Abstract
Hunter's syndrome is a member of a group of recessively inherited metabolic disorders termed mucopolysaccharidoses, caused by deficiency of lysosomal enzymes required for degradation of mucopolysaccharides or glycosaminoglycans, leading to accumulation of partially degraded glycosaminoglycans in various tissues. This leads to various anatomical abnormalities and systemic involvement, posing a challenge to an anesthetist. We present the anesthetic management of a 4-year old child with Hunter's disease with anticipated difficult airway, who presented for adenotonsillectomy and repair of umbilical and inguinal hernia.
Keywords: Difficult airway, Hunter's syndrome, mucopolysaccharidoses, pediatric patient
Introduction
Hunter's syndrome is a X linked recessive disorder, characterized by deficiency of lysosomal enzymes required for degradation of mucopolysaccharides or glycosaminoglycans, leading to systemic involvement and various anatomical abnormalities. Patients usually present with difficult airway because of MPS tissue infiltration in the upper and middle airways. We present our experience of managing anticipated difficult airway of a known case of Hunters syndrome.
Case Report
A 4-year-old, 14 kg child, diagnosed case of Hunter's syndrome was scheduled for surgery. He had history of breathlessness, easy fatiguability, mouth breathing, snoring, episodes of sleep apnea, and hearing loss. There were episodes of cyanosis and loss of consciousness in past one year. His milestones were delayed. There was history of repeated chest infections needing hospitalization. Examination revealed a short-statured child with normal intelligence. Respiratory and cardiovascular systems were unremarkable. He had clover shaped skull, coarse facies, macroglossia, receding mandible, and a short neck, with restricted neck movements [Figure 1]. There were generalized stiff joints, particularly shoulder joints. Mouth opening and Mallampatti grading could not be assessed as the child was uncooperative. Preoperative laboratory tests including hemotology and urinalysis were normal. Ultrasound of abdomen showed mild hepatomegaly. Echocardiography was unremarkable. High risk consent was taken in view of difficult airway and in anticipation of respiratory problems in postoperative period.
Figure 1.
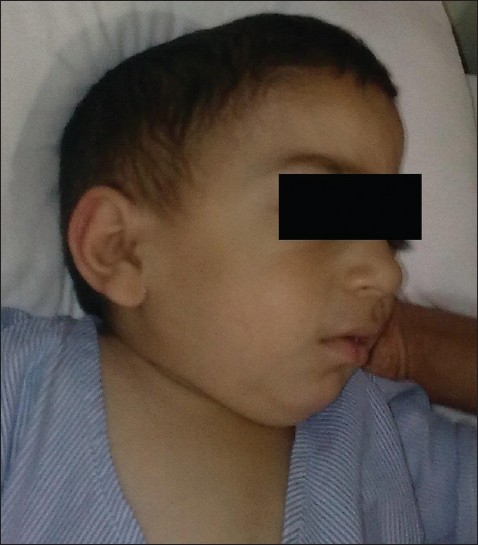
Child with Hunter's syndrome
In the operation theatre, difficult airway cart was prepared, which included different sizes of endotracheal tubes (ETT), laryngeal mask airways (LMA), and laryngoscope blades (both Macintosh and Miller). Basic monitors (electrocardiogram, noninvasive blood pressure, and pulse oximeter) were attached, and intravenous (IV) access was obtained. Midazolam 0.5 mg, glycopyrrolate 0.15 mg, and ketamine 15 mg IV were given for induction of anesthesia. Anesthesia was deepened with 1.5–2% sevoflurane in 100% oxygen, titrated to maintain spontaneous respiration. SpO2 and EtCO2 were constantly monitored. Check laryngoscopy was done with Macintosh blade, which revealed large tongue, hypertrophied palate and pharyngeal structures, and Cormack Lehane grade IV. Laryngeal and pharyngeal structures were non-compliant and intra-oral space was limited. LMA size 2.0 was inserted and ventilation continued with LMA. A trial of tracheal intubation was planned after initial check ventilation with LMA before and after giving muscle relaxant. Succinylcholine 30 mg IV was given. We were able to ventilate the lungs adequately through the LMA after giving relaxant. Then, we removed the LMA and tracheal intubation was attempted. The grade of laryngoscopy changed from grade IV to grade III after giving muscle relaxant. Tracheal intubation with 4.0 mm ETT was not successful. Mask ventilation was possible in between the intubation attempts and the relaxation was maintained with a repeat dose of succinylcholine. With the use of Miller blade and stylet, tracheal intubation was successful with a 3.5 mm ID ETT, in the third attempt. ETT was fixed at 12 cm mark at angle of mouth. During this whole period, the SpO2 remained above 95%. Dexamethasone 2 mg IV was given because of prolonged airway handling.
