Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common forms of cutaneous T cell lymphoma (CTCL), and most frequently manifest as CD4+ CD45RO+ malignant T cells with T cell receptor clonality [1]. MF and SS are often refractory to standard chemotherapeutic treatments, which expose patients to toxicity often without substantial benefit. Because of the limited efficacy of existing treatments, several novel immune-modulating therapies for MF and SS are under investigation, including imiquimod and oligodeoxy-nucleotides (ODNs), ligands for Toll-like receptor 7 (TLR7) and TLR9, respectively. Imiquimod is already Food and Drug Administration (FDA) approved for the treatment of basal cell carcinoma, actinic keratoses and condyloma.
In a published case report, administration of the synthetic TLR7 ligand imiquimod successfully treated a cancerous skin plaque that was resistant to standard therapy, resulting in disease remission for at least 12 months [2]. Imiquimod treatment resulted in complete clearance of skin plaques in a patient with stage 1A CTCL with a 10-year history of disease [3]. Imiquimod treatment also eliminated skin involvement in a patient with B cell chronic lymphocytic leukemia (B-CLL) [4]. A preliminary study of imiquimod in patients with MF likewise showed a clinical response rate of 50% as measured by the clearance of plaques [5]. Both imiquimod and a synthetic CpG-containing ODN TLR9 agonist have shown the ability to enhance the host immune response in patients with CTCL [6,7]. Kim et al. recently reported the results of a phase I clinical trial of ODN 2006 (CpG 7909) in the treatment of a small cohort of patients with CTCL with refractory disease, showing that treatment was well tolerated and demonstrated anti-tumor activity in patients [8]. The ability of imiquimod to alter cell viability has precedent in studies of other skin cancers, where imiquimod has been found to induce apoptosis of malignant skin cells [9].
