diabetes (45). The second explanation could be the dose used for Ang-(1–7) and length of treatment in this study, which was chosen based on unpublished in vivo dose escalation studies in our laboratory examining progenitor cell counts in diabetic bone marrow. These studies were performed using doses ranging from 100 to 1000 μg/kg·d for 14 d, in which 500 μg/kg·d was shown to be the most effective at increasing progenitor cell numbers. However, the effects of various doses of Ang-(1–7) on oxidative stress markers were not determined. In vitro studies from other groups have shown that at higher doses, Ang-(1–7) may have some agonist activity at AT1 receptors (27). Additional studies will have to be performed to thoroughly investigate the effects of various Ang-(1–7) doses and study durations on RAS expression and oxidative stress markers in diabetic bone marrow. In addition, Ang-(1–7)-associated increases in bone marrow nitrite levels were not significantly blocked by coadministration with icatibant, although icatibant did block the effects of all other changes in oxidative stress markers caused by Ang-(1–7). Based on other published studies and our data, the bradykinin B2 receptor may play an integral role in the mechanism of action of Ang-(1–7), and the results in this study may be a reflection of the high variation of nitrite levels often seen in tissue samples from in vivo studies.
In conclusion, our findings demonstrate that sc Ang-(1–7) treatment for 14 d decreased oxidative stress in the bone marrow in a murine model of type 2 diabetes and had significant effects on cellular components, including NADPH oxidase and SOD. Although additional in vitro and in vivo studies will need to be undertaken to further dissect these mechanisms and signaling pathways, pharmacological treatment with Ang-(1–7) along with other first-line therapies may prove useful to treat diabetes-induced oxidative stress and prevent its associated long-term complications.
Supplementary Material
Acknowledgments
We thank Dr. Enrique Cadenas for his expertise and intellectual contributions to this paper. Author contributions included the following: N.M.M. researched the data and wrote the manuscript; C.J.M. researched the data and reviewed/edited the manuscript; T.E. researched the data; N.R. researched the data; S.S.J. researched the data; G.S.D. reviewed/edited the manuscript; S.G.L. researched the data and reviewed/edited the manuscript; and K.E.R. researched the data and reviewed/edited the manuscript.
This work was supported by National Institutes of Health Grant 5R01HL082722-02.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- Ang-(1–7)
- Angiotensin-(1–7)
- Ang II
- angiotensin II
- AT1
- angiotensin type 1 receptor
- AT2
- angiotensin type 2 receptor
- eNOS
- endothelial nitric oxide synthase
- iNOS
- inducible NOS
- L-NAME
- nitro-l-arginine methyl ester
- NADPH
- nicotinamide adenine dinucleotide phosphate oxidase
- nNOS
- neuronal NOS
- NO
- nitric oxide
- NOS
- NO synthase
- RAS
- renin-angiotensin system
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase.
References
- 1. Maritim AC, Sanders RA, Watkins JB., 3rd 2003. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38 [DOI] [PubMed] [Google Scholar]
- 2. Kocic R, Radenkovic S, Mikic D, Kocic G, Cvetkovic T, Pavlovic D. 1998. Oxidative stress in the development of diabetes during hypothyroidism. Postgrad Med J 74:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ceriello A, Motz E. 2004. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 24:816–823 [DOI] [PubMed] [Google Scholar]
- 4. Radi R. 2004. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA 101:4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zou MH, Cohen R, Ullrich V. 2004. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium 11:89–97 [DOI] [PubMed] [Google Scholar]
- 6. Bitar MS, Wahid S, Mustafa S, Al-Saleh E, Dhaunsi GS, Al-Mulla F. 2005. Nitric oxide dynamics and endothelial dysfunction in type II model of genetic diabetes. Eur J Pharmacol 511:53–64 [DOI] [PubMed] [Google Scholar]
