Abstract
Background:
Glaucoma drainage implants (GDIs) are used for managing recalcitrant glaucoma and are usually placed in the anterior chamber. This approach may lead to complications such as corneal decompensation, and so a pars plana approach is used in at risk eyes.
Aims:
To compare functional outcomes and complications of 250 mm2 and 350 mm2 pars plana Baerveldt tube insertion with pars plana vitrectomy (PPV) (both 20- and 23-gauge) for managing refractory glaucoma.
Settings and Design:
A retrospective chart review of 38 patients (39 eyes) undergoing combined PPV-Baerveldt procedure for glaucoma recalcitrant to maximal medical treatment or previous filtering procedures with >6 weeks of follow-up.
Materials and Methods:
Main outcome measures were visual acuity, intraocular pressure (IOP), number of glaucoma medications, and postoperative complications.
Statistical Analysis Used:
A paired ‘t’ test was used to evaluate changes in IOP and glaucoma medications, Fisher's exact test was used to compare complication rates, and Kaplan-Meier survival curves were constructed for comparison of overall outcomes.
Results:
Mean patient age was 62.2 years. Mean follow-up period was 33.7 months, with 36 (92%) eyes followed for ≥6 months. Mean±SD preoperative IOP and number of glaucoma medications were significantly reduced by the combined procedure (P<0.05). Thirty-five (90%) eyes maintained final IOP between 6 and 21 mmHg. Vision improved by ≥2 lines in 10 (26%) eyes, remained stable in 15 (38%) eyes, and decreased in 14 (36%) eyes. Two (5.1%) eyes developed no light perception vision, with one (2.6%) eye becoming phthisical. Twenty-four (62%) eyes developed complications managed with conservative measures. Five (13%) eyes required ≥1 surgeries within a year of the combined procedure.
Conclusions:
Pars plana Baerveldt tube implantation with PPV can preserve vision, reduce IOP, and decrease the number of glaucoma medications necessary to achieve target IOP in patients with recalcitrant glaucoma.
Keywords: Baerveldt drainage device, pars plana vitrectomy, refractory glaucoma
Introduction
Glaucoma drainage implants (GDIs) are reserved for the management of recalcitrant glaucoma in which intraocular pressure (IOP) control cannot be achieved by medications or laser.[1] They also are indicated after a failed trabeculectomy, with or without an antimetabolite, or if there is extensive conjunctival scarring, iridocorneal endothelial syndrome, or intraocular inflammation.[2] Glaucoma drainage devices play an important role in the management of congenital, neovascular, uveitic, and trauma-related glaucoma.[3] Traditionally, GDIs are inserted into the anterior chamber. This approach may result in tube-cornea touch or endothelial decompensation,[4,5] the latter particularly after corneal transplantation.[6] Pars plana placement of GDIs is considered when such complications are anticipated.[7–10] Tube placement in the vitreous cavity (vs. the anterior chamber) requires a concurrent pars plana vitrectomy (PPV) to prevent tube occlusion with vitreous.[11]
We report our experience with 39 eyes of 38 patients who underwent combined pars plana Baerveldt tube insertion with PPV (herein referred to as ‘combined procedure’) and our evaluation of surgical complications and functional outcomes. We compared the changes in IOP, number of glaucoma medications, and visual acuity as well as rate and type of complications for 250 mm2 vs. 350 mm2 Baerveldt drainage devices combined with 20- or 23-gauge PPV.
Materials and Methods
Our retrospective study identified patients that underwent both PPV and pars plana Baerveldt tube placement at the Institute of Ophthalmology and Visual Science, New Jersey Medical School between 1997 and 2010. The University of Medicine and Dentistry of New Jersey Institutional Review Board committee approved the study. Only patients with >6 weeks of follow-up were included. We did not include patients who had undergone a previous PPV. One patient who underwent PPV with Baerveldt tube revision (i.e., the tube was moved from the anterior chamber to the vitreous cavity through the pars plana) was also included in this series. Indications for Baerveldt tube implantation were determined by glaucoma specialists (RDF or PJL) based on individual needs of patients to control IOP and slow glaucoma progression. These indications included: 1) IOP>21 mmHg despite maximal tolerated medical therapy (30 patients); 2) previously failed Express shunt placement (two patients); and 3) previously failed trabeculectomy with mitomycin C (two patients). A Baerveldt tube was placed in the other five patients with advanced glaucoma to attempt to attain long-term IOP control below a target value to decrease glaucoma progression.
