Abstract
Background
The effect of exercise on sleep-disordered breathing is unknown. While diet and weight loss have been shown to reduce the severity of sleep-disordered breathing, it is unclear whether exercise has an independent effect.
Methods
A population-based longitudinal epidemiologic study of adults measured the association between exercise and incidence and severity of sleep-disordered breathing. Hours of weekly exercise were assessed by two mailed surveys (1988 and 2000). Sleep-disordered breathing was assessed by 18-channel in-laboratory polysomnography at baseline and at follow up.
Results
Associations were modeled using linear and logistic regression, adjusting for body mass index, age, sex, and other covariates. Hours of exercise were associated with reduced incidence of mild (Odds Ratio = 0.76, p= 0.011) and moderate (Odds Ratio = 0.67, p= 0.002) sleep-disordered breathing. Also a decrease in exercise duration was associated with worsening sleep-disordered breathing, as measured by the apnea-hypopnea index, AHI (ß = 2.368, p= 0.048). Adjustment for body mass index attenuated these effects.
Conclusions
Exercise is associated with a reduced incidence of mild and moderate sleep-disordered breathing and decreasing exercise is associated with worsening of sleep-disordered breathing. The effect of exercise on sleep-disordered breathing appears to be largely, but perhaps not entirely, mediated by changes in body habitus.
Keywords: Exercise, Sleep Apnea
Introduction
Sleep-disordered breathing is associated with increased risk of cardiovascular disease and all-cause mortality.1-7 Treatment with continuous positive airway pressure may reduce this risk,8 though long-term adherence is suboptimal.9 Thus, although less efficacious, other treatment modalities often are recommended especially for mild to moderate sleep disordered breathing.10-12 Inasmuch as obesity is one of the most powerful risk factors for the development of sleep disordered breathing,13-16 and is potentially modifiable, dieting and increased exercise are commonly recommended as means of achieving weight reduction. Although weight loss accomplished through dieting has been shown to decrease the severity of sleep disordered breathing,17-21 it is unclear whether exercise exerts an independent beneficial effect on reducing sleep disordered breathing severity.
In epidemiologic cross sectional studies, increased physical activity is associated with a reduced prevalence of sleep disturbance.13-16,22 More recently, cross sectional studies have shown reduced odds of sleep disordered breathing with increasing physical activity independent of body habitus.23,24 In addition, small short-term clinical trials have demonstrated that exercise programs can reduce the severity of sleep disordered breathing without clinically significant changes in weight.25,26 However, whether increased exercise confers a beneficial effect on the development or severity of sleep disordered breathing over an extended time period is unknown. In addition, causal associations between exercise and sleep disordered breathing may be complex and it is plausible that daytime symptoms from sleep disordered breathing, such as sleepiness, might inhibit exercise rather than, or in addition to, exercise improving sleep disordered breathing.
Therefore, the objective of this study is to use long-term longitudinal data to determine whether changes in physical activity affect the severity and incidence of sleep disordered breathing in a large general population cohort. Our study design allows us to test the hypothesis that increasing exercise can reduce the incidence of new onset sleep disordered breathing. We further hypothesized that exercise would have a beneficial impact on sleep disordered breathing beyond those associated with weight loss.
RESEARCH DESIGN AND METHODS
Study protocols and informed consent documents were approved by the institutional review board of the University of Wisconsin. The sampling and data collection methods have been described previously.14,15,24
The Wisconsin Sleep Cohort is comprised of 1521 randomly-selected adults who were 30-60 year-old employees of state agencies in 1988. All subjects have had at least one overnight polysomnography (PSG). Participants are invited for follow-up PSGs every 4-years (participation rates for follow-up are ~79%). Exercise data were collected by mailed surveys in 1988 and 2000.
