Abstract
Aims and Objectives:
To analyze testicular histopathology in men diagnosed as idiopathic obstructive azoospermia (IOA) based on normal spermatogenesis on fine needle aspiration cytology (FNAC) but with absence of sperms in the epididymis during surgical exploration.
Materials and Methods:
Men presenting with infertility due to IOA during the study period from July 2008 to July 2010 were prospectively evaluated. Clinical examination, semen analysis, serum follicle stimulating hormone (FSH), and testicular FNAC were done. Men with normal volume azoospermia with normal spermatogenesis on FNAC and palpable vas were offered scrotal exploration for microsurgical vasoepididymal anastomosis (VEA). Patients in whom reconstruction was not feasible intraoperatively were analyzed for the causes of failure. The FNAC, FSH and biopsy of these patients were compared.
Results:
77 men fulfilled the inclusion criteria. In 38 men, sperm was present in the epididymal fluid and VEA was performed. In 39 men, reconstruction was not feasible. Thirty-four of these 39 men had normal FSH and testicular volume. In 5 of these 39 men, serum FSH was high (mean 17.48 mIU/ml) and testes were small in size (mean volume 14.5 ml). Testicular biopsy in two of these five men showed patchy areas of atrophy, while the other three men had hyalinized seminiferous tubules with thickened basement membrane, maturation arrest and normal spermatogenesis, respectively.
Conclusion:
FNAC was discordant with histopathological examination (HPE) in four out of five patients of negative surgical exploration with raised FSH. Therefore, among men with idiopathic azoospermia, only those with both normal FSH and normal FNAC should be diagnosed as obstructive azoospermia and explored.
Keywords: Fine needle aspiration cytology, follicle stimulating hormone, infertility, obstructive azoospermia
INTRODUCTION
Azoospermia is the cause of infertility in about 15% of men. Ductal obstruction is responsible for about 40% of azoospermia cases.[1] Obstructive azoospermia due to an anatomical block in the epididymis or the vas deferens is a surgically correctable cause of male infertility and has a good outcome. Patients with idiopathic obstructive azoospermia (IOA) have normal semen volume, testicular size, presence of the vas deferens, normal levels of serum follicle stimulating hormone (FSH) and histological evidence of normal spermatogenesis. Since patients with non-obstructive azoospermia (NOA) do not benefit from surgery, it is recommended that normal testicular spermatogenesis and thus an obstructive etiology for azoospermia be established before surgery.[2]
Open testicular biopsy had been the standard method for preoperative assessment of spermatogenesis but is now being largely replaced by fine needle aspiration cytology (FNAC) of the testis, which is minimally invasive.[3] FNAC serves as a screening method for the presence or absence of sperm. The correlation between FNAC and biopsy of the testis has been reported to be 91.9%.[4]
There are occasions where azoospermic patients have high FSH levels and are clinically NOA but FNAC shows the presence of normal spermatogenesis. This discordance between clinical and histology information results in a dilemma about the etiology of azoospermia and feasibility of surgical reconstruction. Since surgical reconstruction – if feasible – is preferred over in vitro fertilization (IVF), we explore such patients for performing vasoepididymal anastomosis (VEA). The outcomes in these patients are reported in this study.
MATERIALS AND METHODS
All men presenting with infertility due to IOA during the study period from July 2008 to July 2010 were prospectively evaluated. A detailed clinical examination was done to note the secondary sexual characteristics, testicular size, presence of the vas, epididymal fullness and presence of varicocele. Testicular size was measured using Prader orchiometer. At least two semen samples, 4 weeks apart, were obtained from each patient to confirm normal semen volume, presence of fructose and absolute azoospermia. Serum FSH was estimated using microparticle enzyme immunoassay. Bilateral testicular FNAC was done under local infiltrative anesthesia. FNAC was done using a 23-G needle on 10 ml syringe, which was passed multiple times into the testicular tissue through a single skin puncture. The aspirate was considered as adequate when at least 2000 cells or 100 clusters of 20 cells each were obtained. The aspirate was air-dried on slides and stained with the May–Grunwald–Giemsa stain for microscopic examination. Mature spermatozoa were characterized on the basis of identification of the sperm head with cap. The identification of a tail would have required for the majority of mature spermatazoa. But in the right milieu, the sperm heads without visible tail were also characterized as mature sperms.
