Abstract
Aim:
Exposure to UV light is the major risk factor in the development of age-related cataract (ARC). UV filters produced during tryptophan catabolism maintain the transparency of the lens and protect retina from photo damage. Indoleamine 2, 3-dioxygenase (IDO), the first rate-limiting enzyme in the tryptophan catabolism, is up regulated by interferon-gamma (IFN-g) which harbors single nucleotide polymorphisms (SNPs). The T allele of SNP at +874 position of the IFN-g is known to be associated with the up regulation of IDO than the allele A. Hence, we attempted to study the IFN-g+874(T/A) polymorphism for its association with ARCs.
Materials and Methods:
A total of 680 cataract cases [199 nuclear (NC), 175 cortical (CC), 174 posterior subcapsular (PSC), and 132 mixed types (MT)] and 210 healthy controls were genotyped for +874(T/A) polymorphism using amplification refractory mutation system-polymerase chain reaction on 2% agarose gel stained with ethidium bromide.
Results:
There was increased risk for CC and PSC when the patients happened to be females, with low body mass index and with early onset. Considering the IFN-g polymorphism, a high risk was observed for CC and PSC in female patients of AA genotype with significant protection for those with TT genotypes.
Conclusion:
Present results indicate that +874(T/A) polymorphism may be considered as one of the biomarkers to distinguish between the CC and PSC types of cataracts for risk estimations. The study appears to be the first of its kind reporting an association of IFN-g+874(T/A) polymorphism with ARCs.
Keywords: Age-related cataracts, indoleamine 2, 3-dioxygenase, interferon-gamma, tryptophan metabolism
Introduction
Age-related cataract (ARC) is a progressive opacification of ocular lens leading to visual impairment and blindness. The causes for ARCs are manifold and include gene defects, oxidative stress, cross-linking of macromolecules, truncation, and aggregation of lenticular proteins.[1,2] The lack of protein turnover, accumulation of modified proteins with age, exposure to UV light, and factors that generate free radicals lead to lens opacification.[3] While accumulation and generation of free radicals can be prevented by scavenging agents and action of inhibitors, presence of low-molecular-weight compounds that act as UV filters help in preventing photo damage to the retina.[4] With increasing age decrease in the levels of free UV filters are seen in the lens affecting normal vision.[5]
UV filters are derived from tryptophan catabolism and indoleamine 2, 3-dioxygenase (IDO; EC 1.13.11.42) is the first rate-limiting enzyme of this pathway whose production is induced by several inflammatory mediators including the protein mediator, interferon-gamma (IFN-g).[6] Expression of IFN-g is genetically controlled and the presence of alleles T and A at +874 position from the translation start site is related to high and low IFN-g expression, respectively, which in turn influences the activity of IDO.[7–9] Because of this relationship between the genes IFN-g and IDO, the present study was undertaken to assess the risk of +874(T/A) polymorphism in the development of different types of ARCs.
Materials and Methods
Venous blood samples of 680 cataract cases [199 nuclear (NC), 175 cortical (CC), 174 posterior subcapsular (PSC), and 132 mixed type (MT)] and 210 healthy, normal individuals were collected in the EDTA vaccutainers. The study patients were recruited from inpatients of the Sarojini Devi Eye Hospital and Institute of Ophthalmology, Hyderabad, India. The type of cataract was determined by ophthalmologists concerned following LOCS III (Lens opacities classification system III) classification.[10] Controls were selected at random by personal contacts, by house visits, and from among the employees of Government and private organizations with the provision for annual health checkup. The patients and controls were explained about the purpose and outcome of the study and only those who gave their consent to participate in the investigations by providing the blood samples and demographic history were considered. The study was approved by the Sarojini Devi Eye Hospital and Institute of Ophthalmology and also by the Institutional Ethical Committee.
Inclusion and exclusion criteria
Only patients with primary cataracts were included in the present study. Those arising due to trauma, action of toxins, inflammations, and degenerative ocular diseases were excluded. In addition, patients with associated conditions such as diabetes, hypertension, myopia, glaucoma, thyroid syndromes, and cataract-inducing medications (such as steroids) were eliminated. Control subjects were without cataract or history of diabetes, hypertension, thyroid, and other ocular diseases.
From all the patients and controls, information pertaining to occupation, sex, age, age at onset, duration of disease, type of cataract, information on habits, diet, and detailed medical history along with three-generation pedigree were collected using a specified proforma prepared for the study.