Two sub-samples of participants were used to address the primary research questions. First, initial levels of exercise were used to predict incident sleep disordered breathing among participants who: were free of sleep disordered breathing at baseline PSG (AHI<5/h, no continuous positive airway pressure—CPAP—use), had exercise data within 2 years of a baseline PSG, and had ≥1 follow-up PSG. There were n=959 available for incident sleep disordered breathing analysis. Second, changes in exercise habits were used to predict changes in sleep disordered breathing severity among participants who had: initial exercise data within 2 years of a baseline PSG and follow-up exercise data within 2 years of a follow-up PSG. There were n=478 available for sleep disordered breathing-change analysis.
Data Collection
Exercise information was obtained by the question—“About how many hours per week—if any—do you spend at regular planned exercise (such as jogging, sports, exercise class, workouts at home or a gym)?”—on both surveys.
Prior to overnight PSG, medical history and questionnaire data were collected. Body mass index (BMI) was calculated from measured height and weight (kg/m2). PSG was performed and measured the apnea-hypopnea index (AHI, the average number of apneas and hypopneas per hour of objectively measured sleep).
Data Analysis
Analyses were performed with SAS software, release 9.2 (SAS Institute, Inc., Cary, NC). Generalized linear models were used to measure the association of initial exercise and risk of subsequent development (over 8±2 years of follow-up) of at least mild sleep disordered breathing (AHI≥5/h or CPAP use) or at least moderate sleep disordered breathing (AHI≥15/h or CPAP use) among subjects without sleep disordered breathing at baseline. Initial exercise, modeled categorically (0 hrs/wk, 1-3 hrs/wk, or ≥4 hrs/wk), was the primary predictor variable.
For models predicting change in sleep disordered breathing severity, SAS PROC MIXED was used to analyze change in AHI from baseline to follow-up PSGs. Change in AHI was the outcome variable and change in exercise (modeled categorically) was the primary predictor variable. Both untransformed AHI change (follow-up - baseline) and loge((follow-up AHI+1)/(baseline AHI+1))—as a measure of relative change—were examined.
Change in exercise was categorized as changing from one category to another: no change, decrease (≥4 to 0 or 1-3 hrs/wk, 1-3 hrs/wk to 0 hrs/wk), or increase (0 to 1-3 or ≥4 hrs/wk, 1-3 to ≥4 hrs/wk).
All models included age and sex as covariates and then added BMI. Smoking status, alcohol use, chronic obstructive pulmonary disease, diabetes, and cardiovascular disease were investigated as confounding factors. Only BMI substantially altered the models.
RESULTS
Table 1 provides summary statistics for all study participants, categorized by statistical model used (i.e., incident sleep disordered breathing or change in sleep disordered breathing severity as outcome variable). The cohort exercised an average of 2 hours per week. Participants generally were overweight at baseline and obese at follow up. The majority (78-80%) had no clinically significant sleep disordered breathing (AHI<5/h) at baseline, while at follow up severity of sleep disordered breathing had worsened. The first column of Table 1 provides values of baseline variables for all participants who had baseline PSG study and exercise information. Key variables—mean age, gender distribution, mean AHI, mean hours of exercise—did not differ appreciably at baseline for all participants compared to participants—second column—who provided both baseline and follow-up PSG evaluations and exercise information (with the requirement that baseline measures of sleep and exercise occurred within a 2-year interval, and a similar requirement for follow-up PSG and exercise measures). Baseline values for some factors were different for all baseline participants compared to those that were analyzed for incident moderate or worse sleep disordered breathing (forth column of Table 1); this was expected as only participants with AHI<5/h were included in that analysis and thus the lower mean baseline AHI.
Table 1.
