Abstract
Emerging evidence supports the hypothesis that the skeleton is an endocrine organ that regulates energy metabolism through the release of the osteoblast-derived hormone, osteocalcin (Ocn). This bone-pancreas endocrine network is controversial because important gaps remain to be filled in our knowledge of the physiological effects of Ocn in multiple organs and the complex alterations in other hormonal networks induced by Ocn administration. A key step toward understanding the integrative regulation of energy metabolism by bone is the identification of GPCR family C group 6 member A (GPRC6A) as the Ocn receptor. GPRC6A is an amino acid-sensing G protein-coupled receptor highly expressed in β-cells and is activated by recombinant Ocn in vitro and in vivo but that is widely expressed in tissues other than the pancreas and is capable of sensing multiple structurally unrelated ligands, including l-amino acids, cations, and anabolic steroids in addition to Ocn. The broad expression and multiligand specificity of GPRC6A is identifying both systemic and paracrine regulation of seemingly disparate biological processes, ranging from energy metabolism, sexual reproduction, hypothalamic-pituitary function, bone formation, and prostate cancer. Consistent with the existence of more complex endocrine networks, ablation of GPRC6A in Gprc6a−/− mice results in complex metabolic abnormalities, including obesity, glucose intolerance, hepatic steatosis, insulin resistance, hyperphosphatemia, osteopenia, plus several hormonal abnormalities, including decreased circulating testosterone, IGF-I, and insulin and increased estradiol, LH, GH, and leptin. Recombinant Ocn also regulates testosterone production by the testes and male fertility through a GPRC6A-dependent mechanism, and testosterone regulation of LH secretion is abnormal in Gprc6a−/− mice. Thus, GPRC6A, as the biologically relevant receptor for Ocn, defines not only a molecular mechanism for linking bone metabolism with metabolic regulation of β-cells and sexual reproduction but also as a receptor shared by testosterone and dietary factors, and it is also involved in multiple endocrine networks integrating the functions of pancreas, muscle, liver, fat, testes, bone, and the hypothalamic-pituitary axis with alterations in both environmental and endogenous ligands.
Stimulation of hormone secretion from endocrine organs and activation of hormone receptors in target tissues comprise negative or, less commonly, positive feedback loops that coordinate biological functions to maintain systemic homeostasis. Hormone ligand-receptor interactions tend to control a limited number of biological processes through their restricted tissue expression and activation by a specific ligand. For example, increments in serum glucose lead to glucose uptake by β-cells and release of insulin into the circulation. Circulating insulin targets insulin receptors (IR) in liver to decrease glucose production and in muscle and fat to increase glucose uptake and use that restores serum glucose levels to normal. Complex hierarchical endocrine networks, however, are being identified through cross talk between known and newly discovered endocrine pathways. Indeed, fat cells secrete hormones, such as leptin, adiponectin, and resistin as well as fatty acids that target distinct receptors in diverse tissues to regulate food intake, energy expenditure, and insulin sensitivity. Similarly, nutrient intake stimulates incretin hormones, such as glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide, ghrelin, cholecystokinin, and gastrin, which regulate insulin secretion and β-cell mass through specific G protein-coupled receptors (GPCR) (1, 2). Thus, regulation of glucose homeostasis involves multiple interrelated physiological networks that act in a coordinated manner to control overall energy metabolism. Our understanding of glucose and energy metabolism has recently expanded to include involvement of bone in regulating glucose homeostasis, both as a target for circulating insulin (3) and as an endocrine organ that releases undercarboxylated osteocalcin (Ocn) into the circulation, which acts as a hormone to regulate glucose homeostasis and energy metabolism (3–8). The discovery of the Ocn-sensing receptor (OcnR), which is a multiligand GPCR (9), has further expanded these potential networks.
Bone regulation of energy metabolism: initial hypothesis
This new paradigm of endocrine function (Fig. 1) proposes that insulin activates IR in osteoblasts to increase Ocn secretion and bioactivity; in turn, circulating underdecarboxylated Ocn activates a putative OcnR in β-cells (4, 10) to regulate insulin secretion and other β-cell functions (11).
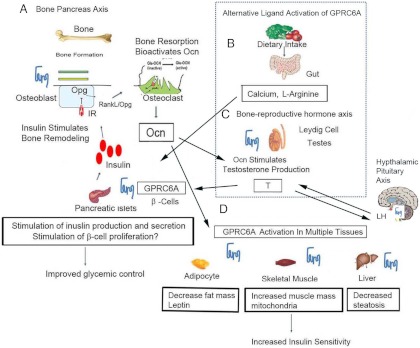
Fig. 1.
