Abstract
A case of endophthalmitis following uneventful phacoemulsification and posterior chamber intraocular lens (IOL) implantation in a 77-year-old diabetic man was culture-positive for Enterococcus faecalis. After successful treatment with intravitreal, topical, and systemic antibiotic agents, the infection seemed to clear and the patient achieved a corrected visual acuity of 20/25. Four months after the initial presentation, the patient again developed signs and symptoms of endophthalmitis, with regrowth of E faecalis. The antibiotic therapy was repeated. One month later, the IOL was removed surgically and found to harbor a biofilm of the strain demonstrated by DNA analysis. The microbiologic and DNA analyses support that a biofilm on an IOL could be a vector for a cause of recurrent endophthalmitis. Intraocular lens exchange in cases of postoperative endophthalmitis caused by E faecalis may be considered to decrease the risk for recurrent infection.
Endophthalmitis caused by Enterococcus faecalis is an infrequent and unusually virulent infection,1,2 with a typically poor visual prognosis even with aggressive treatment. Recurrent E faecalis endophthalmitis after presumed clearing of the infection has been described.3–5 We present a case of late recurrent postoperative endophthalmitis caused by E faecalis. We have included microbiologic, microscopic, and DNA analyses as supporting evidence that the same strain of E faecalis caused both occurrences of endophthalmitis and survived therapy through the formation of a bacterial biofilm on an IOL.
CASE REPORT
A 77-year-old man with a history of well-controlled type II diabetes mellitus without retinopathy had uneventful phacoemulsification in the left eye at the Veterans Administration Pittsburgh Healthcare System (VAPHS) hospital. A standard IOL (SN60WF, Alcon Laboratories, Inc.) was implanted in the intact capsular bag. Although preoperative moxifloxacin was administered, no intracameral antibiotic agents were given at the time of surgery because of the surgeon’s preference.
On postoperative day 1, the uncorrected visual acuity was 20/80, the intraocular pressure was 14 mm Hg, and the cornea showed 2+ to 3+ Descemet membrane folds. The red reflex was intact, and the patient denied any pain or discomfort. He was started on the surgeon’s standard postoperative regimen of moxifloxacin 4 times daily and prednisolone acetate 1% every 2 hours. On postoperative day 3, the patient called the surgeon to say his vision had become increasingly blurred since the surgery and he was encouraged to return to the clinic. Because he lived 4 hours away, the patient did not present to the clinic until the morning of postoperative day 4. At the time of this examination, the visual acuity had decreased to hand motions (HM) and the examination demonstrated a classic picture of acute postoperative endophthalmitis. There was a 1.2 mm hypopyon and 4+ cells in the anterior chamber. The wound was Seidel negative. After evaluation, anterior chamber tap, and administration of intravitreal vancomycin (1 mg/0.1 mL) and ceftazidime (2.25 mg/0.1 mL), the patient was admitted to the VAPHS for frequent monitoring and frequent administration of topical antibiotic agents. Oral moxifloxacin was also administered. A transpars plana vitreous needle tap was attempted at presentation, but an adequate specimen could not be obtained safely.
The anterior chamber was culture positive for a light amount of E faecalis (E616) on trypticase soy agar medium supplemented with 5% sheep’s blood (TSA), chocolate agar, and enriched thioglycolate liquid media. The bacteria appeared as white shiny 1.0 mm colonies with no hemolysis on TSA. Gram-positive coccoids were observed on Gram stain, catalase testing was minimally positive, and testing for pyrrolidonyl peptidase activity was positive. Kirby-Bauer disk diffusion antibiotic susceptibility determined susceptibility to vancomycin, ampicillin, gatifloxacin, and moxifloxacin.
The patient was monitored daily while admitted and was discharged home on postoperative day 11. Throughout his hospital course, he received 2 additional intravitreal injections of vancomycin and 1 injection of preservative-free triamcinolone, as well as topical fortified vancomycin eye-drops every 1 to 2 hours. At home, he continued treatment with topical antibiotic and steroid agents. By postoperative week 6, the corrected visual acuity was 20/25 and the anterior chamber and vitreous were quiet. The view to the posterior pole was clear, but some vitreous debris that was not visually significant remained.
