Abstract
Although having the capacity to grow in response to a stimulus that perturbs the pituitary-thyroid axis, the thyroid gland is considered not a regenerative organ. In this study, partial thyroidectomy (PTx) was used to produce a condition for thyroid regeneration. In the intact thyroid gland, the central areas of both lobes served as the proliferative centers where microfollicles, and bromodeoxyuridine (BrdU)-positive and/or C cells, were localized. Two weeks after PTx, the number of BrdU-positive cells and cells with clear or faintly eosinophilic cytoplasm were markedly increased in the central area and continuous to the cut edge. Clear cells were scant in the cytoplasm, as determined by electron microscopy; some retained the characteristics of calcitonin-producing C cells by having neuroendocrine granules, whereas others retained follicular cell-specific features, such as the juxtaposition to a lumen with microvilli. Some cells were BrdU-positive and expressed Foxa2, the definitive endoderm lineage marker. Serum TSH levels drastically changed due to the thyroidectomy-induced acute reduction in T4-generating tissue, resulting in a goitrogenesis setting. Microarray followed by pathway analysis revealed that the expression of genes involved in embryonic development and cancer was affected by PTx. The results suggest that both C cells and follicular cells may be altered by PTx to become immature cells or immature cells that might be derived from stem/progenitor cells on their way to differentiation into C cells or follicular cells. These immature clear cells may participate in the repair and/or regeneration of the thyroid gland.
The thyroid gland is a dormant organ with very slow turnover with cells dividing approximately five times during adult life (1). The adult thyroid gland maintains its size with a slow cell turnover, whereas the capacity to grow through cell hypertrophy and proliferation in response to a stimulus is retained. A stimulus can be various xenobiotics or physiological alterations that perturb the pituitary-thyroid axis (2, 3). The major pathogenic mechanisms responsible for development of thyroid hyperplasia include iodide deficiency, iodide excess, goitrogenic compounds, and/or genetic enzyme defects that interfere with the biosynthesis and secretion of thyroid hormone (2, 3). Surgical partial thyroidectomy (PTx) also induces hypothyroidism, to which the thyroid responds and undergoes hyperplasia to sustain adequate thyroid hormone production.
PTx has been used to produce hypothyroidism to study the effect of decreased endogenous thyroid hormone levels or exogenously administered thyroid hormone on liver regeneration (4) or the function and/or changes in enzyme activities or levels of thyroid hormone-regulated molecules in the brain, hypothalamus, pituitary, and liver (5–9). Despite the occasional use of this technique, few studies have been performed on the effect of PTx on the thyroid gland itself. An established technique similar to PTx is a partial hepatectomy that is frequently used to study liver regeneration (10–12). Partial hepatectomy is a type of liver injury, where after two-thirds removal, the remaining one-third of the liver regenerates within 1–2 wk, in the case of rodents, to reach the original size proportional to total body weight (12). By analogy to partial hepatectomy, PTx may be considered as a type of thyroid injury that could provide a model to study thyroid repair and/or regeneration, even though the gland does not recover its normal size (1).
Gene expression profiling has been extensively used in all areas of research, including the thyroid. In particular, it was used as a tool to diagnose and identify molecular targets to treat thyroid carcinomas (13–15). However, no study has been carried out to describe changes in gene expression patterns after PTx. In this study, mouse thyroid glands before and after PTx were subjected to histological and immunohistochemical examinations and microarray analysis in conjunction with laser capture microdissection. Serum TSH and T4 levels were also determined before and at different times after PTx. The possible implication of up-regulated serum TSH levels in the current findings is discussed. The results revealed that PTx may provide a model to study the process and/or mechanisms underlying development, repair, regeneration, and/or goitrogenesis (hypertrophy and hyperplasia) of the thyroid gland.
Materials and Methods
Animals
C57BL/6 mice, both males and females, aged 6–8 wk, were subjected to PTx, and 2 wk later, the thyroid glands were subjected to histological analysis or laser capture microdissection followed by isolation of RNA for microarray analysis. PTx consisted of the removal of one whole thyroid lobe and approximately 2/5 caudal segment of the other lobe, leaving the central area of the lobe intact. Age-matched, not operated mice were used as controls for all experiments. All animal studies were performed in accordance with the Using Animals in Intramural Research Guidelines (National Institutes of Health Animal Research Advisory Committee, National Institutes of Health, Bethesda, MD) and after approval of the institutional Animal Care and Use Committee. For bromodeoxyuridine (BrdU) labeling, mice were injected ip with BrdU (20 mg/kg) at the time of PTx, followed by daily consecutive injection starting 2 d after the surgery until 1 d before killing.
Histological examination
A cervical region of a mouse containing the thyroid gland, larynx, and trachea was dissected, fixed in 4% paraformaldehyde in 0.1 m PBS at 4 C overnight, dehydrated, and embedded in paraffin. Serial sections of 3-μm thickness were treated with xylene and graded ethanol and then stained with hematoxylin (Mayer's) and eosin (H&E). For immunohistochemistry, sections were treated with 1% hydrogen peroxide in methanol for 30–45 min to block endogenous peroxidase activity, followed by rinsing three times for 10 min each with PBS. Epitope retrieval was carried out by heating sections at 100 C for 3 min, five times using a microwave oven in 10 mm citrate buffer (pH 6.0), followed by cooling for 30 min at room temperature, and washing in PBS. Sections were then treated with 5% skim milk in PBS for 15 min to block nonspecific protein binding. Incubation with primary antibody was carried out overnight at 4 C in a humidified chamber using the following antibodies: anticalcitonin (rabbit polyclonal, 1:1000 dilution; ICN Biomedicals, Irvine, CA), antithyroglobulin (rabbit polyclonal, 1:400 dilution; Biomeda Corp, Foster City, CA), anti-BrdU (rat polyclonal, 1:200 dilution; Serotec, Oxford, UK), anticytokeratin 14 (Krt14) (rabbit polyclonal, 1:1000 dilution; Covance, Emeryville, CA), and anti-Foxa2 (rabbit polyclonal, 1:2000–5000 dilution; Seven Hills Bioreagents, Cincinnati, OH). After washing in 0.01 m PBS, the sections were treated using horseradish peroxidase-conjugated rabbit antirat IgG (Serotec) or the Avidin Biotin Complex method with a commercially available kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's instructions. Immunocomplexes were visualized with 3,3′-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO). The immunostaining condition was determined for each antibody using various tissue sections where the protein in question is expressed as a positive control and those tissues/areas where the protein is not expressed as a negative control.
Electron microscopy (EM)
Tissue sample preparation for EM analysis was described in great detail (16). Briefly, mouse tissue was fixed with formaldehyde (4%) and glutaraldehyde (2%) in cacodylate buffer [0.1 m (pH 7.4)] (Tousimis, Rockville, MD) followed by postfixation in 1% osmium in same buffer. The tissue was en bloc stained in 0.5% uranyl acetate in acetate buffer [0.1 m (pH 4.5)] and dehydrated in a graded ethanol (35, 50, 70, 95, and 100%) and propylene oxide. The infiltration was done in an equal mixture of epoxy resin (Embed 812; Electron Microscope Sciences, Fort Washington, PA) and propylene oxide overnight. The tissue was embedded in a pure epoxy resin and cured in a 55 C oven. Thin sections were mounted on copper grids and stained in uranyl acetate and lead citrate. The grids were examined in the EM (Hitachi H7600, Tokyo, Japan) operated at 80 kV, and digital images were taken by charge-coupled device camera (AMT, Danvers, MA).