A, Ocn released from bone resorption activates GPRC6A to regulate insulin secretion in β-cells. Insulin stimulates bone resorption releasing bioactive Ocn. GPRC6A may also be a receptor shared by nutrients (B) and testosterone, which stimulates insulin secretion (C). In addition, Ocn may target GPRC6A in other tissues to regulate testosterone production (C) and to promote insulin sensitivity (D). GPRC6A ligands may also regulate LH production.
The existence of a bone-pancreas endocrine network is supported by several observations. First, osteoblast-specific deletion of IR results in loss of insulin-mediated release of bioactive Ocn from bone (4). Second, ablation of osteocalcin (Ocn−/−) leads to glucose intolerance in mice and compound heterozygous mutant IRob-cko+/−/Ocn+/− mice display glucose intolerance similar to IRob-cko mice or Ocn[minus]/[minus] mice (12). Third, genetically modified mice with an increase in uncarboxylated osteocalcin are protected from type 2 diabetes (T2DM) and obesity (13) and the administration of recombinant Ocn to mice stimulates β-cell functions, including increase in β-cell mass and insulin secretion (10, 12).
Many questions regarding the endocrine functions of Ocn remain, including the identity of the receptor mediating the effects of Ocn in the efferent limb of these circuits, understanding direct organ-specific effects of Ocn, and unraveling the biological and clinical relevance of these new endocrine networks that appear to coordinate the activities of organs not previously recognized as being physiologically linked.
GPCR family C group 6 member A (GPRC6A), a nutrient GPCR activated by Ocn
An important step toward addressing these questions is the discovery of GPRC6A is an Ocn-sensing receptor (9). GPRC6A is a nutrient sensing receptor belonging to family C of seven-transmembrane (7-TM) receptors, which also includes metabotropic glutamate receptors, the γ-aminobutyric acid type B receptor, the calcium-sensing receptor, and taste receptors (14). Several findings support the conclusion that GPRC6A is the biologically relevant OcnR. First, Ocn activates GPRC6A in a dose-dependent fashion in the presence of calcium (9). Second, Ocn directly binds to wild-type cells expressing Gprc6a but not Gprc6a-deficient cells (12). Third, Gprc6a is expressed tissues involved in regulating energy metabolism that are affected by recombinant Ocn administration (10, 15). GPRC6A is highly expressed in mouse pancreatic tissue and in the mouse TC-6 pancreatic β-cell line and recombinant Ocn (rOcn) stimulates ERK activity and insulin secretion in the pancreas (10). Fourth, compound heterozygous mice lacking one copy of Ocn and GPRC6A display additive effects on glucose metabolism (12). Finally, ablation of Gprc6a in Gprc6a−/− mice results in glucose intolerance, impaired insulin secretion, and undermasculinization in vivo (15), a phenotype that resembles that of Ocn−/− mice. The effect of recombinant Ocn administration to stimulate ERK activity in the pancreas and increase serum insulin levels are also markedly attenuated in Gprc6a−/−mice.
Broad expression and multiligand specificities of GPRC6A defines additional endocrine networks
GPRC6A is capable of sensing multiple structurally unrelated ligands and is widely expressed. GPRC6A has unique structure/functional characteristics (Fig. 2) that permits it to sense dissimilar ligands. GPRC6A evolved from the fusion of a periplasmic nutrient venus fly trap motif (VFTM) and a classical 7-TM domain. This fusion of the VFTM and 7-TM creates the structural basis for both independent biological and pharmacological actions of othosteric ligands and allosteric modulators with different affinities and efficacies but also for cooperative interactions between the orthosteric and allosteric ligand binding sites.
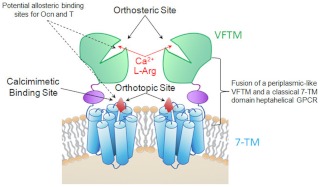
Fig. 2.
Hypothetical model of ligand activation and allosteric modulation of GPRC6A. GPRC6A consists of a VFTM for sensing nutrients and the 7-TM domain and like other members of this family may function as a dimer. The orthotopic agonists, such as L-Arg and Ca2+, which are likely to be the natural ligands, binding sites are located adjacent to each other in the VFTM, whereas binding sites for allosteric modulators, such as calcimimetics, are located in the 7-TM region, possibly representing a retained (cryptic) ligand binding site in the classical GPCR region. We hypothesize that Ocn and testosterone (T) are also allosteric activators of GPRC6A with mechanism of activation similar to calcimimetics because they require the presence of a threshold concentration of Ca2+ for signaling to occur. Other amino acids and cations activate GPRC6A (61).