TLRs are most widely known for their role in pathogen recognition during the innate immune response. Upon detection of TLR agonists, antigen presenting cells (APCs) up-regulate costimulatory molecules and become primed to activate other immune cells. Anti-cancer studies employing TLR agonists are centered on the hypothesis that stimulation through TLR7 or TLR9, respectively, in APCs will enhance APC uptake and presentation of cancer antigens to other cells of the immune system. The alternative hypothesis that TLR ligands may directly affect the cancerous T cells themselves has not been investigated, and may have important implications for continued investigation of TLR ligands as therapeutic agents in the treatment of T cell malignancies. Several studies have demonstrated the ability of TLR ligands to affect malignant B cells with both pro- and anti-cancer outcomes, providing precedent for such a hypothesis [10].
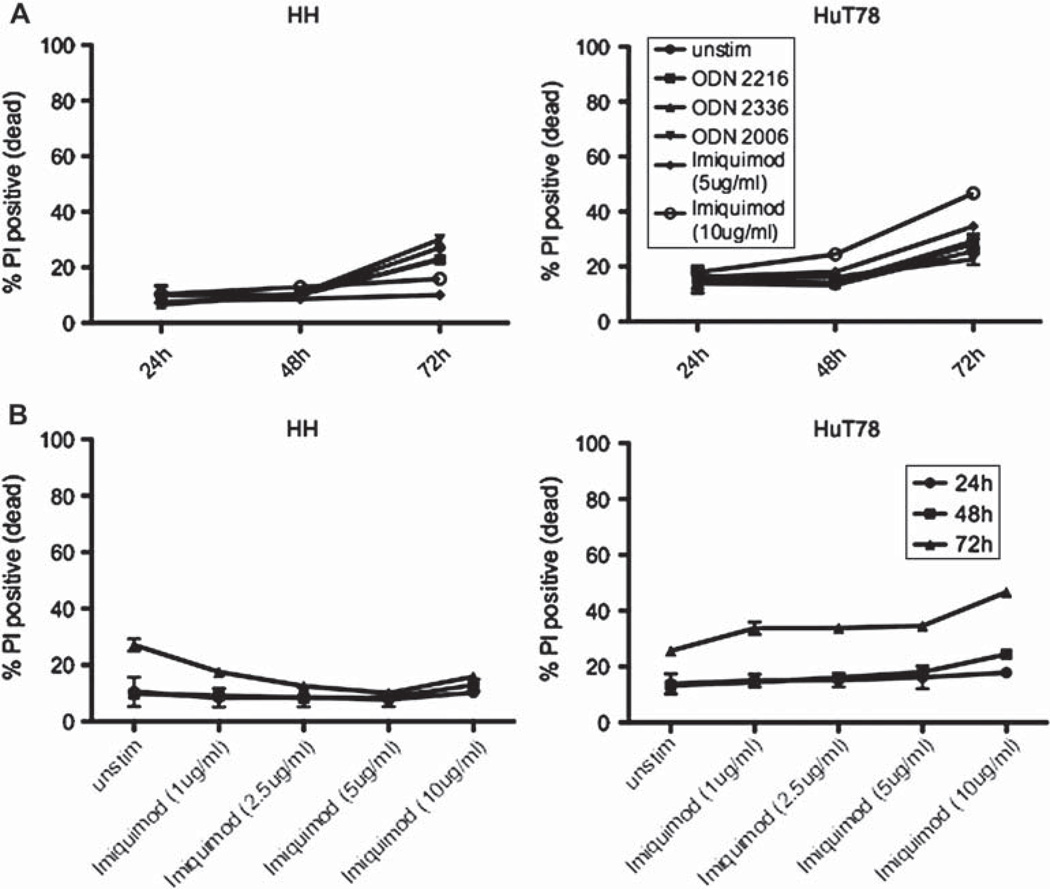
We tested the hypothesis that TLR agonists directly alter T cell lymphoma cell biology, first using mouse T cell lymphoma cell lines as a model system (data not shown), and ultimately confirming these findings on cell lines derived from human patients with CTCL. We focused these studies by stimulating HH, a non-MF/non-SS CTCL cell line, and HuT78, a SS cell line, with ODNs and imiquimod. Cells were stimulated with these ligands for 24, 48 or 72 h and assessed for viability by propidium iodide (PI) stain. By 72 h, the cell line HH demonstrated increased culture viability upon incubation with imiquimod, whereas imiquimod increased cell death in HuT78 cell cultures [Figure 1(A)]. A dose titration of imiquimod revealed maximal viability of HH cells at 5 µg/mL imiquimod, and maximal death induction of HuT78 cells was observed with 10 µg/mL imiquimod [Figure 1(B)]. In contrast, ODNs only minimally altered cell line viability [Figure 1(A)]. These human data, taken together with mouse T cell line viability data, indicate that the TLR7 agonist imiquimod has the greatest potential to influence malignant T-cell viability.
Figure 1.
Imiquimod alters human CTCL cell line viability. Cells were stimulated for 24, 48 or 72 h and stained with propidium iodide (PI) to measure the percentage of dead cells by flow cytometry. Error bars represent the standard deviation of values from three independent experiments.
