SUMMARY
Mouse models of chronic obstructive pulmonary disease (COPD) focus on airway inflammation and lung histology, but their use has been hampered by the lack of pulmonary function data in their assessment. Systemic effects such as muscle dysfunction are also poorly modeled in emphysematous mice. We aimed to develop a cigarette-smoke-induced emphysema mouse model in which serial lung function and muscular dysfunction could be assessed, allowing the disease to be monitored more appropriately. C57Bl6 mice were nose-only exposed to cigarette smoke or filtered air for 3–6 months. Lung function tests were repeated in the same mice after 3 and 6 months of cigarette smoke or air exposure and compared with lung histological changes. Contractile properties of skeletal muscles and muscle histology were also determined at similar time points in separate groups of mice. Serial lung function measurements documented hyperinflation after 3 and 6 months of cigarette smoke exposure, with a significant 31–37% increase in total lung capacity (TLC) and a significant 26–35% increase in compliance (Cchord) when compared with animals exposed to filtered air only (P<0.001 after 3 and after 6 months). These functional changes preceded the changes in mean linear intercept, which became only significant after 6 months of cigarette smoke exposure and which correlated very well with TLC (r=0.74, P=0.004) and Cchord (r=0.79, P=0.001). After 6 months of cigarette smoke exposure, a significant fiber-type shift from IIa to IIx/b was also observed in the soleus muscle (P<0.05), whereas a 20% reduction of force was present at high stimulation frequencies (80 Hz; P=0.09). The extensor digitorum longus (EDL) muscle was not affected by cigarette smoke exposure. These serial pulmonary function variables are sensitive outcomes to detect emphysema progression in a nose-only cigarette-smoke-exposed animal model of COPD. In this model, muscular changes became apparent only after 6 months, particularly in muscles with a mixed fiber-type composition.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a slowly progressive disease characterized by airflow limitation that is not fully reversible and by an abnormal inflammatory response in the lung (Pauwels and Rabe, 2004; Rabe et al., 2007). Besides the presence of lung inflammation, COPD is also characterized by several systemic consequences, including weight loss, skeletal muscle wasting and muscle dysfunction (Pauwels and Rabe, 2004; Decramer et al., 2005; Gea and Barreiro, 2008). Skeletal muscle dysfunction is accepted as a very important systemic consequence of COPD because it is associated with exacerbations of the disease, reduced quality of life and decreased prognosis (Pitta et al., 2005; Swallow et al., 2007). Moreover, rehabilitation programs have clearly established that muscle dysfunction is susceptible to intervention, with beneficial effects of training on general outcomes (Paz-Diaz et al., 2007; Troosters et al., 2010a; Baltzan et al., 2011). Although the mechanisms that contribute to muscle dysfunction are not completely understood, multiple factors are implicated, such as systemic inflammation, oxidative stress and nutritional deficits (Decramer et al., 2005). Furthermore, physical inactivity is likely to be the most important factor for developing muscle dysfunction because it sneaks in at the very early stages of COPD and further develops as the disease progresses (Pitta et al., 2005; Watz et al., 2008; Watz et al., 2009; Troosters et al., 2010b). Muscle dysfunction in individuals with COPD is characterized by structural and biochemical alterations in lower limb muscles, with fiber-type redistribution (muscle type shift from type I to type II) (Whittom et al., 1998; Maltais et al., 1999) and loss of muscle mass (Schols et al., 1991; Engelen et al., 1994), but the exact mechanisms and the exact role of the different factors involved remains unclear. For a better understanding of the mechanisms that lead to disease progression, including the development of muscle dysfunction, improving existing animal models is fundamental.
Cigarette smoke (CS) exposure is the most appropriate model to study emphysema in mice and several exposure systems are available (Wright et al., 2008; Luthje et al., 2009; Stevenson and Birrell, 2010). The nose-only exposure system probably best resembles the human situation (Wright et al., 2008; Stevenson and Birrell, 2010). Until now, only a few studies have used nose-only exposure systems to induce emphysema and these studies mainly focused on alterations in the lungs (Takubo et al., 2002; Guerassimov et al., 2004; Bracke et al., 2006). To explore the mechanisms that contribute to muscle dysfunction in COPD, skeletal muscle fiber changes and muscle contractile properties need to be determined. Some studies investigated peripheral skeletal muscle fiber-type changes and enzyme content in elastase-induced emphysema in hamsters (Mattson and Martin, 2005; Mattson et al., 2008) and in CS-induced emphysema models by whole-body exposure (Ardite et al., 2006; De Paepe et al., 2008; Tang et al., 2010; Barreiro et al., 2010), but so far no studies are available exploring contractile properties and histological changes in a nose-only CS exposure model. Theoretically, the latter model is superior for such a purpose because it avoids systemic effects by the uptake of tar substances or nicotine through the skin. Another important drawback of the current models is that they all monitor disease progression on histopathological changes. However, similar to individuals with COPD, a variety of pulmonary function variables can now be measured in small animal models. For instance, we recently demonstrated that invasive lung function measurements are useful to distinguish between different respiratory diseases in mice (Vanoirbeek et al., 2010) and we have also developed a technique allowing repeated invasive pulmonary function tests in healthy mice (De Vleeschauwer et al., 2011). It is now to be investigated to what extent the serial measurement of pulmonary function can detect histopathological changes in the lungs over time.
Until now, no studies have ever determined disease progression while measuring lung function over time in the same animal, although this is commonly performed in COPD patients. Whether skeletal muscle contractile dysfunction develops after CS exposure is not known and also the time course of this dysfunction has not yet been examined. Therefore, the aim of our study was first to assess disease progression in a nose-only CS-induced emphysema model by consecutive lung function measurements over time. Secondly, we explored whether peripheral skeletal muscle dysfunction was present in our animal model by determining skeletal muscle function and fiber-type distribution at different time points during disease progression. To achieve these goals, two series of animals were used: in series 1, repeated lung function measurements were assessed in the same animal after 3 and 6 months of CS or air exposure and muscle function was determined at 6 months of exposure; in series 2, measurements were made after 3 months of exposure.