Laser capture microdissection
Laser capture microdissection was carried out with the PixCell II Arcturus Laser Capture Microdissection system (Arcturus Engineering, Carlsbad, CA) using frozen thyroid sections of 10 μm, after quick staining with hematoxylin (Mayer's) (17, 18). Cells collected were from the following four areas of thyroid tissues: 1) central proliferative area of intact mouse thyroid lobe where microfollicles are present [tentatively named intact central (IC)], 2) peripheral area of intact mouse thyroid lobe [tentatively named intact peripheral (IP)], 3) proliferative areas from the center and near the cut edge of partially thyroidectomized thyroid lobe, in which many microfollicles and clear cells are found [tentatively named dissected central (DC)], and 4) peripheral area of partially thyroidectomized thyroid lobe [tentatively named dissected peripheral (DP)].
RNA preparation and microarray analysis
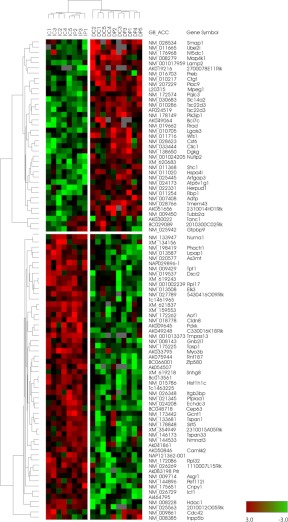
Total RNA was extracted from approximately 50 laser capture microdissected thyroid cells using TRIzol reagent (Life Technologies, Grand Island, NY), followed by purification using RNeasy Micro kit (MinElute spin column) (QIAGEN, Valencia, CA) according to the manufacturer's instructions. The yield was estimated to be approximately 50–100 pg. The purified RNA was amplified twice with Arcturus RiboAmp PLUS amplification kit (Applied Biosystems, Foster City, CA) and labeled with Arcturus Turbo Labeling kit (Applied Biosystems). The final amount of purified RNA was just about enough to carry out microarray analysis as follows. Dye-coupled antisense RNA were hybridized to an Agilent 44 K mouse 60-mer oligo microarray (Agilent Technologies, Santa Clara, CA). The slides were washed, dried, and scanned using Agilent G2565AA microarray scanner (Agilent Technologies). The procedures were repeated for replicate experiments with independent hybridization and processing. The data were processed by Genespring GX software package (version 10; Agilent Technologies) and were excluded if their t test P value was greater than 0.05. The t test P value gives a confidence measure on how reproducible an expression level measurement is. For the retained confidence signals, they were transformed into the log2 ratio relative to the pooled intact thyroid reference sample. Genes differentially expressed at P ≤ 0.05 in each condition were filtered by Bootstrap t test with 6000 repetitions (19). Data mining was performed using the Ingenuity Pathway Analysis (IPA) tool (Ingenuity Systems, Redwood City, CA). The significance of each network, function, and pathway was determined by the scoring system provided by the Ingenuity Systems. All effective genes were submitted to the Gene Expression Omnibus (ID GSE25934; http://www.ncbi.nlm.nih.gov/geo).
With differently expressed genes of interest, the Gene Ontology (GO) term analysis was conducted from Lewis-Sigler Institute at Princeton University (Princeton, NJ; http://go.princeton.edu/). The P value was calculated based on hypergeometric distribution, and the value less than 0.05 was considered to have a statistical significance (20). Hierarchical cluster analysis was performed with Cluster 3.0, and microarray image analysis was performed with TreeView 1.60 (Michael Eisen Laboratory, Lawrence Berkeley National Laboratory and University of California, Berkeley; http://rana.lbl.gov/eisen/).
Serum T4 and TSH assays
Serum total T4 concentrations were measured by coated tube RIA (Siemens Medical Solutions Diagnostics, Los Angeles, CA) adapted for mouse using 25 μl of serum. TSH was measured in 50 μl of serum using a sensitive, heterologous, disequilibrium, double-antibody precipitation RIA (21).
Results
Histological changes after PTx
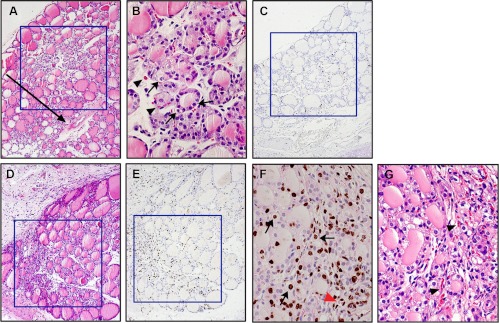
Histological analysis revealed that in intact thyroid tissue, microfollicles were frequently found in the central area of the right or left lobes (Fig. 1A). Occasionally, relatively large cells were observed that had enlarged nuclei with clear or faintly eosinophilic large/wide cytoplasm (Fig. 1B, arrows). Microfollicles and/or cells with clear cytoplasm were surrounded by capillary vessels (Fig. 1, B and G, arrowheads). BrdU labeling experiments demonstrated BrdU-positive cells, either follicular cells or C cells, in the central area of intact thyroid lobes (Fig. 1C). PTx was carried out to remove one thyroid lobe (right or left) and approximately 2/5 of the lower part of the other lobe, leaving 3/5 of the upper part intact, including the central area. Two weeks after PTx, the number of microfollicles and/or cells with clear or faintly eosinophilic cytoplasm markedly increased in the area corresponding to the central part of the intact thyroid lobe and the area near the cut edge that extended to and overlapped with the central area of the lobe (Fig. 1D). Some of these changes became apparent 1 wk after PTx (data not shown). BrdU-positive cells were markedly increased, particularly in the area near the cut edge (Fig. 1E). Some cells with clear cytoplasm and capillary endothelial cells were also BrdU-positive (Fig. 1F). These results suggested that 1) the central part of the intact thyroid lobe where many microfollicles are present may generally serve as a center for proliferation, 2) the proliferative center extends to the area near the cut edge after PTx, 3) PTx stimulates cell proliferation, 4) the number of cells with clear or faintly eosinophilic cytoplasm as well as capillary vessels increase after PTx, and 5) some clear cells actively participate in proliferation.
Fig. 1.
Central proliferative area of the thyroid. A–C, Normal thyroid. D–G, Two weeks after PTx. A, B, D, and G, H&E staining. C and E, BrdU immunostaining. F, BrdU immunostaining counterstained with light H&E. The arrow in A indicates the approximate position where the thyroid was cut. The arrows in B and F indicate cells with clear cytoplasm. The arrowheads in B and G indicate capillary vessels, and red arrowhead in F indicates capillary endothelial cells, which are BrdU-positive. Boxes in A and C, and D and E indicate, respectively, the central proliferative area and the proliferative area near the cut edge and the overlapping central area after PTx. Original magnification, ×100 (A, and C–E) and ×200 (B, F, and G).
