Abstract
Purpose:
To evaluate risk factors for pediatric microbial keratitis and to describe the clinical picture, microbial spectrum, treatment modalities, posttreatment sequelae, and visual outcome in cases with pediatric microbial keratitis.
Materials and Methods:
All cases of microbial keratitis that occurred in children 16 years or younger who had an initial examination between January 2000 and December 2010 at a tertiary referral eye hospital in Riyadh, Saudi Arabia, were identified. A retrospective review of medical records was conducted using a computer-based diagnosis code. Demographic data, predisposing factors, clinical course, microbial culture results, and visual outcomes were recorded.
Results:
Sixty-eight eyes were included in this study. Predisposing factors were identified in 63 eyes (92.6%). All patients had unilateral microbial keratitis. The mean±SD age was 4.5 ± 4.8 years and 57.4% were male. Trauma was the leading cause [27 eyes (39.7%)], followed by systemic diseases [14 eyes (20.6%)], contact lens wear [11 eyes (16.1%)], and ocular diseases [11 eyes (16.1%)]. Corneal scraping was performed in all cases. Five patients needed general anesthesia to carry out the corneal scraping. Thirty-four (50.0%) eyes showed positive cultures. Gram-positive bacteria accounted for 67.8% and gram-negative bacteria for 38.2% of isolates. Streptococcus pneumoniae was the most commonly isolated organism [8 eyes (25.8%)], followed by Staphylococcus epidermidis [7 eyes (22.7%)]. Pseudomonas aeruginosa was the most commonly isolated gram-negative [6 eyes (17.6%)] organism. One eye had corneal perforation and required surgical intervention. Forty-five of 68 eyes (66.2%) had a best-corrected visual acuity evaluation at the last follow-up and 28 eyes (62.2%) of them had a best-corrected visual acuity of 20/40 or better.
Conclusion:
Children with suspected microbial keratitis require comprehensive evaluation and management. Early recognition, identifying the predisposing factors and etiological microbial organisms, and instituting appropriate treatment measures have a crucial role in outcome. Ocular trauma was the leading cause of childhood microbial keratitis in our study.
Keywords: Bacteria, children, keratitis, trauma
Introduction
Microbial keratitis in children is a serious, vision-threatening condition associated with high incidence of amblyopia.[1] Although microbial keratitis occurs infrequently in childhood, a study in southern California showed that pediatric keratitis accounted for 11% of all microbial keratitis cases.[1] Children with keratitis differ from adult keratitis patients in many ways, due to difficulty in patient examination, level of inflammation in children being higher compared with adults, and difficulty in administering topical medications.[2] In our study, we aimed to evaluate risk factors for pediatric microbial keratitis and to describe the clinical picture, microbial spectrum, treatment modalities, posttreatment sequelae, and visual outcome in cases of pediatric microbial keratitis in Saudi Arabia.
Materials and Methods
All cases of microbial keratitis that occurred in children 16 years or younger who had an initial examination between January 2000 and December 2010 at our center were identified. A retrospective review of medical records was conducted with a computer-based diagnosis code. Institutional review board approval was obtained. The inclusion criteria for the study were a diagnosis of microbial (nonviral) keratitis based on clinical findings and microbiologic investigations such as corneal epithelial defect and stromal infiltrate. Patients who received topical antibiotics before referral with negative culture results were included if the clinical findings were suggestive of the diagnosis of an infectious ulcer. The medical records were reviewed using a standardized protocol with emphasis on predisposing factors to corneal infection. Clinical evaluation included visual acuity (VA) assessment using age-appropriate methods and slit-lamp biomicroscopy examination to determine the size and location of corneal infiltrates. Corneal scraping (with general anesthesia where indicated) and cultures for bacteria, mycobacteria, and fungi were performed in all cases. Using standard techniques, scrapings were inoculated in blood, chocolate, and modified Sabouraud agar, Lowenstein-Jensen agar, and thioglycate broth. Culture for Acanthamoeba was carried out as indicated by the clinical need. Size and location of corneal infiltrates were reported in the charts using the midpoint of the ulcer to characterize location. Ulcers with a midpoint within 2 mm of geometric center of the cornea were categorized as central. Midpoints between 2 and 4 mm of the geometric center were considered paracentral and the remainders were considered as peripheral. Statistical analysis was performed using Student's t-test to compare the mean difference between groups while Chi-square test and comparison of proportions test were used for categorical variables.