At postoperative week 17, the patient presented to the clinic with complaints of worsening vision and eye pain in the operative eye that began the previous evening. The visual acuity was HM, with Descemet membrane folds in the cornea and a 1.0 mm hypopyon. The patient again had evaluation, anterior chamber tap, and intravitreal injections of vancomycin and ceftazidime by a retina specialist, as well as admission to the VAPHS for frequent monitoring and antibiotic treatment. Repeat intravitreal vancomycin injections were performed twice, and the patient received frequent topical antibiotic and steroid agents in addition to oral moxifloxacin and prednisone (Figure 1). The anterior chamber culture was again culture-positive for E faecalis (E623) but only in the enriched thioglycolate liquid medium. Laboratory studies including the antibiotic susceptibilities indicated that the E faecalis isolate was identical to the previously isolated E faecalis. The patient was discharged home on his fourth day of admission on topical vancomycin eyedrops and prednisolone eyedrops. The anterior inflammation gradually cleared, but the vision remained poor.
Figure 1.
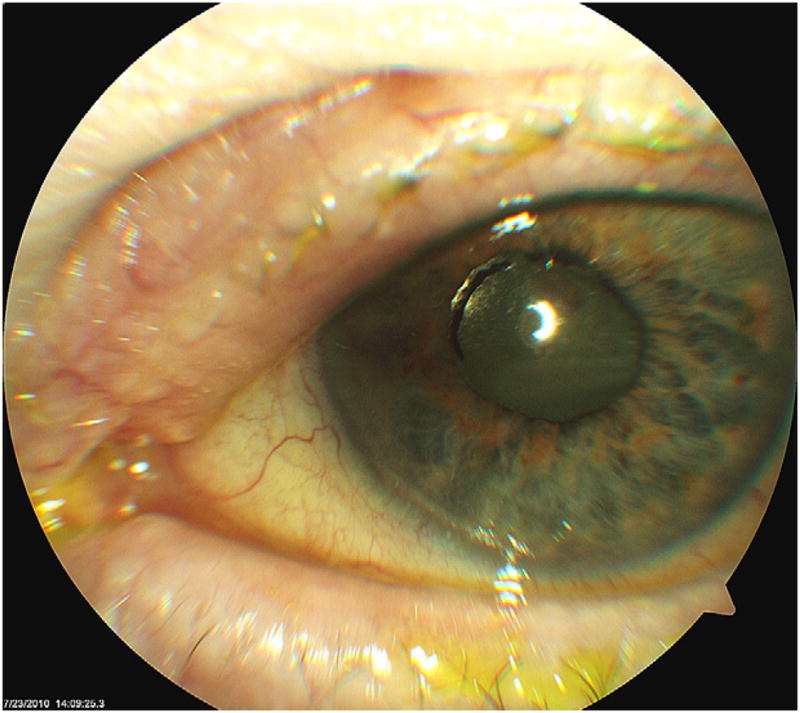
Clinical photograph of the patient’s left eye at postoperative week 19. Most of the anterior chamber inflammation has resolved after treatment of the recurrent E faecalis endophthalmitis, but the white plaque on the posterior surface of the IOL could represent a bacterial biofilm.
On postoperative week 21, the patient had synechialysis, IOL exchange with explantation of the original posterior chamber IOL, removal of the capsular bag, placement of an anterior chamber IOL, and anterior vitrectomy. Unfortunately, the patient’s vision did not have as favorable an outcome after the recurrence of endophthalmitis. After the infection had cleared and the patient recovered from the IOL exchange surgery, the vision remained at the counting fingers level. The examination demonstrated severe retinal hemorrhages in all quadrants without significant ocular ischemia on fluorescein angiography, and macular and foveal atrophy were seen on optical coherence tomography, which appeared to limit the final visual outcome.
Laboratory Analyses
After the posterior chamber IOL was removed from the capsular bag, it was placed in enriched thioglycolate liquid medium, as positive growth in enriched thioglycolate liquid medium would pinpoint the location of growth on the IOL. Three months later, a thin layer of turbidity was observed on the IOL. The IOL was carefully touched with a soft-tipped applicator and cultured aerobically on TSA and chocolate agar media and anaerobically on chocolate agar incubated in an anaerobic bag. The material obtained from the soft-tipped applicator was positive for Gram-variable pleomorphic coccoids, some in short chains, on Gram stain (Figure 2, A). Growth was positive 24 hours later on chocolate agar medium but not on TSA. Growth was subsequently passed on TSA. Colony morphology and biochemical testing were consistent for the identification of E faecalis (E633). Susceptibility of E633 to vancomycin, ampicillin, gatifloxacin, and moxifloxacin was the same as that of the previous 2 isolates (E616, E623).
