Abstract
Flupirtine is neither an opioid nor a non steroidal anti-inflammatory drug (NSAID) producing its analgesic action through blockade of glutamate N-methyl-D-aspartate receptor. It is devoid of adverse effects of routinely used analgesic drugs, but is equally efficacious in reducing pain sensation. It has a distinctive mechanism of action, exerting a dual therapeutic effect with both analgesic and muscle relaxant properties that has utility in the treatment of pain, including that associated with muscle tension.
Keywords: Flupirtine, N-methyl-D-aspartate receptor, non-steroidal anti-inflammatory drug, opioids
Introduction
Pain is the most miserable symptom of disease presentation. Treatment of this noxious sensation is a challenge for clinicians. There are a number of medications available for the treatment of pain such as non-steroidal anti-inflammatory drugs (NSAID), opioids, and their derivatives. Flupirtine is in use for the last 25 years for the management of pain following surgery, trauma, dental extraction, pain associated with muscle spasm, cancer, degenerative joint diseases, and conditions such as headache and dysmenorrhoea in Europe. It is a nonopioid, non-NSAID analgesic, and is devoid of common adverse effects seen with NSAIDs and opioids. Additional effects identified include muscle relaxation, neuroprotection, and antiapoptotic action. Although it is not yet approved by United States Food and Drug Administration (US FDA), it is still used in many countries including India. Recently, US FDA granted permission to carry out phase II clinical trial with flupirtine for the treatment of fibromyalgia.
Chemistry
Flupirtine is a triaminopyridine derivative having a chemical structure - 2-amino-3-ethoxy-carbonylamino-6-4-fluoro-benzylamino-pyridine [Figure 1]. The basic molecule used for synthesis of flupirtine was 2, 6-dichoro 3-nitropyridine.[1] It was first synthesized in 1980s in Germany and was marketed by Degussa Pharma.[2]
Figure 1.
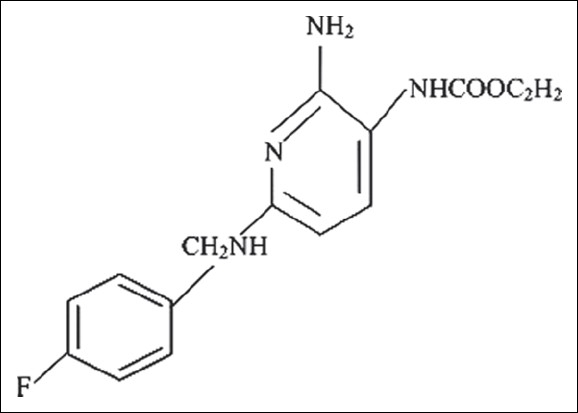
Structure of flupirtine
Pharmacodynamics
Analgesic action
Flupirtine was introduced as an alternative analgesic to opioids and NSAIDs. Subsequently, multiple other actions such as muscle relaxation and neuroprotective activity were identified. Flupirtine acts indirectly as N-methyl-D-aspartate (NMDA) receptor antagonist by activation of K+ channels[3] Flupirtine causes a dose-dependent reduction of NMDA receptor mediated glutamate induced rise in intracellular Ca++ concentration.[4] It binds to and activates G- protein coupled inwardly rectifying K+ channels. Activation of this channel leads to hyperpolarization of neuronal membrane and the neuron becomes less excitable; thus, there is stabilization of resting neuronal membrane.[5] The drugs activating this channel are called as selective neuronal potassium channel openers (SNEPCO) and flupirtine is the prototype.[3] Experimental evidence suggests that flupirtine might suppress channel opening by acting as an oxidizing agent at the redox site of the NMDA receptor.[6] This action inhibits the transmission of nociceptive impulses during neuronal excitation.
