Abstract
Objective
To examine whether mood symptoms increased more for women in the years after hysterectomy with or without bilateral oophorectomy relative to natural menopause.
Methods
Using data from the Study of Women’s Health Across the Nation (n=1,970), depression and anxiety symptoms were assessed annually for up to 10 years with the Center for Epidemiological Studies Depression Index and four anxiety questions, respectively. Piecewise hierarchical growth models were used to relate natural menopause, hysterectomy with ovarian conservation, and hysterectomy with bilateral oophorectomy to trajectories of mood symptoms before and after the final menstrual period or surgery. Covariates included educational attainment, race, menopausal status, age the year prior to final menstrual period or surgery, and time-varying body mass index, self-rated health, hormone therapy, and antidepressant use.
Results
By the 10th annual visit, 1,793 (90.9%) women reached natural menopause, 76 (3.9%) reported hysterectomy with ovarian conservation, and 101 (5.2%) reported hysterectomy with bilateral oophorectomy. For all women, depressive and anxiety symptoms decreased in the years after final menstrual period or surgery. These trajectories did not significantly differ by hysterectomy or oophorectomy status. The Center for Epidemiological Studies Depression Index means were .72 standard deviations lower, and anxiety symptoms .67 standard deviations lower, five years after final menstrual period or surgery.
Conclusion
In this study, mood symptoms continued to improve after the final menstrual period or hysterectomy for all women. Women who undergo a hysterectomy with or without bilateral oophorectomy in midlife do not experience more negative mood symptoms in the years after surgery.
Introduction
More than one third of women in the United States have had a hysterectomy by the age of 60. This common surgery is performed on six hundred thousand women in the United States each year, (1) accompanied by bilateral oophorectomy in 55–80% of cases. (2) An association between negative mood and hysterectomy is supported by cross-sectional studies, which suggest that women with a hysterectomy, regardless of oophorectomy status, are more distressed than age-matched peers without a hysterectomy. (3,4)
Investigations of the potential influence of hysterectomy and oophorectomy on mood are limited and mixed. (5–7) Existing studies do not generally account for mood in the years prior to presentation for surgery, and typically compare the impact of hysterectomy with bilateral oophorectomy only to hysterectomy alone. Follow-up is generally short, providing mixed results about the effects of oophorectomy or hysterectomy on mood in the months and initial years after surgery. (5,6) It therefore remains unclear whether any change in mood is attributable to hysterectomy or oophorectomy, and whether any changes are different than would be expected with natural menopause. In this study, we sought to examine the course of depressive and anxiety symptoms among women in midlife before and after natural menopause, hysterectomy with ovarian conservation, and hysterectomy with bilateral oophorectomy.
Materials and Methods
Participants
Participants were from the Study of Women’s Health Across the Nation, a multi-site community-based prospective study designed to examine the physical and psychological health of women as they undergo the menopausal transition. At baseline, all participants had an intact uterus and at least one ovary and met the additional eligibility criteria: aged 42–52 years, not pregnant, not using reproductive hormones, and having one or more menstrual cycles in the three months prior to the interview. Details of the design and recruitment procedures have been reported elsewhere. (8) Seventy-three percent of the women selected were contacted and provided information to determine eligibility; 51% (n = 3,302) of eligible women enrolled. The study enrollment period was from 1996–1997, and participants continue to return to their local site facility annually for interviewer- and self-administered questionnaires, a fasting blood draw, and reassessments of physical measures. The study was approved by the institutional review boards at each site, and each participant provided written, informed consent.
Design and procedures
Depressive symptoms
Depressive symptoms were assessed by trained interviewers at each annual visit using the Center for Epidemiological Studies Depression Scale, a 20-item depression symptom scale. (9,10)
Anxiety
Anxiety was assessed at each annual visit with four questions asking about the number of days in the two weeks prior to the baseline visit in which participants reported “irritability or grouchiness”, “feeling tense or nervous”, “heart pounding or racing”, or “feeling fearful for no reason”.