Men with normal volume azoospermia with normal spermatogenesis on FNAC and palpable vas were offered scrotal exploration for microsurgical VEA. Those patients who had ejaculatory duct obstruction/Congenital bilateral absence of vas deferens as a cause of obstructive azoospermia, presence of varicocele and those who had a history of previous vasoepididymal reconstructive surgery were excluded.
In patients opting for surgery, scrotal exploration for the possibility of reconstructive surgery was done under general anesthesia after taking informed consent. The testes were exposed through a scrotal incision and the epididymis was examined under an operating microscope to look for dilated tubules. Any epididymal fluid present was examined under a light microscope for the presence of sperm. In the presence of sperm in epididymal fluid, VEA was performed using a two-suture microsurgical intussusception technique with longitudinal suture placement.[5] In the absence of sperm in the epididymal fluid even up to the caput, a biopsy of the testis was taken and preserved in Bouin's fluid for histopathological examination (HPE).
Patients in whom reconstruction was not possible on surgical exploration were analyzed for the causes of failure of reconstruction. The FNAC, serum FSH and biopsy of these patients were compared.
RESULTS
Clinical and Intraoperative Findings
During the study period, 77 men fulfilled the inclusion criteria and were taken for scrotal exploration for microsurgical reconstruction.
In 38 men, sperm was present in the epididymal fluid and microscopic VEA was performed using our previously described technique.[5] Their mean FSH levels were 5.96 mIU/ml (range 1.75–11.28 mIU/ml) and testicular volume was 22.25 ml (range 10–30 ml).
In 39 men (50.64%), reconstruction could not be performed due to either dense adhesions precluding identification of epididymal tubules or collapsed tubules with no fluid or sperm even till the caput. Thirty-four of these 39 men had normal FSH (range 2.24–10.34 mIU/ml, mean 6.62 mIU/ml) and normal testicular volume (range 15–25 ml, mean 20.45 ml). In the remaining 5 of these 39 men, serum FSH was elevated (range 13.67–22.61 mIU/ml, mean 17.48 mIU/ml) [Table 1] with mean testicular size being 14.5 ml (range 5-20 ml). These five patients had collapsed epididymal tubules with no evidence of sperms in the epididymal fluid. Reconstruction was therefore not attempted.
Table 1.
Clinical and biochemical parameters of patients
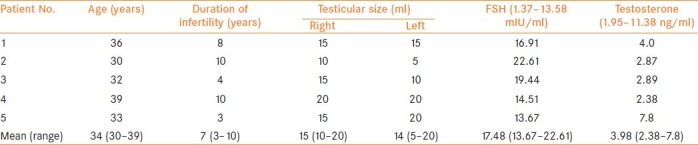
None of the 77 patients had any history of vasectomy. Out of 39 patients in whom reconstruction was not feasible, 9 had a previous hydrocelectomy, 2 had pyocele and 17 were from endemic filariasis regions. In all of these 39 patients, testicular biopsy was taken. Out of 38 successful reconstructions, 4 patients had H/O hydrocelectomy and 5 patients were from endemic filariasis region. Rest of the 29 patients had idiopathic etiology for obstruction.
The FNAC, intraoperative findings and histopathology of all the five patients were analyzed [Table 2]. While all five patients with a high FSH had normal spermatogenesis on preoperative FNAC [Figure 1a], biopsies in two of them showed patchy areas of atrophy and hyalinization of seminiferous tubules with interspersed tubules showing normal spermatogenesis including mature spermatozoa [Figure 1b]. One patient showed hyalinized and thickened seminiferous tubules with basement membrane (BM) thickening; however, some tubules showed the presence of spermatogenesis including mature spermatozoa [Figure 1c]. One patient showed mild BM thickening and maturation arrest [Figure 1d]. One patient had normal spermatogenesis on biopsy.
