Abstract
Objective:
Headache is not an uncommon complication of spinal anesthesia. The efficacy of intravenous (IV) hydrocortisone in treating the patients with postdural puncture headache was observed in this study.
Materials and Methods:
Sixty patients with headache following spinal anesthesia were randomly allocated into two groups. Thirty patients received only conventional therapy (recumbent positioning, IV or oral hydration, analgesics with caffeine, stool softeners, and soft diet) plus 2 ml of normal saline IV (placebo) 8 hourly for 48 h. Thirty other patients received conventional therapy plus hydrocortisone IV (100 mg in 2 ml 8 hourly for 48 h). Headache intensity was measured using visual analogue scale.
Results:
No significant difference was observed in baseline headache intensity between the two groups (P = 0.6642) before beginning of treatment. After 6 h, the mean headache intensity in patients treated conventionally was 6.02 ± 2.46, while it was 2.06 ± 1.98 in other patients who received additional hydrocortisone IV (P < 0.0001). After 24 h, headache intensity was 3.77 ± 1.85 in conventionally treated group versus 0.94 ± 2.67 in hydrocortisone group (P < 0.0001), while it was 1.95 ± 1.12 in conventionally treated group versus 0.69 ± 1.64 in hydrocortisone group (P = 0.001) after 48 h.
Conclusions:
Very short-term use of IV hydrocortisone was found effective in reducing headache following spinal anesthesia. However, its clear mechanism of action is yet to be determined. Large-scale studies are recommended to consider the steroid therapy as a standard treatment for postdural puncture headache.
Keywords: Corticosteroids, headache, hydrocortisone, postdural puncture headache, spinal anesthesia
Introduction
Postdural puncture headache (PDPH) is not an uncommon following subarachnoid block (SAB) and accidental dural puncture (ADP) during attempting epidural anesthesia. PDPH might also occur following lumbar puncture and myelography. This headache is usually a severe, dull, nonthrobbing pain, often fronto-occipital, which is aggravated in upright and diminished in supine position. It may be accompanied by nausea, vomiting, or visual disturbances. Headache begins 1 or 2 days after spinal anesthesia and if left untreated, usually relieves spontaneously within a week, but the patients suffer miserably during this period. In case headache is severe, other causes of headache must be looked for such as migraine, hypertension, meningitis, cortical vein thrombosis, pneumocephalus, sinusitis, nonspecific headache, and intracranial pathology.[1]
Cerebrospinal fluid (CSF) leakage following dural puncture is the most accepted mechanism of this headache. Reduction in CSF pressure lessens the cushioning effect of brain, allowing it to sag within the intracranial vault, and stimulates dural pain receptors especially in upright position. Headache continues until dural hole repairs and it is relieved when CSF volume and pressure return to normal.[2]
Current conventional and symptomatic treatments for PDPH include bed rest, hydration, analgesics, caffeine, sumatriptan, aminophyline, and ACTH. But none of these therapeutic approaches can relieve this headache completely and just help the patients to endure it. An epidural blood patch (EBP) is performed if the headache is too severe to limit one's activity or if any evidence of cranial nerve involvement is noted.[3]
The role of corticosteroids in management of postoperative pain and cancer pain[4] has been discussed in the recent years. Some authors have used these drugs to treat PDPH[5] and syndrome of spontaneous intracranial hypotension.[6] There is limited literature on the role of steroids in PDPH. The present study was designed to observe the efficacy of intravenous (IV) hydrocortisone in reducing headache intensity after spinal anesthesia.
Materials and Methods
Sixty adult patients (ASA I and II) who developed PDPH after nonobstetric surgery were observed between July 2010 and June 2011. A prior approval was obtained from the ethical committee of the hospital. Patients with history of cluster headache, convulsion, cerebrovascular accident, preeclampsia, eclampsia, coagulopathy, or previous neurological diseases were excluded. The modus of treatment was thoroughly explained to selected patients and informed consents were taken.
All SABs were performed by anesthesiologists not involved in this study. The procedures were done in sitting position through midline approach with 25-gauge Quincke needle and 0.5% bupivacaine heavy. Patients with PDPH were allocated in two groups by computer generated randomization: 30 patients were treated conventionally: Recumbent positioning, IV or oral hydration, analgesics with caffeine, stool softeners, and soft diet plus 2 ml of normal saline IV (placebo) 8 hourly for 48 h. Other 30 patients were treated conventionally and received 100 mg hydrocortisone, diluted in 2 ml, IV 8 hourly for 48 h. The patients and the single observer were blinded to this study.
The mean of headache intensity was measured in all 60 patients after 1 min in upright position. A trained observer asked the patients about severity of headache at 0, 6, 24, and 48 h after beginning of treatment. Using a 10 cm visual analogue scale (VAS), the intensity of headache (0–1, no headache; 2–4, mild headache; 5–7, moderate headache; and 8–10, severe headache) were recorded at 6, 24, and 48 h after commencement of treatment in two groups. Statistical analysis was done using Student's t-test to compare the mean (±SD) of headache intensities between the two groups and P value < 0.05 was considered significant.