We have found that Ocn activation of GPRC6A requires the presence of extracellular calcium (9), suggesting that Ocn is binding to a site distinct from the orthosteric cation binding pocket in GPRC6A. Calcium needs only to be present, as evidence by Ocn enhancing the activation of GPRC6A in the presence of threshold calcium concentrations (9). Because allosteric modulators likely bind to sites in GPRC6A other than the orthosteric domain, they can be structurally diverse (i.e. there is no selective pressure to maintain uniformity of nonessential allosteric binding sites). In addition to Ocn, GPRC6A is activated by cations (such as calcium and zinc) and amino acids [such as l-arginine (L-Arg,) and l-lysine] as well as mediates the nongenomic membrane actions of testosterone (9, 16–19). GPRC6A senses basic l-amino acids and extracellular cations via orthosteric binding sites in the VFTM. Allosteric modulators, such as calcimimetics, possibly Ocn and testosterone, may bind to distinct sites in the transmembrane domain (20). Thus, rather than the typical ligand-receptor interaction that characterizes most endocrine networks, multiple ligands or possibly mixtures of ligands may work in concert to modulate GPRC6A activity.
There are many gaps in our knowledge of GPRC6A downstream signaling pathways. GPRC6A is purported to be coupled to Gαq and possibly Gαi; indeed, we (9) and others (18) have shown that activation of GPRC6A results in increased intracellular calcium and ERK activation and can be inhibited by β-arrestins. However, GPRC6A is atypical in that, although ERK activation is inhibited by a pertussis toxin, GPRC6A fails to inhibit cAMP production in osteoblasts (9, 19), and in some tissues, such as Leydig cells, it stimulates cAMP production (12), suggesting the capacity to also couple to Gαs.
Moreover, GPRC6A is present in nearly all tissues tested so far (except the small and large intestines and parathyroid gland) (9, 17, 18), suggesting that GPRC6A may regulate multiple biological processes. In addition to GPRC6A-regulating insulin secretion, there is emerging evidence that this nutrient receptor may regulate testosterone production in Leydig cells (3, 12), LH release from the pituitary (16), and possibly gastrin release from the stomach (21) as well as regulate prostate cell functions (22). The promiscuity of GPRC6A with regard to both expression and ligand specificity may provide a molecular explanation for the multiple well-known associations between nutrients, sex hormones, and metabolic functions.
The multiple ligand specific and broad tissue distribution of GPRC6A suggest an even more complex communication between various organs and other physiological networks. With regard to the biological function of GPRC6A in other organs, Gprc6a−/− mice, in addition to glucose intolerance, have complex metabolic abnormalities, including obesity, hepatic steatosis, insulin resistance, hyperphosphatemia, and osteopenia as well as several hormonal abnormalities, including decreased serum testosterone, IGF-I, and insulin and increased estradiol, LH, GH, and leptin (15). More work is needed to understand the full extent of the cross talk between various organs mediated by GPRC6A and its multiple ligands, but several potential endocrine networks are emerging from the detailed analysis of GPRC6A−/− mice and the multiligand specificity of GPRC6A.
Role of GPRC6A in insulin resistance
Ocn and GPRC6A regulate biological processes beyond β-cell function. In this regard, Gprc6a−/− mice have complex metabolic abnormalities affecting liver, fat, and muscle, including hepatic steatosis, increase adipose tissue and insulin resistance. Conversely, Ocn enhances insulin sensitivity (12) (Fig. 1D) through possible direct effects to activate GPRC6A in skeletal muscle, liver, and adipocytes (9, 17, 18, 23, 24). In this regard, Ocn administration to mice reduces visceral fat and insulin resistance, increases mitochondrial content of skeletal muscle and energy expenditure, and induces hepatic alterations that protects mice from high fat diet-induced hepatic steatosis (12, 13). Recombinant Ocn administration is also protective against obesity and T2DM induced by diet or hyperphagia in various animal models (13, 25). Moreover, the ability of a high-fat diet to induce glucose intolerance in mice is attenuated by receptor activator of nuclear factor-κB ligand-mediated increases in bone resorption and release of bioactive Ocn.