Summary of key variables.
| Characteristic | Baseline: Any Sleep Study |
Baseline: Change Models |
Followup: Change Models |
Baseline: Incidence Models |
Followup: Incidence Models |
|---|---|---|---|---|---|
| N (total sample) | 1488 | 478 | 959 | ||
| Men, % | 55 | 56 | 53 | ||
| Age, y | 44 (8) | 45 (8) | 54 (8) | 50 (9) | 58 (9) |
| BMI (kg/m2) | 27 (6) | 27 (5) | 30 (6) | 28 (6) | 31 (7) |
| AHI, events/h | 5 (11) | 5 (10) | 7 (10) | 3 (4) | 6 (8) |
| 0/h ≤ AHI < 5/h, % | 76 | 80 | 61 | 80 | 60 |
| 5/h ≤ AHI < 15/h, % | 14 | 12 | 26 | 20 | 28 |
| AHI ≥ 15/h, % | 10* | 8 | 13 | 0 | 12* |
| Exercise, hrs/week | 2 (3) | 2 (3) | 3 (3) | 2 (3) | |
| Exercise 0 hrs/week, % | 40 | 37 | 34 | 35 | |
| Exercise 1-3 hrs/week, % | 38 | 42 | 35 | 39 | |
| Exercise ≥ 4 hrs/week, % | 22 | 21 | 32 | 26 | |
| Current Smoker, % | 18 | 16 | 12 | ||
| Diabetes, % | 3 | 3 | 4 | ||
| Education beyond high school, % | 75 | 79 | 78 |
Unless otherwise indicated, all data are presented as mean (SD).
Includes participants on CPAP
Table 2 provides the results of logistic regression models of incident mild and moderate sleep disordered breathing as a function of baseline exercise. For both mild (AHI≥5/h) and moderate (AHI≥15/h) the incidence of sleep disordered breathing declined with increasing exercise irrespective of whether exercise duration was analyzed as an ordinal variable with increasing duration or a dichotomous variable. However, the effect is attenuated after adjustment for BMI, although a non-statistically significant trend remained.
Table 2.
Association of exercise and incidence of mild and moderate SDB. The Odds Ratio predicts incident SDB for a higher category of exercise hours per week relative to the next lower category of exercise.
| Incidence of AHI ≥ 5/h* | |||||
|---|---|---|---|---|---|
| Adjusted† | BMI-Adjusted‡ | ||||
| N | Odds Ratio (CI) | p-value | Odds Ratio (CI) | p-value | |
| Baseline exercise (trend)§ |
763 | 0.76 (0.62, 0.94) | 0.011 | 0.86 (0.69, 1.07) | 0.177 |
| Baseline exercise (≥ 4 hrs/week vs no exercise) |
763 | 0.59 (0.39, 0.89) | 0.012 | 0.74 (0.48, 1.15) | 0.186 |
| Incidence of AHI ≥ 15/hδ | |||||
|---|---|---|---|---|---|
| Adjusted† | BMI-Adjusted‡ | ||||
| N | Odds Ratio (C) | p-value | Odds Ratio (CI) | p-value | |
| Baseline exercise (trend)§ |
959 | 0.67 (0.51, 0.87) | 0.002 | 0.79 (0.60, 1.04) | 0.089 |
| Baseline exercise (≥ 4 hrs/week vs no exercise) |
959 | 0.47 (0.28, 0.79) | 0.004 | 0.65 (0.38, 1.11) | 0.117 |
Adjusted for age and gender.
Adjusted for age, gender, and BMI.