Muscle relaxant action
The muscle relaxation is due to inhibition of both mono- and polysynaptic reflexes. The spinal polysynaptic flexor reflex, mediated by NMDA receptors, was depressed by flupirtine, whereas the monosynaptic Hoffmann reflex (H-reflex), mediated by non-NMDA receptors, was not influenced.[7] Healthy human subjects responded with a significant reduction of both the early phase of the electrically elicited polysynaptic flexor reflex of pretibial muscles and the medium latency response of the toe-up paradigm after 2 h of 200 mg of flupirtine. Flupirtine possesses analgesic as well as muscle-relaxing effect in same dose ranges; thus, it can be used in the treatment of painful diseases of the motor system presenting with spasticity and chronic musculoskeletal pain.[8]
Neuroprotective action
Apoptosis, a programmed cell death, is caused by increased intracellular Ca++ levels, mitochondrial dysfunction, cell membrane disruption, and finally, nucleolysis. In vitro studies with primary cortical neurons from rat embryos have shown that lead acetate, prions like PrPsc, HIV coat protein gp120, and β amyloid peptide will cause apoptotic cell death. But if preincubated with flupirtine, it completely protects apoptotic cell death caused by above agents in the neurons.[2] It has been found that flupirtine also antagonizes both glutamate and NMDA induced increase in intracellular levels of Ca++, as observed in in vitro cultures of cortical and hippocampal neurons.[9]
The expression of Bcl-2, an antiapoptotic agent, and glutathione, a scavenger of reactive oxygen, are reduced during glutamate or NMDA-induced apoptosis in cells. Flupirtine is found to increase the levels of Bcl-2 and glutathione in glutamate or NMDA-induced apoptosis of human Ntera/D1 (hNT) neurons as well as cultured retinal pigment cells.[2,5] Flupirtine reduced the expression of oncogenes and formation of reactive oxygen radicals in experimental models which explains its action of preventing ischemia-induced apoptosis. This explains the role of flupirtine in future for treatment of neuroinfections such as immune deficiency syndrome (AIDS), prion diseases, and neurodegenerative disease such as Alzheimer's.[3]
Antiparkinsonian action
Flupirtine has an NMDA receptor antagonistic action and hence it was studied for its antiparkinsonian effect as an adjuvant to L-3,4-dihydroxyphenylalanine (L-DOPA). Akinesia and muscular rigidity were produced in rats by giving reserpine and α methyl p-tyrosine. Flupirtine was given alone and in combination with L-DOPA, it strongly reduced muscle rigidity and increased the ability of L-DOPA to reverse akinesia.[10]
In haloperidol-induced catalepsy, which is considered as a model of Parkinson's disease, flupirtine alone and in combination with L-DOPA exerted a potent anticataleptic effect.[7,10] However, human studies are not available till date to support this evidence. If studies are done to prove effectiveness in Parkinson's disease, it can be combined with L-DOPA.
Pharmacokinetics
Absorption
Flupirtine is a hydrophilic compound. It is completely absorbed from gastrointestinal tract with a bioavailability of 90% by oral route and 70% by rectal route.[11] 100 mg of oral flupirtine in normal healthy volunteers reached a peak plasma concentration of 773 μg/L at 1.6 h and rectal administration 890 μg/L. Steady-state concentrations were achieved after 2 days in four healthy volunteers who received 75 mg of flupirtine orally at 12 h intervals.[12]
Distribution
Flupirtine has large volume of distribution (Vd) and gets equally distributed into both extra and intravascular compartments. Vd of 100 mg flupirtine is 154 L, 212 L, and 195 L in healthy volunteers, patients with renal impairment, and elderly patients, respectively.[2,11] Flupirtine is 80–84% bound to human albumin. Concentration in CSF is same as that in plasma and higher concentration was observed in liver and exocrine glands, whereas lower concentration was observed in the kidney.[12] The half-life of flupirtine on oral, intravenous, and rectal administration with 100 mg is 6.5, 8.5, and 10.7 h, respectively, in healthy volunteers. The clearance of 100 mg of flupirtine after oral administration is 275, 263, and 161 ml/min in healthy volunteers, renal impairment patients, and elderly people, respectively.[2,11]
Metabolism and elimination
Flupirtine is metabolized in liver to 4-Fluorohippuric and N-acetylated analogue D13223 by peroxidase enzymes [human myeloperoxidase and horse radish peroxide (HRP)]. The N-acetylated metabolite D13223 retains 20–30% of activity of its parent compound. The two metabolites are further oxidized and then conjugated with glycine to form inactive metabolites.[1,13] 72% of the total dose administered appears in urine as parent drug and its metabolites, whereas 18% is excreted in feces.[14]
Dosage and formulation
Flupirtine can be administered by oral and rectal routes. It is available as 50 and 100 mg for oral administration. Adult dose is 300–400 mg per day and can be increased to 600 mg per day. Dose in children is 150–200 mg per day in 3–4 divided doses.[1,2,15] Rectal suppositories are administered in the dose range of 450–600 mg per day in adults and 150–250 mg per day in children.[15] In India, it is being marketed under the trade name KETADOL as 100 mg tablet (Lupin Pharmaceuticals). Drug Controller General of India (DCGI) has approved flupirtine maleate 100 mg for treatment of acute and chronic pain, i.e., for painful increased muscle tone of the posture and motor muscles, primary headache, tumor pain, dysmenorrheal, and pain after trauma/orthopedic operations and injuries.[16]
Special group
Safety of flupirtine in pregnant, lactating women, and children less than 6 years is not established. If indicated in lactating women, breastfeeding should be stopped. Dose of flupirtine should be reduced to 50% in elderly patients and those with renal and hepatic impairment.[1,15]
Drug interactions
Flupirtine has shown to increase warfarin toxicity but the mechanism is not clear; hence, patients on oral anticoagulant therapy should be monitored for prothrombin time. It also increases hepatotoxic potential of paracetamol; thus, hepatic transaminases levels should be monitored when both the drugs are given concomitantly. Alcohol and other sedatives including benzodiazepines potentiate tiredness and dizziness due to flupirtine.[15] Coadministration with carbamazepine is not advisable as carbamazepine induces hepatic enzymes. Not much information is available regarding interaction with other drugs.