Implications of nutrient-sensing functions of GPRC6A
Nutrients regulate the function of peripheral organs in a coordinated fashion that are mediated through the release of hormones and complex integration of signaling pathways. The GPRC6A map provides a metabolic sensor that enables the organism to adapt to both external and internal environmental changes. Indeed, GPRC6A may also be a receptor shared by multiple ligands that mediates responses to nutrients and endogenous ligands that are known to regulate energy metabolism, but heretofore their mechanism of action was poorly understood (Fig. 1, B–D). In this regard, GPRC6A may provide a common mechanism in β-cells for diverse ligands to regulate insulin secretion (9). Amino acids directly modulate insulin secretion and contribute to the maintenance of β-cell function, resulting in an improvement of insulin release (26). Indeed, L-Arg administration stimulates insulin secretion, and this action has been used diagnostically to evaluate β-cell function. L-Arg effects on insulin secretion in β-cell appear to be due in part to activation of GPRC6A. Whether amino acids also affect insulin resistance in liver, muscle, and fat through a GPRC6A-dependent mechanism is not clear. There are examples, however, of L-Arg and l-ornithine supplementation enhancement of muscle response to exercise, but increased serum amino acids have also been associated with insulin resistance (27). Dietary supplementation of L-Arg reduces fat deposition and increases lean body mass and/or protein accretion in rats and pigs (28, 29). The multiligand specificity of GPRC6A could also potentially explain a wide range of factors associated with metabolic syndrome, such as the association between low Ocn and metabolic syndrome (30) as well as the salutary effects of a high-protein diet on metabolic parameters in obesity via amino acids (31) and mechanistic insights into the adaptive mechanisms involved in insulin secretion in malnutrition (32).
Anabolic steroid-sensing functions of GPRC6A
GPRC6A appears to mediate the nongenomic effects of testosterone as evidence by the loss of the rapid signaling responses to testosterone administration in Gprc6a−/− mice (16). Testosterone also has rapid effects on insulin secretion in β-cells (33), which also appear to be mediated through activation of GPRC6A (16). Testosterone is known to have a role in glucose homeostasis as well as obesity and lipid metabolism (9–10, 16, 34, 35). Thus, testosterone may have both effects on insulin sensitivity as well as a direct effect on insulin secretion that are at least in part mediated by GPRC6A (36). Because insulin has a suppressive effect on testicular steroidogenesis (36), theoretically a positive feedback loop may exist linking testosterone production by Leydig cells with testosterone stimulation of insulin release from the pancreas. Ocn-mediated activation of GPRC6A also affects testosterone production by the Leydig cells in the testes (Fig. 1C) through GPRC6A (12, 15), which in turn may exert effects on muscle, and adipocyte function through androgen receptors activation or through nongenomic, membrane effects via GPRC6A (12, 16). GPRC6A may also regulate the aromatization of androgens to estrogens, thereby accounting for the increased estrogen levels in Gprc6a−/− mice. If so, Ocn, testosterone, and GPRC6A may create a bone-testes-β-cell endocrine loop (Fig. 1C), controlling male fertility, as has been recently proposed by others (6). This network might also link anabolic steroids to energy metabolism via regulation of β-cell functions via GPRC6A (Fig. 1C). This axis may be particularly relevant to skeletal growth during male puberty. During rapid skeletal growth, increments in testosterone levels initiated by alterations in the hypothalamic-pituitary axis may be further augmented by increasing Ocn due to skeletal growth, thereby increasing bone size in males (37).
The potential relevance of GPRC6A to human diseases involving energy metabolism is also supported by several observations. Clinical studies show that undercarboxylated Ocn levels are inversely associated with glycemic status and insulin resistance in humans (38). In addition, Ocn is lower in patients with diabetes, who have a low-turnover bone disease, and Ocn is inversely correlated with body mass index, fasting glucose and insulin, triglycerides, and leptin and positively correlated with adiponectin (39). There is also an association between obesity and reduction in undercaboxylated Ocn (40). Genome-wide association studies show that GPRC6A is a genetic locus highly associated with C-reactive protein levels (41), a heritable marker of chronic inflammation that is strongly associated with diabetes mellitus (42) and cardiovascular diseases (43, 44). However, despite human Ocn to activate GPRC6A, given the differences in the mouse and human Ocn genes (45), additional data are needed to confirm the relevance of Ocn activation of GPRC6A in humans.
Role of GPRC6A in hypothalmic-pituitary function
GPRC6A function to mediate the nongenomic effects of testosterone also may provide new insights in to regulation of LH secretion. GPRC6A may be expressed in both the hypothalamus and anterior pituitary gland. In addition, Gprc6a−/− mice exhibit paradoxical testosterone-mediated stimulation of LH secretion rather than suppression, suggesting additional abnormalities of the hypothalamic-pituitary-sex hormone axis (16). This suggests that GPRC6A may be mediating the rapid signaling responses between the testes and hypothalamic/pituitary axis, although additional studies are needed to determine whether GPRC6A functions at the level of the hypothalamus or anterior pituitary. Elucidation of the role of GPRC6A in the hypothalamic-pituitary-testicular endocrine axis could provide insights into effects of nutrients on sexual reproduction as well as provide possible pathways that may disrupted in diseases such as polycystic ovary disease, which like Gprc6a−/− null mice display a paradoxical increase in LH secretion in response to testosterone administration (46). In addition, regulation of LH secretion by testosterone via GPRC6A and activation of GPRC6A by Ocn released from bone and regulation of testosterone production by Ocn may provide a linkage between skeletal growth and alterations in endocrine functions during puberty in males (37). Because the insulin secretion in response to amino acids is less severely affected in pancreatic islets derived from T2DM donors compared with glucose sensing (47), GPRC6A may also be an alternative therapeutic target for treatment of T2DM.