For each patient, age at time of combined procedure, race, gender, past ocular history, past surgical history, type of glaucoma, indications for combined procedure, operative factors, status of lens, presence or absence of iris or angle neovascularization, complications, length of follow-up, and preoperative (i.e., exam prior to combined procedure) and final (i.e., last recorded follow-up visit) best-corrected visual acuity (BCVA), IOP, and number of glaucoma medications were recorded. The type of PPV procedure (20- or 23-gauge) was identified.
Visual acuity (VA) was considered “improved” if the final BCVA was two or more Snellen lines or one low-vision category [e.g., change from hand motion (HM) vision to finger counting (CF)] better than the preoperative BCVA. VA was considered “same” if preoperative and final BCVA were within two Snellen lines or the same low-vision category. VA was considered “decreased” if the final BCVA was two or more lines or one low-vision category worse than the preoperative BCVA.
Definitions of “success”, “qualified success” and “failure” were used as previously defined in literature.[10,12,13] Briefly, success was defined as a reduction in IOP to less than 22 mmHg and greater than 5 mm Hg without medication; qualified success was defined using the same IOP criteria but with the patient using glaucoma medication(s); and failure was defined as IOP of 22 mmHg or greater with medication, IOP of 5 mmHg or less or 22 mmHg or greater with the need for glaucoma reoperation, progression to VA of no light perception (NLP), phthisis, or enucleation. Only eyes with ≥6 months of follow-up were included in categorization of functional outcome.
The change from preoperative to final IOP and the number of glaucoma medications were analyzed for statistical significance (P<0.05) using a paired t-test after passing the Shapiro-Wilks normality test and the Equal Variance test (SigmaPlot 11, Systat Software, Inc., San Jose, CA). Fisher's exact test was used to compare rates of complications and Kaplan-Meier survival curves were constructed using JMP statistical software (version 9; SAS Institute Inc., Cary, NC).
Surgical technique
The surgical procedures were performed by one of two glaucoma specialists (RDF or PJL) and one of two retina specialists (NB or MAZ). Twenty-one (54%) 350 mm2 and 18 (46%) 250 mm2 Baerveldt drainage devices (Abbott Medical Optics, Inc., Abbott Park, IL) were used. The procedure consisted of a superior and temporal conjunctival peritomy followed by anchoring of the implant to the episclera in the superotemporal quadrant (8-10 mm posterior to the limbus). PPV was performed with scleral depression in 31 (79%) 20-gauge eyes and eight (21%) 23-gauge eyes, followed by placement of the Baerveldt tube through either the 23-gauge sclerotomy (in 23-gauge PPV) or a separate 23-gauge incision (in 20-gauge PPV). For 23-gauge vitrectomies, the sclerotomies were beveled and biplanar except for the supero-temporal one, which was used to place the Baerveldt tube and was created perpendicular to the sclera. All sclerotomies (except for the one with the Baerveldt tube through it) were closed with a suture at the end of the vitrectomy; the conjunctiva was sutured to the sclera using one 9-0 vicryl suture. The conjunctiva over the two sclerotomies was barely disturbed with this technique and was sutured with the sclerotomy at the end of the surgery. As noted above, the conjunctiva over the temporal sclerotomy was undermined and draped back by the glaucoma surgeon before the PPV to place the Baerveldt shunt. Twenty (51%) nylon (PJL) and 19 (49%) prolene (RDF) 4-0 stents were placed in the tube, and the tube was ligated with a 6-0 or 7-0 (20-gauge PPV) or an 8-0 (23-gauge PPV) vicryl suture near the tube-plate junction in 30 (77%) eyes at the discretion of the surgeon. Pericardium (five [13%] eyes) or an irradiated scleral graft (34 [87%] eyes) was used to cover the tube insertion site.
