SUMMARY
We described previously a screening protocol in Drosophila melanogaster that allows us to identify small molecules that increase the killing effect of ionizing radiation in vivo in a multicellular context. The ability of this screen to identify agents that enhance the effect of radiation in human cancer models has been validated in published proof-of-concept studies. Here we describe an agent, identified by screening through two National Cancer Institute (NCI) small molecule libraries in Drosophila, that increases the effect of radiation. This agent, Bouvardin (NSC 259968), inhibits the elongation step of protein synthesis. We find that Bouvardin enhances the killing effect of X-rays in both Drosophila larvae and in human cancer cells. More detailed analysis showed that Bouvardin also increases the effect of radiation in clonogenic assays and in human cancer xenografts in mice. Finally, we present data that Bouvardin can also increase the efficacy of taxol. Regulation of translation is important to cancer biology. Current therapies target every aspect of cancer cell proliferation from growth factor signaling to cell division, with the exception of translation elongation. Our identification of Bouvardin as an enhancer of radio- and chemo-therapeutic agents suggests that targeting this niche has the potential to improve existing cancer therapies.
INTRODUCTION
Combination therapy, in which two or more anti-cancer agents are applied in combination, has more promise than monotherapy and is being assessed in multiple clinical trials (www.clinicaltrials.gov). Most new combinations being assessed, however, were identified empirically and might not be the best combinations available. We developed a proprietary screen (US Patent No. 7,695,899) to identify from the onset molecules that are effective in vivo in combination with radiation, a standard cancer therapy. This screen takes advantage of similarities in radiation biology between mammalian tumors and organ primordia of Drosophila larvae. Both are capable of regeneration through ‘accelerated repopulation’, in which surviving cells after radiation treatment proliferate even faster than before (Jaklevic and Su, 2004; Hall and Giaccia, 2005; Jaklevic et al., 2006). The screen searches for small molecules that inhibit this or any other process that confers radio-resistance. Briefly, in the screen, Drosophila larvae were irradiated with a dose of X-rays to produce partial lethality and were then cultured, post-irradiation, on food containing different small molecules. Thus, we were not searching for classical radiation sensitizers that are typically administered prior to or concurrently with radiation and act to increase the damaging effect of radiation. Instead, the screen is designed to uncover molecules that act on processes that occur following radiation exposure and are important for the regeneration and survival of multicellular tissues. Survival of larvae was measured 10 days later by quantifying the fraction of animals that successfully emerged as viable adult flies. Molecules that reduced the survival by more than two standard deviations from the mean of the population were carried forward into further characterization (Jaklevic et al., 2006).
We previously reported proof-of-concept data that the Drosophila screen can identify known enhancers of radiation therapy against human cancers (Jaklevic et al., 2006; Edwards et al., 2011). Here, we describe the results of screens through two molecule libraries from the National Cancer Institute Developmental Therapeutics Program (NCI-DTP). Besides known agents used in combination therapy with radiation, the screens identified inhibitors of translation elongation.
Protein synthesis is, of course, essential for the growth and proliferation of cells. Translation is therefore a fundamental cellular process much like DNA replication or transcription. Numerous agents that interfere with DNA replication, transcription or other fundamental cellular processes are used to inhibit the growth of rapidly proliferating cancer cells and are among US Food and Drug Administration (FDA)-approved cancer drugs. Yet, inhibitors of translation are underrepresented among approved anti-cancer agents. In fact, the only agent closest to a translation modulator that is approved for clinical use is a derivative of rapamycin, which inhibits TOR, a component of the PI3K signaling pathway that impinges upon ribosomal protein S6 and initiation of translation (Ciuffreda et al., 2010; Dancey, 2010). Even for rapamycin, inhibition of PI3K signaling is generally recognized as being relevant for cancer therapy, rather than the inhibition of translation.
The Drosophila screen for agents that enhance the effect of ionizing radiation (IR) yielded three inhibitors of translation elongation. We report here a study of one of these, Bouvardin, that shows that it enhances the effect of radiation in preclinical models of human cancer. Specifically, Bouvardin synergizes with radiation in several human cancer cell lines and enhances the effect of radiation in a xenograft model of human lung cancer. We discuss these results in the context of what is known about two translation inhibitors currently in clinical trials: Aplidin (by PharmaMar), which is in Phase III clinical trials for multiple myeloma; and Homoharringtonine (by ChemGenex), which is in clinical trials for several cancer types. We propose that inhibitors of translation elongation might be a new class of molecules with potential to improve the outcome of radiation therapy in clinical settings.