Role of GPRC6A and prostate cancer
There are also compelling data that GPRC6A is involved in the pathogenesis of prostate cancer. Genome-wide association studies have identified GPRC6A as one of five novel genetic loci highly associated with prostate cancer in the Japanese and Chinese population (48, 49). In addition, GPRC6A is expressed at higher levels in human prostate cancer cells and prostate cancer tissues and small interfering RNA knockdown of GPRC6A attenuates these response in human prostate cancer cell lines (22). GPRC6A is also coupled to signaling pathways, such as phosphatidylinositol 3-kinase that are known to be deregulated in prostate cancer (16, 50). Finally, transfer of global Gprc6a deficiency onto a transgenic adenocarcinoma of mouse prostate mouse model of prostate cancer significantly retards prostate cancer progression and improved survival of compound Gprc6a−/−/transgenic adenocarcinoma of mouse prostate mice (22). Interestingly, Ocn is expressed in prostate cancer cells (51, 52) and elevated in the serum of prostate cancer patient is a predictor of metastasis in prostate cancer (53) and tumor progression (51). Dietary proteins and minerals are contributors to the development and progression of prostate cancer (54). For example, Ca and arginine supplements are associated with an increased risk of prostate cancer (55–58). Based on these observations, and the multiligand specificity of this receptor, GPRC6A may account for some of the associations between nutrient factors (e.g. dietary calcium, zinc, protein intake, etc.) and internal factors (e.g. osteocalcin and sex steroids) on human prostate cancer (9, 10, 16, 59).
Local actions of GPRC6A in bone?
GPRC6A is also expressed in osteoblasts. In addition, Gprc6a−/− mice also exhibit impaired mineralization and defects in osteoblast function in vivo and ex vivo (15, 59). Ocn released from bone resorption may stimulate osteoblast-mediated bone formation and mineralization through activation of GPRC6A in bone. Others, however, have failed to find evidence for a function of GPRC6A in osteoblasts (60). The related calcium-sensing receptor is also purported to be present in bone, which does not sense Ocn. Further studies are needed to determine the separate functions of calcium-sensing receptor and GPRC6A in regulating bone metabolism.
GPRC6A is also expressed the brain, heart, and kidney as well as other tissues, in which its function remains to be elucidated.
Summary and conclusions
In summary, GPRC6A has been shown to directly regulate signaling in osteoblasts (59), prostate cells (22), Leydig cells (7, 12), and pancreatic β-cells (10).
GPRC6A identifies a key mechanism that integrates bone, sex steroid, and nutrient factors in the regulation of glycemic control and energy metabolism. GPRC6A appears to be the OcnR that mediates the effects of Ocn to regulate insulin secretion and production and β-cell proliferation. GPRC6A likely has broader ligand specificities and tissue functions. GPRC6A may also provide a mechanism to modulate insulin resistance in liver, muscle, and adipose tissues. If so, agonists to GPRC6A may coordinately improve both resistance to insulin in peripheral tissues and β-cell functions. Such dual actions would distinguish targeting GPRC6A from pharmacological approaches that regulate insulin secretion but have counteractive effects on peripheral tissues that make them problematic as therapeutic agents. GPRC6A also has indirect effects through regulation of other hormones that affect energy metabolism and other metabolic processes. Both direct and indirect actions would generate specific pathways about the ways in which a number of endocrine systems involved in intermediary metabolism may be integrated through this receptor. Growing new knowledge of endocrine networks linking metabolic processes across multiple tissues, such as those involving GPRC6A, will likely improve our understanding of the complex factors contributing to the pathogenesis of a wide variety of endocrine disorders. The integrative physiological activity whereby single receptor activated by multiple, distinct ligands links together the function of multiple organs also provides a new conceptual framework to explain how functional related metabolic pathways related to diet, skeletal health, and reproduction are interconnected.
Acknowledgments
This work was supported by National Institutes of Health grant 5R01AR037308.
Disclosure Summary: The authors have nothing to disclose.