Contraindications
Flupirtine is avoided in patients with history of hypersensitivity to flupirtine, hepatic encephalopathy, cholestasis, myasthenia gravis, chronic alcoholism, primary biliary cirrhosis, and liver disease.
Uses
Musculoskeletal pain
Flupirtine has been compared with placebo and standard analgesics to determine the analgesic efficacy and tolerability. A post marketing surveillance of flupirtine 200–300 mg/day for 1 week has shown improvement in response rate as assessed by visual analogue pain scale. It was observed that the response rates were 94%, 89.4%, and 85.9% for patients with acute, subacute, and chronic pain, respectively.[1,17] A randomized, double blind study revealed that flupirtine 300 mg/day was as effective as tramadol 150 mg/day in reducing subacute low-back pain.[18] Comparative studies with diclofenac 150 mg/day has also shown that flupirtine 300 mg/day significantly reduced postoperative pain equally. The pain reduction was significant after 60 min of oral administration of both the drugs.
Headache
Patients who had insufficient response to conventional analgesics for chronic tension headache showed better response to flupirtine.[17] Comparative studies of flupirtine 100 mg three times daily with that of placebo in chronic headache of 2 weeks have shown significant reduction in pain intensity.[1,17] In acute migraine attacks, flupirtine 100 mg has shown better efficacy in terms of pain relief, restriction in working ability with less adverse effects when compared to paracetamol 1 gm orally.
Neurogenic pain
Flupirtine 380 mg/day was found to be effective in reducing lumbar and cervical spinal root pain in comparison with aspirin 1800 mg/day. The study also showed that 25% of flupirtine-treated patients reported no pain and 46% reported improvement, but in those who received aspirin only 35% reported an improvement.[1,19]
Cancer pain
Studies have shown that flupirtine reduced cancer pain more effectively than tramadol and pentazocine. Also, the adverse reaction profile was similar in both flupirtine and tramadol treated patients.[20] But, with an increased incidence of central nervous system (CNS) side effects to pentazocine when compared with flupirtine.[21]
Fibromyalgia
Flupirtine has been found to reduce the pain of fibromyalgia better than other available drugs. ADEONA, a pharmaceutical company, was granted permission by US FDA to conduct randomized, double blind, placebo-controlled phase II clinical trial of flupirtine in Fibromyalgia syndrome.[21]
Postoperative pain
Pain during postoperative period is most distressing to the patient. Flupirtine is found to have better analgesic effect with minimal side effects compared to standard drugs. In postepisiotomy pain, flupirtine showed 69% reduction in pain score 6 h after administration when compared to placebo which had only 26% reduction. Flupirtine also had greater efficacy in postepisiotomy pain reduction when compared to suprofen and paracetamol.[22]
A parallel group control study comparing flupirtine with diclofenac was done in 40 orthopedic patients to evaluate the efficacy in reducing postoperative pain. This study concluded that flupirtine and diclofenac were equally effective in reducing postoperative pain.[23] Flupirtine was also equally effective as that of pentazocine in reducing postoperative pain but with less adverse effects.[24] In a double blind parallel group study, 50 women who underwent abdominal hysterectomy were given 100 mg of flupirtine and 60 mg of dihydrocodeine for 3 days. Both the drugs produced equal efficacy in reducing postoperative pain.[25]
Adverse effects
In long-term trials done for rheumatic disease, majority of adverse reactions occurred within 6 months of treatment, among which most common were dizziness (11%), drowsiness (9%), pruritis (9%), dry mouth and gastric fullness (5%), nausea, and muscle tremor (2%).[26] Other side effects were heart burn, vomiting, disturbed sleep, sedation, headache, fatigue, and mood elevation. All these effects were dose dependent. A dose of 100 and 200 mg caused an insignificant increase in systolic blood pressure with not much alteration in heart rate and other hematological parameters.[27] Rare and serious side effects were increased transaminases levels, drug-induced hepatitis, ataxia, tremors, restlessness, and nervousness.