RESULTS
Survival and weight evolution
Survival rate was 92% in series 2 after 3 months of CS or air exposure. None of the animals died with intubation and only one mouse died during a CS exposure session. In series 1, survival rate was 75% after 3 months of CS or air exposure and remained as such until sacrifice. In this series, one control died after the first intubation, and three controls and two CS-exposed mice died after the second intubation. Death was due to the fact that animals did not awake after anesthesia or had difficulties to breathe spontaneously. Mortality in controls was twice the mortality in CS-exposed animals.
Starting body weight was similar between the two groups in each series. After 3 months of CS or air exposure, body weight increases were significantly lower in the CS-exposed group compared with controls (e.g. for series 1: 6±1% vs 11±10%; P<0.001). After 6 months of CS or air exposure, these differences were more pronounced (series 1: 5±5 vs 23±10%; P<0.001). Finally, intubation resulted in a transient drop of body weight in both groups and each series (e.g. series 1: 6±3%), which restored after 4 days.
Pulmonary disease
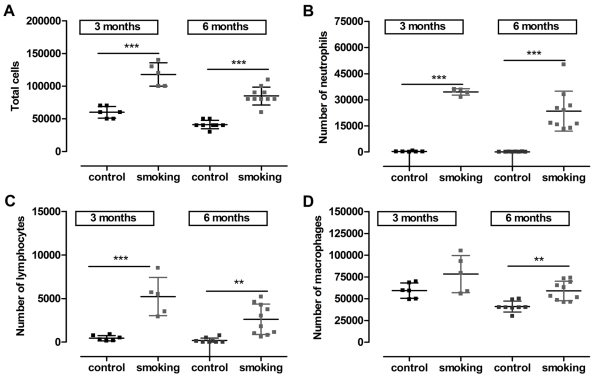
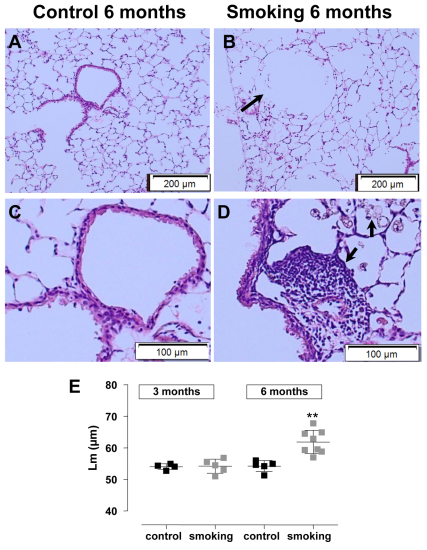
Hyperinflation of the lung was demonstrated by a statistically significant increase in total lung capacity (TLC; series 1: 31% and series 2: 23%) and compliance (Cchord; series 1: 26% and series 2: 24%) in CS-exposed animals after 3 months of exposure when compared with age-matched controls (series 1: P<0.001 and series 2: P<0.01). After 6 months of CS exposure (series 1), lung volumes were even more distended, with a 37% increase in TLC and 35% increase in Cchord compared with age-matched controls (P<0.001; Fig. 1A,B). Additionally, CS-exposed animals in both series showed pressure-volume curves that typically shifted upwards and leftwards, indicative of enhanced Cchord. The forced-expiration maneuvers did not result in changes in forced expiratory volume at 100 ms (FEV0.1) or the FEV0.1:forced vital capacity (FVC) ratio between two groups in any series (Fig. 1C for series 1). The total cell counts on bronchoalveolar lavage (BAL) fluid increased substantially with smoking (Fig. 2A) and shifted towards an abundant presence of neutrophils, lymphocytes and macrophages as early as 3 months after CS exposure in both series (Fig. 2B–D). Despite these pro-inflammatory changes, no statistically significant differences in mean linear intercept (Lm) was observed after 3 months of CS exposure (series 2: 54.2±2.2 μm vs control 54.0±0.9μm, P>0.05) (Fig. 3E). Only after 6 months of CS exposure did a statistically significant increase in Lm become clear (61.9±3.7 μm vs control 54.2±1.8 μm, P=0.001; Fig. 3E). Strong correlations were found after 6 months of CS exposure between Lm and TLC (r=0.7, P=0.004) and between Lm and Cchord (r=0.8, P=0.001). Similarly, peribronchovascular inflammation with foamy macrophages and lymphoid aggregates were already present in the smoking groups after 3 months but were far more extended after 6 months of CS exposure (Fig. 3).
Fig. 1.
Repeated lung function measurements assessed over time in the same animal at baseline (0), and after 3 and 6 months of CS or air exposure using a nose-only exposure system. Lung hyperinflation is shown by the increase in total lung capacity (TLC; A) and compliance (Cchord; B) in the mice exposed to CS (solid line) compared with controls (dashed line). This increase is already present after 3 months of CS exposure. The marker of airway obstruction, FEV0.1:FVC (ratio forced expiratory volume at 100 ms to forced vital capacity; C) remained unchanged over time. Data represent data of series 1 and are presented as mean and standard deviation. ***P<0.001. At baseline, n=12 for controls and smoking group; at 3 months, n=11 for controls and n=10 for smoking group; at 6 months, n=8 for controls and n=10 for smoking group.
Fig. 2.
Total and differential cell counting in the broncho-alveolar lavage of mice exposed to CS or air during 3 or 6 months. Total (A) and differential (B–D) cell counting. Notice that the data at 3 and 6 months refer to data of separate groups of animals. Total cell counts increased with CS exposure and this effect was already present after 3 months of exposure (A). This was associated with a shift towards an abundant number of neutrophils (B), lymphocytes (C) and macrophages (D). Each square represents data of an individual mouse; horizontal line represents mean and vertical lines the standard deviation. **P<0.01; ***P<0.001.
Fig. 3.
Representative illustrations of a sagittal lung section stained with H&E in a control and a CS-exposed mouse after 6 months. Control (A,C) and CS-exposed (B,D) mice. Notice the alveolar destruction after CS exposure in B (arrow). Presence of foamy macrophages and lymphocytes were observed around the airways (arrow in D) after 6 months of CS exposure. Airspace enlargement was quantified by measuring mean linear intercept (Lm). Notice that Lm was significantly increased in the CS-exposed animals but only after 6 months of exposure (E). In E, each square represents data of an individual mouse; horizontal line represents mean and vertical lines the standard deviation. **P<0.01.