Menopause and Hysterectomy status
Menopausal status and the occurrence of hysterectomy and oophorectomy were assessed annually. Women were categorized as naturally postmenopausal if they reported a complete absence of menstrual bleeding in the previous 12 months. Participants were categorized as having had a hysterectomy with ovarian conservation if they reported hysterectomy without oophorectomy or with one ovary removed before becoming naturally postmenopausal. Participants were categorized as having had a hysterectomy with bilateral oophorectomy if they reported a hysterectomy and both ovaries removed, or both ovaries removed without hysterectomy, before becoming naturally postmenopausal.
Covariates
All covariates were selected on the basis of previously documented associations with negative mood symptoms in midlife, and included race, educational attainment, menopausal status the year prior to final menstrual period or surgery, and age the year prior to final menstrual period or surgery, as well as annual measurements of body mass index, self-rated health, hormone therapy use, and antidepressant use. Race and educational level were self-reported in the screening interview. Final menstrual period date was based on participant self-report; if final menstrual period date was unknown, it was set as 12 months prior to the date of the annual visit participants were first categorized postmenopausal. Menopausal status was assessed by self-reported bleeding patterns annually, and drawn from the annual visit prior to each participant’s final menstrual period or surgery date. Age was calculated from the participant’s date of birth and date of final menstrual period or surgery. Body mass index (BMI) was calculated at each annual visit using weight (kg) and height (m)2. Self-rated health was assessed at each annual visit by response to the following question: “In general, would you say your health is excellent, very good, good, fair or poor?”. (11) Antidepressant and hormone therapy use were self-reported at each annual visit.
Analytic sample
A total of 1,970 women, including 1,793 women who reached natural menopause, 76 women who had a hysterectomy with ovarian conservation, and 101 women who had a hysterectomy with bilateral oophorectomy comprised the analytic sample. Data from women who did not report hysterectomy or reach natural menopause (n=920) during their participation in SWAN, those who had a hysterectomy after having been categorized naturally postmenopausal (n=32), those who reported hysterectomy in the presence of known or suspected endometrial, uterine, or ovarian cancer (n=21), those who did not have data regarding depressive or anxiety symptoms from at least one annual visit post-final menstrual period or surgery (n=51), and women from the New Jersey site, subject to systematically fewer annual observations due to an early discontinuation for reasons unrelated to scientific integrity (n=131), were also excluded from this analysis. Compared to the women who were excluded from analysis, women in analytic sample were older (mean 46.3, SD 2.6 vs. mean 45.2, SD 2.7, p<.001), more educated (46.5% v. 37.3% college or post-college, p<.001), reported better self-rated health (61.3% v. 52.6% excellent or very good health, p<.001), and had a lower mean BMI (28.0, SD 7.3, v. 28.8, SD 7.1, p<.01) at baseline (data not shown in tables).
Racial differences in the prevalence and age at hysterectomy are documented, (1) though racial differences in the impact of hysterectomy and oophorectomy have not been well-studied. In order to assess potential interactions between hysterectomy status and race, a secondary analysis was performed in a sample restricted to only African American and Caucasian women (n=1, 581). The restricted sample included 1,422 women who reached natural menopause, 71 women who had a hysterectomy with ovarian conservation, and 88 women who had a hysterectomy with bilateral oophorectomy.
Statistical analyses
Baseline characteristics were compared using chi-square and multinomial regression for categorical variables, and ANOVA and Bonferroni correction for continuous variables using SPSS v.17. (SPSS for Windows, Rel 17.0.0, 2008.) Site, race and ethnicity, age at final menstrual period or surgery, educational attainment, and menopausal status the year prior to final menstrual period or surgery were included as covariates in final models based on a priori decisions. Preliminary analyses were conducted to assess independent associations between annual observations of antidepressant use, hormone therapy use, self-rated health, and BMI with depressive symptoms and anxiety. These time-varying covariates were retained in final models if they were associated with depressive symptoms or anxiety in otherwise unadjusted models at p<.05.