Results
Before beginning of treatment all 60 patients had severe headache. The demographic characteristics including age, sex, height, and weight are shown in Table 1. The mean (±SD) baseline intensity of headache was 9.17 ± 1.69 in the conventionally treated group and 9.32 ± 0.83 in hydrocortisone group as per VAS. The headache intensities (mean ± SD) at 6, 24, and 48 h after commencement of treatment in two groups were tabulated [Table 2] and the means of headache intensities were plotted in Figure 1.
Table 1.
Demographic characteristics
Table 2.
Headache intensities (mean ± SD) following the beginning of treatment in the conventionally treated and conventional treatment + intravenous hydrocortisone groups
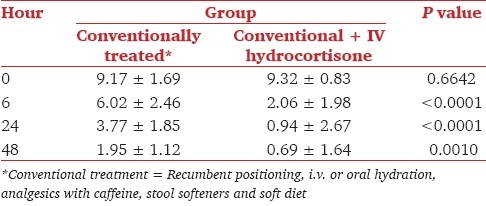
Figure 1.
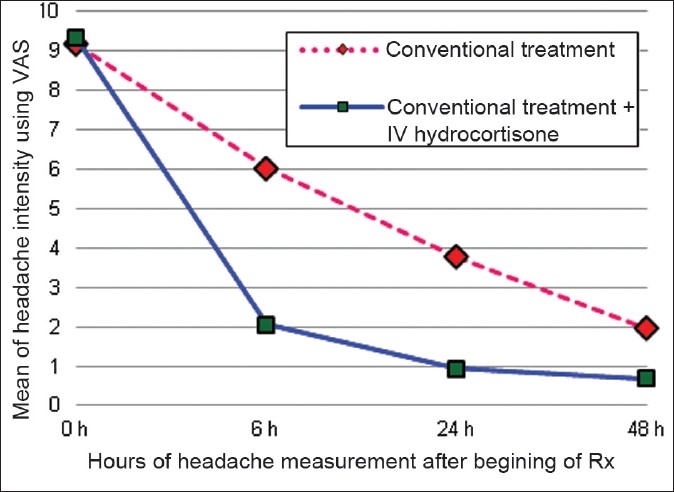
Comparison of headache during to 48 h after beginning of treatment
Eleven (11/30, 36.66%) patients in conventional treatment plus IV hydrocortisone group required three doses of hydrocortisone to get complete relief of symptoms. But one patient with severe headache from each group required EBP whose symptoms were not resolved even after 3 days therapy. No adverse effects were noted and no patient was re-referred to hospital for relapse of headache.
Discussion
Toriel et al. proposed the use of IV hydrocortisone in the treatment of PDPH in 2002.[7] In patients with severe PDPH after cesarean section received 100 mg hydrocortisone IV 8 hourly for 3 days, in addition to the conventional therapy, headache disappeared completely 12 h after the last dose.[8] The technique of anesthesia and needle gauge was not the same in these patients. Ashraf et al. studied patients with PDPH following cesarean section under SAB.[9] We administered hydrocortisone to the patients with PDPH who underwent various types of surgery under SAB, using the same needle gauge for all patients. Addition of hydrocortisone IV with conventional treatment for patients with PDPH reduced headache significantly.
Efficacy of corticosteroids to treat the syndrome of spontaneous intracranial hypotension has been demonstrated.[10] The syndrome is characterized by low CSF pressure, usually severe fronto-occipital headache which is aggravated in upright position and not relieved by analgesics. The quality of headache and response to therapy is very similar to PDPH. Headache of patients with spontaneous intracranial hypotension disappeared in 2–4 weeks after treatment with oral prednisolone.[11] Other researchers have reported the similar results.[12]
The mechanism by which corticosteroids resolve PDPH and headache of intracranial hypotension has not been elucidated. Patients with headache associated with spontaneous low CSF pressure, present a variable amount of CSF in the spinal extradural space with associated dilated epidural veins in the high cervical portion. CSF hypovolemia, and not the intracranial hypotension, may be the pathogenetic mechanism of this symptom.[13] Steroids may exert their clinical effects by favoring the reabsorption of CSF from the extradural space and thus increase CSF volume.[14] Steroids suppress arachidonic acid production through lipocortin-induced phospholipase inhibition, which ultimately inhibits production of algogenic prostaglandins (PGE2 and PGI2) and leukotrienes (LT-B4).[15] Corticosteroids block production of proinflammatory cytokines such as IL-1, IL-2, and TNF-α.[16] It seems that analgesic action of steroids in PDPH may relate to their anti-inflammatory effects at dural puncture site. During the healing process of dural puncture site, inflammatory mediators those are secreted from immune cells, spread in CSF, stimulate pain receptors, and cause headache. Steroids may suppress production of these algogenic mediators and relieve headache.[15] Since this very short-term use of steroids may have only hypersensitivity reaction like all other drugs[17] and no persisting adverse effects,[18] its administration can be adopted safely.