or CPAP use
Comparison for trend: no exercise to 1-3 hrs/week to ≥4 hrs/week
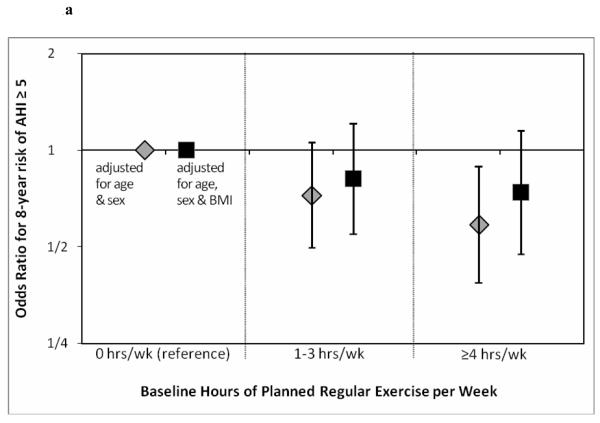
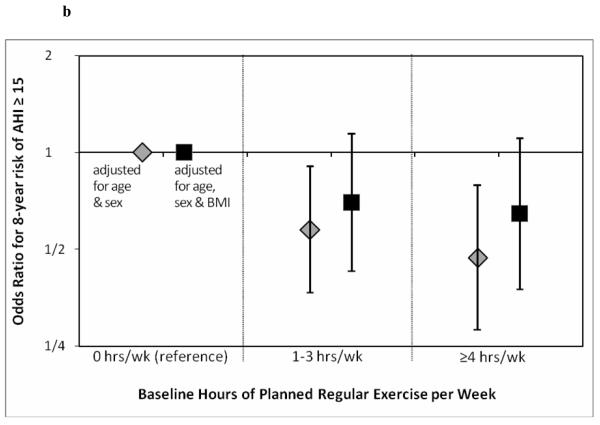
Figure 1a demonstrates the odds ratio for 8 year risk of mild sleep disordered breathing (AHI≥5/h). As noted, ≥4hrs/wk of exercise was associated with a significantly reduced odds ratio. Once again this is attenuated when adjusted for BMI, though a non-statistically significant trend remained. Figure 1b is similar, showing the odds ratio for 8 year risk of moderate sleep disordered breathing (AHI≥15/h). Odds ratio was reduced for 1-3 hrs/wk and ≥4hrs/wk of exercise (BMI attenuated the effect).
Figure 1.
a. Odds ratios estimating the relative reduction in ~8-year odds of developing at least mild SDB in participants who exercised at baseline vs. participants who did not exercise. N=763 follow-up periods among participants with baseline AHI<5/h.
b. Odds ratios estimating the relative reduction in ~8-year odds of developing at least moderate SDB in participants who exercised at baseline vs. participants who did not exercise. N=959 follow-up periods among participants with baseline AHI<15/h.
Table 3 shows whether modifying the amount of exercise is associated with a change in AHI. Participants who increased exercise did not have significantly different average change in AHI compared to participants who had no changes in exercise, irrespective of whether exercise was defined as an ordinal variable or as a dichotomous variable. However, decreased exercise duration was significantly associated with increased AHI using either an absolute or logarithmic transformed AHI (Absolute AHI: ß =2.4, p=0.048; Logarithmic AHI: ß =0.26, p=0.02). Adjustment for BMI attenuated this association, but again a non-statistically significant trend remained.
Table 3.
Effect of modifying exercise on AHI. The beta-coefficients are estimates of the absolute change in AHI (or change in loge(AHI)) associated with an increase in category of exercise hours per week.
| Absolute Change in AHI | log transformed Change in AHI* | |||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted† | BMI-adjusted‡ | Adjusted† | BMI-adjusted‡ | |||||
| Change in exercise (trend)§ |
Beta | −0.487 | Beta | −0.238 | Beta | −0.05 | Beta | −0.025 |
| SE | 0.651 | SE | 0.649 | SE | 0.061 | SE | 0.061 | |
| n=478 | p-value | 0.454 | p-value | 0.714 | p-value | 0.412 | p-value | 0.686 |
| Absolute Change in AHI | log transformed Change in AHI* | |||||||
| Adjusted† | BMI-adjusted‡ | Adjusted† | BMI-adjusted‡ | |||||
| Increase in exercise (as compared to no change) |
Beta | 1.025 | Beta | 0.968 | Beta | 0.117 | Beta | 0.112 |
| SE | 1.033 | SE | 1.025 | SE | 0.097 | SE | 0.096 | |
| n=478 | p-value | 0.322 | p-value | 0.345 | p-value | 0.228 | p-value | 0.247 |
| Absolute Change in AHI | log transformed Change in AHI* | |||||||
| Adjusted† | BMI-adjusted‡ | Adjusted† | BMI-adjusted‡ | |||||
| Decrease in exercise (as compared to no change) |
Beta | 2.368 | Beta | 1.77 | Beta | 0.259 | Beta | 0.198 |
| SE | 1.192 | SE | 1.198 | SE | 0.112 | SE | 0.113 | |
| n=478 | p-value | 0.048 | p-value | 0.140 | p-value | 0.021 | p-value | 0.080 |
Adjusted for age and gender.