Contractile properties of muscles
The data of muscle weight and contractile properties of the soleus and extensor digitorum longus (EDL) muscle at 3 months after CS or air exposure were obtained in a separate group of animals (series 2) than those obtained at 6-month exposure (series 1).
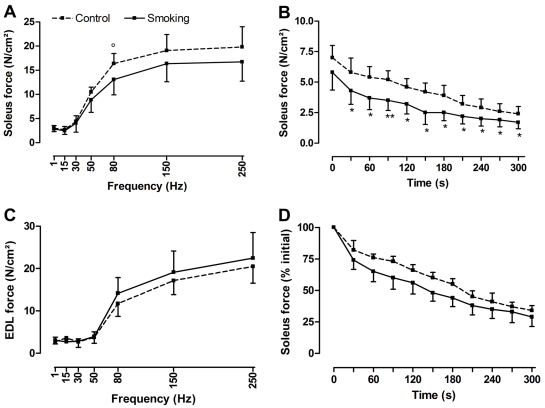
The right soleus muscle mass was similar between both groups after 3 (10.4±2.0 mg vs control 11.0±1.6 mg) and 6 (10.6±1.2 mg vs control 10.2±1.8 mg) months of CS or air exposure. No differences between groups were found for maximal tetanic tension of soleus muscle after 3 months (23.5±8.8 N/cm2 vs control 22.3±2.3 N/cm2) or after 6 months (22.8±4.8 N/cm2 vs control 26.4±3.4 N/cm2) of exposure. The force generated by the soleus muscle during the force-frequency curve did not change after 3 months of CS exposure but tended to be lower at high stimulation frequencies after 6 months of CS exposure (20% decrease at 80 Hz, P=0.087; Fig. 4A). Although the absolute force of the soleus muscle generated during the fatigue run was lower in the CS-exposed group after 6 months compared with controls, no difference in fatigability was observed at the end of the fatigue run between both groups (Fig. 4B,D).
Fig. 4.
In vitro contractile properties of skeletal muscles in mice exposed to CS or air after 6 months. In vitro force-frequency curve (A,C) and fatigue run (B,D) of the soleus muscle (A,B,D) and extensor digitorum longus muscle (C) in mice exposed to CS (solid line) and in controls (dashed line) after 6 months of exposure. Notice the trend for the soleus muscle to generate lower force after CS exposure especially at high frequency (e.g. 20% decrease at 80 Hz, P=0.087) (A). For the fatigue run, although the soleus muscle generated lower force over time (B), no differences in fatigability were observed between the two groups at the end of the fatigue run (D). Force of EDL muscle was not affected by CS exposure (C). Data are represented as mean and standard deviation. Force is expressed in absolute values (A–C) or as a percentage of initial value (D). For A, n=4 controls and n=10 for smoking group; for B, n=5 controls and n=10 for smoking group; for C and D, n=4 controls and n=8 for smoking group. °P=0.087; *P<0.05; **P<0.01.
The right EDL mass did not change after 3 months (13.0±1.3 mg vs control 12.1 ± 0.6 mg) or after 6 months (11.2±1.5 mg vs control 10.7±1.2 mg) of CS or air exposure. The maximal tetanic tension of EDL muscle was also not different after 3 months (26.3±6.6 N/cm2 vs control 24.7±6.5 N/cm2) or after 6 months (31.3±5.8 N/cm2 vs control 29.4±2.6 N/cm2) of CS or air exposure, nor did the force-frequency curve (Fig. 4C) or the fatigability change between groups.
Muscle fiber-type shift and atrophy (both series)
As for contractility, the data of muscle histology after 3 or 6 month of CS exposure pertain to a separate group of animals.
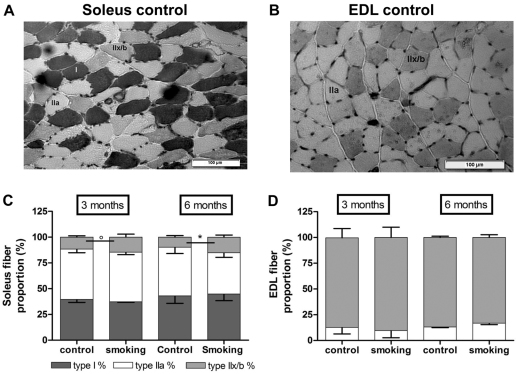
Morphological analysis of soleus and EDL muscle stained with hematoxylin and eosin (H&E) did not show any structural abnormalities. Typical ATPase staining of soleus and EDL muscles is shown in Fig. 5A,B. After 3 and 6 months of CS or air exposure, there were no changes in fiber-type proportions of the EDL muscle (Fig. 5D). However, after 3 months of CS exposure, there was a trend for fiber-type redistribution in the soleus muscle with a shift from IIa to IIb/x fibers (14.5±1.7% vs control 11.6±0.6%, P=0.09) that became significant after 6 months of CS exposure (15.1±1.2% vs control 9.8±0.7%, P=0.01; Fig. 5C). No changes in cross-sectional area (CSA) were observed in EDL or soleus muscle after 3 months or after 6 months of CS exposure.
Fig. 5.
Histochemical analysis of the soleus and EDL in mice exposed to CS or air. Representative myofibrillar ATPase staining of a soleus (A) and EDL (B) cross-section in controls after 6 months. Based on their histochemical reactions, fibers were classified as slow-oxidative type I, fast-glycolytic and oxidative type IIa, and fast-glycolytic type IIx/b fibers. A shift from type IIa to type IIx/b fibers was present in the soleus after 3 months of CS exposure and this shift became significant after 6 months of exposure (C). No changes in fiber proportion were found in the EDL muscle (D). Notice that the data at 3 and 6 months refer to data of separate groups of animals. Data are presented as mean and standard deviation. n=4/group at 3 months of exposure; n=5/group at 6 months of exposure. °P=0.09; *P<0.05.
DISCUSSION
Importantly, the present study demonstrated that emphysema progression in mouse models can be monitored over a prolonged period of time by serial invasive measurements of TLC and Cchord within the same animal. Importantly, the obtained pulmonary function parameters were found to be more sensitive than morphological changes of the lung because they picked up differences in lung recoil earlier than the corresponding histological quantifications. These data suggest that early emphysema with loss of elasticity was already present after 3 months of CS exposure while using a nose-only exposure system.