Quinke needles were used in the subjects of this study (they are mostly used in our country). It was preferred to observe the outcome of PDPH in the prevailing perspective. A limitation of this study was that we did not consider the relation between body mass index and headache and that a wide interpersonal variation in presentations of PDPH was noted.
Conclusion
The efficacy of IV hydrocortisone in reducing the headache following dural puncture has been demonstrated in this study. Large-scale studies are recommended to establish steroid therapy as a standard treatment for PDPH.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rabiul MA, Raheen MR, Iqbal KM, Chowdhury RA. Headache following Spinal Anaesthesia: A Review on Recent Update. J Bangladesh Coll Phys Surg. 2011;29:32–40. [Google Scholar]
- 2.Munnur U, Suresh MS. Backache, headache, and neurologic deficit after regional anesthesia. Anesthesiol Clin North America. 2003;21:71–86. doi: 10.1016/s0889-8537(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull DK, Shepherd DB. Post-dural puncture headache: Pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718–29. doi: 10.1093/bja/aeg231. [DOI] [PubMed] [Google Scholar]
- 4.Vyvey M. Steroids as pain relief adjuvants. Can Fam Physician. 2010;56:1295–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Chohan U, Hamdani GA. Post Dural Puncture Headache. J Pak Med Assoc. 2003;53:246–55. [PubMed] [Google Scholar]
- 6.Gentile S, Giudice RL, Martino PD, Rainero I, Pinessi L. Headache attributed to spontaneous low CSF pressure: report of three cases responsive to corticosteroids. Eur J Neurol. 2004;11:849–51. doi: 10.1111/j.1468-1331.2004.00898.x. [DOI] [PubMed] [Google Scholar]
- 7.Turiel M, Simón MO, Lastra J, Pascual JA. Treatment of post-dural-puncture headache with intravenous cortisone. Rev Esp Anestesiol Reanim. 2002;49:101–4. [PubMed] [Google Scholar]
- 8.Neves JF, Vieira VL, Saldanha RM, Fde A, Neto M, Magalhães MG, Neves MM, Araújo FP. Hydrocortisone treatment and prevent post-dural puncture headache: Case reports. Rev Bras Anestesiol. 2005;55:343–9. doi: 10.1590/s0034-70942005000300011. [DOI] [PubMed] [Google Scholar]
- 9.Ashraf MA, Sadeghi A, Azarbakht Z, Salehi S, Hamediseresht E. Hydrocortisone in post-dural puncture headache. ME J Anesth. 2007;19:415–22. [PubMed] [Google Scholar]
- 10.Hong M, Shah GV, Adams KM, Turner RS, Foster NL. Spontaneous intracranial hypotension causing reversible fronto-temporal dementia. Neurology. 2002;58:1285–7. doi: 10.1212/wnl.58.8.1285. [DOI] [PubMed] [Google Scholar]
- 11.Silberstein SD. Syndrome of spontaneous intracranial hypotension. Cephalalgia. 1999;19:73–4. doi: 10.1046/j.1468-2982.1999.019002073.x. [DOI] [PubMed] [Google Scholar]
- 12.Pascual LF, Santos S, Escalza I, Iñiguez C, Morales-Asín F. Spontaneous intracranial hypotension: Quick clinical and magnetic resonance imaging response to corticosteroids.A case report. Headache. 2002;42:359–61. doi: 10.1046/j.1526-4610.2002.02108.x. [DOI] [PubMed] [Google Scholar]
- 13.David B, Richard B, Lipton, Sait A. Post-Dural Puncture Headache: Diagnosis, Epidemiology, Etiology, and Pathophysiology. Headache. 2010;50:1144–52. doi: 10.1111/j.1526-4610.2010.01699.x. [DOI] [PubMed] [Google Scholar]
- 14.Miyazawa K, Shiga Y, Hasegawa T, Endoh M, Okita N, Higano S, et al. CSF hypovolemia vs.intracranial hypotension in “spontaneous intracranial hypotension syndrome”. Neurology. 2003;60:941–7. doi: 10.1212/01.wnl.0000049933.51044.81. [DOI] [PubMed] [Google Scholar]
- 15.Gilron I. Corticosteroids in postoperative pain management: Future research directions for a multifaceted therapy. Acta Anaesthesiol Scand. 2004;48:1221–2. doi: 10.1111/j.1399-6576.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 16.Elenkov IJ, Chrousos GP. Stress Hormones, Pro-inflammatory and Anti-inflammatory Cytokines, and Autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 17.Graeme PC, Paterson E, Keenan F. An unexpected response to intravenous hydrocortisone succinate in an asthmatic patient. Br J Clin Pharmacol. 2005;60:342. doi: 10.1111/j.1365-2125.2005.02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra R, Jones S. Adjuvant Analgesics in Cancer Pain: A Review. Am J Hosp Palliat Care. 2012;29:70–9. doi: 10.1177/1049909111413256. [DOI] [PubMed] [Google Scholar]