Methods
Genomic DNA was isolated by using salting out method.[11] Amplification refractory mutation system-polymerase chain reaction (ARMS-PCR) was carried out for amplifying the interferon gamma gene by using two different PCR reactions; each reaction involving a generic antisense primer: F 5’-tcaacaaagctgatactcca-3’ and one of the two allele-specific sense primers: 5’- ttcttacaacacaaaatcaaatct-3’ for the detection of allele T or 5’-ttcttacaacacaaaatcaaatca-3’ for the detection of allele A.[6] PCR was performed with initial denaturation at 95°C for 5 m, followed by (a) 10 cycles of 95°C for 1 min, 62°C for 1 min, and 72°C for 1 min and (b) 20 cycles of 95°C for 45 s, 56°C for 1 min, and 72°C for 2 min with a final extension at 72°C for 5 min. The amplified products of 261 bp were separated by electrophoresis on a 2% agarose gel stained with ethidium bromide. Genotypes were typed as TT and AA depending on the presence of the bands when primers specific for allele T (as TT) or allele A (as AA) were used. Samples were typed as heterozygotes (AT) when bands were seen with both the primers [Figure 1].
Figure 1.
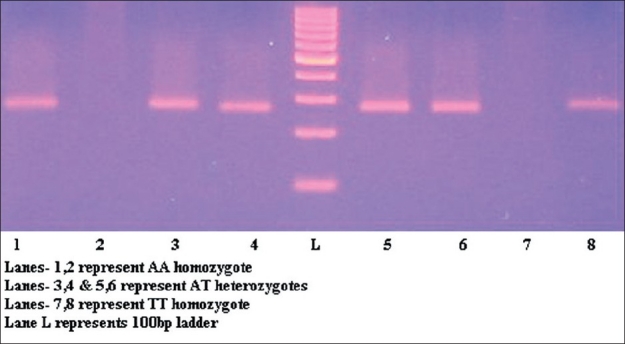
ARMS-PCR analysis for genotyping IFN-g+874(T/A) polymorphism
Data analysis
SPSS (version 17) package was used to analyze the data for descriptive statistics and computation of means and standard error. χ2 statistics was computed for significance of differences in the distribution of genotypes between cases and controls and also between different types of cohorts (sex, family history, obesity, and habits such as smoking and alcohol consumption) in the two groups. The frequencies of the marker alleles were estimated by allele counting method and tested for Hardy–Weinberg equilibrium. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed for different combinations of genotypes to estimate the risk contributing to cataract development.
Results
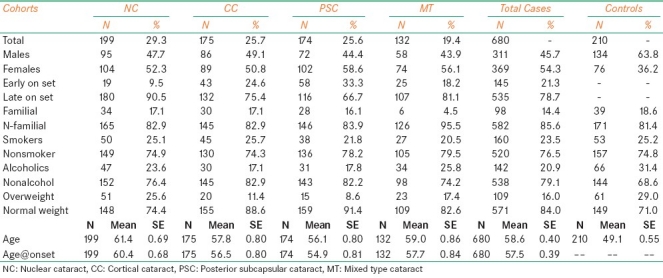
Table 1 shows the baseline characteristic features of the cataract and control subjects studied. There was preponderance of female patients (54.3%) when compared with males (45.7%), the frequency being highest in cases of PSC (58.6%) when compared with other types of cataracts (NC 52.3%, CC 50.8%, and MT 56.1%). This indicates high risk for females for developing ARCs, especially the PSC type. The ages ranged from 35 to 85 years in patients and 40 to 80 years in the controls. The frequency of cases with early age at onset (<50) was high in PSC (33.3%) followed by CC (24.6%), MT (18.2%), and NC (9.5%). The frequency of overweight patients was nearly 50% lesser in cases (16.0%) when compared with controls (29.0%). In general, the incidence of smokers was slightly less in cataract patients (23.5%) when compared with controls (25.2%). Similarly, the frequency of alcoholics was lesser in patients (20.9%) when compared with controls (31.4%). Occupationally about 40% of the patients were associated with outdoor work and exposure to sunlight. The present findings are in compliance with the earlier reports from Framingham, Beaver Dam and Barbodas Eye studies and also other studies from various geographical regions suggesting association of different types of cataracts with female sex.[12–16]
Table 1.