Piecewise hierarchical linear growth models were used to estimate the mean rate of change in depressive and anxiety scores leading up to and after final menstrual period or surgery, with annual observations nested within women. (HLM for Windows, version 6.08, 2010) Hierarchical linear modeling was used due to its utility in accounting for the dependence of repeated, correlated observations within individuals. Piecewise hierarchical linear growth models were used to allow for the possibility of different mean growth trajectories before versus after final menstrual period or surgery. (12) The intercept was set as the first annual visit after final menstrual period or surgery, with estimates of the mean rate of change in mood symptoms modeled from baseline to intercept, and from intercept to the end of observations. Depressive symptoms and anxiety were modeled separately. The intercept and each growth trajectory were modeled as a function of hysterectomy status (hysterectomy with ovarian conservation or hysterectomy with bilateral oophorectomy), with natural menopause set as the referent. All predictors and covariates were entered simultaneously in the final multivariate models. For all analyses, p values less than 0.05 (two-tailed) were considered statistically significant.
Results
Characteristics of sample
Participants were followed for up to 10 years after baseline, with observations from up to 9 years before and after final menstrual period or surgery. Characteristics of the sample can be found in table 1. Women who reported hysterectomy with or without ovarian conservation during the observed period differed significantly at baseline from women who reached natural menopause and did not have a hysterectomy over the observed period (table 1). Naturally postmenopausal women were less likely to be African American, less likely to ever use hormone therapy over the observed period, and more likely to be older at baseline. Naturally postmenopausal women were also less likely than women with hysterectomy with ovarian conservation to have used an antidepressant over the observed period and had a lower BMI at baseline. No significant differences were seen at baseline between women who subsequently had a hysterectomy with versus without ovarian conservation.
Table 1.
Characteristics of Analytic Sample at Time of Final Menstrual Period or Hysterectomy With or Without Bilateral Oophorectomy
| Hysterectomy With Ovarian Conservation (n=76, 3.9%) n(%) |
Hysterectomy With Bilateral Oophorectomy (n=101, 5.2%) n(%) |
Natural Menopause (n=1793, 90.9%) n(%) |
P | |
|---|---|---|---|---|
| Race or ethnicity | <.001 | |||
| White | 31 (40.8) | 42 (41.6) | 881 (49.1) | |
| African American | 40 (52.6) | 46 (45.5) | 541 (30.2) | |
| Chinese | 0 (.0) | 6 (5.8) | 172 (9.6) | |
| Japanese | 5 (6.6) | 7 (6.9) | 199 (11.1) | |
| Prior menopausal status | <.001 | |||
| Premenopausal | 44 (57.9) | 46 (45.5) | 323 (18.0) | |
| Perimenopausal | 2 (2.6) | 3 (3.0) | 847 (47.2) | |
| Unknown | 9 (11.8) | 16 (15.8) | 286 (16.0) | |
| Missing | 21 (27.6) | 36 (35.6) | 337 (18.8) | |
| Education | .21 | |||
| Less than High school | 13 (17.1) | 24 (23.8) | 372 (20.9) | |
| Some college | 26 (34.2) | 41 (40.6) | 572 (32.1) | |
| College or more | 37 (48.7) | 36 (35.6) | 838 (47.0) | |
| Self-rated health | .42 | |||
| Excellent or very good | 33 (46.5) | 46 (48.4) | 924 (52.9) | |
| Good, fair, or poor | 38 (53.5) | 49 (51.6) | 824 (47.1) | |
| Postfinal menstrual period or surgery hormone therapy use | 37 (48.68) | 80 (79.21) | 430 (24.00) | <.001 |
| Age, mean (SD) | 48.00 (2.85) | 49.07 (2.74) | 51.71 (2.54) | <.001 |
| Body mass index, mean (SD) | 30.92 (8.03) | 29.60 (6.72) | 28.53 (7.24) | .01 |
| Duration of hormone therapy use, mean (SD) | 1.22 (1.76) | 3.48 (2.97) | .72 (1.58) | <.001 |
| Number of days between final menstrual period or surgery and index visit, mean (SD) | 196.62 (135.67) | 242.08 (268.49) | 173.17 (151.92) | <.01 |
SD, standard deviation.