Results
Demographics
Sixty-eight patients (57.4% male) were recruited in this study. All patients had unilateral effect of the disease, with total of 68 eyes. Infection was more common in the left eye (67.6%) than the right eye (32.4%). The mean±SD age was 4.5 ± 4.8 years (11 days to 16 years). For the purpose of comparison, all of the patients were divided into two age groups: 37 eyes in group 1 (ages ≤4 years) and 31 eyes in group 2 (ages >4 years).
Predisposing factors
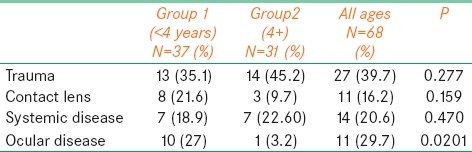
Predisposing factors were identified in 63 (92.6%) of 68 eyes. The most common predisposing factor for pediatric microbial keratitis was trauma, which was associated in 27 (39.7%) of the eyes. Other predisposing factors included systemic disease association in 14 eyes (20.5%), contact lens wear in 11 eyes (16.1%), and ocular disease in 11 eyes (16.1%). The prevalence of trauma, systemic disease, and contact lens wear was distributed among all of the ages [Table 1]. There was no statistically significant difference between both groups except for ocular diseases where group 1 showed significantly (P<0.0201) greater predisposition to microbial keratitis than group 2 [Table 2]. Meanwhile, there was no statistically significant association between vision improvement and Trauma, Wearing Contact Lens, and having other systemic comorbidity (P values: 0.507, 221, and 0.180, respectively). However, there was a significant association between having ocular disease and nonimprovement of vision (P=0.012). Three of the 11 contact lens wearers used extended-wear soft contact lens for myopia. Two patients wore extended-wear contact lens with removal every week. Six patients used cosmetic lenses.
Table 1.
Predisposing factors for microbial keratitis in children
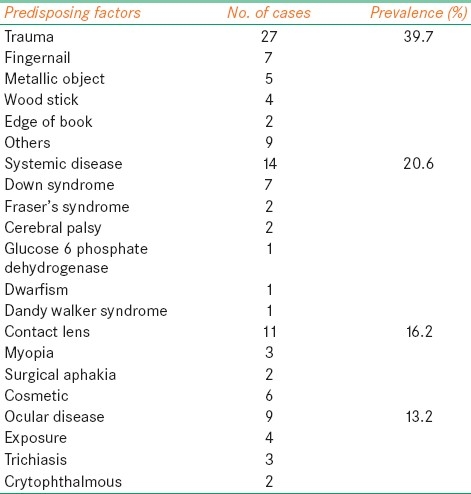
Table 2.
Comparison between both groups in terms of trauma, contact lens, and ocular disease
Clincal findings
The location of the corneal ulcer, as defined earlier, was central in 40 (58.8%) of the eyes peripheral in 20 eyes (29.4%), and paracentral in 8 eyes (11.7%). The area of the infiltrate was small (<2 mm) in 42 eyes (61.7%), medium (2–6 mm) in 24 eyes (35.2%), and large (>6 mm) in 2 eyes (2.9%). Hypopyon was present in three eyes. Perforation was present in one eye at the time of admission.
Microbiological analysis
General anesthesia was required in five patients. Thirty-four eyes (50%) showed positive cultures. Overall, gram-positive cocci accounted for 21 (61.7%) of all bacterial isolates. S. pneumoniae was the most commonly isolated organism in 8 eyes (23.5%), followed by S. epidermidis in 7 eyes (20.5%), and S. aureus in 6 eyes (17.6%) [Table 3].
Table 3.
Organisms identified in cultures of corneal scrapings in children
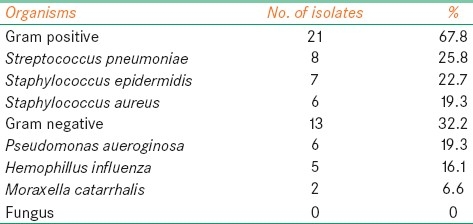
Gram-negative bacteria accounted for 13 eyes (38.2%) of the isolates. P. aeruginosa was the most common gram-negative bacteria accounting for 6 eyes (17.6%), followed by Haemophilus influenza in 5 eyes (14.7%) and Moraxella catarrhalis in 2 eyes (5.8%). Five of the six eyes of P. aeruginosa were associated with contact lens wear.