Distribution of baseline characteristic features observed in different types of cataracts and controls
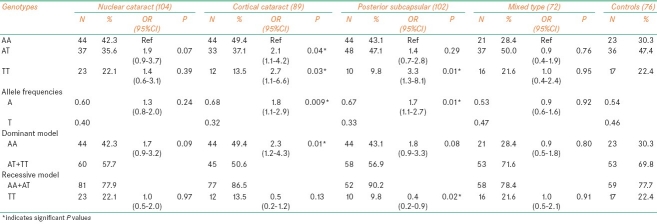
IFN-g+874(T/A) polymorphism
The genotype distribution of +874(T/A) polymorphism did not differ significantly between the patients and controls in general. Considering different types of cataracts, the genotype distribution of +874(T/A) polymorphism in cases of NC was more or less similar to that found in the controls, while the frequency of AA genotype was higher in patients of CC (44.6%) and PSC (40.8%) with corresponding decrease in the frequency of TT (CC: 13.1% and PSC: 13.2%) when compared with controls (AA: 38.1% and TT: 17.1%). In cases with PSC, the frequency of AT (46.0%) genotype was also found to be higher when compared with controls (44.8%). In MT type, the frequency of AT (48.5%) and TT (19.7%) was higher and that of AA (31.8%) lesser when compared with controls (AT: 44.8%; TT: 17.1%, and AA: 38.1%). The allele and genotypic frequencies, in general, in all types of cataracts and controls remained in Hardy–Weinberg equilibrium.
Considering the genderwise distribution of +874(T/A) polymorphism, no statistically significant difference was found between male patients and male controls. But female CC (49.4%) and PSC (43.1%) patients showed a significant increase in the frequency of AA with corresponding decrease in the frequency of TT (CC: 13.5% and PSC: 9.8%) genotype when compared with female controls (AA: 30.3%; TT: 22.4%). Between the cataract types, females with NC showed high frequency of TT (22.1%) genotype compared with females of CC (13.5%) and PSC (9.8%) types. Allele frequencies in female patients of different types of cataracts and female controls remained in Hardy–Weinberg equilibrium [Table 2].
Table 2.
The genotype distribution and the risk estimates for +874 (T/A) polymorphism of IFN-g gene in female patients of ARCs
The OR estimates did not show any significant results in the distribution of +874(T/A) polymorphism in cataract patients in general, and when each type was compared with the controls. However, female cataract patients with AA genotype showed 2 to 3 folds increase in the risk for developing CC (AA/AT: OR=2.1; 95% CI=1.1–4.2; AA/TT: OR=2.7; 95% CI=1.1–6.6) and PSC (AA/TT: OR=3.3; 95% CI=1.3–8.1) types of cataracts. Further computations for risk estimates made under dominant and recessive model showed a significantly high risk for AA females with CC at 1% (OR=2.3, 95% CI=1.2–4.3; P=0.01); with PSC at 8% (OR=1.8, 95% CI=0.9–3.3; P=0.08); and with NC at 9% level of significance (OR=1.7, 95% CI=0.9–3.2; P=0.09). Under recessive model, ORs showed significantly high protection for TT individuals against developing PSC (OR=0.4, 95% CI=0.2–0.9; P=0.02), while for other cataracts the results were insignificant. Considering allele frequencies, similar results were obtained showing nearly 2 folds increase in risk for A allele in female patients with CC (OR=1.8, 95% CI=1.1-2.9; P=0.009) and PSC (OR=1.7, 95% CI=1.1-2.7; P 0.01) [Table 2]. Patients with other confounding factors such as BMI, smoking, alcohol consumption, age at onset, and family history were not found to be associated with the IFN-g+874(T/A) polymorphism.