Results
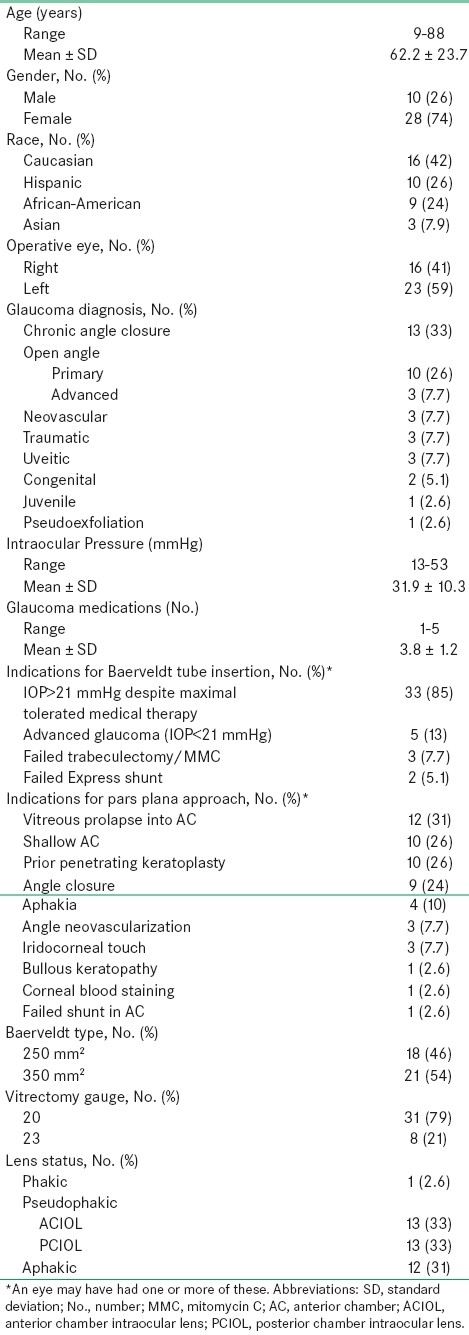
A total of 38 patients (39 eyes) were included in this evaluation. The demographics and preoperative characteristics of the cohort are shown in Table 1.
Table 1.
Patient demographics and preoperative data
Intraocular pressure
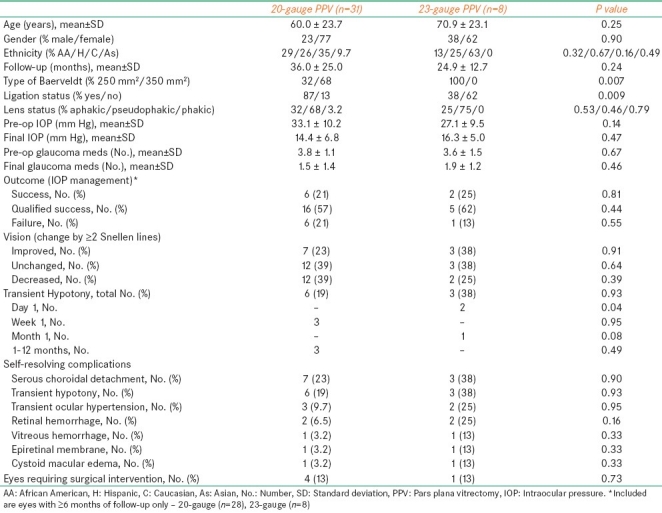
The changes in mean±standard deviation (SD) IOP and number of glaucoma medications with respect to the follow-up period are outlined in Table 2. At each selected time point (from 6 to 60 months), mean preoperative IOP and number of glaucoma medications were significantly (P<0.05) reduced. Kaplan-Meier survival curve analysis of overall success for the 36 eyes with follow-up ≥6 months is shown in Figure 1a. Median period of successful IOP control was 32.5 months (95% C.I., 24-44 months). The changes in IOP and number of glaucoma medications were both statistically significant (P<0.05) irrespective of the size of the Baerveldt implant [Table 3] or vitrectomy gauge [Table 5].
Table 2.
Mean±SD intraocular pressure and number of glaucoma medication as a function of follow-up time
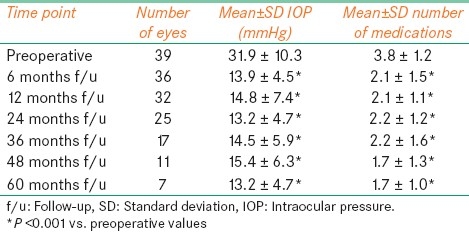
Figure 1.
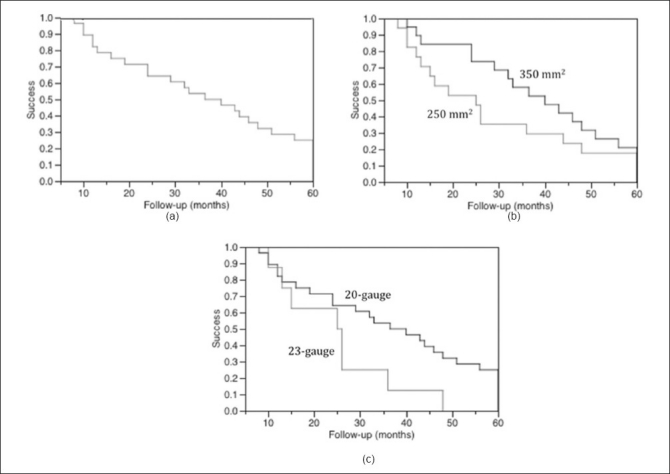
Kaplan-Meier survival graphs up to the 60-month follow-up time point. “Success” includes patients defined as “success” and “qualified success”, i.e., maintenance of intraocular pressure of 6-21 mmHg, with or without medications, without the need for glaucoma reoperation, progression to no light perception, phthisis, or enucleation. (a) Overall success (median, 32.5 months; 95% C.I., 24-44 months) for 36eyes with ≥6 months of follow-up. (b) 250 mm2 (n=17; median, 25 months; 95% C.I., 12-44 months) vs. 350 mm2 (n=19; median, 40 months; 95% C.I., 24-51 months) Baerveldt tube [Log-rank test (P=0.62), Wilcoxon test (P=0.13)]. (c) 23-gauge (n=8; median, 25.5 months; 95% C.I., 10-36 months) vs. 20-gauge (n=28; median, 38.25 months; 24-48 months) pars plana vitrectomy [Log-rank test (P=0.05), Wilcoxon test (P=0.14)]
Table 3.