Data do not add to 100% because of missing data.
Depressive symptoms before and after final menstrual period or surgery
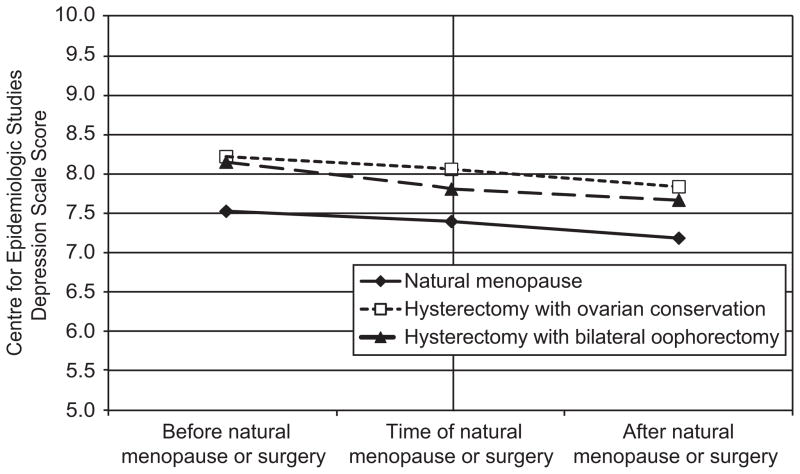
Depressive symptoms declined before final menstrual period or surgery and continued to decline after final menstrual period or surgery (Table 2; row for slopes). The Center for Epidemiological Studies Depression Index means were .72 standard deviations lower five years after final menstrual period or surgery. Regardless of whether ovaries were conserved, hysterectomy status had no effect on depressive symptoms at the first annual visit after final menstrual period or surgery or on the trajectory of depressive symptoms leading up to or after final menstrual period or surgery (Figure 1). There was no significant difference in the mean rate of change in depressive symptoms in these groups relative to natural menopause. Depressive symptoms at the first annual visit after final menstrual period or surgery varied by site, were higher among women with missing menopausal status in visit prior to final menstrual period or surgery, and were lower among women with at least a 4 year college education and older age at final menstrual period or surgery. The Center for Epidemiological Studies Depression Index scores were higher at visits when women were using antidepressant medications and reported poorer health, and were lower at visits when women used hormone therapy.
Table 2.
Mean Change in Depressive and Anxiety Symptoms Before and After Natural Menopause, Hysterectomy With Ovarian Conservation, and Hysterectomy With Bilateral Oophorectomy
| Depressive Symptoms | Anxiety Symptoms | |||
|---|---|---|---|---|
| Coefficient (SE) | P | Coefficient (SE) | P | |
| Mean mood scores at index visit | 7.39 (.54) | <.001 | 1.86 (.14) | <.001 |
| Hysterectomy status (Referent: natural menopause) | ||||
| Hysterectomy with ovarian conservation (n=76) | .66 (1.08) | .54 | .18 (.27) | .51 |
| Hysterectomy with bilateral oophorectomy (n=101) | .41 (.84) | .63 | .14 (.21) | .51 |
| Number of days between final menstrual period or surgery and index visit | 0.00 (.00) | .27 | 0.00 (.00) | .23 |
| Race or ethnicity (Referent: Caucasian) | ||||
| African American | .03 (.38) | .94 | −.27 (.10) | .01 |
| Chinese | −1.20 (.69) | .08 | −.41 (.18) | .02 |
| Japanese | 1.11 (.60) | .07 | −.11 (.14) | .45 |
| Education (Referent: High school or less) | ||||
| Some college | −.75 (.42) | .07 | .03 (.10) | .80 |
| College or more | −1.74 (.39) | <.001 | .00 (.10) | .96 |
| Menopausal status prior to final menstrual period or surgery (Referent: Premenopausal) | ||||
| Perimenopausal | −.11 (.38) | .78 | .05 (.10) | .61 |
| Unknown: Hormone therapy/other | .14 (.44) | .75 | .18 (.11) | .12 |
| Missing | 1.62 (.51) | <.01 | .39 (.12) | .01 |
| Age at final menstrual period or surgery | −.23 (.05) | <.001 | −.03 (.01) | .06 |
| Mean rate of change in mood symptoms from baseline to final menstrual period or surgery | −.13 (.03) | <.001 | 0.00 (.00) | .97 |