For the first time, a CS-exposed mouse model was also used to determine skeletal muscle function at different time points during the development of emphysema. Our study shows that CS-induced emphysema with nose-only exposure associates with mild skeletal muscle dysfunction in terms of high frequency force and small morphological changes with fiber-type redistribution in the soleus muscle after 6 months of exposure. Together, the present findings allow further research on skeletal muscle dysfunction in a more clinical model of COPD.
Invasive pulmonary function measurements are not routinely assessed in mouse models of emphysema and when used they only refer to one time point measurement. In nose-only CS-exposed mice, Guerassimov et al. (Guerassimov et al., 2004) and Takubo et al. (Takubo et al., 2002) could not show significant changes in tissue elastance and compliance after 6 months of CS exposure in C57Bl6 mice, despite significant increases in linear intercept (13% and 18%, respectively). Although their changes of linear intercept were in line with those observed in our study, we found significant differences in TLC and compliance in our model. Moreover, in our study there was a discrepancy between linear intercept and lung function measurements to detect emphysema after 3 months of CS exposure, with linear intercept being less sensitive than lung function technique. This discrepancy disappeared at 6 months of CS exposure such that TLC and Cchord correlated well with measures of lung morphology (Lm) at that time point. Because changes were detected earlier by pulmonary function, this suggests that Cchord and TLC were not only accurate but also more sensitive outcomes. At least, it emphasizes that caution should be taken in studies where only Lm is measured. The discrepancies between our data and previous studies might be attributed to a different smoking protocol (two 2R1 cigarettes/day in the other studies) or to different equipment used to monitor pulmonary function. Indeed, we have previously shown that semi-quasistatic measurements of compliance with Scireq-Flexivent, as used by others, are less reliable than compliance (Cchord) measurements obtained with the Buxco as in the present study, and that measures of compliance and TLC are probably the best markers for mouse models of emphysema (Vanoirbeek et al., 2010).
Another advantage of our approach is that serial measurements in the same mice allow respiratory disease monitoring without sacrifice. This is particularly interesting to monitor the effects of long-term intervention in chronic labor-intensive models such as smoking mice. This is the first time that such an approach has been used in an animal model of CS-induced emphysema, especially with a nose-only exposure system. It should be stressed, however, that the typical marker for airway obstruction (the FEV0.1:FVC ratio) was not affected in our model. In contrast to what is seen in humans with COPD or emphysema, FEV0.1 even increased with disease progression. The explanation might be found in the fact that mice have a very compliant chest wall with an unlimited hyperinflation of the lungs and subsequent higher flows during expiration (Vanoirbeek et al., 2010). Additionally, airflow limitation might be limited because small airways are lacking in the mouse and the disease model basically represents alveolar destruction or emphysema instead of chronic bronchitis. Despite these inherent shortcomings in the pathophysiology of our model, we should stress the fact that, after 3 months of CS exposure, the inflammatory pattern of neutrophils and lymphocytes in the bronchoalveolar lavage fluid clearly corresponded to what is seen in human COPD. Similarly, the presence of lymphocyte aggregates around the distal airways, which have been equally observed in other smoking mouse models (Bracke et al., 2006; van der Strate et al., 2006; Maeno et al., 2007; Harrison et al., 2008), is also a feature of human COPD.
Besides the presence of lung disease, we also investigated body weight evolution and muscle function over time in our model. The present study shows that body weight gain was reduced after 3 months of CS exposure, an effect that was exacerbated after 6 months of CS exposure. Our findings are in line with the results of previous studies using nose-only exposure (Takubo et al., 2002; Guerassimov et al., 2004; Ardite et al., 2006; Barreiro et al., 2010) or a whole-body exposure system (De Paepe et al., 2008; Gosker et al., 2009; Tang et al., 2010). Body weight loss in our model was not attributed to a lower muscle weight in the smoking animals. So far, there are no data reported on muscle weight in a CS-induced emphysema model by nose-only exposure. However, with whole-body exposure, results are controversial, with some studies showing a decrease in skeletal muscle weight (De Paepe et al., 2008; Tang et al., 2010), whereas another study did not (Gosker et al., 2009).
Measurement of contractile properties of two skeletal muscles at two different time points during the course of the disease is new. In fact, contractile properties of skeletal muscles in CS models have never been assessed before. This study reveals that, after 3 months of CS exposure, no changes were found in contractile properties of the soleus and EDL muscle. However, after 6 months of CS exposure, a trend towards lower force generation at high stimulation frequencies (80 Hz) was found in the soleus muscle. Interestingly, the contractile properties of the EDL, a fast-twitch muscle, were not affected. Moreover, our data suggest that skeletal muscles with an oxidative profile (such as the soleus) are preferentially affected by CS exposure. This is in agreement with studies in human subjects comparing skeletal muscle function in smokers and non-smokers (Orlander et al., 1979; Montes de Oca et al., 2008). In addition, our data showed that skeletal muscle dysfunction might develop over time during CS exposure but this dysfunction is not present after 3 months of CS exposure and remains mild after 6 months of CS exposure. This has never been demonstrated before. In individuals with COPD, loss of muscle function is, however, present in the early stage of the disease (Seymour et al., 2010). Because smoking results in only mild skeletal muscle dysfunction in our model, this suggests that additional factors related to COPD (including oxidative stress, sedentarism, exacerbations, etc.) might contribute to more severe muscle dysfunction in humans with COPD. In our study, the muscle fatigue remained unchanged in the soleus and the EDL muscle after CS exposure at both time points. Skeletal muscle fatigue during CS-induced emphysema has been poorly investigated and data refer to a one time point measurement. For the EDL muscle, our results are similar to those obtained by Tang et al. after 16 weeks using a whole-body CS-exposure model (Tang et al., 2010). For the soleus muscle, our data differ from those obtained by Tang et al. However, these discrepancies are likely to be related to differences in stimulation protocol and the way fatigability was assessed rather than to real differences. In the study of Tang et al. (Tang et al., 2010), stimulation rate varied every 2 minutes and fatigue protocol lasted 8 minutes, whereas, in our study, stimulation rate was constant during the 5-minute fatigue run. In addition, we assessed fatigability by force reduction at the end of the fatigue run (compared with baseline), as commonly done by others (Derave et al., 2005; Arbogast et al., 2007; Mador et al., 2010), whereas Tang et al. determined fatigability as the time to reach 50% of initial force (Tang et al., 2010). As such it remains difficult to compare both studies. Overall, these data show that fatigue in the soleus muscle after CS exposure in the available mouse models is not as severe as in humans with COPD. This suggests that skeletal muscle fatigue might be caused by other factors than CS alone.