Comparison of eyes with a 250 mm2 vs. a 350 mm2 Baerveldt drainage tube
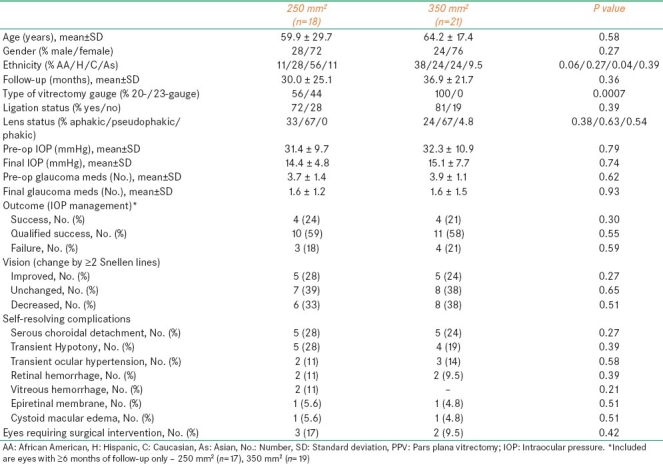
Table 5.
A comparison of eyes undergoing 20- vs. 23-gauge pars plana vitrectomy
In the 36 eyes with ≥6 months of follow-up, eight (22%) eyes were a “success” with a mean±SD IOP of 14.9 ± 2.5 mmHg (range, 11-19 mmHg), 21 (58%) eyes were a “qualified success” with a mean±SD IOP of 13.5 ± 3.4 mmHg (range, 8-20 mmHg) on mean±SD 2.6 ± 1.2 (range, 1-5) glaucoma medications, and 7 (19%) eyes were a “failure” with a mean±S.D. IOP of 18.1 ± 13.8 mmHg (range, 4-38 mmHg) on mean±SD 1.1 ± 1.5 (range, 0-3) glaucoma medications. Of the failed eyes, one (14%) eye had an IOP of 30 mmHg on two glaucoma medications, two (29%) eyes were hypotonous (IOP=4 and 5 mmHg on zero glaucoma medications), two (29%) eyes had NLP vision (one became phthisical), and two (29%) eyes required GDI explantation for aqueous leakage (IOP=6 mmHg on zero glaucoma medications) and refractory diplopia (IOP=27 mmHg on two glaucoma medication). Six (86%) of seven failed eyes underwent a 20-gauge vitrectomy.
Nine (22%) eyes developed transient hypotony (IOP<6 mmHg) within 12 months of the combined surgery (range, 1 day-12 months); six (67%) occurred within the first postoperative month (two on postoperative day-1 and five during postoperative week-1). One eye (11%) developed hypotony after stent removal. Hypotony was associated with shallow serous choroidal detachment in six (67%) eyes, in three (50%) of which the Baerveldt tube was not ligated. Macular retinal folds were noted in two (22%) of the nine eyes. Seven (78%) hypotonous eyes recovered normal IOP within 3 weeks. Two (22%) hypotonous eyes developed rebound intraocular hypertension one and one-and-a-half months after becoming hypotonous. The pressures were successfully stabilized in both eyes with medication. Overall, five (13%) eyes had a transient hypertensive episode (IOP>21 mmHg) after the combined surgery (range, 6 days-3.5 months), four (80%) of which had a ligated Baerveldt tube. A final VA of 20/200 or better was achieved in five (56%) hypotonous eyes. Transient corneal edema developed in five (56%) eyes (range, 3 weeks-11.5 months).
Visual acuity
Twenty-four (62%) eyes had a preoperative VA of 20/200 or worse. Ten (26%) eyes experienced an improvement in their VA, 15 (38%) remained unchanged, and 14 (36%) had decreased vision. Two (14%) of the 14 eyes with diminished vision achieved a final VA of NLP. The first was due to neovascular glaucoma (NVG) with uncontrolled high IOP, and the second was due to progressive congenital glaucoma with increased IOP refractory to glaucoma medications and 270-degree transscleral diode cyclophotocoagulation. The eye with NVG became phthisical.