Characterization of clear cells
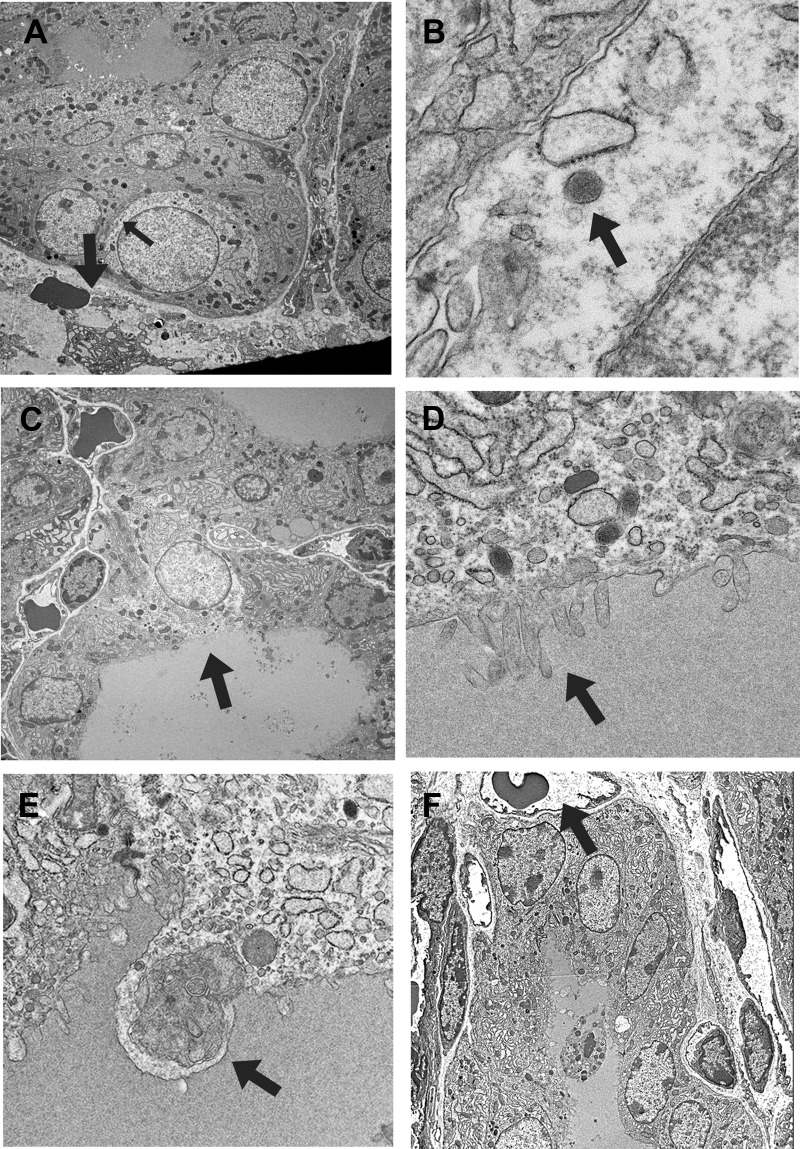
EM analysis showed that cells with clear cytoplasm had little cytoplasmic components, such as rough endoplasmic reticulum and fewer numbers of Golgi apparatus (Fig. 2, A and C). Some clear cells had dispersed chromatin and a few dense neuroendocrine-like granules (Fig. 2B), reminiscent of immature C cells, and were surrounded by follicular cells (Fig. 2A). Another class of clear cells retained characteristics of follicular cells, such as those facing the lumen and having microvilli at their apical side (Fig. 2, C and D). Capillary blood or lymphoid vessels were arranged next to follicular or clear cells. Some cells with follicular characteristics appeared to undergo cell death through autophagy (Fig. 2E). EM further demonstrated that dead cells were occasionally seen in the colloid lumens of many microfollicles, which were about to form a follicle (Fig. 2F). This appeared to be a way to generate a follicle in vivo.
Fig. 2.
EM analysis of the proliferative area of the thyroid. A, Clear cell that retains characteristics of C cells such as being surrounded by other cells and the presence of neuroendocrine granules (representative, indicated by a small arrow). The thick arrow indicates a capillary vessel with erythrocytes. B, High magnification of a neuroendocrine granule shown in A (indicated by an arrow). C, Clear cell that retains follicular cell characteristics such as those facing the lumen and the presence of microvilli at the apical side of the cell (indicated by an arrow). D, High magnification of microvilli shown in C (indicated by an arrow). E, Cell undergoing autophagy. Various cytosolic vesicles are found inside lysosome (shown by an arrow). F, Dying cells and cells surrounding them, which are about to form a lumen and a follicle, respectively. An arrow indicates capillary vessel with erythrocytes. Original magnification: ×1000 (A, C, and F), ×20,000 (B), ×10,000 (D), and ×5000 (E).
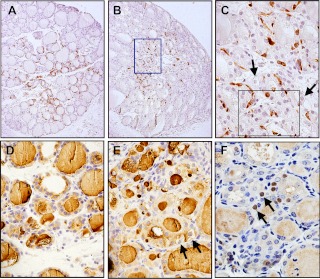
Immunohistochemical analysis demonstrated that the expression of C cell-specific calcitonin was mainly localized in the proliferative area of the thyroid lobe regardless of PTx (Fig. 3, A and B). Most of C cells are surrounding microfollicles, whereas some C cells intermingle within small epithelial cell nests (Fig. 3C). No calcitonin expression was found in cells with clear cytoplasm (Fig. 3C). In contrast, thyroglobulin was expressed in almost all cells of the thyroid gland with and without PTx (Fig. 3, D and E). Interestingly, low amounts of thyroglobulin were noted in some of clear cells in partially thyroidectomized glands (Fig. 3E).
Fig. 3.
Immunohistochemical analysis. A and B, Calcitonin-positive C cells are found in the central proliferative area of the thyroid lobe in both intact (A) and 2 wk after PTx (B). C, Magnified image of the boxed area in B. Slides were counterstained with hematoxylin. Arrows show clear cells. An epithelial cell nest with trabecular pattern is seen within the black box. D and E, Thyroglobulin immunostaining of normal intact thyroids (D) and those of 2 wk after PTx (E). Slides were counterstained with hematoxylin. Clear cells are indicated by an arrow in E. F, Immunostaining for Foxa2. Some clear cells are positives for Foxa2 (shown by arrows). Original magnification, ×100 (A and B) and ×200 (C–F).
Alteration of T4 and TSH levels after PTx
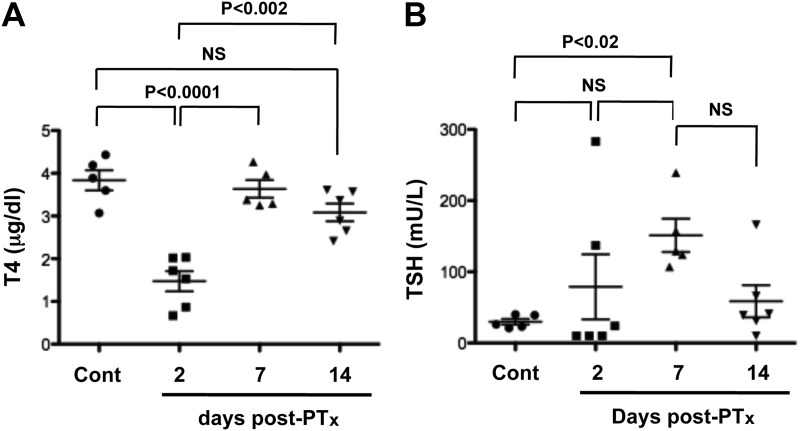
It is well known that hypothyroidism after thyroidectomy is due to the sudden loss of thyroid tissue. Accordingly, T4 and TSH levels were determined in mice at various time points after PTx (Fig. 4). T4 levels were markedly reduced 2 d after PTx but returned to baseline levels after 7 d (Fig. 4A). In contrast, serum TSH levels tended to increase at 2 d and were clearly high at 7 d after PTx (Fig. 4B). At 2 wk after PTx, TSH levels declined but did not reach baseline levels, whereas T4 levels were slightly, but not significantly, lower.