- 7. Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T. 2008. Angiotensin metabolites can stimulate receptors of the Mas-related genes family. Mol Cell Biochem 319:115–123 [DOI] [PubMed] [Google Scholar]
- 8. Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. 2003. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 100:8258–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sabuhi R, Ali Q, Asghar M, Al-Zamily NR, Hussain T. 2011. Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: opposing effects in lean and obese Zucker rats. Am J Physiol Renal Physiol 300:F700–F706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li P, Chappell MC, Ferrario CM, Brosnihan KB. 1997. Angiotensin-(1–7) augments bradykinin-induced vasodilation by competing with ACE and releasing nitric oxide. Hypertension 29(1 Pt 2):394–400 [DOI] [PubMed] [Google Scholar]
- 11. Ferrario CM, Averill DB, Brosnihan KB, Chappell MC, Iskandar SS, Dean RH, Diz DI. 2002. Vasopeptidase inhibition and Ang-(1–7) in the spontaneously hypertensive rat. Kidney Int 62:1349–1357 [DOI] [PubMed] [Google Scholar]
- 12. Heitsch H, Brovkovych S, Malinski T, Wiemer G. 2001. Angiotensin-(1–7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension 37:72–76 [DOI] [PubMed] [Google Scholar]
- 13. Benter IF, Ferrario CM, Morris M, Diz DI. 1995. Antihypertensive actions of angiotensin-(1–7) in spontaneously hypertensive rats Am J Physiol 269(1 Pt 2):H313–H319 [DOI] [PubMed] [Google Scholar]
- 14. Benter IF, Diz DI, Ferrario CM. 1995. Pressor and reflex sensitivity is altered in spontaneously hypertensive rats treated with angiotensin-(1–7) Hypertension 26(6 Pt 2):1138–1144 [DOI] [PubMed] [Google Scholar]
- 15. Roks AJ, van Geel PP, Pinto YM, Buikema H, Henning RH, de Zeeuw D, van Gilst WH. 1999. Angiotensin-(1–7) is a modulator of the human renin-angiotensin system. Hypertension 34:296–301 [DOI] [PubMed] [Google Scholar]
- 16. Dias-Peixoto MF, Santos RA, Gomes ER, Alves MN, Almeida PW, Greco L, Rosa M, Fauler B, Bader M, Alenina N, Guatimosim S. 2008. Molecular mechanisms involved in the angiotensin-(1–7)/Mas signaling pathway in cardiomyocytes. Hypertension 52:542–548 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y, Qian C, Roks AJ, Westermann D, Schumacher SM, Escher F, Schoemaker RG, Reudelhuber TL, van Gilst WH, Schultheiss HP, Tschöpe C, Walther T. 2010. Circulating rather than cardiac angiotensin-(1–7) stimulates cardioprotection post myocardial infarction. Circ Heart Fail 3:286–293 [DOI] [PubMed] [Google Scholar]
- 18. Schmaier AH. 2003. The kallikrein-kinin and the renin-angiotensin systems have a multilayered interaction. Am J Physiol Regul Integr Comp Physiol 285:R1–R13 [DOI] [PubMed] [Google Scholar]
- 19. Shen B, El-Dahr SS. 2006. Cross-talk of the renin-angiotensin and kallikrein-kinin systems. Biol Chem 387:145–150 [DOI] [PubMed] [Google Scholar]
- 20. Gorelik G, Carbini LA, Scicli AG. 1998. Angiotensin 1–7 induces bradykinin-mediated relaxation in porcine coronary artery. J Pharmacol Exp Ther 286:403–410 [PubMed] [Google Scholar]
- 21. Fernandes L, Fortes ZB, Nigro D, Tostes RC, Santos RA, Catelli De Carvalho MH. 2001. Potentiation of bradykinin by angiotensin-(1–7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension 37(2 Part 2):703–709 [DOI] [PubMed] [Google Scholar]
- 22. Ueda S, Masumori-Maemoto S, Wada A, Ishii M, Brosnihan KB, Umemura S. 2001. Angiotensin(1–7) potentiates bradykinin-induced vasodilatation in man. J Hypertens 19:2001–2009 [DOI] [PubMed] [Google Scholar]
- 23. Rodgers KE, Xiong S, diZerega GS. 2002. Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol 49:403–411 [DOI] [PubMed] [Google Scholar]
- 24. Rodgers K, Xiong S, DiZerega GS. 2003. Effect of angiotensin II and angiotensin(1–7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol 51:97–106 [DOI] [PubMed] [Google Scholar]
- 25. Heringer-Walther S, Eckert K, Schumacher SM, Uharek L, Wulf-Goldenberg A, Gembhardt F, Schultheiss HP, Rodgers K, Walther T. 2009. Angiotensin-(1–7) stimulates hematopoietic progenitor cells in vitro and in vivo. Haematologica 94:857–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Costa MA, Lopez Verrilli MA, Gomez KA, Nakagawa P, Peña C, Arranz C, Gironacci MM. 2010. Angiotensin-(1–7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 299:H1205–H1211 [DOI] [PubMed] [Google Scholar]