Complications
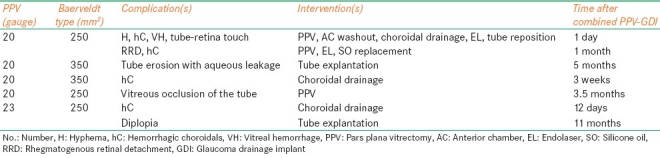
Postoperative complications not requiring surgical intervention occurred in 24 (62%) eyes. These included 10 (26%) eyes with serous choroidal detachment, two of which were large, non-appositional choroidals with overlying shallow exudative retinal detachments; nine (23%) eyes with transient hypotony; five (13%) eyes with transient ocular hypertension; four (10%) eyes with retinal hemorrhage; two (5.1%) eyes with vitreous hemorrhage; two (5.1%) eyes with cystoid macular edema; two (5.1%) eyes with epiretinal membrane formation; and one (2.6%) eye with vitreous partially occluding the Baerveldt tube. The eye with partial occlusion had an IOP of 21 mmHg on zero glaucoma medications before being lost to follow-up 2 months after the combined procedure. Five (13%) eyes underwent additional surgery within 12 months of the combined surgery [Table 4]. Intraoperative hyphema was encountered in an eye with florid rubeosis with neovascular glaucoma.
Table 4.
Complications requiring additional surgery within 12 months of combined vitrectomy and glaucoma drainage implant placement
250 mm2 vs. 350 mm2 Baerveldt drainage device
Eighteen eyes underwent 250 mm2 while 19 underwent 350 mm2 Baerveldt tube placement [Table 3]. Significant differences included percent Caucasian ethnicity (P=0.04) and type of vitrectomy used (P=0.0007). No differences were elucidated in terms in reduction in preoperative IOP and number of glaucoma medications, effects in VA, IOP control, or rate of complications. Although not statistically significant, the time to failure was longer while using the 350 mm2 vs. 250 mm2 Baerveldt tube (median, 40 vs. 25 months, respectively; Figure 1b).
20- vs. 23-gauge vitrectomy
23-gauge vitrectomy was performed in eight (21%) eyes [Table 5]. Open-angle glaucoma was diagnosed in five (50%) eyes, traumatic glaucoma in one (13%) eye, angle-closure glaucoma in one (13%) eye, and uveitic glaucoma in one (13%) eye. All eight eyes received a 250 mm2 Baerveldt drainage tube (P=0.0007 vs. 20-gauge vitrectomy eyes). Mean±SD preoperative IOP and number of glaucoma medications were significantly (P<0.05) decreased by the combined procedure. Effects on visual acuity and IOP were similar to eyes undergoing 20-gauge vitrectomy [Table 5]. Although not statistically significant, the time to failure was longer in 20- vs. 23-gauge vitrectomy eyes (median, 38.25 vs. 25.5 months, respectively; Figure 1c). While the rate of postoperative day-1 hypotony was significantly higher (P=0.04) in 23- vs. 20-gauge eyes, these eyes also had a significantly (P=0.009) lower rate of tube ligation [Table 5].
Extensive, nonappositional hemorrhagic choroidal detachments were identified in one eye on postoperative day-1 with an IOP of 42 mmHg. This complication was treated successfully with choroidal drainage on postoperative day-12. However, 11 months after the combined surgery, the patient requested GDI removal due to diplopia [Table 4, case 5]. Six weeks later, IOP rose to 31 mmHg (on zero glaucoma medications). At last follow-up, 4 months after GDI explantation, the IOP was 27 mmHg on three glaucoma medications with subjective improvement in diplopia.
Discussion
Combined pars plana GDI tube insertion and PPV can preserve vision, reduce IOP, and reduce the number of glaucoma medications needed to achieve target IOP in selected patients with refractory glaucoma. Traditionally, GDI tubes have been placed in the anterior chamber. While the overall rate of functional success (i.e., reduction in IOP and number of glaucoma medications) with this approach is similar to that of pars plana Baerveldt tube placement, posterior chamber GDI tube placement is indicated for patients with anterior segment abnormalities.[4,5] Tube-cornea touch and endothelial decompensation has been reported in 8-20% and 17-19% of GDIs placed in the anterior chamber, respectively.[4,5] The risk of corneal graft failure is increased, possibly due to bidirectional flow of antigenic materials between the anterior chamber and subconjunctival space.[6] Therefore, a different surgical approach in which one places the GDI tube away from the cornea in eyes that have primary corneal disease may have merit. Alternative approaches to inserting GDI have been explored. Rumelt and associates reported three successful cases of placing a GDI tube into the ciliary sulcus, without posterior segment complications or corneal decompensation.[14] A summary of published series on functional outcomes and complications after pars plana GDI placement in combination with PPV is provided in Table 6.