Fig. 4.
Serum T4 (A) and TSH levels (B) measured at various time points after PTx. Cont means serum obtained from nonoperated normal mouse. Each dot indicates a mouse. Statistical analysis was carried out by ANOVA, and P < 0.05 was considered as statistically significant. NS, Not significant.
Gene expression in the proliferative area vs. peripheral nonproliferative area
Microarray analysis was carried out on laser capture microdissected thyroid cells from four areas of the thyroid. Microarray analysis of the intact thyroid revealed that expression of 474 and 281 genes were higher and lower, respectively, in the central proliferative area of the thyroid lobe compared with the peripheral area (IC vs. IP). After PTx, 353 genes were higher and 392 genes lower in the proliferative areas covering both the central part of the lobe and the area near the cut edge compared with the peripheral area of the thyroid lobe (DC vs. DP). When IC and DC, and IP and DP were combined, the expression of 137 genes was higher and 12 was lower in the proliferative area compared with the peripheral nonproliferative area of the lobe (Table 1 and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Endocrine System Development and Function was identified by the IPA as the top Physiological System Development and Function (P value of 3.31E-05–2.55E-02). Many highly up-regulated genes were identified included calcitonin/calcitonin-related polypeptide, α (Calca), Syt13, Scg3, Pcsk1, Pcsk2, Chga, Chgb, and Resp18 (Table 1). Calca encodes calcitonin, the major protein produced in the C cells of the thyroid gland. All other genes are also expressed in C cells (22–24). The results of gene expression patterns are in good agreement with the immunohistochemical results, in which calcitonin expression was mainly found in the central proliferative area of the thyroid lobe regardless of PTx (see Fig. 3, A and B). Other genes of interest identified by microarray that are highly up-regulated in the proliferative area included Foxa2 (Table 1). Foxa2 is a gene encoding a transcription factor responsible for the formation and maintenance of the definitive endoderm lineage, including the thyroid (25, 26). Interestingly, immunohistochemistry demonstrated that some cells with clear cytoplasms were positive for Foxa2 (Fig. 3F). These results, together with EM analysis, suggested that some clear cells may be considered as immature cells.
Table 1.
Commonly up-regulated genes in the central area vs. the peripheral area of the thyroid
| GenBank | Gene symbol | Description | Fold change | P value |
|---|---|---|---|---|
| NM_018866 | Cxcl13 | Chemokine (C-X-C motif) ligand 13 (Cxcl13) | 4.8165902 | 0.0004 |
| NM_007587 | Calca | Calcitonin/calcitonin-related polypeptide, α | 4.5885531 | 0.0024 |
| NM_030725 | Syt13 | Synaptotagmin XIII (Syt13) | 4.3638275 | 0.001 |
| NM_009130 | Scg3 | Secretogranin III (Scg3) | 4.2596294 | 0.0002 |
| NM_013628 | Pcsk1 | Proprotein convertase subtilisin/kexin type 1 | 4.0102517 | 0.0016 |
| NM_007694 | Chgb | Chromogranin B (Chgb) | 3.9725184 | 0.0003 |
| NM_007693 | Chga | Chromogranin A (Chga) | 3.9549664 | 0.0005 |
| NM_152915 | Dner | δ/Notch-like EGF-related receptor (Dner) | 3.9470842 | 0.0013 |
| NM_010446 | Foxa2 | Forkhead box A2 (Foxa2) | 3.9079868 | 0.0004 |
| AK129109 | Trim9 | mKIAA0282 protein | 3.8122757 | 0.0041 |
| NM_010461 | Hoxb8 | Homeo box B8 (Hoxb8) | 3.5424765 | 0.0074 |
| NM_008792 | Pcsk2 | Proprotein convertase subtilisin/kexin type 2 | 3.425516 | 0.0001 |
| BC042620 | Fbxl16 | F-box and leucine-rich repeat protein 16 | 3.4076878 | 0.0072 |
| NM_011510 | Abcc8 | ATP-binding cassette, subfamily C (CFTR/MRP), member 8 (Abcc8) | 3.2616852 | 0.0008 |
| NM_010932 | Pnoc | Prepronociceptin | 3.257574 | 0 |
| NM_001024698 | Cpa2 | Similar to Carboxypeptidase A2 precursor (MGC107514) | 3.1251535 | 0.0214 |
| NM_007467 | Aplp1 | Amyloid β (A4) precursor-like protein 1 | 3.091298 | 0.0003 |
| NM_007709 | Cited1 | Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 1 | 3.0654408 | 0.0003 |
| AK030625 | Gpr179 | Adult male pituitary gland cDNA, RIKEN full-length enriched library, clone:5330439C02 product:hypothetical protein | 3.0615862 | 0.0001 |
| NM_027539 | Dclk2 | Doublecortin and CaM kinase-like 2 (Dcamkl2) | 2.9675104 | 0.0009 |
| NM_144926 | Sez6l2 | Seizure related 6 homolog (mouse)-like 2 | 2.928459 | 0.0004 |
| NM_007675 | Ceacam10 | CEA-related cell adhesion molecule 10 | 2.8990497 | 0.0076 |
| NM_009049 | Resp18 | Regulated endocrine-specific protein 18 | 2.8595104 | 0.0007 |
| NM_144891 | Gdap1l1 | Ganglioside-induced differentiation-associated protein 1-like 1 | 2.8063135 | 0.001 |
| AK220449 | Lhfpl4 | mKIAA4027 protein | 2.7361495 | 0.0012 |
| NM_178676 | Entpd3 | Ectonucleoside triphosphate diphosphohydrolase 3 | 2.6888210 | 0.0017 |
| NM_009513 | Nrsn1 | Vesicular membrane protein p24 (Vmp) | 2.6829494 | 0.0003 |
| NM_007866 | Dll3 | δ-Like 3 (Drosophila) (Dll3), transcript variant 1 | 2.6579014 | 0.0005 |
| NM_009022 | Aldh1a2 | Aldehyde dehydrogenase family 1, subfamily A2 | 2.5968126 | 0.0012 |
| NM_025869 | Dusp26 | Dual specificity phosphatase 26 (putative) | 2.5442488 | 0.0003 |
| NM_008101 | Gcgr | Glucagon receptor | 2.5138046 | 0.0006 |
Only genes with known gene symbols that were up-regulated more than 2-fold in log2 scale in the center parts of both normal and dissected thyroids as compared with the peripheral areas are listed. Values shown are those obtained with dissected thyroids.