Anesthesia was maintained with 1% sevoflurane in oxygen-nitrous oxide mixture (50:50) and intermittent boluses of atracurium. Patient was put on pressure-controlled ventilation, as airway pressures were high due to the small ETT. Peak airway pressures around 25 mmHg and respiratory rate of 16/min were kept to achieve EtCO2 between 32 and 35 mmHg. For analgesia, fentanyl 30 mcg and paracetamol 200 mg IV were given. The umbilical and inguinal hernia surgeries were successfully done, but the ENT surgeon could only do adenoidectomy and right tonsillectomy, due to difficulty in negotiating his instruments in narrow oral cavity.
Caudal block with 7 ml of 0.25% bupivacaine was given before tracheal extubation for postoperative analgesia. Trachea was extubated on table after reversal of neuromuscular blockade with neostigmine 0.7 mg with glycopyrrolate 0.15 mg IV, in lateral position after ensuring adequate respiration, with the child fully awake and responding to verbal commands. Postoperatively, the child was observed in a high dependency unit for 24 h. Paracetamol 200 mg IV was given every 6 hourly for postoperative analgesia. Child remained comfortable and pain free and did not have any respiratory difficulty.
Discussion
Hunter's syndrome (mucopolysaccharidoses type II) is an X-linked recessive disorder that primarily affects males and is characterized by deficiency of lysosomal enzymes required for degradation of mucopolysaccharides (MPS) or glycosaminoglycans. The chronic progressive course is caused by the accumulation of partially degraded GAG's, causing thickening of tissue and compromising cell and organ function over time. Disease presentation is usually between 2 and 4 years of age. Presentation may include coarse facies, short stature, skeletal deformities, joint stiffness, and mental retardation. Hepatomegaly, neurological involvement, cardiac valvular defects, myocardial disease, and carpal tunnel syndrome are other features of the syndrome. Presence of urine MPS is used as a screening test and the diagnosis is confirmed by direct enzymatic (iduronate-2-sulfatase) assay in leukocytes or fibroblasts.
Patients usually present with difficult airway because of MPS tissue infiltration in the upper and middle airways, limited TM joint movement, macroglossia, and frequent upper respiratory tract infections. Cardiac, liver and renal functions also may be deranged. Patients present for surgical interventions like adenotonsillectomy, chronic hydrocephalus, nerve entrapment (carpal tunnel syndrome), abdominal wall hernias, tracheostomy, and joint contractures.
Local or regional anesthetic techniques are unsuitable as the sole form of anesthesia in these young children. Regional techniques may fail because of deposition of MPS in the nervous system, which have direct toxic effects on the neurons.[1] Inhalational induction has been used as a preferred technique because of potential for airway obstruction.[2] IV induction with ketamine or low-dose thiopentone to maintain spontaneous ventilation, until definite airway control is achieved, has been used in uncooperative patients.[3] We used ketamine for induction and supplemented it with sevoflurane, maintaining spontaneous respiration.