Time course evolution of muscle fiber dimensions during CS-induced emphysema has not yet been studied. In the present study, no changes in fiber dimensions were observed in the soleus and EDL muscles after 3 or 6 months of CS exposure. Our results are in line with other studies using a whole-body exposure system (De Paepe et al., 2008; Barreiro et al., 2010) or elastase (Mador et al., 2010) to induce emphysema. In our study, fiber proportions of the EDL did not change after CS exposure, whereas there was a trend towards a fiber-type shift from IIa to IIx/b fibers in the soleus muscle after 3 months of CS exposure, which was significant after 6 months of CS exposure. Other studies with a CS-exposure model also reported a shift from IIa to IIb fibers in the soleus muscle (Gosker et al., 2009; Tang et al., 2010), with no changes in fiber-type composition of the EDL muscle (Tang et al., 2010). Our data of the soleus muscle, where oxidative fiber proportion (IIa) was reduced whereas proportion of glycolytic fibers was increased (IIx/b), are in line with the loss of peripheral muscle oxidative phenotype reported in COPD patients (Whittom et al., 1998; Maltais et al., 1999; Barreiro et al., 2010). Although muscle fiber changes were already present after 3 months and were more pronounced after 6 months of CS exposure, muscle fatigue was not yet affected in our animal model.
In conclusion, this study shows for the first time that disease progression can be assessed by measuring lung function over time in the same animal. It shows that emphysema was already established after 3 months of CS exposure and pulmonary function variables such as TLC and Cchord were more sensitive outcomes to detect early emphysema than was lung morphology (Lm). Emphysema after 6 months of CS exposure was associated with mild skeletal muscle dysfunction in the soleus muscle. Our nose-only CS-exposure model provides a good model for further research in skeletal muscle dysfunction in emphysema. Future research should investigate, in particular, whether the changes in muscle fiber type have an impact on exercise capacity and are linked to alterations in systemic mediators and signaling pattern.
METHODS
All experimental procedures were approved by the Ethical Committee of Animal Experiments of the University of Leuven.
Animals
A total of 36 male C57Bl6 mice (∼25 g, 8-weeks old) were obtained from Harlan (The Netherlands). Animals were housed in a conventional animal house and maintained on a 12-hour light-dark cycle. The animals were placed in cages with filter top and supplied with pelleted food and water ad libitum. Following 1 week of acclimatization, animals were randomly divided into two groups: control group (n=18) and CS-exposed group (n=18). Animals were exposed to CS (3R4F research cigarettes with filter purchased from Kentucky Tobacco Research and Development Center, University of Kentucky) using a nose-only exposure system (InExpose System, Scireq). Briefly, mice were placed in soft restraints and connected to the exposure tower. A computer-controlled puff was generated every minute, leading to 10 seconds of CS exposure followed by 50 seconds of fresh air. Mice were acclimatized to CS by exposure to two cigarettes per day during the first week of the experiment. Afterwards, animals were exposed daily to four cigarettes, twice a day, 5 days per week, over 3 or 6 months. The CS challenge was based on a pilot study in which different regimens were tested (three, four, five or six cigarettes a day, twice a day) and daily particulate density concentration was measured. The CS challenge chosen in the present study was associated with a good tolerance of mice to the CS sessions without mortality, and an acceptable level of particulate density concentration according to literature. Control animals were treated similarly and were exposed to filtered air for the same duration. The total particulate density concentration of CS was measured weekly and indicated an average of 149.5 mg total particulate matter per m3 (TPM/m3) in the tower. Mice were weighed weekly to determine health conditions of animals.
Protocol
In a first series, disease progression was assessed in both the control group (n=12) and the CS-exposed group (n=12) by performing repeated lung function measurements after 3 and 6 months of CS or air exposure. After 6 months of CS exposure, the animals were sacrificed to determine emphysema levels and contractile properties of peripheral muscles. In a second series of experiments, control (n=6) and CS-exposed animals (n=6) were sacrificed after 3 months to determine whether emphysema and muscle dysfunction were present.
Lung function measurements
In the first series, repeated lung function measurements were determined in the same animals (n=12/group) at baseline, after 3 months and after 6 months. At 24 hours after the last CS or air exposure, mice were anesthetized with a mixture of medetomidine (1 mg/kg, Domitor, Pfizer Animal Health, Belgium) and ketamine (75 mg/kg, Ketalar, Pfizer, Belgium) intraperitoneally. Intubation was performed with a 20G, 30 mm catheter (BD Insyte, Spain) through direct visualization of the vocal cords as described previously (Brown et al., 1999). After intubation, mice were placed in a body plethysmograph and connected to a computer-controlled ventilator (Buxco-Force Pulmonary Maneuvers) to measure lung volumes such as total lung capacity (TLC), lung compliance (Cchord), and forced expiratory volumes such as forced vital capacity (FVC), forced expiratory volume at 100 ms (FEV0.1) and the FEV0.1:FVC ratio. After pulmonary function measurements, mice received an antidote with atipamezole (0.5 mg/kg Antisedan, Pfizer Animal Health).
In the second series, pulmonary function was determined after 3 months (n=6/group) and animals did not receive antidote because they were sacrificed to study skeletal muscle function.