Genes affected in a whole thyroid gland after PTx
To further determine the genes affected after PTx, cluster analysis was carried out for all genes in both DC and DP areas (Fig. 5). Thirty-four genes were clustered that were up-regulated in dissected thyroids (DC and DP combined) vs. intact thyroid (IC and IP combined), whereas 53 genes were clustered that were down-regulated in dissected thyroids (DC and DP combined) vs. intact thyroid (IC and IP combined) (Supplemental Table 2). Three genes, Tanc1, 2010300C02Rik, and Gtpbp9, were up-regulated in the central area of dissected thyroids whereas down-regulated in the peripheral area of dissected thyroids vs. the respective intact thyroids. On the other hand, two genes, Cdc42 and Inpp5b, were up-regulated in the peripheral area of dissected thyroids and down-regulated in the central area of dissected thyroid vs. the respective intact thyroids. IPA identified Endocrine System Disorders as the top Diseases and Disorders Bio Functions (P value of 7.18E-04–9.88E-03) and Embryonic Development as the top Physiological System Development and Function (P value of 1.17E-03–2.90E-02). GO term analysis demonstrated that most affected genes by PTx are involved in cellular and/or metabolic processes and their regulation (Table 2). The affected genes included those involved in cellular signaling, such as Camkk2, Dgkg, Shc1, Cdc42, and Phactr1, those involved in regulation of transcription, such as Elk3, Sirt5, Tsc22d3, and Hdac1, and mitogen Ctgf and the ubiquitination-related gene Ube2i. These results demonstrated that PTx severely affected a whole thyroid lobe, resulting in markedly disorganized physiology of cells with altered cellular and/or metabolic status, and some of the changes may resemble to those seen during thyroid gland development.
Fig. 5.
Clustering analysis of genes affected after PTx. Supervised clustering analysis was carried out for all genes for dissection effect in DC + DP area vs. IC + IP area.
Table 2.
GO terms for affected genes after partial thyroidectomy
| GO term | Cluster frequency (%) | Genome frequency | Corrected P value | FDR (%) | False positive |
|---|---|---|---|---|---|
| Cellular process | 51.3 | 31.0 | 0.04406 | 0.80 | 0.12 |
| Metabolic process | 39.7 | 20.0 | 0.0147 | 0.00 | 0 |
| Cellular metabolic Process | 34.6 | 17.0 | 0.03719 | 0.46 | 0.06 |
| Regulation of metabolic process | 23.1 | 8.0 | 0.01117 | 0.00 | 0 |
| Regulation of cellular metabolic process | 21.8 | 7.5 | 0.01746 | 0.29 | 0.02 |
| Protein metabolic process | 20.5 | 6.8 | 0.01671 | 0.33 | 0.02 |
| Unannotated | 19.2 | 3.2 | 5.90E-06 | 0.00 | 0 |
| Cellular protein metabolic process | 17.9 | 5.4 | 0.01945 | 0.22 | 0.02 |
| Intracellular signaling pathway | 12.8 | 2.8 | 0.01786 | 0.25 | 0.02 |
| Regulation of molecular function | 11.5 | 1.7 | 0.00192 | 0.00 | 0 |
| Regulation of catalytic activity | 10.3 | 1.4 | 0.00428 | 0.00 | 0 |
| Regulation of phosphorylation | 7.7 | 0.9 | 0.0238 | 0.20 | 0.02 |
| Regulation of phosphate metabolic process | 7.7 | 0.9 | 0.02914 | 0.18 | 0.02 |
| Regulation of phosphorus metabolic process | 7.7 | 0.9 | 0.02914 | 0.17 | 0.02 |
| Positive regulation of molecular function | 7.7 | 1.0 | 0.03889 | 0.43 | 0.06 |
GO terms analysis was carried out using gene probes identified in clustering analysis. FDR, False discovery rate.
Genes affected in the proliferative area after PTx
Genes affected in the proliferative area after PTx were analyzed by comparing the expression of genes in the central area with and without PTx. Three hundred fifty-three genes were up-regulated, whereas 392 were down-regulated in DC compared with IC (Supplemental Table 3). The IPA for Top Bio Functions revealed that approximately 120 genes are involved in Cancer in Diseases and Disorders category, and Cellular Growth and Proliferation and Cell Death in Molecular and Cellular Function category (Table 3). As expected from the IPA results, among the top genes affected (Supplemental Table 4), many are involved in cancers, such as Bcan in glioma (27), Epor in thyroid cancers (28), and Cpne3 in prostate, breast, and ovarian cancers (29). Any combinatorial analysis of microarray data did not reveal any differences in the expression levels of TSH receptor (Tshr), Nanog, and Oct 4 between intact and partially thyroidectomized thyroid glands (data not shown). When expression of Oct 4 was examined by immunohistochemistry, the expression was not detected in either intact or partially thyroidectomized thyroid glands (data not shown).
Table 3.
Top Bio Functions identified in the proliferative area of partially thyroidectomized thyroid vs. intact thyroid
| Name of functions | P value | No. of molecules |
|---|---|---|
| Diseases and disorders | ||
| Cancer | 8.71E-04–3.47E-02 | 121 |
| Hematological disease | 8.71E-04–3.47E-02 | 31 |
| Renal and urological disease | 1.63E-03–3.47E-02 | 34 |
| Dermatological diseases and conditions | 1.90E-03–3.47E-02 | 22 |
| Cardiovascular disease | 2.04E-03–3.47E-02 | 13 |
| Molecular and cellular functions | ||
| Cellular growth and proliferation | 2.54E-04–3.47E-02 | 125 |
| Cell-to-cell signaling and interaction | 3.88E-04–3.47E-02 | 20 |
| Cell death | 4.54E-04–3.47E-02 | 121 |
| Cell signaling | 6.29E-04–3.05E-02 | 39 |
| Lipid metabolism | 7.67E-04–3.47E-02 | 26 |
Appearance of Krt14 expression after PTx
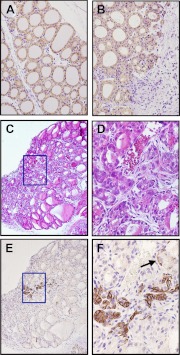
To obtain a further insight into the possible altered condition of cells other than epithelial cells of microfollicles and/or follicles after PTx, genes whose expression is not restricted to thyroid follicular cells, such as cytokeratins, were examined. Several cytokeratins are expressed in the thyroid gland (30, 31). Among them, Krt19 is known to be highly expressed in human papillary thyroid carcinomas (31). In our immunohistochemical study, Krt19 expression did not differ between intact thyroid glands and those after PTx, although the expression pattern after PTx appeared slightly diffused (Fig. 6, A and B). Krt14 is known as a liver progenitor marker (32) and is expressed in immature and/or stem/progenitor cells, such as taste buds (33) and prostate (34). Immunohistochemistry demonstrated that Krt14 expression was barely detected in normal thyroid tissue (data not shown), whereas after PTx, intense staining for Krt14 was identified in a restricted area within the proliferative area of the thyroid lobe (Fig. 6, C–F). Krt14-positive cells presented both mesenchymal and epithelial cell features; some had trabecular and/or nested patterns, whereas others showed a basal cell pattern with spindle shapes (Fig. 6F, arrow). In addition, they had narrow cytoplasm and a high N/C ratio, features distinct from those of thyroid follicular and/or clear cells.
Fig. 6.
Cytokeratin expression after PTx. A and B, Immunohistochemistry for Krt19 of intact (A) and 7 d after partially thyroidectomized thyroids (B). C and D, H&E staining. E and F, Immunohistochemistry for Krt14 counterstained with hematoxylin. C and E are from serially prepared sections. Box in C corresponds to the area where Krt14 is expressed in E, and the magnified images of the boxed areas are shown in D and F, respectively. The arrow in F indicates that only basal cell cytoplasm is positively stained for Krt14 (basal cell pattern), whereas the upper cells (luminar cells) are negative for Krt14 expression. Original magnification: ×100 (C and E), ×200 (A and B), and ×400 (D and F).