Adjusted for age, gender, and BMI.
loge((AHI at follow-up + 1)/(AHI at baseline + 1))
Comparison for trend: decrease in exercise to no change in exercise to increase in exercise
DISCUSSION
This study demonstrates that exercise is associated with a reduced incidence of sleep disordered breathing. Reductions in body habitus appear responsible for much of this effect. Nevertheless, controlling for BMI did not explain all the exercise-sleep disordered breathing association and there remained a non-significant trend in reduced incidence of sleep disordered breathing. This finding suggests that exercise may also affect sleep disordered breathing via pathways other than weight loss.
Our findings are consistent with prior cross sectional analyses that found an inverse association between AHI and exercise, which remained significant even after adjusting for body habitus.23,24 They also are concordant with two small interventional studies demonstrating that exercise can reduce AHI without a change in body habitus.25,26 In one study of 11 subjects, there was a 27% reduction in AHI despite no change in BMI.25 This reduction was attained after a 6-month protocol of 2 hours of weekly aerobic exercise plus 2 hours of weekly weight lifting. In another study, 10 subjects in the exercise group had a 27.5% reduction in AHI despite no change in body habitus measures.26 Subjects underwent breathing and aerobic exercises 5 hours per week for 12 weeks. Finally, one other interventional study showed that with a minor BMI change (5%), exercise led to a decrease in AHI by 45%.27
There are several possible mechanisms by which exercise can modulate sleep disordered breathing other than through weight loss. First, exercise may increase upper airway muscle tonus. Three randomized controlled trials have assessed this hypothesis with inconsistent results. In one trial, participants played a didgeridoo 6 days a week for 25 minutes for a total of 4 months, leading to a decrease in AHI by 6/h (26% reduction), compared to controls.28 There were several limitations to the study, including a questionable control group (who were simply placed on a waiting list), no follow up anthropomorphic measurements, and a subjective primary outcome of daytime sleepiness, not AHI. Another trial used a set of complicated oropharyngeal exercises for 30 minutes daily for a total of 3 months and found that AHI decreased from 22.4 to 13.7/h (~39% reduction).29 Though there were no changes in BMI, neck circumference was decreased in subjects undergoing oropharyngeal exercises. Finally, intraoral electrical neurostimulation for 20 minutes, twice a day for a total of 8 weeks resulted in no improvement in AHI.30 Although the pathogenesis of sleep apnea is thought to involve a state-related decrease in upper airway muscular tone during sleep, the possibility exists that exercise during wakefulness may improve upper airway dilator function (e.g. motor tone or strength) during sleep.
Other than leading to increased muscle tonus of the upper airway, a second possible explanation for our findings is that exercise could affect the distribution of body fat. As such, even if there was no major change in BMI, the parapharyngeal fat may be redistributed. Other speculative possibilities include exercise-induced alterations in control of breathing and arousal threshold. However, there is little current evidence to support these latter potential mechanisms. Further work is clearly required in this area.