In vitro muscle contractile properties
After the pulmonary function measurements, muscle contractile properties were analyzed in a subgroup of animals after 3 months (second series) or after 6 months (first series) of CS or air exposure. Muscle force of two skeletal muscles, the soleus and EDL muscle, was investigated. The right soleus muscle and right EDL muscle of each animal were removed for measurements of in vitro contractile properties as previously described (Matuszczak et al., 2004). Both ends of each muscle were tied with silk sutures to serve as anchoring points. The muscles were suspended in a tissue bath containing Krebs solution (in mM/l: NaCl 137, KCl 4, CaCl2 2, MgCl2 1, KH2PO4 1, NaHCO3 12, glucose 6.5 and 0.03 d-tubocurarine chloride), equilibrated with 5% CO2 and 95% O2. The temperature in the tissue bath was maintained at 37°C using a thermostatically controlled water pump. The muscles were placed in between two zig-zag platinum stimulating electrodes, anchored at the bottom to a rigid support and at the top fastened to an isometric force transducer (Maywood, Hampshire, UK) connected to a micrometer. The optimal length (Lo) of each muscle was determined as the length at which peak twitch force was obtained. After 15 minutes of thermo-equilibration, the muscles were stimulated with a pulse duration of 0.2 ms and train duration of 500 ms for soleus muscle and 250 ms for EDL muscle. Maximal tetanic force was determined at 300 Hz for both muscles. The force frequency curve was obtained by stimulating at 1, 15, 30, 50, 80, 150, 250 and 300 Hz. Each stimulus was applied every 2 minutes. After the force frequency curve, muscles were fatigued by 250 ms stimulation at 80 Hz for EDL and by 500 ms at 40 Hz for soleus, each stimulus being applied every 3 seconds during 5 minutes. Fatigability of the muscles was measured by calculating the percentage decrease in force between the start of stimulation (F0s) and the end of stimulation (F300s). All stimulations were delivered through a home-made stimulator. Signals were amplified and recorded on computer via analog to digital conversion (DT-2801A) using Labdat (Labdat/Anadat, RHT-Infodat, Montreal, Canada). The muscles were blotted dry and weighed. CSA was calculated by muscle weight divided by specific density (1.056 g/cm3) and muscle Lo. All measured forces were normalized for CSA.
Bronchoalveolar lavage and histopathology of the lung
After muscle dissection and for each series, mice were euthanized with an overdose of pentobarbital. The lungs were lavaged three times with 0.7 ml sterile saline. The total cell counting was performed using a Bürker hemocytometer and cytospins (Shandon) were colored with May-Grünwald-Giemsa staining to determine differential cell counts. For the differential cell counting, 200 cells per mouse were counted to determine the number of macrophages, neutrophils and lymphocytes. After BAL, the heart-lung block was excised and fixed in 6% paraformaldehyde at a constant hydrostatic pressure of 20–25 cm fluid column for 24 hours. After dehydration and embedding in paraffin, sagittal sections were stained with H&E to evaluate lung inflammation and airspace enlargement. Airspace enlargement was quantified by measuring mean linear intercept (Lm). This was measured for each animal in 20 randomly selected fields per slide, at 200× magnification in a blinded manner. The Lm was calculated as the total length of the grid lines × random fields divided by the sum of the alveolar intercepts (Dunnill, 1964).
Histochemistry
After 3 (series 2) and 6 (series 1) months of CS or air exposure, skeletal muscle (left EDL muscle and left soleus muscle) were quickly frozen in isopentane cooled with nitrogen. Serial cross-sections of each muscle were stained with H&E to determine structural changes and with myofibrillar adenosine triphosphatase (ATP-ase) after preincubation at pH 4.5 to identify the different fiber types. On the basis of their histochemical reactions, fibers were classified as slow-oxidative type I, fast-glycolytic and oxidative type IIa, and fast-glycolytic type IIx/b fibers. The proportion and CSA were determined from the pixel number within the outlined borders at 10× magnification using a microscope, which was connected to a computerized image system. Around 150 fibers were used to measure CSA and the proportion of the different fibers.
Statistical analyses
All statistical analyses were performed with GraphPad Prism Software. To determine whether parameters were normally distributed, the D’Agostino and Pearson normality test was applied. All statistical analyses were performed using the unpaired t-test for differences between groups. A Pearson correlation test was performed to relate the results of lung function measurement and lung histology. P-values <0.05 were considered as significant. All the data in figures and graphs were reported as mean ± s.d.
TRANSLATIONAL IMPACT.
Clinical issue
Chronic obstructive pulmonary disease (COPD) is a respiratory disease characterized by airflow limitation that is not fully reversible, and by an abnormal inflammatory response in the lung. In addition to pulmonary disease, COPD is characterized by several systemic consequences, including weight loss, skeletal muscle wasting and muscle dysfunction. Skeletal muscle dysfunction is considered an important systemic consequence of COPD because it is associated with disease exacerbations, reduced quality of life and poor prognosis. The mechanisms that contribute to muscle dysfunction in humans with COPD are not completely understood. To better understand these systemic effects, particularly muscle dysfunction, improving existing animal models is fundamental. Most animal models of COPD represent limited features of human COPD and mainly focus on the lung disease. Some recent studies investigated peripheral muscle dysfunction in emphysematous mice but, until now, the effect of muscle dysfunction in a nose-only cigarette-smoke-exposed mouse model for COPD (the most relevant model of human COPD) had not been examined. Furthermore, serial lung function measurements have not been applied to such a model of COPD, which would provide important information about the progression of the disease over time.
Results
In this paper, the authors monitor disease in a nose-only cigarette-smoke-exposed mouse model of COPD by performing serial tests of lung function as well as assessing muscular dysfunction. Previously, they optimized a new method to perform repeated invasive lung function measurements in healthy mice. Here, they apply this method in a mouse model of COPD to detect changes in functional parameters such as total lung capacity and lung compliance. These parameters are impaired in the mice after 3 months of cigarette smoke exposure and become more pronounced after 6 months. These functional changes precede changes in lung histology, which become significant only after 6 months of cigarette smoke exposure. Furthermore, the authors provide evidence of dysfunction and a significant fiber-type shift from IIa to IIx/b in the soleus muscle after 6 months of cigarette smoke exposure.
Implications and future directions
These findings indicate that serial pulmonary function parameters are sensitive outcomes to monitor emphysema progression in a nose-only cigarette-smoke-exposed animal model of COPD. In addition, muscular changes and dysfunction, particularly in muscles with a mixed fiber-type composition, were observed after 6 months of cigarette-smoke exposure, showing that this model led to systemic problems resembling those seen in humans with COPD, in addition to inducing emphysema. This study supports the use of the nose-only cigarette-smoke-exposed mouse model of COPD to mimic both pulmonary and systemic features of the disease in humans, and suggests that it could be useful for studying therapeutic interventions.