Discussion
To our knowledge, this is the first in depth analysis of the effect of PTx on the thyroid gland. The intact mouse thyroid gland was found to have a proliferative focal point near the center of the thyroid lobes, where immature microfollicles, with C cells and capillary vessels, are abundant in well-ordered arrangement. Microarray analysis was carried out using thyroid epithelial cells obtained by laser capture microdissection from the proliferative area vs. nonproliferative peripheral area of the thyroid gland. The results demonstrated that the proliferative areas express many genes involved in the development and function of the endocrine system as analyzed by the IPA. Among them is Calca expressed in C cells, one of the critical components of thyroid tissue. Immunohistochemical analysis revealed that C cells are mainly localized in the central proliferative area of the lobe, regardless of PTx. Accordingly, many genes specifically expressed in C cells are also overexpressed in the central proliferative area compared with the peripheral nonproliferative area. C cells originate from the ultimobranchial body (UBB) and are disseminated within the thyroid gland after the UBB meets with the thyroid primordium around mouse embryonic d 14.5 (35). The current results indicate that C cells do not disseminate evenly throughout the mouse thyroid but rather stay in the central proliferative area of the lobe. This centralized location of C cells was previously described by McMillan et al. (36).
The presence of immature microfollicles, clear cells, and BrdU-positive cells was occasionally noted in the central proliferative area of intact thyroid lobe. The differences in follicular diameter were not related to a specific slide, and microfollicles were observed in the central area of all analyzed thyroid glands using serially sectioned specimens. After PTx, the central proliferative area extended to the cut edge, and the number of immature microfollicles and clear epithelial cells drastically increased. Many BrdU-positive cells were found near the cut edge, suggesting that cells in this area actively proliferate after PTx. The clear cells had scant cytoplasm as demonstrated by EM and were divided into two types of cells: immature follicular cells and immature C cells. Thus, some cells retained the characteristics of the C cell, such as dispersed chromatin and the presence of dense neuroendocrine-like granules, whereas others showed follicular cell specific features, such as the juxtaposition to a lumen and having microvilli at the apical side of the cells, and residual thyroglobulin expression. Autophagy was sometimes found in follicles, suggesting that some cells are in the process of dying (see below). Cell death in the proliferative area is compatible with the up-regulated expression pattern of genes involved in cell death as shown in Table 3. This finding is in agreement with the notion that thyroid glands do not recover their normal size after PTx due to the compensated increased cell death rate (1).
Some clear cells were BrdU-positive and expressed Foxa2, the definitive endoderm lineage marker (25, 26). Microarray clustering analysis revealed that many genes involved in cellular and metabolic processes and the regulation of these processes were severely affected throughout the tissue after PTx. Based on Pathway Analysis, the genes affected after PTx were involved in Endocrine System Disorders and Embryonic Development. Limited to the proliferative area only, genes affected after PTx were categorized as Cancer and Cellular Growth and Proliferation by the Pathway Analysis (Table 3). These results suggest that many cellular and metabolic pathways may have been set to restore normal thyroid function and homeostasis after PTx through activating cell regeneration that resembles the process that occurs during thyroid development and/or cancer development. The involvement of stem cells in the development of a normal organ as well as cancer is well established (37, 38). Thus, clear cells may be immature cells that were previously C cells or follicular cells that participate in thyroid tissue repair and/or regeneration after PTx. Alternatively, clear cells may be immature cells derived from thyroid stem/progenitor cells, which have the potential of becoming either C cells or follicular cells and are on their way to maturation. The possible presence of stem/progenitor cells in mouse thyroid has been demonstrated (39). Further, it was previously proposed that the solid cell nest, the embryonic remnant of UBB that is the precursor to C cells, might be a source for both follicular cells and C cells (35, 40, 41). UBB is thought to be derived from the neural crest, although recent results demonstrated that the fourth pharyngeal pouch is the origin of the UBB but not the neural crest (42). Consistent with this hypothesis, clear immature cells may be derived from the UBB, suggesting a critical role for remnant UBB or C cells in thyroid regeneration. The fact that many genes expressed in neuroendocrine cells as well as C cells are up-regulated in the central proliferative area is in support of this hypothesis. Pale-staining cells, similar to what was observed as clear cells in the thyroid gland, were observed in mammary gland explants, which give rise to mammary epithelial outgrowths with complete developmental capacity (43). These pale-staining cells were considered to represent a latent epithelial stem cell population. Further studies are required to understand the nature and origin of clear cells and their relationship to the UBB and to C cells and/or follicular cells.
Microarray analysis was carried out using cells collected by laser capture microdissection. Although laser capture microdissection was performed with great care to capture only epithelial cells within microfollicles and/or follicles that have typical characteristics of epithelial cells, C cells were inevitably included in the collection, because clear cells and C cells are difficult to differentiate by microscopy during laser capture microdissection, especially given that they were minimally stained, as required for microdissection. It is well known that hypothyroidism arises after thyroidectomy and serum TSH increases by positive feedback, which results in goitrogenesis (hypertrophy and hyperplasia). In fact, that is what was observed in the current study. Two days after PTx, an approximately 2.5-fold reduction in serum T4 was followed, 5 d later, by a 5-fold increase in serum TSH. These drastic changes were almost restored to baseline levels 2 wk after PTx. TSH was shown to be critical for embryonic stem cells, stem cells derived from adult goiters, and dedifferentiated follicular cells to be transformed into mature follicular cells (44–46). Further, it was reported that the TSH receptor is present on follicular cells and C cells (47), and up-regulated serum TSH levels in hypothyroid rats directly and indirectly induces proliferation of follicular cells and C cells (48). Thus, it is very likely that the temporary increase in TSH participates in thyroid regeneration, which involves immature follicular cells, C cells and capillary vessels, and cell differentiation and proliferation. It is also possible that the changes in morphology and gene expression patterns observed after PTx might have included the process of goitrogenesis (hypertrophy and hyperplasia).
In the current study, the expression of Krt14 appeared only after PTx in a restricted area within the proliferative area of the thyroid gland. Krt14-positive cells possessed both epithelial and mesenchymal features that are distinct from those of epithelial cells found in microfollicles and/or follicles. Laser capture microdissection collected only the latter cell types, which were then subjected to microarray analysis. This was the reason that no changes were detected for Krt14 expression levels in microarray analysis. Although the thyroid gland is known to express several cytokeratins, their expression was reported mainly in thyroid tumors of humans (30, 31). Among them, the most studied is Krt19 (49, 50). In the current study, expression of Krt19 was observed at similar levels and patterns in intact as well as postpartially thyroidectomized thyroid glands. On the other hand, Krt14 expression has not been demonstrated in the normal thyroid gland (30, 31). Krt14 is known as a liver lineage marker and is expressed in immature and/or stem/progenitor cells of taste buds (33) and prostate (34). Based on these studies, it is tempting to speculate that Krt14 may be transiently expressed to participate in regeneration and/or restoration of thyroid physiology and function after PTx. How Krt14 cells contribute to thyroid regeneration if any and their relationship to clear cells are not known. Further studies are required to address these questions.