While we observed that baseline levels of exercise predicted subsequent development of sleep disordered breathing in participants initially free of sleep disordered breathing, we did not find a significant relation between overall change in exercise category (as measured by the within-participant differences in exercise from information initially collected in 1988, and then again in 2000) and change in sleep disordered breathing severity (the first model presented in Table 3). One possible explanation for positive results for the incidence models, but not for the change vs. change model, is that the limited exercise information we have may be considerably better at making coarse distinctions between, e.g., “exercisers” and “non-exercisers” compared to our ability to measure more subtle changes in exercise within subjects. In addition, whereas we were able to compare substantial proportions of the sample at the extremes of the exercise distribution (e.g., 37% of the baseline sample exercised 0 h/wk, and 21% of the sample that exercised ≥4 h/wk) in incidence models, a smaller proportions of the sample changed from 0 h/wk to ≥4 h/wk (17.5% of the sample) or from ≥4 h/wk to 0 h/wk (13% of the sample) over the follow-up period. Thus, the incidence models had considerable more statistical leverage to compare high levels of exercise vs. no exercise, than did the change-in-exercise models. We did find, however, that decreasing exercise over time was significantly associated with worsened AHI (the third model presented in Table 3). This observation is consistent with an effect of reducing or quitting exercise on increasing sleep disordered breathing severity, as well as a potential reverse causation association such that onset of sleep apnea symptoms may contribute to daytime symptoms which reduce propensity for exercise.
Our findings are consistent with a long-term effect of exercise on sleep disordered breathing; exercise was associated with incident sleep disordered breathing over 8 years of follow-up (as demonstrated in table 2). However, our design cannot readily distinguish between longer- and shorter-term effects of exercise on sleep disordered breathing severity. Potential mechanisms by which exercise putatively mitigates sleep disordered breathing——such as weight change, fat redistribution, increased muscle tonus, or altered chemosensitivity—may develop slowly. Shorter-term processes may also link exercise to sleep disordered breathing, as suggested by the 3-4 months trials described above (28, 29). Nevertheless, clinically it may be prudent to counsel patients that potential improvements in sleep disordered breathing severity resulting from initiation or intensification of an exercise program may not occur in the short term.
There are several limitations to this study. Exercise was measured by a simple question (hours of weekly exercise). Intensity of the exercise was not assessed, which is an important factor although difficult to quantify accurately. The higher the intensity, the shorter time of exercise is needed to be performed.31 Participants, as well, likely had varying interpretations of exercise (e.g., performing leisurely activity to some might be considered exercise). It is also possible that reverse causation may occur, in that increasing AHI can lead to decreasing exercise due to the confounding effect of increased sleepiness. However, in one of our models, baseline exercise was related to incidence of sleep disordered breathing 8 years later. As such reverse causation is not plausible. CPAP users were also not included in the study, because treating sleep disordered breathing may potentially mask the effects of exercise. Given the nature (i.e., it was not sampled out of clinical population with more severe sleep disordered breathing) of our cohort, the effects of exercise on more severe cases of sleep disordered breathing could not be fully assessed. Thus, our models included primarily participants with mild to moderate sleep disordered breathing. Despite these limitations, we believe that our findings are have clinical relevance and could lead to future mechanistic research and/or clinical trials.
In conclusion, we found that exercise is associated with reduced incidence of sleep disordered breathing. This outcome is largely mediated by changes in body habitus, though exercise may have other beneficial effects. We also found that decreasing exercise is associated with worsening of AHI. Taken together, these findings suggest that exercise programs, along with diet, may play an important role in modulating the severity and preventing the incidence of sleep disordered breathing.
Acknowledgments
We are grateful for the advice and expertise of Terry Young, PhD, K. Mae Hla, MD MHS, F. Javier Nieto, MD, PhD, Erika Hagen, PhD, Laurel Finn, Kathryn Pluff, Amanda Rasmuson, Nicole Salzieder, Kathy Stanback, Robin Stubbs, and Mary Sundstrom.
Funding Sources: Supported by grants R01HL62252 and 1UL1RR025011from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that they have no conflict of interest.