Acknowledgments
We thank Petra Weckx for cutting and staining the histological sections of the muscles.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
M.R. participated in the study design, carried out the experiments, conducted data analysis and drafted the manuscript. K.M. performed the muscle contractile properties in this study. S.D.V. intubated the animals and measured lung function. D.T. isolated and fixated the skeletal and respiratory muscles. E.K.V. performed the lung histology and analyzed the results. M.D., W.J. and G.N.G.-R. conceived the study, participated in the study design and helped to draft the manuscript. All authors read and approved the final manuscript.
FUNDING
This work was supported by the ‘Fonds voor Wetenschappelijk Onderzoek Vlaanderen’ [FWO-Flanders G.0687.09]; and by AstraZeneca Pharmaceuticals. K.M. (postdoctoral research fellow) and W.J. (clinical investigator) are funded by FWO-Flanders.
REFERENCES
- Arbogast S., Smith J., Matuszczak Y., Hardin B. J., Moylan J. S., Smith J. D., Ware J., Kennedy A. R., Reid M. B. (2007). Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J. Appl. Physiol. 102, 956–964 [DOI] [PubMed] [Google Scholar]
- Ardite E., Peinado V. I., Rabinovich R. A., Fernandez-Checa J. C., Roca J., Barbera J. A. (2006). Systemic effects of cigarette smoke exposure in the guinea pig. Respir. Med. 100, 1186–1194 [DOI] [PubMed] [Google Scholar]
- Baltzan M. A., Scott A. S., Wolkove N., Bailes S., Bernard S., Bourbeau J., Maltais F. (2011). Fatigue in COPD: Prevalence and effect on outcomes in pulmonary rehabilitation. Chron. Respir. Dis. 8, 119–128 [DOI] [PubMed] [Google Scholar]
- Barreiro E., Peinado V. I., Galdiz J. B., Ferrer E., Marin-Corral J., Sanchez F., Gea J., Barbera J. A. (2010). Cigarette smoke-induced oxidative stress: A role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am. J. Respir. Crit. Care Med. 182, 477–488 [DOI] [PubMed] [Google Scholar]
- Bracke K. R., D’hulst A. I., Maes T., Moerloose K. B., Demedts I. K., Lebecque S., Joos G. F., Brusselle G. G. (2006). Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J. Immunol. 177, 4350–4359 [DOI] [PubMed] [Google Scholar]
- Brown R. H., Walters D. M., Greenberg R. S., Mitzner W. (1999). A method of endotracheal intubation and pulmonary functional assessment for repeated studies in mice. J. Appl. Physiol. 87, 2362–2365 [DOI] [PubMed] [Google Scholar]
- De Paepe B., Brusselle G. G., Maes T., Creus K. K., D’hose S., D’Haese N., Bracke K. R., D’hulst A. I., Joos G. F., De Bleecker J. L. (2008). TNFalpha receptor genotype influences smoking-induced muscle-fibre-type shift and atrophy in mice. Acta Neuropathol. 115, 675–681 [DOI] [PubMed] [Google Scholar]
- De Vleeschauwer S. I., Rinaldi M., De Vooght V., Vanoirbeek J. A., Vanaudenaerde B. M., Verbeken E. K., Decramer M., Gayan-Ramirez G. N., Verleden G. M., Janssens W. (2011). Repeated invasive lung function measurements in intubated mice: an approach for longitudinal lung research. Lab. Anim. 45, 81–89 [DOI] [PubMed] [Google Scholar]
- Decramer M., De Benedetto F., Del Ponte A., Marinari S. (2005). Systemic effects of COPD. Respir. Med. 99 Suppl. B, S3–S10 [DOI] [PubMed] [Google Scholar]
- Derave W., Eijnde B. O., Ramaekers M., Hespel P. (2005). Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp. Gerontol. 40, 562–572 [DOI] [PubMed] [Google Scholar]
- Dunnill M. S. (1964). Evaluation of a simple method of sampling the lung for quantitative histological analysis. Thorax 19, 443–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen M. P., Schols A. M., Baken W. C., Wesseling G. J., Wouters E. F. (1994). Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur. Respir. J. 7, 1793–1797 [DOI] [PubMed] [Google Scholar]
- Gea J., Barreiro E. (2008). Update on the Mechanisms of Muscle Dysfunction in COPD. Arch. Bronconeumol. 44, 328–337 [PubMed] [Google Scholar]
- Gosker H. R., Langen R. C., Bracke K. R., Joos G. F., Brusselle G. G., Steele C., Ward K. A., Wouters E. F., Schols A. M. (2009). Extrapulmonary manifestations of chronic obstructive pulmonary disease in a mouse model of chronic cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 40, 710–716 [DOI] [PubMed] [Google Scholar]
- Guerassimov A., Hoshino Y., Takubo Y., Turcotte A., Yamamoto M., Ghezzo H., Triantafillopoulos A., Whittaker K., Hoidal J. R., Cosio M. G. (2004). The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am. J. Respir. Crit. Care Med. 170, 974–980 [DOI] [PubMed] [Google Scholar]
- Harrison O. J., Foley J., Bolognese B. J., Long E., III, Podolin P. L., Walsh P. T. (2008). Airway infiltration of CD4+ CCR6+ Th17 type cells associated with chronic cigarette smoke induced airspace enlargement. Immunol. Lett. 121, 13–21 [DOI] [PubMed] [Google Scholar]
- Luthje L., Raupach T., Michels H., Unsold B., Hasenfuss G., Kogler H., Andreas S. (2009). Exercise intolerance and systemic manifestations of pulmonary emphysema in a mouse model. Respir. Res. 10, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mador M. J., Mogri M., Lewis M., Fournier M., Ray A. D., Michlin C., Farkas G. (2010). Exercise capacity in hamsters with elastase-induced emphysema compared to normal controls. Respir. Physiol. Neurobiol. 173, 16–22 [DOI] [PubMed] [Google Scholar]
- Maeno T., Houghton A. M., Quintero P. A., Grumelli S., Owen C. A., Shapiro S. D. (2007). CD8+ T cells are required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J. Immunol. 178, 8090–8096 [DOI] [PubMed] [Google Scholar]