Discussion
UV filters are known to play a pivotal role in the maintenance of transparency of lens and prevention of cataract development.[4] Indoleamine 2,3-dioxygenase is the first rate-limiting enzyme that catalyzes the production of UV filter molecules such as kynurenine and 3-OH kynurenine by tryptophan catabolism through kynurenine pathway. IDO needs several inflammatory mediators for its production including interferons such as IFN-g.[6] The two polymorphisms, 12 CA repeat and+874(T/A) at IFN-g, have been associated with risk for certain conditions such as acute kidney rejection, aplastic anemia, tuberculosis, symptomatic parovirus infection, and recurrent pregnancy loss.[9,17–22] IFN-g+874(T/A) polymorphism lies within the transcription factor binding site of NF-kB, which shows an allele-specific binding pattern. NF-kB site induces IFN-g expression and the presence of T and A allele of +874(T/A) polymorphisms are related to high and low IFN-g expression, respectively, which in turn influences the IDO activity proportionally. This observation was found in females without any clinical condition by Raitala et al.[6]
Studies have confirmed that opacity of the ocular lens is caused by the elevation of free radicals in the lenticular tissue with increasing age and accumulation of noxious factors. IDO enzyme is also involved in O2 radical scavenging,[23] thus helping in the prevention of cataract formation. Hence, the study of this enzyme and its association with genes that mediate its induction like IFN-g seems pertinent to understand in more detail the mechanism of cataract formation.
A high risk was observed in this study for AA genotype in female patients with CC and PSC and with significant protection for TT genotypes against PSC. Keeping in view the high induction of IDO gene by allele T of +874(T/A) polymorphism by Raitala et al.,[6] the reduced frequency of genotype TT found in the present cases of PSC may indicate upregulation of IDO facilitating increase in the production of UV filters and also efficient scavenging of free radicals together playing a role in delaying the onset of lens opacification. The high risk found for allele A carriers of +874(T/A) polymorphism in the CC cases of this study may be associated with low IDO activity affecting the formation of UV filters required for the maintenance of lens transparency.
The present results indicate that +874(T/A) polymorphism, specifically in females, may be considered as one of the biomarkers to distinguish between the CC and PSC types of cataracts as far as risk estimations are concerned. The study appears to be the first of its kind to be reported with reference to ARCs. Estimation of IDO levels along with genotyping will throw more light on understanding the functional aspects of the association found.
Acknowledgments
This work has been funded by Department of Biotechnology Grant (DBT), New Delhi (BT/PR9540/AAQ/03/351/2007) to T. Nagaraju and T. Padma. Ms. M. Mamata, and G. Sridhar acknowledge the Department of Biotechnology, Government of India, for granting fellowship to carryout the study. We are grateful to all the doctors and all the subjects who have participated in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Truscott RJ. Age–related nuclear cataract–oxidation is the key. Exp Eye Res. 2005;80:709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Snytnikova OA, Kopylova LV, Chernyak EI, Morozov SV, Kolosova NG, Tsentalovich YP. Tryptophan and kynurenine levels in lenses of Wistar and accelerated-senescence OXYS rats. Mol Vis. 2009;15:2780–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Wannemacher CF, Spector A. Protein synthesis in core of calf lens. Exp Eye Res. 1968;7:623–5. doi: 10.1016/s0014-4835(68)80018-1. [DOI] [PubMed] [Google Scholar]
- 4.Bova LM, Wood AM, Jamie JF, Truscott RJ. UV filter compounds in human lenses: The origin of 4-[2-amino-3-hydroxyphenyl]-4-oxobutanoic acid O-β-D-glucoside. Invest Ophthalmol Vis Sci. 1999;40:3237–44. [PubMed] [Google Scholar]