RESULTS
Drosophila screen identifies 16 natural products that enhance the effect of radiation
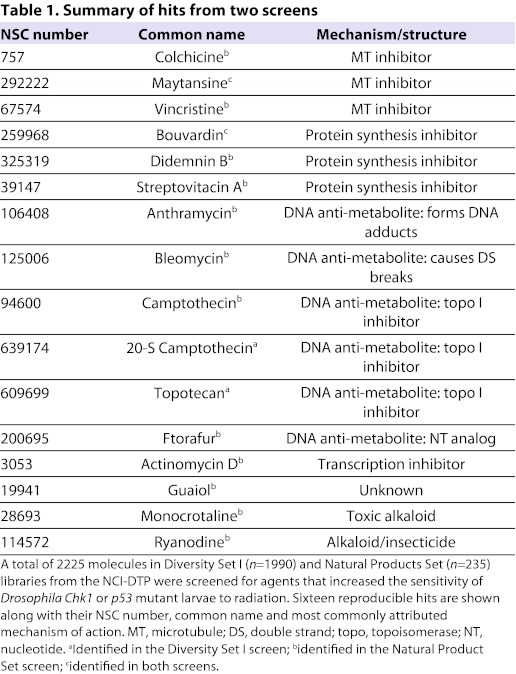
We began by screening through Diversity Set I from the NCI-DTP, which consists of 1990 molecules chosen for chemical structural diversity and not for biological activity. It yielded four molecules that enhanced the killing effects of radiation in Drosophila Chk1 homozygous null mutants (Jaklevic et al., 2006). Diversity Set I includes 42 molecules of natural origin (2% of the total), yet all four molecules identified in the Drosophila screen were of natural origin (Table 1). This led us to hypothesize that compounds of natural origin might be particularly efficacious in our screening model. Therefore, we next chose the Natural Product Set, also from the NCI-DTP, which contains 235 small molecules derived from natural sources. These molecules were identified initially in extracts from plants, microbes and marine organisms, but are now synthesized to sufficient purity for distribution by the NCI as plated sets. We used Drosophila Chk1 and p53 homozygous null mutant larvae in the screen, in order to mimic the loss of p53 and checkpoint controls that are frequently found in human cancers. The screen through the Natural Product Set yielded 14 molecules, translating into a hit rate of 6% (Edwards et al., 2011) (and this report). This is higher than the hit rate from Diversity Set I (4/1990 or 0.2%), which includes compounds of both natural and synthetic origins. These results support the hypothesis that compounds of natural origin are particularly efficacious in the Drosophila screening model.
Table 1.
Summary of hits from two screens
The 16 hits from the two screens (two were found in both screens) fall into three major classes according to their mode of action (Table 1): microtubule depolymerizing agents, DNA anti-metabolites (topoisomerase I inhibitors, nucleotide analogs, DNA-damaging agents, etc.) and protein synthesis inhibitors. Several microtubule poisons and DNA anti-metabolites are among FDA-approved anticancer agents. More relevant to our studies, microtubule poisons and DNA anti-metabolites are also among agents known to synergize with radiation in preclinical models of human cancer. For example, Topotecan and its analogs synergize with radiation in cells from human squamous cell carcinoma, melanoma, non-small cell lung carcinoma (NSCLC), malignant gliomas and small cell lung cancer (Boscia et al., 1993; Hennequin et al., 1994; Lamond et al., 1996a; Lamond et al., 1996b; Lamond et al., 1996c; Marchesini et al., 1996; Kohara et al., 2002). Synergy between Topotecan and radiation has been confirmed in xenografts of rhabdomyosarcoma (Chastagner et al., 2000). Microtubule poisons and DNA anti-metabolites are also among agents used in combination therapy with radiation to treat human cancers. These lines of evidence support the idea that Drosophila can be used to find agents that are efficacious in human cancers.
The third class of hits from the Drosophila screen consists of three inhibitors of protein synthesis that are not structurally related. Didemnin B (NSC 325319) and its analogs were originally extracted from tunicates or ‘sea squirts’, a marine invertebrate. Streptovitacin A (NSC 39147) and its analogs were originally extracted from soil bacteria. Bouvardin (NSC 259968) and its analogs were originally extracted from the plant Bouvardia ternifolia and related species (Jolad et al., 1977). All three inhibit the elongation step of translation but possibly by different mechanisms (Felicetti et al., 1966; Zalacain et al., 1982; SirDeshpande and Toogood, 1995; Ahuja et al., 2000).
Protein synthesis remains under-utilized as a drug target in cancer therapy. Therefore, we assessed the efficacy of the protein synthesis inhibitors found in the Drosophila screen in preclinical mammalian cancer models. Streptovitacin A, an analog of cycloheximide, has been assessed in clinical trials, in which it showed dose-limiting toxicity (Delta et al., 1961; Dederick et al., 1963; Field, 1963). Didemnin B is being actively pursued in the industry, and an analog from a related tunicate, Aplidin, is already in Phase III clinical trials for relapsed/refractory multiple myeloma (www.pharmamar.com/aplidin.aspx). Therefore, we chose to focus our studies on Bouvardin.