In elderly healthy individuals, it was reported to cause transient faintness, dizziness, and lethargy, whereas in patients with renal dysfunction, it has produced transient light headedness and headache. But in liver disease patients, it produced serious adverse effects such as encephalopathy due to increased plasma concentration of the drug; so, the drug was discontinued.[12]
Advantages over NSAIDs
NSAIDs is frequently associated with symptoms of dyspepsia, heartburn, and epigastric pain leading to reduced compliance especially among patients on long-term treatment. A randomized open label study has shown that flupirtine was better tolerated and had better patient compliance compared to diclofenac.[23] It has been documented that NSAIDs are associated with endoscopically diagnosed ulcers, serious upper gastrointestinal side effects including bleeding, perforation, and obstruction. In contrast, these are not seen with flupirtine; hence, it can be used as an alternate analgesic.
Advantages over OPIOIDs
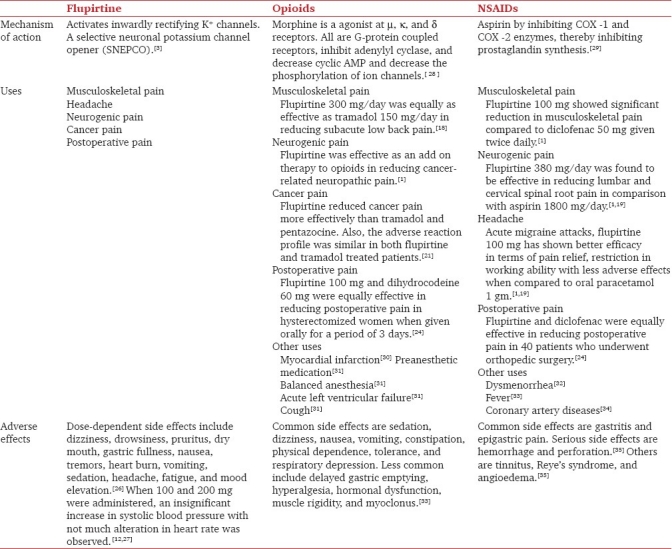
Long-term use of opioids for treatment of pain leads to constipation, nausea, sedation, confusion, pruritis, urinary retention, tolerance, and dependence. These adverse effects were not seen with flupirtine when given daily for 12 months in chronic pain. Comparative studies of flupirtine with tramadol and pentazocine showed that it is equally efficacious but without adverse effects as that of opioids. Hence, flupirtine could be a better alternate to opioids. Comparison of flupirtine with opioids and NSAID s is enumerated in Table 1.
Table 1.
Comparison of flupirtine with opioids and NSAIDs
Conclusion
Studies suggest flupirtine as an effective analgesic for treatment of acute pain states such as postoperative pain, traumatic injury, headache, and migraine, as well as chronic pain such as musculoskeletal pain. It is effective and better tolerated for treatment of cancer pain. It has been observed to be equally effective like pentazocine, diclofenac, naproxen, ketoprofen, and paracetamol. It lacks anti-inflammatory effect which limits its use in inflammatory conditions such as rheumatism, rheumatic fever, and osteoarthritis. Its antiapoptotic, cytoprotective, and antioxidant properties can be used in treatment of neurodegenerative diseases. It is well tolerated with mild and infrequent adverse effects. No effects on respiration, lack of tolerance, or physical dependence are added advantage over opioids. Evidence of flupirtine's efficacy from clinical trials and well-established use in European countries for more than 2 decades suggests that it has a unique and important place in pain management.