| Hysterectomy status (Referent: Natural menopause) | ||||
| Hysterectomy with ovarian conservation (n=76) | −.03 (.21) | .90 | −.02 (.05) | .76 |
| Hysterectomy with bilateral oophorectomy (n=101) | −.21 (.17) | .21 | −.01 (.04) | .84 |
| Mean rate of change in mood symptoms from final menstrual period or surgery to end of observations | −.21 (.03) | <.001 | −.05 (.01) | <.001 |
| Hysterectomy status (Referent: Natural menopause) | ||||
| Hysterectomy with ovarian conservation (n=76) | −.01 (.14) | .93 | .06 (.05) | .24 |
| Hysterectomy with bilateral oophorectomy (n=101) | .07 (.13) | .59 | .02 (.03) | .59 |
| Time-varying covariates (reported annually) | ||||
| Body mass index | 0.00 (.02) | .93 | ||
| Antidepressant use | 1.87 (.25) | <.001 | .41 (.07) | <.001 |
| Self-rated health (Referent: excellent or very good) | ||||
| Good, fair, or poor | 1.65 (.13) | <.001 | .36 (.03) | <.001 |
| Hormone therapy use | −.38 (.18) | .03 | −.09 (.05) | .04 |
SE, standard error.
Final models show mean rate of change in depressive and anxiety symptoms before and after final menstrual period or surgery in women with natural menopause, hysterectomy with ovarian conservation, and hysterectomy with bilateral oophorectomy. Intercept adjusted by hysterectomy status, time elapsed since final menstrual period or surgery, site, race/ethnicity, education, prior menopausal status, and age; body mass index (depressive symptoms only), self-rated health, antidepressant use, and hormone use at each annual visit included as time-varying covariates. Models also adjusted by site (data not shown).
Figure 1.
Depressive symptom scores in years before and after final menstrual period or surgery. No significant differences in intercept or time slopes between groups.
Anxiety symptoms before and after final menstrual period or surgery
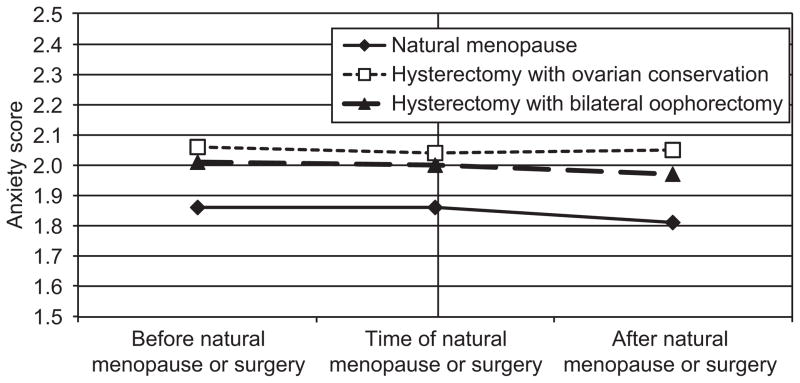
Anxiety scores did not change significantly in the years leading up to final menstrual period or surgery, but decreased during the period after final menstrual period or surgery (Table 2, row for slopes). Regardless of whether ovaries were conserved or not, hysterectomy status had no effect on anxiety symptoms at the first annual visit after final menstrual period or surgery or on the trajectory of anxiety symptoms after final menstrual period or surgery (Figure 2). Mean anxiety scores were .67 standard deviations lower five years after final menstrual period or surgery. Anxiety symptoms at the first annual visit after final menstrual period or surgery varied by site, were higher among women with missing menopausal status in the visit prior to final menstrual period or surgery, were lower among African American women and Chinese women, and tended to be lower among women who were older at final menstrual period or surgery. Anxiety scores increased concurrently with antidepressant use and poorer self-rated health, and decreased with hormone therapy use.