We next determined whether TLR ligands alter cytokine secretion by human CTCL cell lines. Human CTCL cell lines were stimulated for 72 h with ODNs or imiquimod, and supernatants were subjected to a bead-based cytokine assay to detect the presence of 51 cytokines, with comparison to TLR ligand-stimulated peripheral blood mononuclear cells (PBMCs) from healthy human blood as a control for ligand activity and assay function (Table I). Soluble vascular cell adhesion molecule 1 (sVCAM-1), regulated upon activation, normal T cell expressed and secreted (RANTES), soluble intercellular adhesion molecule 1 (sICAM-1), interferon inducible protein 10 (IP10) and vascular endothelial growth factor (VEGF) were among the cytokines and chemokines secreted at the highest levels by the human CTCL cell lines, with plasminogen activator inhibitor 1 (PAI-1), interleukin 10 (IL-10) and sFas ligand also secreted by the HuT78 cell line but not the HH cell line. ODNs and imiquimod altered secretion of each of these cytokines to varying degrees. As we observed with the mouse cell lines (data not shown), imiquimod lowered the baseline level of VEGF secreted by the human cell lines (HH: 30% decrease, HuT78: 27% decrease). These results are consistent with published findings that imiquimod behaves as an anti-angiogenic agent [11]. The greatest fold change observed was the seven-fold induction of PAI-1 by ODN 2006 in HuT78 cells, which was also observed in PBMCs. This soluble factor is associated with poor prognosis in cancer, where it is thought to promote metastasis and cancer cell growth [12]. This result suggests that clinical treatment of CTCL with ODN 2006 (CpG 7909) may benefit from concurrent treatment with a PAI-1 inhibitor to improve efficacy. ODNs and imiquimod lowered the level of secretion of the adhesion molecules sICAM-1 and sVCAM-1, as well as the chemokine RANTES. The secreted forms of sICAM-1 and sVCAM-1 have been implicated in the loss of epidermotropism seen in advanced cases of CTCL [13]. Malignant cells from patients with CTCL have previously been found to secrete elevated levels of sICAM-1 [14]. RANTES has been implicated in chemoattraction of healthy monocytes to the tumor environment [15]. ODNs and imiquimod modulated IP10 secretion distinctly for HH and HuT78, and all ligands decreased the levels of IL-10 and sFas ligand secreted by the HuT78 cell line, with the exception of ODN 2336 increasing the secretion of IL-10. IP10, increased upon treatment with ODN 2006, is a chemoattractant capable of recruiting immune cells to sites of inflammation, and like sICAM-1 and sVCAM-1 is thought to play a role in CTCL epidermotropism [16]. IL-10, decreased by ODN 2006 and ODN 2216 and increased by ODN 2336, inhibits anticancer functions of APCs by promoting their sustained immaturity [15], and is associated with disease progression in cutaneous lymphomas [17]; and sFas ligand, decreased by ODN 2216, ODN 2006 and imiquimod, is elevated in lymphoproliferative disorders where it is thought to inhibit apoptosis, resulting in poor clinical outcome [18]. These results show that TLR agonists under clinical investigation directly influence cytokine secretion patterns by malignant T cells themselves, and therefore may influence disease progression in this manner.
Table 1.
Multiplex cytokine analysis of human CTCL cell lines stimulated with TLR ligands*.
| V-CAM-1 | RANTES | ICAM-1 | IP10 | VEGF | PAI-1 | IL-10 | sFas-L | IL-13 | LIF | TRAIL | Resistin | IL-lα | GM-CSF | MIP1α | TNFβ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HH, unstimulated | 6603 | 1213 | 737 | 189 | 199 | 6 | 1 | 23 | 0 | 0 | 15 | 1 | 8 | 10 | 26 | 0 |
| HH, ODN2216 | 4541 | 772 | 598 | 88 | 180 | 5 | 1 | 15 | 0 | 0 | 13 | 1 | 6 | 6 | 19 | 0 |
| HH, ODN2336 | 3570 | 838 | 462 | 135 | 186 | 4 | 0 | 15 | 0 | 0 | 11 | 1 | 8 | 4 | 10 | 0 |
| HH, ODN2006 | 4762 | 467 | 624 | 341 | 189 | 6 | 1 | 18 | 1 | 0 | 16 | 1 | 19 | 7 | 45 | 0 |