Figure 2.
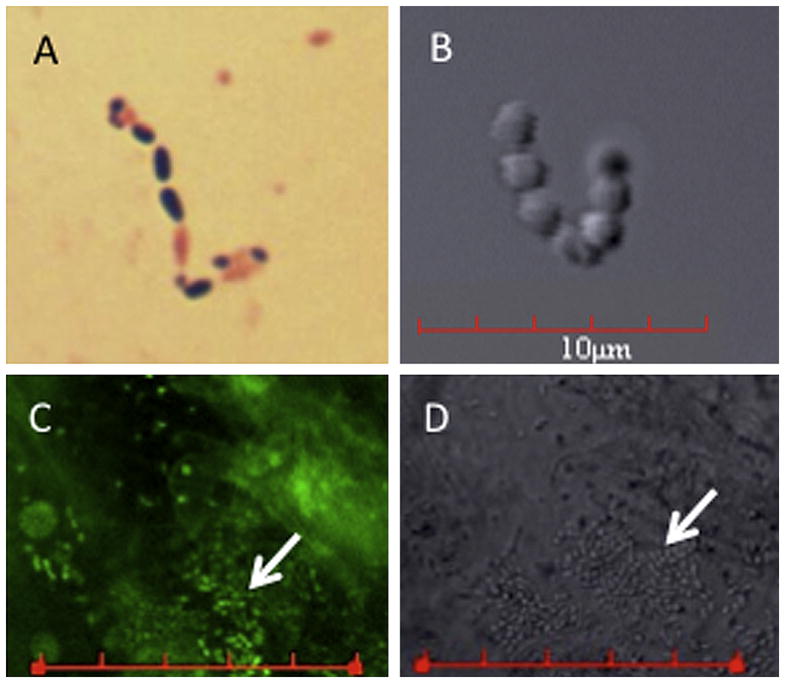
Microscopic analysis of Enterococcus isolates. A: Gram stain of the E633 E faecalis isolate (IOL isolate from this case). B: Differential interference contrast photomicrograph taken of IOL showing coccus-shaped bacteria. C: Confocal laser scanning microscopic image of the removed IOL that had been incubated with a DNA-fluorescent dye. D: The differential interference contrast image of the same region of the IOL shown in C. These data support that strain E633 formed a biofilm on the IOL (bars in C and D = 30 μm; arrows indicate bacterial biofilm).
The antibiotic susceptibility patterns of the 3 isolated bacteria indicated that they were the same strain. To confirm that the isolates were the identical strain, random amplified polymorphic DNA typing (RAPD) analysis using 2 different primer sets and conditions was performed. Both primer sets showed that E616, E623, and E633 exhibited the identical RAPD patterns, whereas 3 other Enterococcus endophthalmitis isolates and 1 Serratia marcescens strain (negative control) showed highly different patterns (Figure 3). Together these data support that the E616, E623, and E633 isolates were the same strain isolated at different times.
Figure 3.