- 27. Gurzu B, Costuleanu M, Slatineanu SM, Ciobanu A, Petrescu G. 2005. Are multiple angiotensin receptor types involved in angiotensin-(1–7) actions on isolated rat portal vein. J Renin Angiotensin Aldosterone Syst 6:90–95 [DOI] [PubMed] [Google Scholar]
- 28. Jujo K, Ii M, Losordo DW. 2008. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol 45:530–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cosentino F, Hishikawa K, Katusic ZS, Lüscher TF. 1997. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96:25–28 [DOI] [PubMed] [Google Scholar]
- 30. Hohenstein B, Hugo CP, Hausknecht B, Boehmer KP, Riess RH, Schmieder RE. 2008. Analysis of NO-synthase expression and clinical risk factors in human diabetic nephropathy. Nephrol Dial Transplant 23:1346–1354 [DOI] [PubMed] [Google Scholar]
- 31. Kamata K, Kobayashi T. 1996. Changes in superoxide dismutase mRNA expression by streptozotocin-induced diabetes. Br J Pharmacol 119:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguvhi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. 2003. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol 14(8 Suppl 3):S227–S232 [DOI] [PubMed] [Google Scholar]
- 33. Xia L, Wang H, Munk S, Kwan J, Goldberg HJ, Fantus IG, Whiteside CI. 2008. High glucose activates PKC-ζ and NADPH oxidase through autocrine TGF-β1 signaling in mesangial cells. Am J Physiol Renal Physiol 295:F1705–F1714 [DOI] [PubMed] [Google Scholar]
- 34. Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. 2001. Mechanisms underlying endothelial function in diabetes mellitus. Circ Res 88:E14–E22 [DOI] [PubMed] [Google Scholar]
- 35. Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. 2003. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem 278:33972–33977 [DOI] [PubMed] [Google Scholar]
- 36. Chi Q, Wang T, Huang K. 2005. Effect of insulin nitration by peroxynitrite on its biological activity. Biochem Biophys Res Commun 330:791–796 [DOI] [PubMed] [Google Scholar]
- 37. Haznedaroglu IC, Beyazit Y. 2010. Pathobiological aspects of the local bone marrow renin-angiotensin system: a review. J Renin Angiotensin Aldosterone Syst 11:205–213 [DOI] [PubMed] [Google Scholar]
- 38. Rodgers KE, Oliver J, diZerega GS. 2006. Phase I/II dose escalation study of angiotensin 1–7 [A(1–7)] administered before and after chemotherapy in patients with newly diagnosed breast cancer. Cancer Chemother Pharmacol 57:559–568 [DOI] [PubMed] [Google Scholar]
- 39. Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. 2005. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension 46:937–942 [DOI] [PubMed] [Google Scholar]
- 40. Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. 2007. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension 50:1093–1098 [DOI] [PubMed] [Google Scholar]
- 41. Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. 2007. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49:185–192 [DOI] [PubMed] [Google Scholar]
- 42. Muñoz MC, Giani JF, Dominici FP. 2010. Angiotensin-(1–7) stimulates the phosphorylation of Akt in rat extracardiac tissues in vivo via receptor Mas. Regul Pept 161:1–7 [DOI] [PubMed] [Google Scholar]
- 43. Lassègue B, Alexander RW, Nickenig G, Clark M, Murphy TJ, Griendlink KK. 1995. Angiotensin II down-regulates the vascular smooth muscle AT1 receptor by transcriptional and post-transcriptional mechanisms: evidence for homologous and heterologous regulation. Mol Pharmacol 48:601–609 [PubMed] [Google Scholar]
- 44. Zong WN, Yang XH, Chen XM, Huang HJ, Zheng HJ, Qin XY, Yong YH, Cao K, Huang J, Lu XZ. 2011. Regulation of angiotensin-(1–7) and angiotensin II type 1 receptor by telmisartan and losartan in adriamycin-induced rat heart failure. Acta Pharmacol Sin 32:1345–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nickenig G. 2002. Central role of the AT(1)-receptor in atherosclerosis. J Hum Hypertens 16(Suppl 3):S26–S33 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.