Bouvardin blocks polypeptide chain elongation by inhibiting both the GTP- and EF-2-dependent translocation of peptidyl-tRNA and the binding of aminoacyl-tRNA to the 80S ribosome (Zalacain et al., 1982). Previous studies have examined the effectiveness of Bouvardin as a single agent on tumor cells in culture as well as in murine tumor models (Tobey et al., 1978; Adwankar et al., 1984). In NCI-60 lines, for example, the IG50 for Bouvardin ranged from approximately 1 nM to 1 μM depending on the cell line (http://dtp.nci.nih.gov/). Bouvardin has also been assessed in murine xenograft models, where it showed activity in some but not all tumor models tested (Geran et al., 1972). Activity is defined by the NCI as <75% in treated (T)/control (C) tumor volume or 125% or more in T/C animal survival. Interestingly, a later finding that Bouvardin can cause arrest throughout the cell cycle marked it as a poor candidate for a useful chemotherapeutic agent (Tobey et al., 1978). Although Bouvardin might not be effective on its own, it still has the potential to synergize with other anti-cancer agents. Identification of Bouvardin in the Drosophila screen suggests that it could increase the effect of radiation in certain contexts. We set out to test this possibility.
To validate the identification of Bouvardin from the primary screen, we assessed the effect of this molecule on Drosophila larvae alone or in combination with radiation (Fig. 1). The screen was performed using irradiated mutant larvae. Hits from the screen were defined as those that reduced the survival of irradiated larvae compared with the population of drugs being tested (approximately 80 molecules at a time) or compared with DMSO controls in the re-test. A hit could therefore act by enhancing the effect of radiation, or by simply killing larvae as a single agent, or both. To distinguish among these possibilities, hits from the screen were re-tested against larvae with and without irradiation. We have shown previously that other hits thus tested (Camptothecin, Camptothecin derivatives and microtubule poisons) were able to enhance the effect of radiation while also having varying degrees of effect on their own (Jaklevic et al., 2006; Edwards et al., 2011).
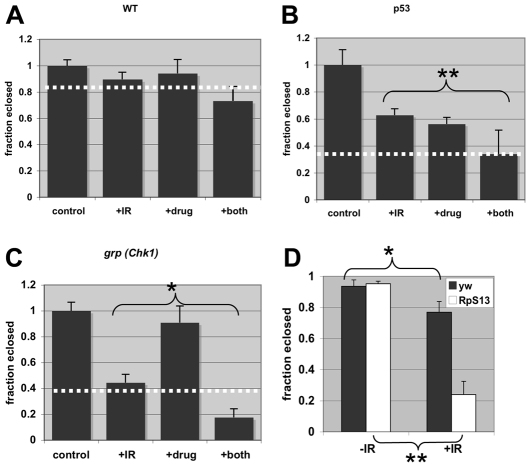
Fig. 1.
Radiation sensitivity of Drosophila larvae in the presence of Minute mutations or Bouvardin. Survival after each treatment is expressed as the fraction of animals that emerged (‘eclosed’) as viable adults. The averages have been normalized to the average fraction eclosed in no IR or drug control for the respective genotype. Error bars extend beyond fractional survival of 1.0 simply because of normalization. (A–C) Fraction eclosed for wild type (WT) and grp and p53 mutants at 16 μM of Bouvardin, with and without IR. The data are averages of three to eight biological replicates per sample. Dotted line on each graph denotes expected ‘fraction eclosed’ if drug and IR act additively. IR doses were 4000 rad for WT and grp, and 3000 rad for p53 because of the higher radiation sensitivity of the latter. (D) Fraction eclosed for the Minute mutant and yw controls after exposure to 0 (–IR) and 2000 (+IR) rad of X-rays. n ranged from 86 to 207 per genotype per treatment, in three biological replicates per sample. *P<0.01; **P<0.001. 100 rad=1 Gy. Error bar=1 s.d.