Treatment and outcome
The antibiotic regimen for each case varied, depending on the clinical manifestation and personal preference of the treating physician. All patients with bacterial keratitis were treated with intensive fortified antibiotics with dosing similar to that used for adults. A combination of cefazolin (50 mg/ml) and gentamycin (14 mg/ml) at the frequency of every 15–30 minutes was the most common therapy, used in 29 eyes (42.6%). A child who developed a corneal perforation was treated with cyanoacrylate glue initially and later penetrating keratoplasty was performed. Thirty-nine (57.3%) eyes developed corneal scar with an average size of 2.1 mm range [0.5–5] mm.
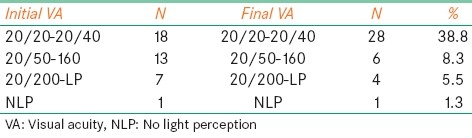
Best-corrected VA (BCVA) using the Snellen chart was recorded in 45 eyes (66.1%) at the time of last follow-up. The remaining was either uncooperative or systematically ill. Twenty-eight eyes (41.2%) had BCVA of 20/40 or better, 6 eyes (8.2%) had BCVA between 20/50 and 20/160, and 5 eyes (7.3%) had BCVA of 20/200 or worse [Table 4]. Comparing best-corrected VA of the affected eyes at presentation to VA at the last follow-up, we found that the average (SD) of the initial VA (transferred into LogMAR) was 0.57 (0.7), which became 0.3 (0.6) at the end of follow-up period. This remarkable improvement in VA was statistically significant as measured by t-test where the mean difference was 0.216 within 95% confidence interval of 0.096–0.337 and a corresponding P value of 0.001.
Table 4.
Visual acuity outcome: Initial visual acuity measured in 39 (60%) patients as follows
Discussion
Risk factors for infectious keratitis in adults include the use of contact lenses, severe systemic illness, trauma, previous corneal surgery, topical corticosteroid medications, bullous keratopathy, exposure keratitis, keratitis sicca, and recurrent erosions.[1,3,4] We found similar predisposing conditions in children. Traumatic corneal injury is an age-independent and leading cause of microbial keratitis in this study (39.7%) and in several previous studies.[3–5] A recent review of microbial keratitis in Taiwanese children[6] revealed that trauma was the most common risk factor in 21.0% of the study group. Many children with severe systemic illnesses had multiple risk factors, especially exposure keratitis. Systemic and ocular diseases were significant predisposing risk factors in pediatric infectious keratitis in this and other studies.[1,4,5] Systemic disease was the second most common risk factor for childhood microbial keratitis in this study and two studies.[6,7] Pediatricians and neonatal intensive care personnel should be alerted to the increased risk of infection and the need for prophylactic lubricating drops or ointment in these children. The results of published studies are summarized in Table 5. The mean age of patients in our study was 4.9 years and the median age was 1.7 years which seems to be lower than that of other studies.[3–6] Ocular diseases and contact lens wear-related infectious keratitis were the third most common risk factor in our series (16.2%). Information on the contact lens cleaning regimen and solution was not available in every case of contact lens-related infectious keratitis. Most of the 11 cases reported improper lens hygiene. Different organisms have been reported in different parts of the world.[8,9] In general, pseudomonal and fungal infections tend to be more prevalent in the southern latitudes (Saudi Arabia is located north of the equator). Staphylococcus and streptococcus infection tend to occur in northern part of the world.[1,2,10]
Table 5.
Comparative analysis of previous case series of microbial keratitis in children

In our study, gram-positive cocci were the most common isolates [21 culture-positive eyes (67.8%)]. Similar results have been reported in two studies[1,5] but differed from a study in Florida,[5] which found gram-negative bacilli (43.2%) to be most prevalent. P. aeruginosa is the most common organism isolated in contact lens-related microbial keratitis, as found in our study. The recovery rate of bacteria from corneal cultures in our study was 50%, which is almost near to the recovery rate found in two studies[4,6] but is lower than the recovery rates found in other studies of microbial keratitis in children.[2,5,11,12] Frequent use of antibiotics [16 eyes (23.5%)] before referral may be the cause of low positive culture in our study.
In our study, 45 eyes (66.1%) had small and central ulcers. This finding contrasts with several studies that reported centrally located, moderately sized corneal ulcers.[2,4,5,11–13] All of eyes in our study were treated empirically with topical fortified antibiotics. Eyes treated with combination of gentamycin and cefazolin were 29 (42.6%). This group showed remarkable improvement in VA compared with other groups who received different combination of fortified antibiotics combination (P value=0.033). The other antibiotics combinations were ceftazidime+vancomycin and cefazoline+ceftazidime.