Inability to ventilate the lungs after giving muscle relaxant has been reported in patients though it could be ventilated with facemask before giving muscle relaxant. We ensured adequate ventilation with classic LMA before giving muscle relaxant. Moreover, we used a short-acting muscle relaxant-succinylcholine, even after establishment of ventilation with LMA, as patients can have obstruction at laryngeal and subglottic levels. There are reports of tracheobronchomalacia with major airway collapse in these patients.[4]
In anticipation of airway narrowing due to MPS deposition, we first tried a 4 mm ID ETT, though 4.5 ID mm was the age appropriate sized ETT for this patient. Tracheal intubation was possible with a 3.5 mm ID ETT with no air leak, which shows that there was airway narrowing in our patient.
Walker et al. found the use of LMA to be extremely useful both to provide a secure airway for short procedures and also to clear the obstructed airway in many cases of mucopolysaccharidoses.[5] Chen CH et al. successfully resuscitated a 16-year old patient of mucopolysaccharidoses with LMA, after failed intubation.[6] However, cases have been reported where LMA was unsuccessful in managing airway obstruction.[7]
Flexible fiberoptic intubation is an option for relatively older children or adults, but our center does not have a pediatric bronchoscope. There are reports of use of airway scope for managing difficult airway in these patients, which may provide better view of glottis but difficulty to negotiate it still remains because of narrow oral cavity.[8] In an emergency situation, tracheostomy may be the choice to secure the airway. Cricothyroidotomy is not recommended for MPS patients whose cricothyroid membrane, cricoid and thyroid cartilages are often thickened and deformed by MPS deposits, making rapid dissection difficult and vocal cord damage likely.[3]
Patients with Hunter's syndrome have a reported perioperative complication rate of 50%.[2] The overall incidence of difficult intubation in MPS has been reported to be 25% and that of failed intubation as 8%.[5] In Hunter's syndrome, the incidence of airway related problems such as narrowing of airway, laryngomalacia, copious secretions, and pulmonary dysfunction has been reported to be as high as 53% because of restrictive lung disease, thoracic dystrophy, pulmonary hypertension, and obstructive sleep apnea.[2]
To conclude, in patients with Hunter's syndrome, a careful consideration of the risks versus benefits involved in doing the surgery, and a careful induction by an experienced anesthesiologist with anticipation of a difficult airway, is recommended.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Vas L. Preanaesthetic evaluation and premedication in Paediatrics. Indian J Anaesth. 2004;48:347–53. [Google Scholar]
- 2.Herrick LA, Rhine EJ. The Mucopolysaccharidoses and anaesthesia: A report of clinical experience. Can J Anaesth. 1988;35:67–73. doi: 10.1007/BF03010548. [DOI] [PubMed] [Google Scholar]
- 3.Diaz JH, Belani KG. Perioperative management of children with Mucopolysaccharidoses. Anesth Analg. 1993;77:1261–70. doi: 10.1213/00000539-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Morehead JM, Parsons DS. Tracheobronchomalacia in Hunter's syndrome. Int J Pediatr Otorhinolaryngol. 1993;26:255–61. doi: 10.1016/0165-5876(93)90096-l. [DOI] [PubMed] [Google Scholar]
- 5.Walker RW, Darowski M, Morris P, Wraith JE. Anaesthesia and mucopolysaccharidoses. A review of airway problems in children. Anaesthesia. 1994;49:1078–84. doi: 10.1111/j.1365-2044.1994.tb04360.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Huang GS, Lee CK, Huang YS, Wong CS. Use of Laryngeal Mask airway for the resuscitation of a Hunter Syndrome patient during general anaesthesia induction. J Med Sci. 2003;23:351–54. [Google Scholar]
- 7.Busoni P, Fognani G. Failure of the laryngeal mask to secure the airway in a patient with Hunter's syndrome (mucopolysaccharidosis type II) Pediatr Anaesth. 1999;9:153–55. doi: 10.1046/j.1460-9592.1999.9220289.x. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi S, Kusunoki S, Fukuda H, Hamada H, Kawamoto M. Difficult tracheal intubation using airway scope in a pediatric patient with Hunter syndrome. Masui. 2009;58:1278–81. [PubMed] [Google Scholar]