For article see page 2070
- GPCR
- G protein-coupled receptor
- GPRC6A
- GPCR family C group 6 member A
- IR
- insulin receptor
- L-Arg
- l-arginine
- Ocn
- osteocalcin
- OcnR
- Ocn-sensing receptor
- T2DM
- type 2 diabetes
- 7-TM
- seven-transmembrane domain
- VFTM
- venus fly trap motif.
References
- 1. Rehfeld JF. 2011. Incretin physiology beyond glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide: cholecystokinin and gastrin peptides. Acta Physiol (Oxf) 201:405–411 [DOI] [PubMed] [Google Scholar]
- 2. Winzell MS, Ahrén B. 2007. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther 116:437–448 [DOI] [PubMed] [Google Scholar]
- 3. Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, Ducy P, Karsenty G. 2010. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clemens TL, Karsenty G. 2011. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res 26:677–680 [DOI] [PubMed] [Google Scholar]
- 5. Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. 2010. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karsenty G. 22 August 2011. Regulation of male fertility by bone. Cold Spring Harb Symp Quant Biol 10.1101/sqb.2011.76.010934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Confavreux CB, Levine RL, Karsenty G. 2009. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol Cell Endocrinol 310:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N, Fontilla-Poole M, Mays RW, Brunden KR, Harrington JJ, Quarles LD. 2005. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J Biol Chem 280:40201–40209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pi M, Wu Y, Quarles LD. 2011. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res 26:1680–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bodine PV, Komm BS. 1999. Evidence that conditionally immortalized human osteoblasts express an osteocalcin receptor. Bone 25:535–543 [DOI] [PubMed] [Google Scholar]
- 12. Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, Hermo L, Suarez S, Roth BL, Ducy P, Karsenty G. 2011. Endocrine regulation of male fertility by the skeleton. Cell 144:796–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. 2012. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone 50:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bräuner-Osborne H, Wellendorph P, Jensen AA. 2007. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets 8:169–184 [DOI] [PubMed] [Google Scholar]
- 15. Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, Faber P, Brunden KR, Harrington JJ, Quarles LD. 2008. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 3:e3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pi M, Parrill AL, Quarles LD. 2010. GPRC6A mediates the non-genomic effects of steroids. J Biol Chem 285:39953–39964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuang D, Yao Y, Lam J, Tsushima RG, Hampson DR. 2005. Cloning and characterization of a family C orphan G-protein coupled receptor. J Neurochem 93:383–391 [DOI] [PubMed] [Google Scholar]
- 18. Wellendorph P, Bräuner-Osborne H. 2004. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene 335:37–46 [DOI] [PubMed] [Google Scholar]
- 19. Christiansen B, Hansen KB, Wellendorph P, Bräuner-Osborne H. 2007. Pharmacological characterization of mouse GPRC6A, an l-α-amino-acid receptor modulated by divalent cations. Br J Pharmacol 150:798–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faure H, Gorojankina T, Rice N, Dauban P, Dodd RH, Bräuner-Osborne H, Rognan D, Ruat M. 2009. Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium 46:323–332 [DOI] [PubMed] [Google Scholar]
- 21. Haid D, Widmayer P, Breer H. 2011. Nutrient sensing receptors in gastric endocrine cells. J Mol Histol 42:355–364 [DOI] [PubMed] [Google Scholar]
- 22. Pi M, Quarles LD. 2012. GPRC6A regulates prostate cancer progression. Prostate 72:399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harno E, Edwards G, Geraghty AR, Ward DT, Dodd RH, Dauban P, Faure H, Ruat M, Weston AH. 2008. Evidence for the presence of GPRC6A receptors in rat mesenteric arteries. Cell Calcium 44:210–219 [DOI] [PubMed] [Google Scholar]