| HH, imiquimod | 4281 | 807 | 419 | 125 | 139 | 4 | 1 | 18 | 0 | 0 | 10 | 1 | 7 | 9 | 7 | 0 |
| HuT78, unstimulated | >70 294 | 866 | 426 | 253 | 218 | 168 | 703 | 267 | 84 | 47 | 29 | 50 | 13 | 29 | 12 | 25 |
| HuT78, ODN 2216 | >70 294 | 409 | 222 | 298 | 193 | 142 | 620 | 199 | 62 | 44 | 23 | 36 | 13 | 19 | 3 | 29 |
| HuT78, ODN 2336 | >70 294 | 581 | 242 | 381 | 284 | 141 | 780 | 247 | 66 | 48 | 27 | 51 | 18 | 23 | 10 | 32 |
| HuT78, ODN 2006 | >70 294 | 500 | 267 | 612 | 214 | 1175 | 283 | 110 | 68 | 51 | 23 | 13 | 26 | 79 | 9 | 21 |
| HuT78, imiquimod | >70 294 | 672 | 259 | 270 | 158 | 115 | 638 | 178 | 92 | 44 | 22 | 45 | 13 | 26 | 3 | 33 |
| PBMC, unstimulated | 1 | 3 | 79 | 23 | 3 | 148 | 1 | 8 | 0 | 0 | 10 | 36 | 1 | 0 | 5 | 1 |
| PBMC, ODN 2216 | 11 | 4 | 106 | 3372 | 3 | 216 | 7 | 19 | 0 | 2 | 56 | 37 | 46 | 1 | 17 | 6 |
| PBMC, ODN 2336 | 14 | 5 | 100 | >5000 | 3 | 211 | 10 | 22 | 0 | 3 | 36 | 31 | 50 | 1 | 19 | 7 |
| PBMC, ODN 2006 | 12 | 43 | 249 | 84 | 8 | 1258 | 50 | 10 | 0 | 4 | 101 | 63 | 6 | 2 | 179 | 36 |
| PBMC, imiquimod | 11 | 4 | 174 | 5 | 27 | 293 | 20 | 8 | 2 | 2 | 187 | 83 | 10 | 4 | 54 | 2 |
CTCL, cutaneous T cell lymphoma; PBMC, peripheral blood mononuclear cell; sVCAM-1, soluble vascular cell adhesion molecule 1; RAN TES, regulated upon activation, normal T cell expressed and secreted; sICAM-1, soluble intercellular adhesion molecule 1; IP10, interferon inducible protein 10; VEGF, vascular endothelial growth factor; PAI-1, plasminogen activator inhibitor 1; IL-10, interleukin 10; sFas-L, soluble Fas ligand; LIF, leukemia inhibitory factor; TRAIL, TNF-related apoptosis-inducing ligand; GM-CSF, granulocyte-macrophage colony stimulating factor; MIP1α, macrophage imflammatory protein 1α; TNFβ, tumor necrosis factor β
Cells were stimulated with Toll-like receptor (TLR) ligands as shown. After 72 h, supernatants were harvested and analyzed for the presence of 51 soluble factors using a bead-based cytokine assay. Only data for soluble factors scoring as detectable (>15 pg/mL above background) for HH and/or HuT78 cells are shown. Note that HuT78 values for VCAM-1 were elevated beyond the scale of the reference standard curve.
We present the first data demonstrating the ability of malignant mouse T cell lymphoma lines and human CTCL cell lines to respond with functional outcomes to direct TLR ligand stimulation. We show that imiquimod had the greatest impact on human CTCL cell line viability, promoting death of HuT78 cells and survival of HH cells. This differential outcome may be reflective of the variation in the CTCL subtypes of the cell lines assayed: HuT78 cells being of the SS type, and HH cells of a non-MF/non-SS type. Future studies on primary patient samples will be needed to test this hypothesis.
These data have implications for the ongoing investigation of TLR ligands as therapeutic agents in the treatment of human T cell malignancies. Specifically, TLR agonists may promote interaction of cancerous T cells with the anti-tumor infiltrating immune system, and knowledge of this interplay may be leveraged in the design of more effective therapeutic strategies. An enhanced understanding of the effects of TLR agonists on malignant T cells may provide additional targets to block, and may allow better understanding of differing patient responses to therapy. These results highlight the importance of dissecting the relationship between cells expressing TLRs and the mechanisms by which these TLR ligands function. They further demonstrate the complexity of the physiological context, revealing a need for a better understanding of how to fully leverage the immunological environment to promote favorable responses.
Acknowledgements
The authors would like to thank R. Levy and J. Aster for mouse cell lines. The authors would also like to thank members of the Utz Laboratory, O. M. Martinez, G. P. Nolan, and R. Levy for helpful discussion.