Table 6.
A summary of published cases of functional outcomes and complications of pars plana glaucoma drainage device placement
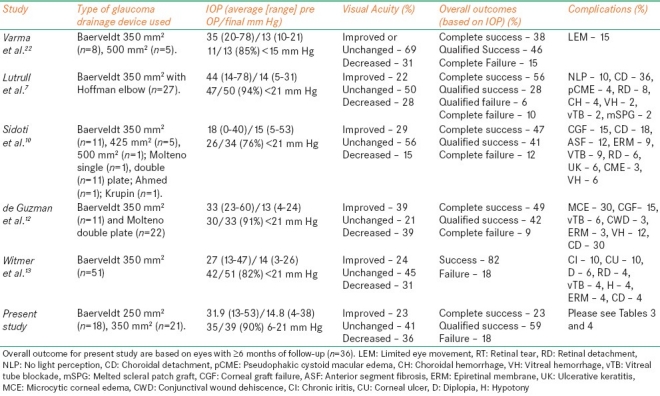
Results of our study suggest that pars plana GDI placement can be an effective treatment in eyes with refractory glaucoma and existing/potential corneal abnormalities. The final IOP and number of glaucoma medications required for IOP management were reduced significantly (P<0.05) by the combined procedure. Thirty-five (90%) eyes had an IOP between 6 and 21 mmHg at last follow-up examination, while success was achieved in 82% of eyes. These rates were similar to those seen in other studies of pars plana GDI placement [Table 6].
Visual acuity improved or remained the same in 25 (64%) eyes. Results from previously published comparable series on pars plana GDI placement reported better or unchanged vision in 60-85% of eyes [Table 6]. In our series, 14 (36%) eyes underwent one or more penetrating keratoplasty procedures for corneal decompensation and graft failure, and eight (21%) eyes had a failed trabeculectomy prior to the combined procedure. Two (6.5%) eyes that underwent 20-gauge vitrectomy had NLP vision at last visit due to advanced glaucoma and uncontrolled high IOP. Advanced corneal and glaucomatous disease status in our cohort, as well as additional surgical procedures required to manage various complications [Table 4], may have precluded the improvement in VA in more eyes, thereby accounting for the difference in stabilization or amelioration of vision between this and previous studies.
The overall rates of various complications reported here are similar to the previously published series [Tables 4 and 5]. A number of studies have reported tube occlusion as a rare complication. Singh and associates[15] reported an 85% success in reopening blocked tube shunts by Nd: YAG laser membranectomy. Subsequent reblockage was seen in 54% of the cases (mean, 2 months). These patients were managed by repeat laser membranectomy, transscleral diode cryophotocoagulation, and/or surgical repositioning of the tube. In our study, vitreous occlusion of the tube was observed in two (5.1%) eyes. One required surgical management (Table 4, case 4), and the other was lost to follow-up 2 months after the combined procedure. At last visit, the eye that underwent a subsequent surgical procedure had an IOP of 17 mmHg on three glaucoma medications with 20/40 vision. The eye that was lost to follow-up had an IOP of 21 mmHg on zero glaucoma medications with HM vision at the last visit. None of the eyes in our study developed corneal decompensation, the prevention of which was one of main reasons for pars plana Baerveldt tube placement.
Recently, a study was published regarding the use of the combined procedure in pediatric population. Banitt et al. showed successful IOP control in 85%, 81%, and 72% of cases at 12-, 24-, and 36-month follow-up time points along with a reduced rate of anterior chamber complications in 30 aphakic and pseudophakic children receiving pars plana Baerveldt tube placement.[16] Although pars plana Baerveldt tube placement is effective in managing refractory pediatric glaucoma, rate of posterior segment complications can be high.[17–19] In the above mentioned study, retinal detachment occurred in four (13%) eyes and hemorrhagic choroidals in one (3.3%) eye. In our study, three (7.7%) eyes developed hemorrhagic choroidals and one of them (2.6%) also developed vitreous hemorrhage and retinal detachment. In addition, the failure rate of successful IOP control in the pediatric population may be inversely related to the patient age at time of surgery.[18] Further studies may be warranted to elucidate this relationship.