- 24. Hovatta I, Zapala MA, Broide RS, Schadt EE, Libiger O, Schork NJ, Lockhart DJ, Barlow C. 2007. DNA variation and brain region-specific expression profiles exhibit different relationships between inbred mouse strains: implications for eQTL mapping studies. Genome Biol 8:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferron M, Hinoi E, Karsenty G, Ducy P. 2008. Osteocalcin differentially regulates β cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newsholme P, Abdulkader F, Rebelato E, Romanatto T, Pinheiro CH, Vitzel KF, Silva EP, Bazotte RB, Procopio J, Curi R, Gorjao R, Pithon-Curi TC. 2011. Amino acids and diabetes: implications for endocrine, metabolic and immune function. Front Biosci 16:315–339 [DOI] [PubMed] [Google Scholar]
- 27. Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, Lim SC, Khoo CM, Shah SH, Newgard CB. 2010. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nall JL, Wu G, Kim KH, Choi CW, Smith SB. 2009. Dietary supplementation of l-arginine and conjugated linoleic acid reduces retroperitoneal fat mass and increases lean body mass in rats. J Nutr 139:1279–1285 [DOI] [PubMed] [Google Scholar]
- 29. He Q, Kong X, Wu G, Ren P, Tang H, Hao F, Huang R, Li T, Tan B, Li P, Tang Z, Yin Y, Wu Y. 2009. Metabolomic analysis of the response of growing pigs to dietary l-arginine supplementation. Amino Acids 37:199–208 [DOI] [PubMed] [Google Scholar]
- 30. Tan A, Gao Y, Yang X, Zhang H, Qin X, Mo L, Peng T, Xia N, Mo Z. 2011. Low serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population survey. Metabolism 60:1186–1192 [DOI] [PubMed] [Google Scholar]
- 31. Clifton P. 2009. High protein diets and weight control. Nutr Metab Cardiovasc Dis 19:379–382 [DOI] [PubMed] [Google Scholar]
- 32. de Oliveira CA, Latorraca MQ, de Mello MA, Carneiro EM. 2011. Mechanisms of insulin secretion in malnutrition: modulation by amino acids in rodent models. Amino Acids 40:1027–1034 [DOI] [PubMed] [Google Scholar]
- 33. Grillo ML, Jacobus AP, Scalco R, Amaral F, Rodrigues DO, Loss ES, Wassermann GF. 2005. Testosterone rapidly stimulates insulin release from isolated pancreatic islets through a non-genomic dependent mechanism. Horm Metab Res 37:662–665 [DOI] [PubMed] [Google Scholar]
- 34. Rasschaert J, Malaisse WJ. 1999. Expression of the calcium-sensing receptor in pancreatic islet B-cells. Biochem Biophys Res Commun 264:615–618 [DOI] [PubMed] [Google Scholar]
- 35. Ozbek M, Erdogan M, Karadeniz M, Cetinkalp S, Ozgen AG, Saygili F, Yilmaz C, Tuzun M. 2009. Evaluation of β cell dysfunction by mixed meal tolerance test and oral l-arginine in patients with newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 117:573–576 [DOI] [PubMed] [Google Scholar]
- 36. Pitteloud N, Hardin M, Dwyer AA, Valassi E, Yialamas M, Elahi D, Hayes FJ. 2005. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J Clin Endocrinol Metab 90:2636–2641 [DOI] [PubMed] [Google Scholar]
- 37. Kirmani S, Atkinson EJ, Melton LJ, 3rd, Riggs BL, Amin S, Khosla S. 2011. Relationship of testosterone and osteocalcin levels during growth. J Bone Miner Res 26:2212–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Iki M, Tamaki J, Fujita Y, Kouda K, Yura A, Kadowaki E, Sato Y, Moon JS, Tomioka K, Okamoto N, Kurumatani N. 2012. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int 23:761–770 [DOI] [PubMed] [Google Scholar]
- 39. Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. 2009. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab 94:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Foresta C, Strapazzon G, De Toni L, Gianesello L, Bruttocao A, Scarda A, Plebani M, Garolla A. 1 February 2011. Androgens modulate osteocalcin release by human visceral adipose tissue. Clin Endocrinol (Oxf) 10.1111/j.1365-2265.2011.03997.x [DOI] [PubMed] [Google Scholar]
- 41. Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, Pellikka N, Wallaschofski H, Kettunen J, Henneman P, Baumert J, Strachan DP, Fuchsberger C, Vitart V, Wilson JF, Paré G, Naitza S, Rudock ME, Surakka I, de Geus EJ, Alizadeh BZ, Guralnik J, Shuldiner A, Tanaka T, Zee RY, et al. 2011. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 123:731–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, Bootsma AH, Stijnen T, Hofman A, Schram MT, Witteman JC. 2007. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 56:872–878 [DOI] [PubMed] [Google Scholar]
- 43. Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. 2004. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 350:1387–1397 [DOI] [PubMed] [Google Scholar]