Poor visual outcome was associated in our study with systemic and ocular disease. Poor visual outcome has been reported with fungal infection.[1,10,14] No case of fungal infection was recorded in our study. The sequelae of corneal ulceration in early childhood put the children at higher risk for anisometropia and amblyopia which may lead to permanent visual loss.[6,14,15]
This study is limited by being retrospective in nature. VA recording is subjected to variations among different examiners during follow-up visits and the level of child cooperation during eye examination. Our hospital is a tertiary care center, and difficult cases are referred to us, a reason that might bias the analysis of clinical features and risk factors. However, to our knowledge, this study represents the first case series review of microbial keratitis in children in Saudi Arabia.
The findings regarding the nature and risk factors for childhood microbial keratitis in our study, mainly with respect to the cause, prevalence of the various causative organisms, and predisposing factors may provide important clinical information to help in the prevention of childhood microbial keratitis complications.
Children with suspected microbial keratitis should receive complete evaluation and management. Difficulties in managing these young, uncooperative patients must not be allowed to compromise management decisions. With early diagnosis and proper management and treatment, a good visual outcome can be achieved in children with microbial keratitis.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Ormerod LD, Hertzmark E, Gomez DS, Stabiner RG, Schanzlin DJ, Smith RE. Epidemiology of microbial keratitis in southern California: A multivariate analysis. Ophthalmology. 1986;94:1322–33. doi: 10.1016/s0161-6420(87)80019-2. [DOI] [PubMed] [Google Scholar]
- 2.Asbell P, Stenson S. Ulcerative keratitis: Survey of 30 years’ laboratory experience. Arch Ophthalmol. 1982;100:77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- 3.Ormerod LD, Murphee AL, Gomez DS, Schanzlin DJ, Smith RE. Microbial keratitis in children. Ophthalmology. 1987;93:449–55. doi: 10.1016/s0161-6420(86)33717-5. [DOI] [PubMed] [Google Scholar]
- 4.Cruz OA, Sabir SM, Capo H, Alfonso EC. Microbial keratitis in childhood. Ophthalmology. 1993;100:192–6. doi: 10.1016/s0161-6420(93)31671-4. [DOI] [PubMed] [Google Scholar]
- 5.Clinch TE, Plamon FE, Robinson MJ, Cohen EJ, Barron BA, Laibson PR. Microbial keratitis in children. Am J Ophthalmol. 1994;117:65–71. doi: 10.1016/s0002-9394(14)73016-8. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao CH, Yeung L, Ma DH, Chen YF, Lin HC, Tan HY, et al. Pediatric microbial keratitis in Taiwanese children: A review of hospital cases. Arch Ophthalmol. 2007;125:603–9. doi: 10.1001/archopht.125.5.603. [DOI] [PubMed] [Google Scholar]
- 7.Kunimoto DY, Sharma S, Reddy MK, Gopinathan U, Jyothi J, Miller D, et al. Microbial keratitis in children. Ophthalmology. 1998;105:252–7. doi: 10.1016/s0161-6420(98)92899-8. [DOI] [PubMed] [Google Scholar]
- 8.Vajpayee RB, Ray M, Panda A, Sharma N, Taylor HR, Murthy GV, et al. Risk factors for pediatric presumed microbial keratitis: A case-control study. Cornea. 1999;18:565–9. [PubMed] [Google Scholar]
- 9.Wong T, Ormonde S, Gamble G, Mcghee CN. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol. 2003;87:1103–8. doi: 10.1136/bjo.87.9.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90:38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 11.Gudmundsson OG, Ormerod LD, Kenyon KR, Glynn RJ, Baker AS, Haaf J, et al. Factors influencing predilection and outcome in bacterial keratitis. Cornea. 1989;8:115–21. [PubMed] [Google Scholar]
- 12.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: The Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93:1319–24. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 14.Parmar P, Salman A, Kalavathy CM, Kaliamurthy J, Thomas PA, Jesudasan CA. Microbial Keratitis at extremes of age. Cornea. 2006;25:153–8. doi: 10.1097/01.ico.0000167881.78513.d9. [DOI] [PubMed] [Google Scholar]
- 15.Singh G, Palanisamy M, Madhavan B, Rajaraman R, Narendran K, Kour A, et al. Multivariate analysis of childhood microbial keratitis in South India. Ann Acad Med Singapore. 2006;35:185–9. [PubMed] [Google Scholar]