Table 2.
Comparisons of operative findings, FNAC and biopsy of the patients with positive FNAC and raised FSH
Figure 1.
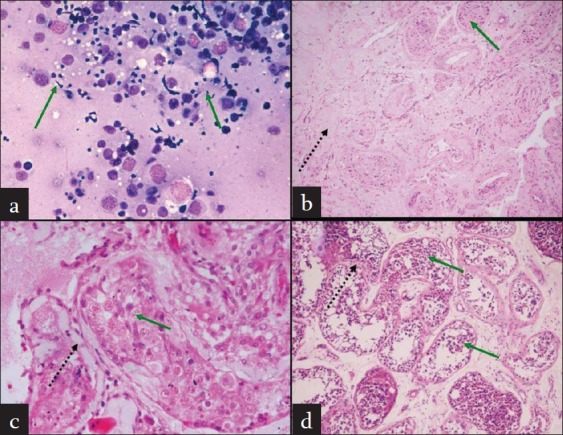
(a) FNAC from testis showing normal spermatogenesis. (b) Testis biopsy showing patchy areas of atrophy and hyalinization of seminiferous tubules (dashed arrow), interspersed tubules showing normal spermatogenesis including mature spermatozoa (green arrow). (c) Testis biopsy showing hyalinized and thickened seminiferous tubules with basement membrane thickening (black dashed arrow); some tubules showed the presence of spermatogenesis including mature spermatozoa (green arrow). (d) Testis biopsy showing mild basement membrane thickening and peritubular fi brosis (black dashed arrow) and maturation arrest till spermatid stage (green arrow)
DISCUSSION
Azoospermia accounts for 15% of male infertility cases. Obstructive azoospermia due to ductal obstruction can be treated surgically, whereas NOA requires IVF. Surgical reconstruction is preferred over IVF if possible.[6] The possibility of surgical reconstruction depends on the presence of normal spermatogenesis in the testis.
FNAC is a minimally invasive procedure for documenting the presence of spermatogenesis in the testis. It can accurately evaluate the testicular pathology and predict if the patient will benefit from surgical exploration and reconstruction.[5] The FNAC finding correlates with biopsy of the testis.[4,5]
There do remain a small proportion of patients in whom FNAC shows normal spermatogenesis but FSH levels are elevated. Previous studies have found that elevated FSH is a good method for exclusion of patients from reconstructive surgery, whereas other authors have used FNAC evidence of spermatogenesis as being sufficient for performing an operative reconstructive procedure without taking FSH levels into account.[7] Another method for assessment is by scrotal exploration, when patients unsuitable for surgery can be excluded by intraoperative microscopic assessment for the presence of sperm in the epididymis. Improving the results of surgical reconstruction requires refined case selection to exclude patients unlikely to benefit from the procedure.
We used both these methods in the present study for the assessment of patients. All patients having normal FSH and normal spermatogenesis on FNAC were considered as suitable candidates for surgical treatment. Patients with elevated FSH levels and abnormal FNAC were excluded from surgical management.
Serum FSH levels have been considered as the best screening method to select patients for reconstruction. We found in this study that despite normal spermatogenesis on FNAC, elevated levels of FSH coincided with absent sperms in the tubules. Serum FSH is therefore an excellent screening test which should be performed in patients even after a normal FNAC, since it picked up all of the cases which had to be abandoned due to absence of sperm in the epididymis.
On biopsy, 3/5 cases showed areas of tubular atrophy interspersed with normal tubules. The discordance between FNAC and open testis biopsy findings is explained by the presence of patchy areas of atrophy with interspersed normal spermatogenesis in the testicular biopsy. Areas of atrophy show fibrosis and hyalinization, which are not properly sampled on FNAC. The pockets of testicular tissue with normal spermatogenesis, when traversed by the fine needle, get over-represented in the aspiration and pick up mature sperms. In this situation, FNAC finding may be misleading and giving a false impression of normal spermatogenesis in the entire testis.