Although larger Baerveldt implants allow better IOP control while using fewer glaucoma medications, they are also associated with higher rates of some complications such as choroidal effusion and strabismus.[20] The decision to place a 250 mm2 vs. a 350 mm2 Baerveldt tube in our series was at the discretion of the surgeon and was driven by maximization of long-term IOP control. In another series by Britt et al.,[17] comparing a 350 mm2 to a 500 mm2 Baerveldt implant in 107 patients, the rate of improvement in visual acuity, complications, and IOP reductions were statistically insignificant between the two cohorts. The observed outcomes in our study closely mirrored the findings in the above-mentioned studies [Table 3]. In addition, the length of time to failure was not significantly different in eyes with 250 mm2 vs. 350 mm2 Baerveldt tube (median, 40 vs. 25 months, respectively; Figure 1b).
Recently reported benefits of small-gauge vitrectomy include reduced ocular inflammation, reduced trauma to ocular tissues, and reduced flow of intraocular fluid.[18] In our series, the overall outcomes of eyes undergoing 20- vs. 23-gauge vitrectomy were very similar [Table 5]. In addition, the length of time to failure was not significantly different in 20- vs. 23-gauge eyes (median, 38.25 vs. 25.5 months, respectively; Figure 1c). The only significant difference, which was likely related to the status of Baerveldt tube ligation, was the higher rate of transient hypotony on post-operative day-1 in 23-gauge eyes. Hypotony resolved within the next two weeks without the need for subsequent surgical intervention. Although transient hypotony was noted in three 23-gauge patients, it unlikely was related to the type of PPV due to the surgical technique. The cannula system used for the 23-gauge PPV may be protective against the risk of causing retinal tears.[19] Also, since the same sclerotomy site is used for vitrectomy and to place the Baerveldt drainage tube in the eye, there may be a decreased risk of vitreous occlusion due to the thoroughness of vitreous base trimming that can be achieved in that localized area. Our study noted no difference between eyes undergoing 20- vs. 23-gauge vitrectomy in terms of functional outcome or the incidence of postoperative complications. Prospective studies with a larger number of eyes undergoing 23-gauge vitrectomy are required to evaluate the potential benefits of this procedure.
The limitations of this study include its retrospective design, variable severity of disease, and involvement of multiple surgeons. Some of the differences in outcomes between subgroups may be related to surgeon rather than surgical technique. Direct comparison of results among studies of GDI is difficult because of differences in study populations, severity of glaucoma, diversity in types of utilized GDI (valved, plate size, etc.), lack of uniformity in surgical procedures (technique for tube ligation, pneumostenting and/or use of antimetabolites), and definition of success.[20] There seems to be general consensus that the success rate, as judged by IOP control and development of complications, is similar among different types of implants; however, the complication profile may differ based on the type of GDI.[14]
Overall, combined GDI implantation through the pars plana and PPV preserved VA, effectively reduced IOP, and produced few complications in patients with refractory glaucoma who were at risk for corneal decompensation. While functional and visual outcomes, and rate and type of complications were similar in eyes receiving a 250 mm2 vs. a 350 mm2 Baerveldt drainage tube, the time to failure was considerably longer in 350 mm2 vs. 250 mm2 Baerveldt tube. Besides the rate of transient hypotony on postoperative day-1, our series did not find differences between eyes undergoing 20- vs. 23-gauge vitrectomy; however, the time to failure was longer in 20- vs. 23-gauge PPV. The results of our study, however, are limited by a small sample size of 23-gauge combined PPVs.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Fechtner RD, Singh K. Maximal glaucoma therapy. J Glaucoma. 2001;10:S73–5. doi: 10.1097/00061198-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 2.Assaad MH, Baerveldt G, Rockwood EJ. Glaucoma drainage devices: Pros and cons. Curr Opin Ophthalmol. 1999;10:147–53. doi: 10.1097/00055735-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Fechter HP, Parrish RK., 2nd Preventing and treating complications of Baerveldt Glaucoma Drainage Device surgery. Int Ophthalmol Clin. 2004;44:107–36. doi: 10.1097/00004397-200404420-00008. [DOI] [PubMed] [Google Scholar]