EM demonstrated the occasional presence of cell death in the area where many microfollicles were present. Cell death was observed in the middle of microfollicles, which appeared to become a lumen, whereas the cells surrounding the dying cells lined up in a circle as if a new follicle was about to develop. The dying cells may be processed by autophagy, because 1) dying cells are found within a follicle (Fig. 2F), 2) they contain enlarged lysosomes, where intracytoplasmic organelles are present, indicative of self-digestion (autodigestion), and 3) follicular cells are connected to the dying cells showing the phagocyte (Fig. 2E). These characteristics are different from those of oncosis or apoptosis. Oncosis is characterized by swelling of both nuclei and intracytoplasmic organelles, whereas apoptosis is characterized by the presence of karyorrhexis (apoptotic bodies), and apoptotic cells are cleared by macrophages. Because no phagocytic cells of macrophages or histicocytes were observed, they are likely not participating in thyroid cell death within the follicular lumen, further suggesting that apoptosis is not involved in this process. Thus, autophagy may be a way of generating a follicle in the thyroid in vivo. The mechanism for thyroid folliculogenesis was extensively studied by Toda et al. (51) using in vitro three dimensional primary culture system. Folliculogenesis consists of three types when thyrocytes proliferate in a sheet-like pattern in vitro: solid nest type, budding type, and lumen-dividing type. In the solid nest type, some thyrocytes form small lumens and the luminal structures grow larger. However, how lumens are initially formed has not been described. The current study may provide a clue that lumens are formed through cell death of centrally located cells within solid nests of thyroid epithelial cells.
In conclusion, this study demonstrates that a proliferative center is present in normal thyroid lobes. PTx-induced thyroid regeneration alters the physiology of cells and patterns of gene expression in this central area and near the cut edge, which resembles those found in thyroid development and cancer. Thyroid gland regeneration is essentially a required process for both thyroid development and cancer in terms of gene expression. Further, this work revealed that thyroid regeneration after PTx involves clear immature cells, cell death of autophagy, and ordered arrangement of follicular cells, C cells, and capillary endothelial cells. TSH might be involved in the regeneration process. PTx provides a model to study the physiological and pathological changes and genes involved in development, repair, regeneration of the thyroid gland, and/or goitrogenesis.
Supplementary Material
Acknowledgments
We thank Frank Gonzalez for his critical review of the manuscript.
This was supported in whole or in part by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research Grant 1Z01BC005522, with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN26120080001E, and by the extramural National Institutes of Health Grant DK 15070.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- Bromodeoxyuridine
- Calca
- calcitonin/calcitonin-related polypeptide, α
- DC
- dissected central
- DP
- dissected peripheral
- EM
- electron microscopy
- GO
- Gene Ontology
- H&E
- hematoxylin and eosin
- IC
- intact central
- IP
- intact peripheral
- IPA
- Ingenuity Pathway Analysis
- Krt14
- cytokeratin 14
- PTx
- partial thyroidectomy
- UBB
- ultimobranchial body.
References
- 1. Dumont JE, Lamy F, Roger P, Maenhaut C. 1992. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 72:667–697 [DOI] [PubMed] [Google Scholar]
- 2. Capen CC, Martin SL. 1989. The effects of xenobiotics on the structure and function of thyroid follicular and C-cells. Toxicol Pathol 17:266–293 [DOI] [PubMed] [Google Scholar]
- 3. Capen CC. 1997. Mechanistic data and risk assessment of selected toxic end points of the thyroid gland. Toxicol Pathol 25:39–48 [DOI] [PubMed] [Google Scholar]
- 4. Biondo-Simoes Mde L, Castro GR, Montibeller GR, Sadowski JA, Biondo-Simoes R. 2007. The influence of hypothyroidism on liver regeneration: an experimental study in rats. Acta Cir Bras 22(Suppl 1):52–56 [DOI] [PubMed] [Google Scholar]
- 5. Smith TJ, Drummond GS, Kourides IA, Kappas A. 1982. Thyroid hormone regulation of heme oxidation in the liver. Proc Natl Acad Sci USA 79:7537–7541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed MT, Sinha AK, Pickard MR, Kim KD, Ekins RP. 1993. Hypothyroidism in the adult rat causes brain region-specific biochemical dysfunction. J Endocrinol 138:299–305 [DOI] [PubMed] [Google Scholar]
- 7. Katakami H, Downs TR, Frohman LA. 1986. Decreased hypothalamic growth hormone-releasing hormone content and pituitary responsiveness in hypothyroidism. J Clin Invest 77:1704–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parmar DV, Khandkar MA, Pereira L, Bangur CS, Katyare SS. 1995. Thyroid hormones alter Arrhenius kinetics of succinate-2,6-dichloroindophenol reductase, and the lipid composition and membrane fluidity of rat liver mitochondria. Eur J Biochem 230:576–581 [DOI] [PubMed] [Google Scholar]
- 9. Ramos S, Goya L, Alvarez C, Martín MA, Pascual-Leone AM. 2001. Effect of thyroxine administration on the IGF/IGF binding protein system in neonatal and adult thyroidectomized rats. J Endocrinol 169:111–122 [DOI] [PubMed] [Google Scholar]
- 10. Duncan AW, Dorrell C, Grompe M. 2009. Stem cells and liver regeneration. Gastroenterology 137:466–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michalopoulos GK. 2007. Liver regeneration. J Cell Physiol 213:286–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oertel M, Shafritz DA. 2008. Stem cells, cell transplantation and liver repopulation. Biochim Biophys Acta 1782:61–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Durand S, Ferraro-Peyret C, Selmi-Ruby S, Paulin C, El Atifi M, Berger F, Berger-Dutrieux N, Decaussin M, Peix JL, Bournaud C, Orgiazzi J, Borson-Chazot F, Rousset B. 2008. Evaluation of gene expression profiles in thyroid nodule biopsy material to diagnose thyroid cancer. J Clin Endocrinol Metab 93:1195–1202 [DOI] [PubMed] [Google Scholar]
- 14. Nikolova DN, Zembutsu H, Sechanov T, Vidinov K, Kee LS, Ivanova R, Becheva E, Kocova M, Toncheva D, Nakamura Y. 2008. Genome-wide gene expression profiles of thyroid carcinoma: identification of molecular targets for treatment of thyroid carcinoma. Oncol Rep 20:105–121 [PubMed] [Google Scholar]
- 15. Pita JM, Banito A, Cavaco BM, Leite V. 2009. Gene expression profiling associated with the progression to poorly differentiated thyroid carcinomas. Br J Cancer 101:1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayat MA. 1970. Principles and techniques of electron microscopy, biological applications. New York: Van Nostranc Reinhold Co [Google Scholar]
- 17. Erickson HS, Albert PS, Gillespie JW, Rodriguez-Canales J, Marston Linehan W, Pinto PA, Chuaqui RF, Emmert-Buck MR. 2009. Quantitative RT-PCR gene expression analysis of laser microdissected tissue samples. Nat Protoc 4:902–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang X, Nakamura M, Mori I, Takeda K, Nakamura Y, Utsunomiya H, Yoshimura G, Sakurai T, Kakudo K. 2004. Calcitonin receptor gene and breast cancer: quantitative analysis with laser capture microdissection. Breast Cancer Res Treat 83:109–117 [DOI] [PubMed] [Google Scholar]