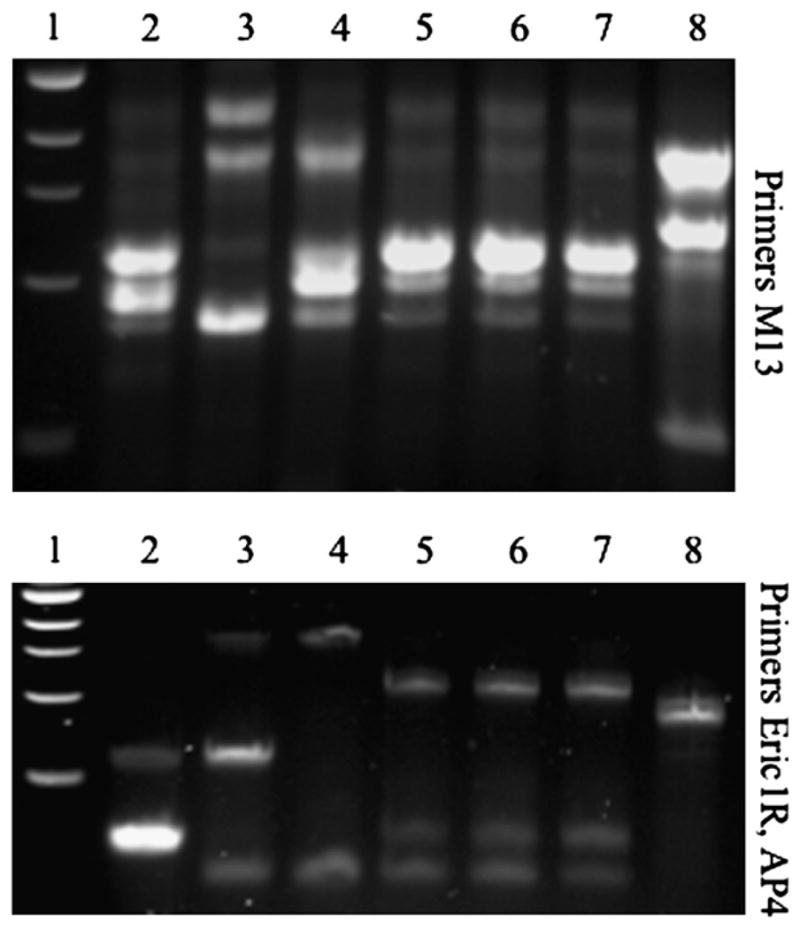
The RAPD DNA analysis of Enterococcus isolates. The RAPD analysis was used to differentiate strains at the genetic level using previously published methods.14 Two different primer sets were used to increase our ability to differentiate strains. The top panel was done using primer M1315 and the bottom panel using primers ERIC1r and AP4.14 Lane 1: 1 KB DNA ladder (New England Biolabs). Lanes 2 to 4: Previously isolated Enterococcus isolates from endophthalmitis patients different from this case serve as controls. Lane 5: E616 E faecalis DNA (first isolate from this case). Lane 6: E623 E faecalis DNA (second isolate from this case). Lane 7: E633 E faecalis DNA (third isolate from this case). Lane 8: Serratia marcescens DNA serves as a non-enterococci control. These data support that E616, E623, and E633 are the same strain.
The IOL was stained with Syto-9 (Invitrogen Corp.) to visualize the presence of bacteria using differential interference contrast and fluorescent confocal laser scanning microscopy with an Fluoview 1000 microscope and Fluo-view 2.1 software (Olympus). Figure 2, B, shows coccoid bacteria consistent with the expected morphology of enterococci. Evidence of bacterial colonization and biofilm formation was observed on the surface of the IOL (Figure 2, C and D). Bacterial biofilms are bacteria living on a biotic or abiotic surface and are commonly associated with device-related infections.
DISCUSSION
Postoperative endophthalmitis occurs in approximately 0.05% to 0.33% of patients after cataract surgery.6–8 Most cases are caused by gram-positive bacteria, with coagulase-negative Staphylococcus causing the majority of these.9 Other pathogens include Staphylococcus aureus, streptococci, enterococci, and gram-positive rods. In the Endophthalmitis Vitrectomy Study,10 4 of 323 (1.2%) culture-positive postoperative endophthalmitis specimens were identified as E faecalis. However, a study done in Japan found E faecalis to be the third most commonly isolated pathogen, accounting for 17% of culture-positive endophthalmitis.11 Enterococcus faecalis is known to be a virulent pathogen associated with endophthalmitis. The visual prognosis of E faecalis endophthalmitis is generally very poor, with almost 50% of final visual outcomes light perception to no light perception and another 35%, 5/200 to HM.12 Visual acuity at presentation is an important factor in determining management in postoperative endophthalmitis. In this case, the decision to treat with antibiotic agents rather than a vitrectomy was based on the results of the Endophthalmitis Vitrectomy Study, which demonstrated that there is no benefit to an immediate vitrectomy in patients with postoperative endophthalmitis who have HM vision.13 There are reports of recurrent endophthalmitis from E faecalis,3–5 but the pathogenesis for recurrence remains unknown.