- 44. Hwang YC, Jeong IK, Ahn KJ, Chung HY. 9 June 2011. Circulating osteocalcin level is associated with improved glucose tolerance, insulin secretion and sensitivity independent of the plasma adiponectin level. Osteoporos Int 10.1007/s00198-011-1679-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Desbois C, Hogue DA, Karsenty G. 1994. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem 269:1183–1190 [PubMed] [Google Scholar]
- 46. Ropelato MG, Rudaz MC, Escobar ME, Bengolea SV, Calcagno ML, Veldhuis JD, Barontini M. 2009. Acute effects of testosterone infusion on the serum luteinizing hormone profile in eumenorrheic and polycystic ovary syndrome adolescents. J Clin Endocrinol Metab 94:3602–3610 [DOI] [PubMed] [Google Scholar]
- 47. Marchetti P, Del Prato S, Lupi R, Del Guerra S. 2006. The pancreatic β-cell in human type 2 diabetes. Nutr Metab Cardiovasc Dis 16(Suppl 1):S3–S6 [DOI] [PubMed] [Google Scholar]
- 48. Takata R, Akamatsu S, Kubo M, Takahashi A, Hosono N, Kawaguchi T, Tsunoda T, Inazawa J, Kamatani N, Ogawa O, Fujioka T, Nakamura Y, Nakagawa H. 2010. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat Genet 42:751–754 [DOI] [PubMed] [Google Scholar]
- 49. Wang M, Liu F, Hsing AW, Wang X, Shao Q, Qi J, Ye Y, Wang Z, Chen H, Gao X, Wang G, Chu LW, Ding Q, Ouyang J, Gao X, Huang Y, Chen Y, Gao YT, Zhang ZF, Rao J, Shi R, Wu Q, Zhang Y, Jiang H, Zheng J, Hu Y, Guo L, Lin X, Tao S, Jin G, Sun J, Lu D, Zheng SL, Sun Y, Mo Z, Yin C, Zhang Z, Xu J. 2012. Replication and cumulative effects of GWAS-identified genetic variations for prostate cancer in Asians: a case-control study in the ChinaPCa consortium. Carcinogenesis 33:356–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clegg NJ, Couto SS, Wongvipat J, Hieronymus H, Carver BS, Taylor BS, Ellwood-Yen K, Gerald WL, Sander C, Sawyers CL. 2011. MYC cooperates with AKT in prostate tumorigenesis and alters sensitivity to mTOR inhibitors. PLoS One 6:e17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang WC, Xie Z, Konaka H, Sodek J, Zhau HE, Chung LW. 2005. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Res 65:2303–2313 [DOI] [PubMed] [Google Scholar]
- 52. Gardner TA, Lee SJ, Lee SD, Li X, Shirakawa T, Kwon DD, Park RY, Ahn KY, Jung C. 2009. Differential expression of osteocalcin during the metastatic progression of prostate cancer. Oncol Rep 21:903–908 [DOI] [PubMed] [Google Scholar]
- 53. Nimptsch K, Rohrmann S, Nieters A, Linseisen J. 2009. Serum undercarboxylated osteocalcin as biomarker of vitamin K intake and risk of prostate cancer: a nested case-control study in the Heidelberg cohort of the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 18:49–56 [DOI] [PubMed] [Google Scholar]
- 54. Lewis JE, Soler-Vilá H, Clark PE, Kresty LA, Allen GO, Hu JJ. 2009. Intake of plant foods and associated nutrients in prostate cancer risk. Nutr Cancer 61:216–224 [DOI] [PubMed] [Google Scholar]
- 55. Gao X, LaValley MP, Tucker KL. 2005. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 97:1768–1777 [DOI] [PubMed] [Google Scholar]
- 56. Kristal AR, Arnold KB, Neuhouser ML, Goodman P, Platz EA, Albanes D, Thompson IM. 2010. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol 172:566–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW. 2010. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer 126:2762–2772 [DOI] [PubMed] [Google Scholar]
- 58. Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Tjonneland A, Johnsen NF, Overvad K, Linseisen J, Rohrmann S, Boeing H, Pischon T, Bueno-de-Mesquita HB, Kiemeney L, Tagliabue G, Palli D, Vineis P, Tumino R, Trichopoulou A, Kassapa C, Trichopoulos D, Ardanaz E, Larrañaga N, Tormo MJ, González CA, Quirós JR, Sánchez MJ, Bingham S, Khaw KT, Manjer J, Berglund G, Stattin P, Hallmans G, Slimani N, Ferrari P, Rinaldi S, Riboli E. 2008. Animal foods, protein, calcium and prostate cancer risk: the European Prospective Investigation into Cancer and Nutrition. Br J Cancer 98:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pi M, Zhang L, Lei SF, Huang MZ, Zhu W, Zhang J, Shen H, Deng HW, Quarles LD. 2010. Impaired osteoblast function in GPRC6A null mice. J Bone Miner Res 25:1092–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wellendorph P, Johansen LD, Jensen AA, Casanova E, Gassmann M, Deprez P, Clément-Lacroix P, Bettler B, Bräuner-Osborne H. 2009. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J Mol Endocrinol 42:215–223 [DOI] [PubMed] [Google Scholar]
- 61. Conigrave AD, Hampson DR. 2010. Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther 127:252–260 [DOI] [PubMed] [Google Scholar]