Contributorship: Conception, design, analysis, interpretation, and drafting the manuscript for important intellectual content: KA, AM, JB, SQ, PP
References
- 1.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: Eighteen-year follow-up of the wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 2.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: A 10-year follow-up. Circulation. 2008;118(9):955–960. doi: 10.1161/CIRCULATIONAHA.108.783290. [DOI] [PubMed] [Google Scholar]
- 4.Sorajja D, Gami AS, Somers VK, Behrenbeck TR, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133(4):927–933. doi: 10.1378/chest.07-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory disturbance index: An independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162(1):81–86. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 6.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: A long-term follow-up. Eur Respir J. 2006;28(3):596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 7.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: Long-term prognosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132(2):693–699. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- 11.Baby BS, Aronow WS, Chandy D. Therapeutic options for obstructive sleep apnea. Am J Ther. 2011 doi: 10.1097/MJT.0b013e318217a59f. [DOI] [PubMed] [Google Scholar]
- 12.Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408–1413. doi: 10.1093/sleep/33.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: The sleep heart health study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 14.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 15.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 16.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: The sleep heart health study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 17.Dixon JB, Schachter LM, O’Brien PE. Polysomnography before and after weight loss in obese patients with severe sleep apnea. Int J Obes (Lond) 2005;29(9):1048–1054. doi: 10.1038/sj.ijo.0802960. [DOI] [PubMed] [Google Scholar]
- 18.Harman EM, Wynne JW, Block AJ. The effect of weight loss on sleep-disordered breathing and oxygen desaturation in morbidly obese men. Chest. 1982;82(3):291–294. doi: 10.1378/chest.82.3.291. [DOI] [PubMed] [Google Scholar]
- 19.Johansson K, Hemmingsson E, Harlid R, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: Prospective observational follow-up study. BMJ. 2011;342:d3017. doi: 10.1136/bmj.d3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med. 2008;4(4):333–338. [PMC free article] [PubMed] [Google Scholar]
- 21.Nahmias J, Kirschner M, Karetzky MS. Weight loss and OSA and pulmonary function in obesity. N J Med. 1993;90(1):48–53. [PubMed] [Google Scholar]
- 22.Sherrill DL, Kotchou K, Quan SF. Association of physical activity and human sleep disorders. Arch Intern Med. 1998;158(17):1894–1898. doi: 10.1001/archinte.158.17.1894. [DOI] [PubMed] [Google Scholar]
- 23.Quan SF, O’Connor GT, Quan JS, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11(3):149–157. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 24.Peppard PE, Young T. Exercise and sleep-disordered breathing: An association independent of body habitus. Sleep. 2004;27(3):480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 25.Giebelhaus V, Strohl KP, Lormes W, Lehmann M, Netzer N. Physical exercise as an adjunct therapy in sleep apnea-an open trial. Sleep Breath. 2000;4(4):173–176. doi: 10.1007/s11325-000-0173-z. [DOI] [PubMed] [Google Scholar]
- 26.Sengul YS, Ozalevli S, Oztura I, Itil O, Baklan B. The effect of exercise on obstructive sleep apnea: A randomized and controlled trial. Sleep Breath. 2011;15(1):49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- 27.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3(3):121–129. [PubMed] [Google Scholar]
- 28.Puhan MA, Suarez A, Lo Cascio C, Zahn A, Heitz M, Braendli O. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: Randomised controlled trial. BMJ. 2006;332(7536):266–270. doi: 10.1136/bmj.38705.470590.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guimaraes KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179(10):962–966. doi: 10.1164/rccm.200806-981OC. [DOI] [PubMed] [Google Scholar]
- 30.Randerath WJ, Galetke W, Domanski U, Weitkunat R, Ruhle KH. Tongue-muscle training by intraoral electrical neurostimulation in patients with obstructive sleep apnea. Sleep. 2004;27(2):254–259. doi: 10.1093/sleep/27.2.254. [DOI] [PubMed] [Google Scholar]
- 31.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: Updated recommendation for adults from the american college of sports medicine and the american heart association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]