It should be noted that E faecalis is not a common contaminant of the ocular surface and is an infrequent etiological agent of endophthalmitis. Although the amount of growth was not abundant, it is highly unlikely that the presence of E faecalis from 3 separate intraocular cultures over a 5-month period from 1 patient was coincidental and not significant.
The primer sets used in the RAPD analysis have been described.14,15 This analysis along with the phenotypic analysis suggest that the same E faecalis strain caused each endophthalmitis episode. To our knowledge, this is the first example of molecular evidence for a recurrent endophthalmitis being caused by the same strain. One scenario for this outcome is that all bacteria were eliminated from the first infection by a combination of antibiotic therapy and the immune system, followed by a second independent infection by the same organism. While the serial infection hypothesis is formally possible, we think it is unlikely given the rarity of endophthalmitis in general and that the Enterococcus genus is a relatively infrequent etiological agent of endophthalmitis.
An alternative hypothesis is that there was a single infection that was incompletely resolved by therapy and the bacteria that survived the initial therapy and immune response slowly grew and reached a sufficient number or other physiological state to again induce an inflammatory response. If this second hypothesis is true, some of the infecting Enterococcus bacteria must have evaded the antibiotic agents, possibly by developing antibiotic resistance. However, our study showed that the 3 isolates of this E faecalis strain were equally susceptible to vancomycin and other agents used for this patient, suggesting that the organism had not mutated to a drug-resistant state. Other than gaining DNA, such as a plasmid, that confers antibiotic resistance or mutation that alters the target site of an antibiotic agent, bacteria can tolerate very high levels of antibiotic agents when they grow in a biofilm. Several active and passive mechanisms by which biofilms confer resistance were recently reviewed by Anderson and O’Toole.16 When bacteria are removed from biofilms, they lose the high antibiotic tolerance, consistent with our in vitro antibiotic susceptibility testing.
Bacterial biofilms are associated with antibiotic-resistant, chronic, recurrent, and implant-related infections. The quiet nature of this infection was a hint that the patient had a biofilm-related infection. Typically, bacteria in biofilms are less pathogenic than their non-biofilm counterparts, difficult to culture, and able to cause chronic infections.17 However, bacteria shed from the biofilm can revert to their more pathogenic phase, explaining the time lapse between virulent infections. In this case, we observed a biofilm on the IOL. One caveat is that the IOL was observed long after it was removed from the eye, so the biofilm could have formed after the IOL was removed. Biofilms can form on tissue as well as foreign-body devices. Although we did observe bacteria on the surface of the IOL, the bacteria could have existed as a biofilm or another state in the capsular bag as well. However, given the history of this case and our findings, the recurrent endophthalmitis by an antibiotic-susceptible organism is likely the result of an Enterococcus biofilm that developed on the IOL.
Given the virulence and biofilm growth of E faecalis on the IOL, we suggest close monitoring for recurrent endophthalmitis. Intraocular lens exchange and possible removal of the capsular bag might offer a superior outcome and reduce the incidence of recurrent endophthalmitis in these cases.
Acknowledgments
Support received from the Charles T. Campbell Foundation; Pennsylvania Lions Club; core grant for Vision Research, National Institutes of Health EY 08098; Research to Prevent Blindness Career Development Award (R.M.Q. Shanks); National Institutes of Health AI085570 (R.M.Q. Shanks); Research to Prevent Blindness; and the Pittsburgh Eye and Ear Foundation.