- 5.Korlimbinis A, Aquilina JA, Truscott RJ. Protein-bound and free UV filter in cataract lenses.The concentration of UV filters is much lower than in normal lenses. Exp Eye Res. 2007;85:219–25. doi: 10.1016/j.exer.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Raitala A, Pertovaara M, Karjalainen J, Oja SS, Hurme M. Association of interferon-gamma þ874[A/T] single nucleotide polymorphism with the rate of tryptophan catabolism in healthy individuals. Scand J Immunol. 2005;61:387–90. doi: 10.1111/j.1365-3083.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- 7.Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, Hutchinson IV. in vitro production of IFN-g correlates with CA repeat polymorphism in the human IFN-g gene. Eur J Immunogenet. 1999;26:1–3. doi: 10.1046/j.1365-2370.1999.00122.x. [DOI] [PubMed] [Google Scholar]
- 8.Pravica V, Perrey C, Stevens A, Lee JH, Hutchinson IV. A single nucleotide polymorphism in the first intron of the human IFN-g gene: Absolute correlation with a polymorphic CA microsatellite marker of high IFN-g production. Hum Immunol. 2000;61:863–6. doi: 10.1016/s0198-8859(00)00167-1. [DOI] [PubMed] [Google Scholar]
- 9.Rossouw M, Nel HJ, Cooke GS, van Helden PD, Hoal EG. Association between tuberculosis and a polymorphic NF-kB binding site in the interferon g gene. Lancet. 2003;361:1871–2. doi: 10.1016/S0140-6736(03)13491-5. [DOI] [PubMed] [Google Scholar]
- 10.Chylack LT, Jr, Leske MC, Sperduto R, Khu P, McCarthy D. Lens opacities classification system. Arch Ophthalmol. 1988;106:330–4. doi: 10.1001/archopht.1988.01060130356020. [DOI] [PubMed] [Google Scholar]
- 11.Lahiri DK, Nurnberger JR. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein BE, Klein R, Linton KL. Prevalence of age-related lens opacities in a population: The Beaver Dam Eye Study. Ophthalmology. 1992;99:546–52. doi: 10.1016/s0161-6420(92)31934-7. [DOI] [PubMed] [Google Scholar]
- 13.Leske MC, Connell AM, Wu SY, Hyman L, Schachat A. Prevalence of lens opacities in the Barbados Eye Study. Arch Ophthalmol. 1997;115:105–11. doi: 10.1001/archopht.1997.01100150107018. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P, Cumming RG, Attebo K, Panchapakesan J. Prevalence of cataract in Australia: The Blue Mountains Eye Study. Ophthalmology. 1997;104:581–8. doi: 10.1016/s0161-6420(97)30266-8. [DOI] [PubMed] [Google Scholar]
- 15.McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128:446–65. doi: 10.1016/s0002-9394(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 16.Delcourt C, Cristol JP, Tessier F, Léger CL, Michel F, Papoz L. Risk factors for cortical, nuclear, and posterior subcapsular cataracts: The POLA Study. Am J Epidemiol. 2000;151:497–504. doi: 10.1093/oxfordjournals.aje.a010235. [DOI] [PubMed] [Google Scholar]
- 17.Asderakis A, Sankaran D, Dyer P, Johnson RW, Pravica V, Sinnott PJ, et al. Association of polymorphisms in the human interferon-gamma and interleukin-10 gene with acute and chronic kidney transplant outcome: The cytokine effect on transplantation. Transplantation. 2001;71:674–7. doi: 10.1097/00007890-200103150-00018. [DOI] [PubMed] [Google Scholar]
- 18.Dufour C, Capasso M, Svahn J, Marrone A, Haupt R, Bacigalupo A, et al. Homozygosis for [12] CA repeats in the first intron of the human IFN-gamma gene is significantly associated with the risk of aplastic anaemia in Caucasian population. Br J Haematol. 2004;126:682–5. doi: 10.1111/j.1365-2141.2004.05102.x. [DOI] [PubMed] [Google Scholar]
- 19.Lio D, Marino V, Serauto A, Gioia V, Scola L, Crivello A, et al. Genotype frequencies of the þ874T-A single nucleotide polymorphism in the first intron of the interferon-gamma gene in a sample of Sicilian patients affected by tuberculosis. Eur J Immunogenet. 2002;29:371–4. doi: 10.1046/j.1365-2370.2002.00327.x. [DOI] [PubMed] [Google Scholar]
- 20.López-Maderuelo D, Arnalich F, Serantes R, González A, Codoceo R, Madero R, et al. Interferon-gamma and interleukin-10 gene polymorphisms in pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;167:970–5. doi: 10.1164/rccm.200205-438BC. [DOI] [PubMed] [Google Scholar]
- 21.Kerr JR, McCoy M, Burke B, Mattey DL, Pravica V, Hutchinson IV. Cytokine gene polymorphisms associated with symptomatic parvovirus B19 infection. J Clin Pathol. 2003;56:725–7. doi: 10.1136/jcp.56.10.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daher S, Shulzhenko N, Morgun A, Mattar R, Rampim GF, Camano L, et al. Associations between cytokine gene polymorphisms and recurrent pregnancy loss. J Reprod Immunol. 2003;58:69–77. doi: 10.1016/s0165-0378(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 23.Taylor MW, Feng GS. Relationship between Interferon γ, indoleamine 2,3 –dioxygenase and tryptophan catabolism. FASEB J. 1991;5:2516–22. [PubMed] [Google Scholar]