Many drugs which were effective in clinical practice like analgin, spiramycin, qing hao (artemsinin) were not popular worldwide for a long time because it was not approved by US FDA; flupirtine is one among them. It is in use in European countries for the last two decades. It is presently under phase II clinical trials for fibromyalgia.[21]
Acknowledgement
We would like to thank Dr. Sarala N, Professor, Department Of Pharmacology, Sri Devaraj Urs Medical College, Tamaka, Kolar 563101, for her help toward preparation of the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Devulder J. Flupirtine in Pain Management Pharmacological Properties and Clinical Use. CNS Drugs. 2010;24:867–81. doi: 10.2165/11536230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Schuster G, Schwarz M, Block F, Pergande G, Schmidt WJ. Flupirtine: A review of its neuroprotective and behavioral properties. CNS Drug Rev. 1998;4:149–64. [Google Scholar]
- 3.Kornhuber J, Maler M, Wiltfang J, Bleich S, Degner D, Rüther E. Neuronal potassium channel opening with flupirtine. Fortschr Neurol Psychiatr. 1999;67:466–75. doi: 10.1055/s-2007-994997. [DOI] [PubMed] [Google Scholar]
- 4.Rupalla K, Weihong C, Krieglstein J. Flupirtine protects neurons against excitotoxic or ischemic damage and inhibits the increase in cytosolic Ca2+ concentration. Eur J Pharmacol. 1995;294:469–73. doi: 10.1016/0014-2999(95)00570-6. [DOI] [PubMed] [Google Scholar]
- 5.Jakob R, Krieglstein J. Influence of flupirtine on a G-protein coupled inwardly rectifying potassium current in hippocampal neurones. Br J Pharmacol. 1997;122:1333–8. doi: 10.1038/sj.bjp.0701519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osborne NN, Cazevieille C, Wood JP, Nash MS, Pergande G, Block F, et al. Flupirtine, a non-opioid centrally acting analgesic, acts as an NMDA antagonist. Gen Pharmacol. 1998;30:255–63. doi: 10.1016/s0306-3623(97)00355-8. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt WJ, Schuster G, Wacker E, Pergande G. Antiparkinsonian and other motor effects of Flupirtine alone and in combination with dopaminergic drugs. Eur J Pharmacol. 1997;327:1–9. doi: 10.1016/s0014-2999(97)89671-9. [DOI] [PubMed] [Google Scholar]
- 8.Nickel B, Jakovlev V, Szelenyi I. Effects of flupirtine, some analgesics, and muscle relaxants on skeletal muscle tone in conscious rats. Arzneim Forsch Drug Res. 1990;40:909–11. [PubMed] [Google Scholar]
- 9.Zimmer G, Balakirev M, Hofmann M, Woodcock BG, Pergande G. Evidence that the cytoprotective action of the triaminopyridine flupirtine involves increases in Ca2+ uptake and ATP synthesis in mitochondria. Br J Pharmacol. 1998;123:1154–8. doi: 10.1038/sj.bjp.0701736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz M, Nolden-Koch M, Purr J, Pergande G, Block F. Antiparkinsonian effect of flupirtine in monoamine-depleted rats. J Neural Transm. 1996;103:581–90. doi: 10.1007/BF01273155. [DOI] [PubMed] [Google Scholar]
- 11.Abrams SM, Baker LR, Crome P, White AS, Johnston A, Ankier SI, et al. Pharmacokinetics of flupirtine in elderly volunteers and in patients with moderate renal impairment. Postgrad Med J. 1988;64:361–3. doi: 10.1136/pgmj.64.751.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heather A, Friedel, Fitton A. Flupirtine: A review of its pharmacological properties, and therapeutic efficacy in pain states. Drugs. 1993;45:548–69. doi: 10.2165/00003495-199345040-00007. [DOI] [PubMed] [Google Scholar]
- 13.Methling K, Reszka P, Lalk M, Vrana O, Scheuch E, Siegmund W, et al. Investigation of the in vitro metabolism of the analgesic flupirtine. Drug Metab Dispos. 2009;37:479–93. doi: 10.1124/dmd.108.024364. [DOI] [PubMed] [Google Scholar]
- 14.Blackburn-Munro G, Dalby-Brown W, Mirza NR. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005;11:1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedel HA, Fitton A. Flupirtine: A review of its pharmacological properties on therapeutic efficacy in pain states. Drugs. 1993;45:548–69. doi: 10.2165/00003495-199345040-00007. [DOI] [PubMed] [Google Scholar]
- 16.List of approved drug from 01.01.2010 to 31.12.2010. [Last accessed on 2011 Nov 18]. Available from: http://cdsco.nic.in/LIST%20OF%20APPROVED%20DRUG%20FROM%2001.htm .