Footnotes
Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
References
- 1.Rishi E, Rishi P, Nandi K, Shroff D, Therese KL. Endophthalmitis caused by Enterococcus faecalis; a case series. Retina. 2009;29:214–217. doi: 10.1097/IAE.0b013e31818eccc7. [DOI] [PubMed] [Google Scholar]
- 2.Chen K-J, Lai C-C, Sun M-H, Chen T-L, Yang K-J, Kuo Y-H, Chao A-N, Wu W-C. Postcataract endophthalmitis caused by Enterococcus faecalis. Ocul Immunol Inflamm. 2009;17:364–369. doi: 10.3109/09273940903105110. [DOI] [PubMed] [Google Scholar]
- 3.Forestier F, Salvanet-Bouccara A, Mohand-Said M, Dublanchet A, Emond JP. Endophtalmie aiguë récurrente à Enterococcus faecalis après chirurgie de la cataracte: un cas à évolution favorable [Recurrent acute Enterococcal faecalis endophthalmitis following cataract surgery: a case report with a favorable outcome] [Accessed April 1, 2011];J Fr Ophtalmol. 2006 29:181–183. doi: 10.1016/s0181-5512(06)73768-8. Available at: http://www.em-consulte.com/showarticlefile/113174/index.pdf. [DOI] [PubMed] [Google Scholar]
- 4.Teoh SCB, Lee J-J, Chee CKL, Au Eong K-G. Recurrent Enterococcus faecalis endophthalmitis after phacoemulsification. J Cataract Refract Surg. 2005;31:622–626. doi: 10.1016/j.jcrs.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 5.Nasrallah FP, Desai SA. Recurrent enterococcal endophthalmitis following cataract surgery: a case report. Ophthalmic Surg Lasers. 1999;30:481–482. [PubMed] [Google Scholar]
- 6.ESCRS Endophthalmitis Study Group. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978–988. doi: 10.1016/j.jcrs.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Wong TY, Chee S-P. The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology. 2004;111:699–705. doi: 10.1016/j.ophtha.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz S, Dick HB, Krummenauer F, Pfeiffer N. Endophthalmitis in cataract surgery; results of a German survey. Ophthalmology. 1999;106:1869–1877. doi: 10.1016/S0161-6420(99)90395-0. [DOI] [PubMed] [Google Scholar]
- 9.Callegan MC, Gilmore MS, Gregory M, Ramaden RT, Wiskur BJ, Moyer AL, Hunt JJ, Novosad BD. Bacterial endophthalmitis: therapeutic challenges and host-pathogen interactions. Prog Retin Eye Res. 2007;26:189–203. doi: 10.1016/j.preteyeres.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, Kelsey SF. Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study; the Endophthalmitis Vitrectomy Study Group. Am J Ophthalmol. 1996;122:1–17. doi: 10.1016/s0002-9394(14)71959-2. correction, 920. [DOI] [PubMed] [Google Scholar]
- 11.Hara J. Relation between causative organism and inflammation onset in pseudophakic endophthalmitis following cataract surgery [Japanese] Atarashii Ganka. 2003;20:657–660. [Google Scholar]
- 12.Scott IU, Loo RH, Flynn HW, Jr, Miller D. Endophthalmitis caused by Enterococcus faecalis; antibiotic selection and treatment outcomes. Ophthalmology. 2003;110:1573–1577. doi: 10.1016/S0161-6420(03)00502-5. [DOI] [PubMed] [Google Scholar]
- 13.Endophthalmitis Vitrectomy Study Group. Results of the Endophthalmitis Vitrectomy Study; a randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. [Accessed April 1, 2011];Arch Ophthalmol. 1995 113:1479–1496. Available at: http://archopht.ama-assn.org/cgi/reprint/113/12/1479. [PubMed] [Google Scholar]
- 14.Barbier N, Saulnier P, Chachaty E, Dumontier S, Andremont A. Random amplified polymorphic DNA typing versus pulsed-field gel electrophoresis for epidemiological typing of vancomycin-resistant Enterococci. [Accessed April 1, 2011];J Clin Microbiol. 1996 34:1096–1099. doi: 10.1128/jcm.34.5.1096-1099.1996. Available at: http://jcm.asm.org/cgi/reprint/34/5/1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mannu L, Paba A, Pes M, Floris R, Scintu MF, Morelli L. Strain typing among enterococci isolated from home-made Pecorino Sardo cheese. [Accessed April 1, 2011];FEMS Microbiol Lett. 1999 170:25–30. doi: 10.1111/j.1574-6968.1999.tb13351.x. Available at: http://onlinelibrary.wiley.com/doi/10.1111/j.1574-6968.1999.tb13351.x/pdf. [DOI] [PubMed] [Google Scholar]
- 16.Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa S, Kuchma SL, O’Toole GA. Keeping their options open: acute versus persistent infections. [Accessed April 1, 2011];J Bacteriol. 2006 188:1211–1217. doi: 10.1128/JB.188.4.1211-1217.2006. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1367219/pdf/1105-05.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]