- Maltais F., Sullivan M. J., LeBlanc P., Duscha B. D., Schachat F. H., Simard C., Blank J. M., Jobin J. (1999). Altered expression of myosin heavy chain in the vastus lateralis muscle in patients with COPD. Eur. Respir. J. 13, 850–854 [DOI] [PubMed] [Google Scholar]
- Mattson J. P., Martin J. C. (2005). Emphysema-induced reductions in locomotory skeletal muscle contractile function. Exp. Physiol. 90, 519–525 [DOI] [PubMed] [Google Scholar]
- Mattson J. P., Poole D. C., Hahn S. A., Musch T. I., Hinkle R. T., Isfort R. J. (2008). Maximal force is unaffected by emphysema-induced atrophy in extensor digitorium longus. Respir. Physiol. Neurobiol. 161, 119–124 [DOI] [PubMed] [Google Scholar]
- Matuszczak Y., Arbogast S., Reid M. B. (2004). Allopurinol mitigates muscle contractile dysfunction caused by hindlimb unloading in mice. Aviat. Space Environ. Med. 75, 581–588 [PubMed] [Google Scholar]
- Montes de Oca M., Loeb E., Torres S. H., De Santis J., Hernandez N., Talamo C. (2008). Peripheral muscle alterations in non-COPD smokers. Chest 133, 13–18 [DOI] [PubMed] [Google Scholar]
- Orlander J., Kiessling K. H., Larsson L. (1979). Skeletal muscle metabolism, morphology and function in sedentary smokers and nonsmokers. Acta Physiol. Scand. 107, 39–46 [DOI] [PubMed] [Google Scholar]
- Pauwels R. A., Rabe K. F. (2004). Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 364, 613–620 [DOI] [PubMed] [Google Scholar]
- Paz-Diaz H., Montes de Oca M., Lopez J. M., Celli B. R. (2007). Pulmonary rehabilitation improves depression, anxiety, dyspnea and health status in patients with COPD. Am. J. Phys. Med. Rehabil. 86, 30–36 [DOI] [PubMed] [Google Scholar]
- Pitta F., Troosters T., Spruit M. A., Probst V. S., Decramer M., Gosselink R. (2005). Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 171, 972–977 [DOI] [PubMed] [Google Scholar]
- Rabe K. F., Hurd S., Anzueto A., Barnes P. J., Buist S. A., Calverley P., Fukuchi Y., Jenkins C., Rodriguez-Roisin R., van Weel C., Zielinski J. (2007). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176, 532–555 [DOI] [PubMed] [Google Scholar]
- Schols A. M., Mostert R., Soeters P. B., Wouters E. F. (1991). Body composition and exercise performance in patients with chronic obstructive pulmonary disease. Thorax 46, 695–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour J. M., Spruit M. A., Hopkinson N. S., Natanek S. A., Man W. D., Jackson A., Gosker H. R., Schols A. M., Moxham J., Polkey M. I., Wouters E. F. (2010). The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur. Respir. J. 36, 81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson C. S., Birrell M. A. (2010). Moving towards a new generation of animal models for asthma and COPD with improved clinical relevance. Pharmacol. Ther. 130, 93–105 [DOI] [PubMed] [Google Scholar]
- Swallow E. B., Reyes D., Hopkinson N. S., Man W. D., Porcher R., Cetti E. J., Moore A. J., Moxham J., Polkey M. I. (2007). Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 62, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo Y., Guerassimov A., Ghezzo H., Triantafillopoulos A., Bates J. H., Hoidal J. R., Cosio M. G. (2002). Alpha1-antitrypsin determines the pattern of emphysema and function in tobacco smoke-exposed mice: parallels with human disease. Am. J. Respir. Crit. Care Med. 166, 1596–1603 [DOI] [PubMed] [Google Scholar]
- Tang K., Wagner P. D., Breen E. C. (2010). TNF-alpha-mediated reduction in PGC-1alpha may impair skeletal muscle function after cigarette smoke exposure. J. Cell Physiol. 222, 320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troosters T., Probst V. S., Crul T., Pitta F., Gayan-Ramirez G., Decramer M., Gosselink R. (2010a). Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 181, 1072–1077 [DOI] [PubMed] [Google Scholar]
- Troosters T., Sciurba F., Battaglia S., Langer D., Valluri S. R., Martino L., Benzo R., Andre D., Weisman I., Decramer M. (2010b). Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respir. Med. 104, 1005–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Strate B. W., Postma D. S., Brandsma C. A., Melgert B. N., Luinge M. A., Geerlings M., Hylkema M. N., Van den Berg A., Timens W., Kerstjens H. A. (2006). Cigarette smoke-induced emphysema: a role for the B cell? Am. J. Respir. Crit. Care Med. 173, 751–758 [DOI] [PubMed] [Google Scholar]
- Vanoirbeek J. A., Rinaldi M., De Vooght V., Haenen S., Bobic S., Gayan-Ramirez G., Hoet P. H., Verbeken E., Decramer M., Nemery B., et al. (2010). Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am. J. Respir. Cell Mol. Biol. 42, 96–104 [DOI] [PubMed] [Google Scholar]
- Watz H., Waschki B., Boehme C., Claussen M., Meyer T., Magnussen H. (2008). Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am. J. Respir. Crit. Care Med. 177, 743–751 [DOI] [PubMed] [Google Scholar]
- Watz H., Waschki B., Meyer T., Magnussen H. (2009). Physical activity in patients with COPD. Eur. Respir. J. 33, 262–272 [DOI] [PubMed] [Google Scholar]
- Whittom F., Jobin J., Simard P. M., LeBlanc P., Simard C., Bernard S., Belleau R., Maltais F. (1998). Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med. Sci. Sports Exerc. 30, 1467–1474 [DOI] [PubMed] [Google Scholar]
- Wright J. L., Cosio M. G., Churg A. (2008). Animal models of chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 295, L1–L15 [DOI] [PMC free article] [PubMed] [Google Scholar]