Figure 2.
Anxiety scores in years before and after final menstrual period or surgery. No significant differences in intercept or time slopes between groups.
Secondary analyses
In the subsample of only African American and Caucasian women, the results were similar to the overall sample results. However, African American women who had a hysterectomy with ovarian conservation experienced a steeper decline in depressive symptoms leading up to surgery than did Caucasian women who had a hysterectomy with ovarian conservation, (estimates = −.20, p<.001, compared to −.47, p-value .03). Though all other women had a decrease in anxiety symptoms leading up to surgery and final menstrual period, (B=−.03, p<.01), African American women with natural menopause had an increase in anxiety symptoms during this time (B=.04, p=.02).
In sensitivity analyses conducted to examine the role of hormone therapy use, the mean rate of change in depressive and anxiety symptoms was equivalent in the full sample, among women who reported hormone therapy use after final menstrual period or surgery, and among women who did not report hormone therapy use after menstrual period or surgery (data not shown).
Discussion
This study assessed change in mood symptoms after surgical menopause relative to natural menopause. We found that both depressive and anxiety symptoms generally improved over the course of the menopausal transition for all women, with no effect of hysterectomy status on this change. Our results suggest that compared to natural menopause, hysterectomy with or without ovarian conservation among women in midlife does not have a lasting negative impact on mood.
These results are consistent with studies examining mood symptoms over a short follow-up period after hysterectomy; some studies, though not all, suggest that mood symptoms improve after surgery. (5,6) In contrast to our data suggesting a continual decline in symptoms over time, Rocca et al. showed an increased long-term risk of de novo anxiety and depressive symptoms among women with hysterectomy with bilateral oophorectomy a median of 24 years post-surgery. (7) However, mood symptoms and their timing in that study were assessed retrospectively from women interviewed years or decades after surgery, and may have been subject to retrospective reporting biases. Women in that study also experienced hysterectomy at a variety of ages, while this investigation examines only women with hysterectomy during midlife and prior to the onset of natural postmenopause. Health and mental health risks of oophorectomy may be limited to younger women who have not already begun to experience hormonal changes related to the progression to natural menopause. (13)
Additional findings about annually measured hormone therapy and antidepressant use are worth mentioning. Use of hormone therapy was concurrently associated with lower levels of anxiety and depressive symptoms. Hormones were used at some point over the observation by the majority of participants, and as expected, were particularly common among women with a hysterectomy and oophorectomy. Excluding women who reported post-final menstrual period or surgery hormone therapy use did not affect the trajectory of changes in depressive or anxiety symptoms, suggesting that while hormone therapy was associated with improvements in mood, it did not account for the general lack of relationship seen between mood trajectories post-surgery and hysterectomy status. Antidepressant or anxiolytic use was strongly and consistently concurrently related to higher anxiety and depressive symptoms, which may highlight the need for clinicians to appropriately monitor treatment efficacy among patients in midlife presenting with mood symptoms.
Several limitations of this study should be noted. These results are based on assessment of anxiety and depressive symptoms, and may not be generalizable to populations with anxiety and depressive disorders. Women who chose to participate in this study and continue to participate in annual visits may differ from women in the general population, and women who reported hysterectomy during the observed period may differ from women with a hysterectomy in the general population. In a survey of 15,160 women screened for study participation, women with prior hysterectomy were more often African American, less educated, older, separated/widowed/divorced, multiparous, current or past smokers, and religious. (14) Thus, the remaining sample of eligible women tended to differ on those potential risk factors for hysterectomy than the sample contacted for survey and eligibility. (8)
Despite these limitations, the study also had considerable strengths. A large, well-characterized, multi-ethnic population-based sample of women in midlife was used, providing information about symptom experience among women from diverse backgrounds. Data were collected over an eleven-year period, allowing for observations across a range of participant ages and stages in the menopausal transition. This study was unique in prospectively evaluating mood and hysterectomy status, providing information on the influence of elective hysterectomy with and without bilateral oophorectomy on depressive and anxiety symptoms over time while accounting for mood symptoms prior to surgery. The comparison of these trajectories to those of naturally postmenopausal women offers needed information not only on the effects of oophorectomy over hysterectomy alone, but also how these differ from general trends experienced over the natural menopausal transition.