- 4.Hill RA, Heuer DK, Baerveldt G, Minckler DS, Martone JF. Molteno implantation for glaucoma in young patients. Ophthalmology. 1991;98:1042–6. doi: 10.1016/s0161-6420(91)32179-1. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd MA, Sedlak T, Heuer DK, Minckler DS, Baerveldt G, Lee MB, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas.Update of a pilot study. Ophthalmology. 1992;99:679–87. doi: 10.1016/s0161-6420(92)31910-4. [DOI] [PubMed] [Google Scholar]
- 6.Sherwood MB, Smith MF, Driebe WT, Jr, Stern GA, Beneke JA, Zam ZS. Drainage tube implants in the treatment of glaucoma following penetrating keratoplasty. Ophthalmic Surg. 1993;24:185–9. [PubMed] [Google Scholar]
- 7.Luttrull JK, Avery RL, Baerveldt G, Easley KA. Initial experience with pneumatically stented baerveldt implant modified for pars plana insertion for complicated glaucoma. Ophthalmology. 2000;107:143–9. doi: 10.1016/s0161-6420(99)00034-2. discussion 149-50. [DOI] [PubMed] [Google Scholar]
- 8.Scott IU, Alexandrakis G, Flynn HW, Jr, Smiddy WE, Murray TG, Schiffman J, et al. Combined pars plana vitrectomy and glaucoma drainage implant placement for refractory glaucoma. Am J Ophthalmol. 2000;129:334–41. doi: 10.1016/s0002-9394(99)00363-3. [DOI] [PubMed] [Google Scholar]
- 9.Schlote T, Ziemssen F, Bartz-Schmidt KU. Pars plana-modified Ahmed Glaucoma Valve for treatment of refractory glaucoma: A pilot study. Graefes Arch Clin Exp Ophthalmol. 2006;244:336–41. doi: 10.1007/s00417-005-0008-5. [DOI] [PubMed] [Google Scholar]
- 10.Sidoti PA, Mosny AY, Ritterband DC, Seedor JA. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology. 2001;108:1050–8. doi: 10.1016/s0161-6420(01)00583-8. [DOI] [PubMed] [Google Scholar]
- 11.Desatnik HR, Foster RE, Rockwood EJ, Baerveldt G, Meyers SM, Lewis H. Management of glaucoma implants occluded by vitreous incarceration. J Glaucoma. 2000;9:311–6. doi: 10.1097/00061198-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 12.De Guzman MH, Valencia A, Farinelli AC. Pars plana insertion of glaucoma drainage devices for refractory glaucoma. Clin Exp Ophthalmol. 2006;34:102–7. doi: 10.1111/j.1442-9071.2006.01170.x. [DOI] [PubMed] [Google Scholar]
- 13.Witmer MT, Tiedeman JS, Olsakovsky LA, Conaway MR, Prum BE. Long-term intraocular pressure control and corneal graft survival in eyes with a pars plana Baerveldt implant and corneal transplant. J Glaucoma. 2010;19:124–31. doi: 10.1097/IJG.0b013e3181a98cc1. [DOI] [PubMed] [Google Scholar]
- 14.Rumelt S, Rehany U. Implantation of glaucoma drainage implant tube into the ciliary sulcus in patients with corneal transplants. Arch Ophthalmol. 1998;116:685–7. doi: 10.1001/archopht.116.5.685. [DOI] [PubMed] [Google Scholar]
- 15.Singh K, Eid TE, Katz LJ, Spaeth GL, Augsburger JJ. Evaluation of Nd: YAG laser membranectomy in blocked tubes after glaucoma tube-shunt surgery. Am J Ophthalmol. 1997;124:781–6. doi: 10.1016/s0002-9394(14)71695-2. [DOI] [PubMed] [Google Scholar]
- 16.Banitt MR, Sidoti PA, Gentile RC, Tello C, Liebmann JM, Rodriguez N, et al. Pars plana Baerveldt implantation for refractory childhood glaucomas. J Glaucoma. 2009;18:412–7. doi: 10.1097/IJG.0b013e31818624bd. [DOI] [PubMed] [Google Scholar]
- 17.Budenz DL, Gedde SJ, Brandt JD, Kira D, Feuer W, Larson E. Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology. 2004;111:2204–10. doi: 10.1016/j.ophtha.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Autrata R, Helmanova I, Oslejskova H, Vondracek P, Rehurek J. Glaucoma drainage implants in the treatment of refractory glaucoma in pediatric patients. Eur J Ophthalmol. 2007;17:928–37. doi: 10.1177/112067210701700610. [DOI] [PubMed] [Google Scholar]
- 19.Rolim de Moura C, Fraser-Bell S, Stout A, Labree L, Nilfors M, Varma R. Experience with the baerveldt glaucoma implant in the management of pediatric glaucoma. Am J Ophthalmol. 2005;139:847–54. doi: 10.1016/j.ajo.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd MA, Baerveldt G, Fellenbaum PS, Sidoti PA, Minckler DS, Martone JF, et al. Intermediate-term results of a randomized clinical trial of the 350- versus the 500-mm2 Baerveldt implant. Ophthalmology. 1994;101:1456–1463. doi: 10.1016/s0161-6420(94)31152-3. discussion 1463-4. [DOI] [PubMed] [Google Scholar]