- 19. Neuhäuser M, Jöckel KH. 2006. A bootstrap test for the analysis of microarray experiments with a very small number of replications. Appl Bioinformatics 5:173–179 [DOI] [PubMed] [Google Scholar]
- 20. Boyle EI, Weng S, Gollub J, Jin H, Botstein D, Cherry JM, Sherlock G. 2004. GO::TermFinder—open source software for accessing gene ontology information and finding significantly enriched gene ontology terms associated with a list of genes. Bioinformatics 20:3710–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. 1999. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- 22. Oczko-Wojciechowska M, Włoch J, Wiench M, Fujarewicz K, Simek K, Gala G, Gubała E, Szpak-Ulczok S, Jarzab B. 2006. Gene expression profile of medullary thyroid carcinoma–preliminary results. Endokrynol Pol 57:420–426 [PubMed] [Google Scholar]
- 23. Schmid KW, Kirchmair R, Ladurner D, Fischer-Colbrie R, Böcker W. 1992. Immunohistochemical comparison of chromogranins A and B and secretogranin II with calcitonin and calcitonin gene-related peptide expression in normal, hyperplastic and neoplastic C-cells of the human thyroid. Histopathology 21:225–232 [DOI] [PubMed] [Google Scholar]
- 24. Kurabuchi S, Tanaka S. 2002. Immunocytochemical localization of prohormone convertases PC1 and PC2 in the mouse thyroid gland and respiratory tract. J Histochem Cytochem 50:903–909 [DOI] [PubMed] [Google Scholar]
- 25. Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. 1993. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development 119:1301–1315 [DOI] [PubMed] [Google Scholar]
- 26. Monaghan AP, Kaestner KH, Grau E, Schütz G. 1993. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3α, β and γ genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development 119:567–578 [DOI] [PubMed] [Google Scholar]
- 27. Nutt CL, Matthews RT, Hockfield S. 2001. Glial tumor invasion: a role for the upregulation and cleavage of BEHAB/brevican. Neuroscientist 7:113–122 [DOI] [PubMed] [Google Scholar]
- 28. Yates CM, Patel A, Oakley K, Helms A, Tuttle RM, Francis GL. 2006. Erythropoietin in thyroid cancer. J Endocrinol Invest 29:320–329 [DOI] [PubMed] [Google Scholar]
- 29. Heinrich C, Keller C, Boulay A, Vecchi M, Bianchi M, Sack R, Lienhard S, Duss S, Hofsteenge J, Hynes NE. 2010. Copine-III interacts with ErbB2 and promotes tumor cell migration. Oncogene 29:1598–1610 [DOI] [PubMed] [Google Scholar]
- 30. Miettinen M, Franssila KO. 2000. Variable expression of keratins and nearly uniform lack of thyroid transcription factor 1 in thyroid anaplastic carcinoma. Hum Pathol 31:1139–1145 [DOI] [PubMed] [Google Scholar]
- 31. Hirokawa M, Carney JA, Ohtsuki Y. 2000. Hyalinizing trabecular adenoma and papillary carcinoma of the thyroid gland express different cytokeratin patterns. Am J Surg Pathol 24:877–881 [DOI] [PubMed] [Google Scholar]
- 32. Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. 1996. Identification of bipotential progenitor cells in human liver development. Hepatology 23:476–481 [DOI] [PubMed] [Google Scholar]
- 33. Asano-Miyoshi M, Hamamichi R, Emori Y. 2008. Cytokeratin 14 is expressed in immature cells in rat taste buds. J Mol Histol 39:193–199 [DOI] [PubMed] [Google Scholar]
- 34. Tokar EJ, Ancrile BB, Cunha GR, Webber MM. 2005. Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation 73:463–473 [DOI] [PubMed] [Google Scholar]
- 35. Kusakabe T, Hoshi N, Kimura S. 2006. Origin of the ultimobranchial body cyst: T/ebp/Nkx2.1 expression is required for development and fusion of the ultimobranchial body to the thyroid. Dev Dyn 235:1300–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McMillan PJ, Heidbüchel U, Vollrath L. 1985. Number and size of rat thyroid C cells: no effect of pinealectomy. Anat Rec 212:167–171 [DOI] [PubMed] [Google Scholar]
- 37. Petersen OW, Polyak K. 2010. Stem cells in the human breast. Cold Spring Harb Perspect Biol 2:a003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vries RG, Huch M, Clevers H. 2010. Stem cells and cancer of the stomach and intestine. Mol Oncol 4:373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoshi N, Kusakabe T, Taylor BJ, Kimura S. 2007. Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology 148:4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harach HR. 1985. Solid cell nests of the thyroid. An anatomical survey and immunohistochemical study for the presence of thyroglobulin. Acta Anat 122:249–253 [PubMed] [Google Scholar]
- 41. Williams ED, Toyn CE, Harach HR. 1989. The ultimobranchial gland and congenital thyroid abnormalities in man. J Pathol 159:135–141 [DOI] [PubMed] [Google Scholar]
- 42. Kameda Y, Nishimaki T, Chisaka O, Iseki S, Sucov HM. 2007. Expression of the epithelial marker E-cadherin by thyroid C cells and their precursors during murine development. J Histochem Cytochem 55:1075–1088 [DOI] [PubMed] [Google Scholar]
- 43. Smith GH, Medina D. 1988. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci 90:173–183 [DOI] [PubMed] [Google Scholar]
- 44. Arufe MC, Lu M, Kubo A, Keller G, Davies TF, Lin RY. 2006. Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology 147:3007–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lan L, Cui D, Nowka K, Derwahl M. 2007. Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require thyrotropin for differentiation into thyrocytes. J Clin Endocrinol Metab 92:3681–3688 [DOI] [PubMed] [Google Scholar]
- 46. Suzuki K, Mitsutake N, Saenko V, Suzuki M, Matsuse M, Ohtsuru A, Kumagai A, Uga T, Yano H, Nagayama Y, Yamashita S. 2011. Dedifferentiation of human primary thyrocytes into multilineage progenitor cells without gene introduction. PLoS One 6:e19354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morillo-Bernal J, Fernández-Santos JM, Utrilla JC, de Miguel M, García-Marín R, Martín-Lacave I. 2009. Functional expression of the thyrotropin receptor in C cells: new insights into their involvement in the hypothalamic-pituitary-thyroid axis. J Anat 215:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martín-Lacave I, Borrero MJ, Utrilla JC, Fernández-Santos JM, de Miguel M, Morillo J, Guerrero JM, García-Marín R, Conde E. 2009. C cells evolve at the same rhythm as follicular cells when thyroidal status changes in rats. J Anat 214:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Choi YL, Kim MK, Suh JW, Han J, Kim JH, Yang JH, Nam SJ. 2005. Immunoexpression of HBME-1, high molecular weight cytokeratin, cytokeratin 19, thyroid transcription factor-1, and E-cadherin in thyroid carcinomas. J Korean Med Sci 20:853–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. de Matos PS, Ferreira AP, de Oliveira Facuri F, Assumpção LV, Metze K, Ward LS. 2005. Usefulness of HBME-1, cytokeratin 19 and galectin-3 immunostaining in the diagnosis of thyroid malignancy. Histopathology 47:391–401 [DOI] [PubMed] [Google Scholar]
- 51. Toda S, Aoki S, Suzuki K, Koike E, Ootani A, Watanabe K, Koike N, Sugihara H. 2003. Thyrocytes, but not C cells, actively undergo growth and folliculogenesis at the periphery of thyroid tissue fragments in three-dimensional collagen gel culture. Cell Tissue Res 312:281–289 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.