In the two cases with relatively normal spermatogenesis on biopsy, FNAC result of normal spermatogenesis correlated well. However, of these, one case had BM thickening. In both the cases, the issue of sampling error in the biopsy cannot be ruled out and it cannot be said with confidence that spermatogenesis was totally normal. Biopsy might have sampled the normal area of testis and missed the atrophic area. The common clinical factors in all these patients were elevated serum FSH level (mean value 17.48) and small testicular size (mean testicular volume 15 ml). The patient having normal spermatogenesis on FNAC with normal biopsy findings showed no dilated epididymal tubules and epididymal fluid was dry.
Mourad et al. have reported a variable histology within the same testis with difference in histological findings and FNAC in 4% of patients. They found that in infertile azoospermic men, with a testicular size of <10 ml combined with a serum FSH level of >19 IU/l, the chances of retrieving sperm are minimal using all three diagnostic modalities (FNAC, wet preparation and biopsy). It was suggested that use of this cut-off point would decrease the number of surgical procedures performed by 30–50%.[8]
However, a study done in azoospermic infertile males by Srivastva et al. has recommended that routine measurement of serum FSH level is not necessary in the preoperative assessment of azoospermic patients with normal testicular FNAC. They concluded that testicular FNAC alone is sufficient to diagnose the testicular function.[7]
It is true that FNAC is less dependable than histopathology. However, it is less invasive and causes less scarring, resulting in easier reconstruction. Even histopathology with biopsy, for that matter, is not 100% accurate for obstruction. It is important to have all parameters such as clinical examination, FSH and FNAC concordant and suggestive of obstruction before considering reconstruction. It is a clinical dilemma whether to offer reconstruction or not to men where FNAC suggests obstruction but FSH is raised. We hope to clarify through this paper that such men do not benefit from reconstruction and should not be explored.
CONCLUSIONS
FNAC was discordant with HPE in 4 out of 5 patients of negative surgical exploration. All these men had raised serum FSH, in concordance with the final HPE. Therefore, among men with idiopathic azoospermia, only those with both normal FSH and normal FNAC should be diagnosed as obstructive azoospermia and explored. This may decrease the total number of negative scrotal exploration as well as the cost, time and patient's anxiety.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Bayasgalan G, Naranbat D, Tsedmaa B, Tsogmaa B, Sukhee D, Amarjargal O, et al. Clinical patterns and major causes of infertility in Mongolia. J Obstet Gynaecol Res. 2004;30:386–93. doi: 10.1111/j.1447-0756.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- 2.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 3.Kurien A, Mammen K, Jacob S. Role of fine needle aspiration cytology (FNAC) of testes in male infertility. Indian J Urol. 2003;19:140–4. [Google Scholar]
- 4.Altay B, Hekimgil M, Cikili N, Turna B, Soydan S. Histopathological mapping of open testicular biopsies in patients with unobstructive azoospermia. BJU Int. 2001;87:834–7. doi: 10.1046/j.1464-410x.2001.02182.x. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Gautam G, Gupta NP, Aron M, Dada R, Kucheria K, et al. Role of testicular fine-needle aspiration cytology in infertile men with clinically obstructive azoospermia. Natl Med J India. 2006;19:18–20. [PubMed] [Google Scholar]
- 6.Kumar R, Mukherjee S, Gupta NP. Intussusception vasoepididymostomy with longitudinal suture placement for idiopathic obstructive azoospermia. J Urol. 2010;183:1489–92. doi: 10.1016/j.juro.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava A, Raghavendran M, Jain M, Gupta S, Chaudhary H. Fine-needle aspiration cytology of the testis: Can it be a single diagnostic modality in azoospermia? Urol Int. 2004;73:23–7. doi: 10.1159/000078799. [DOI] [PubMed] [Google Scholar]
- 8.Mourad WA, Tulbah A, Merdad T, Shoukri M, Al Dayel F, Hanash K. Fine-Needle Aspiration of the Testis in Azoospermic Men: The Value of Measuring Serum Follicle Stimulating Hormone and Testicular Size. Diagn Cytopathol. 2005;32:185–8. doi: 10.1002/dc.20223. [DOI] [PubMed] [Google Scholar]