Overall, the results of this study should provide reassurance to women and their clinicians about the progression of depressive and anxiety symptoms in women both before and after menopause. Clinicians should be aware that negative mood symptoms generally decline over time over the course of the menopausal transition, (15) suggesting that anxiety and depressive symptoms should improve as women enter the postmenopausal years. Lasting effects on both anxiety and depressive symptoms do not appear to be necessary considerations when evaluating decisions surrounding ovarian conservation.
Acknowledgments
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
The authors thank Elizabeth Votruba-Drzal, PhD, for statistical assistance.
Supported by Cardiovascular Behavioral Medicine Training Grant NIH T32 HL 007560.
Footnotes
Presented in part at the 22nd North American Menopause Society Annual Meeting in Washington, DC, September 21-24 2011.
Financial Disclosure: Dr. Joffe has received research support from Bayer HealthCare Pharmaceuticals and has performed advisory and consulting work for Sanofi-Aventis/Sunovion, Pfizer, and Noven. The other authors did not report any potential conflicts of interest.
References
- 1.Keshavarz H, Hillis SD, Kieke BA, Marchbanks PA. Hysterectomy surveillance. United States 1994–1999. MMWR CDC Surveill Summ. 2002;51:1–8. [PubMed] [Google Scholar]
- 2.Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008 Jan;198(1):34.e1–7. doi: 10.1016/j.ajog.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Byles JE, Mishra G, Schofield M. Factors associated with hysterectomy among women in Australia. Health Place. 2000 Dec;6(4):301–8. doi: 10.1016/s1353-8292(00)00011-3. [DOI] [PubMed] [Google Scholar]
- 4.Ceausu I, Shakir YA, Lidfeldt J, Samsioe G, Nerbrand C. The hysterectomized woman. Is she special? The women’s health in the Lund area (WHILA) study. Maturitas. 2006 Jan 20;53(2):201–9. doi: 10.1016/j.maturitas.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar CM, Harvey SA, Yu Y, Sadler L, Stewart AW. A prospective study of 3 years of outcomes after hysterectomy with and without oophorectomy. Am J Obstet Gynecol. 2006 Mar;194(3):711–7. doi: 10.1016/j.ajog.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 6.Aziz A, Brännström M, Bergquist C, Silfverstolpe G. Perimenopausal androgen decline after oophorectomy does not influence sexuality or psychological well-being. Fertil Steril. 2005 Apr;83(4):1021–8. doi: 10.1016/j.fertnstert.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, et al. Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008 Dec;15(6):1050–9. doi: 10.1097/gme.0b013e318174f155. [DOI] [PubMed] [Google Scholar]
- 8.Sowers M, Crawford S, Sternfeld B, et al. Menopause: Biology and pathology. New York, NY: Academic Press; 2000. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition; pp. 175–80. [Google Scholar]
- 9.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385. [Google Scholar]
- 10.Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. Am J Psychiatry. 1983 Jan;140(1):41–6. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- 11.Ware J. Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. SF-36 211 Health Survey. [Google Scholar]
- 12.Bryk A, Raudenbush S. Hierarchical linear models: Applications and data analysis methods. 1992. London: Sage; [Google Scholar]
- 13.Parker WH. Bilateral oophorectomy versus ovarian conservation: effects on long-term women’s health. J Minim Invasive Gynecol. 2010 Apr;17(2):161–6. doi: 10.1016/j.jmig.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Powell LH, Meyer P, Weiss G, Matthews KA, Santoro N, Randolph JF, Jr, et al. Ethnic differences in past hysterectomy for benign conditions. Womens Health Issues. 2005 Aug;15(4):179–86. doi: 10.1016/j.whi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, et al. Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007 Nov;103(1–3):267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]