This work was supported by the US National Institutes of Health grant U19 AI082719, the Canadian Institutes of Health Research grant 20R-92141, The US National Institutes of Health grant U19 AI050864 and a gift from the Floren Family Trust. A.L. was supported by the National Science Foundation Graduate Research Fellowship. G.Y. was funded by the Stanford Medical Scientist Training Program (MSTP). K.A. was funded by the Stanford Institutes of Medical Research (SIMR) Program.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dummer R, Urosevic M, Kempf W, et al. Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology. 2003;207:116–118. doi: 10.1159/000070962. [DOI] [PubMed] [Google Scholar]
- 3.Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138:1137–1139. doi: 10.1001/archderm.138.9.1137. [DOI] [PubMed] [Google Scholar]
- 4.Spaner DE, Miller RL, Mena J, et al. Regression of lymphomatous skin deposits in a chronic lymphocytic leukemia patient treated with the Toll-like receptor-7/8 agonist, imiquimod. Leuk Lymphoma. 2005;46:935–939. doi: 10.1080/10428190500054426. [DOI] [PubMed] [Google Scholar]
- 5.Deeths MJ, Chapman JT, Dellavalle RP, et al. Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol. 2005;52:275–280. doi: 10.1016/j.jaad.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 6.Wysocka M, Newton S, Benoit BM, et al. Synthetic imidazoquinolines potently and broadly activate the cellular immune response of patients with cutaneous T-cell lymphoma: synergy with interferon-gamma enhances production of interleukin-12. Clin Lymphoma Myeloma. 2007;7:524–534. doi: 10.3816/clm.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 7.Wysocka M, Benoit BM, Newton S, et al. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and Il-15. Blood. 2004;104:4142–4149. doi: 10.1182/blood-2004-03-1190. [DOI] [PubMed] [Google Scholar]
- 8.Kim YH, Girardi M, Duvic M, et al. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J Am Acad Dermatol. 2010;63:975–983. doi: 10.1016/j.jaad.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 9.Schon MP, Schon M. Immune modulation and apoptosis induction: two sides of the antitumoral activity of imiquimod. Apoptosis. 2004;9:291–298. doi: 10.1023/b:appt.0000025805.55340.c3. [DOI] [PubMed] [Google Scholar]
- 10.Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, et al. Toll-like receptors: lessons to learn from normal and malignant human b cells. Blood. 2008;112:2205–2213. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li VW, Li WW, Talcott KE, et al. Imiquimod as an antiangiogenic agent. J Drugs Dermatol. 2005;4:708–717. [PubMed] [Google Scholar]
- 12.Gramling MW, Church FC. Plasminogen activator inhibitor-1 is an aggregate response factor with pleiotropic effects on cell signaling in vascular disease and the tumor microenvironment. Thromb Res. 2010;125:377–381. doi: 10.1016/j.thromres.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazawa M, Takahashi S, Kawaguchi H, et al. Low expression of adhesion molecules in a case of cutaneous T-cell lymphoma. J Dermatol. 1995;22:659–664. doi: 10.1111/j.1346-8138.1995.tb03894.x. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Lerma I, Estrach MT. A distinct profile of serum levels of soluble intercellular adhesion molecule-1 and intercellular adhesion molecule-3 in mycosis fungoides and sezary syndrome. J Am Acad Dermatol. 2009;61:263–270. doi: 10.1016/j.jaad.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Wilcox RA, Wada DA, Ziesmer SC, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114:2936–2944. doi: 10.1182/blood-2009-05-220111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarris AH, Daliani D, Ulmer R, et al. Interferon-inducible protein-10 and the pathogenesis of cutaneous T-cell lymphomas. Leuk Lymphoma. 1996;24:103–110. doi: 10.3109/10428199609045718. [DOI] [PubMed] [Google Scholar]
- 17.Urosevic M, Willers J, Mueller B, et al. HLA-G protein up-regulation in primary cutaneous lymphomas is associated with interleukin-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood. 2002;99:609–617. doi: 10.1182/blood.v99.2.609. [DOI] [PubMed] [Google Scholar]
- 18.Hara T, Tsurumi H, Takemura M, et al. Serum-soluble Fas level determines clinical symptoms and outcome of patients with aggressive non-Hodgkin’s lymphoma. Am J Hematol. 2000;64:257–261. doi: 10.1002/1096-8652(200008)64:4<257::aid-ajh4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]