- 17.Mueller-Schwefe G. Flupirtine in acute and chronic pain associated with muscle tenseness: results of a postmarket surveillance study. Fortschr Med Orig. 2003;121:11–8. [PubMed] [Google Scholar]
- 18.Li C, Ni J, Wang Z, Li M, Gasparic M, Terhaag B, et al. Analgesic efficacy and tolerability of flupirtine vs. tramadol in patients with subacute low back pain: A double-blind multicentre trial. Curr Med Res Opin. 2008;24:3523–30. doi: 10.1185/03007990802579769. [DOI] [PubMed] [Google Scholar]
- 19.Heusinger JH. Efficacy and tolerance of flupirtine and pentazocine in two multicentre trials. Postgrad Med J. 1987;63:71–9. [PubMed] [Google Scholar]
- 20.Luben V, Muller H, Lobisch M, Wörz R. Treatment of tumor pain with flupirtine: Results of a double-blind study versus tramadol. Fortschr Med. 1994;112:282–6. [PubMed] [Google Scholar]
- 21.Scheef W. Analgesic efficacy and safety of oral flupirtine in the treatment of cancer pain. Postgrad Med J. 1987;63:67–70. [PubMed] [Google Scholar]
- 22.Ceccarelli G, Ciampini M, Frontespezi S. Flupirtine: The first Italian experience. Postgrad Med J. 1987;63:105–8. [PubMed] [Google Scholar]
- 23.Mastronardi P, D’Onofrio M, Scanni E, Pinto M, Frontespezi S, Ceccarelli MG, et al. Analgesic activity of flupirtine maleate: A controlled double-blind study with diclofenac sodium in orthopaedics. J Int Med Res. 1988;16:338–48. doi: 10.1177/030006058801600503. [DOI] [PubMed] [Google Scholar]
- 24.Riethmüller-Winzen H. Flupirtine in the treatment of post-operative pain. Postgrad Med J. 1987;63:61–5. [PubMed] [Google Scholar]
- 25.Moore RA, Bullingham RE, Simpson SO, Sullivan G, Evans PJ, McQuay HJ, et al. Comparison of flupirtine maleate and dihydrocodeine in patients following surgery. Br J Anaesth. 1983;55:429–32. doi: 10.1093/bja/55.5.429. [DOI] [PubMed] [Google Scholar]
- 26.Herrmann WM, Kenru U, Aigner M. On the adverse reactions and efficacy of long term treatment with flupirtine: Preliminary results of ongoing twelve month study with 200 patients suffering from chronic pain states in arthrosis or arthritis. Postgrad Med J. 1987;63:87–103. [PubMed] [Google Scholar]
- 27.Hummel T, Friedmann T, Pauli E, Niebch G, Borbe HO, Kobal G. Dose-related analgesic effects of flupirtine. Br J Clin Pharmacol. 1991;32:69–76. doi: 10.1111/j.1365-2125.1991.tb05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chahl LA. Opioids – mechanism of action. Aust Presco. 1996;19:63–5. [Google Scholar]
- 29.Cashman JN. The mechanism of action of NSAID s in analgesia. Drugs. 1996;52:13–23. doi: 10.2165/00003495-199600525-00004. [DOI] [PubMed] [Google Scholar]
- 30.Lal S, Savidge RS, Chhabra GP. Cardiovascular and respiratory effects of morphine and pentazocine in patients with myocardial infarction. Lancet. 1969;293:379–81. doi: 10.1016/s0140-6736(69)91351-8. [DOI] [PubMed] [Google Scholar]
- 31.Yaksh TL, Wallace MS. Opioids, analgesia, and pain management. In: Brunton LL, editor. Goodman and Gilman's The pharmacological basis of therapeutics. 12th ed. New York: The Mc Graw Hill Companies; 2011. pp. 481–525. [Google Scholar]
- 32.Dawood MY. Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am J Med. 1988;84:23–9. doi: 10.1016/0002-9343(88)90473-1. [DOI] [PubMed] [Google Scholar]
- 33.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid Complications and Side Effects. Pain Physician. 2008;11:105–20. [PubMed] [Google Scholar]
- 34.Malinin A, Dan Atar D, Callahan KP, McKenzie ME, Serebruany VL. Effect of a single dose aspirin on platelets in humans with multiple risk factors for coronary artery disease. Eur J Pharmacol. 2003;462:139–43. doi: 10.1016/s0014-2999(02)02956-4. [DOI] [PubMed] [Google Scholar]
- 35.Burke A, Smyth E, FitzGerald GA. Analgesic- antipyretic and antiinflammatory agents;pharmacotherapy of gout. In: Brunton LL, editor. Goodman and Gilman's. 11th ed. New York: The Mc Graw Hill Companies; 2006. pp. 671–715. [Google Scholar]