We first assessed the activity of Bouvardin in Drosophila alone or in combination with IR. Drug concentrations of 10–20 μM were used to approximate the conditions of the original screen. Third instar larvae were irradiated and cultured on medium containing drug. The survival of larvae was quantified 10 days later by measuring the rate of eclosion into adulthood. We find that Bouvardin increased the killing effect of radiation in a statistically significant manner in grp and p53 mutants but not wild type (compare ‘+IR’ and ‘+both’ samples in Fig. 1). The degree of the increase is consistent with the drug and radiation acting in an additive manner on p53 mutants and a synergistic manner on grp mutants. For example, IR alone reduces the fraction of p53 mutant flies undergoing eclosion to 0.63±0.05 and 16 μM of drug alone reduces it to 0.56±0.05. The combination of Bouvardin and IR reduces eclosion to 0.34, nearly the exact number expected (0.63±0.05×0.56±0.05=0.35±0.04) if the two treatments produced an additive effect (the formula used to compute the s.d. of the product of two numbers is in the Methods). The expected fraction of eclosion for an additive effect of drug and radiation are denoted with a white dotted line in each graph in Fig. 1. For grp mutants, the observed eclosion is lower than the expected for an additive effect, suggesting synergy between drug and radiation. We conclude that Bouvardin increases the effect of radiation in Drosophila mutants. We note that determination of an additive or synergistic interaction between Bouvardin and IR would require measuring the effect of single agents and the combination at multiple doses of each agent. This is what we did on human cancer cells, as described below.
Bouvardin is reported to inhibit translation elongation (Zalacain et al., 1982). We confirmed that Bouvardin inhibits translation in vitro (supplementary material Fig. S1). Nonetheless, drugs can have off-target effects. To more directly test the idea that inhibition of translation increases sensitivity to radiation, we compared the radiation sensitivity of two Drosophila Minute mutants and wild type. Minute genes are essential genes that encode structural components of the ribosome. We find that Minute heterozygotes with decreased ribosomal protein L36 or S13 gene dosage are more sensitive to radiation than yw controls (Fig. 1D for RpS13 and data not shown). These results support the idea that optimal translation capacity is needed for radiation survival.
Bouvardin synergizes with radiation on human cancer cells
The observation that Bouvardin enhanced the effect of radiation on Drosophila larvae led us to investigate whether this effect would translate to a mammalian system. In order to determine what cell lines should be used to create tumor xenografts in mice, we first used modified tetrazolium salt (MTT) assays for cell viability to test the effects of Bouvardin alone and in combination with radiation in human cancer cells. MTT assays measure cell viability by measuring mitochondrial enzyme function (see Methods for more information). We used 17 cell lines, including head and neck squamous cell carcinoma (HNC) and NSCLC lines.
Bouvardin and radiation synergized in most, but not all, of these cell lines. A representative sample of these data, from five cell lines, are in supplementary material Figs S2 and S3, and Table S1. We show here the data for an NSCLC cell line (H157) (Fig. 2). This line was chosen for further study because we observed synergy over a wide range of doses between Bouvardin and radiation in this cell line and because it is known to be amenable to xenografting in mice (Lee et al., 1992).
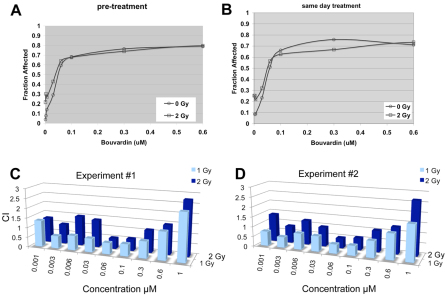
Fig. 2.
Bouvardin synergizes with IR in the human NSCLC line H157. MTT assays were used to determine the effect of Bouvardin in combination with IR. Cells were either treated with drug for 24 hours prior to irradiation (A; pre-treatment) or drug and radiation were applied on the same day (B; same day treatment). (A,B) Fraction of cells killed or otherwise eliminated [fraction affected (Fa)] at different doses of Bouvardin and 0 or 2 Gy of IR. (C,D) CI data from two experiments with pre-treatment protocol, to illustrate reproducibility. CIs below 1 indicate synergy. Bouvardin synergizes with IR under several experimental conditions.
In the absence of radiation, the drug has a half maximal inhibitory concentration [IC50; fraction affected (Fa)=0.5] in the nM range (Fig. 2A,B; ‘0 Gy’). Fa is determined by the formula, 1–(x/y), where x is the MTT signal for the experimental sample and y is the MTT signal for the untreated control. For example, if the MTT signal in the experimental is 30% of the MTT signal in the control, Fa is 0.7. Radiation increased the Fa in a dose-dependent manner in the absence of drug. The addition of the drug further increased the Fa for each dose of radiation. This happened whether the drug was added 24 hours before irradiation (pre-treatment; Fig. 2A) or concurrently (same day treatment; Fig. 2B).
The efficiency of Bouvardin in increasing the effect of radiation on cancer cells is measured in terms of combination index (CI) as determined by CalcuSyn software according to the method of Chou and Talalay (Chou and Talalay, 1983). This method is widely used in biomedical literature and has been applied to combinations of anti-tumor drugs (Chou and Talalay, 1987). CI values are calculated from Fa values for multiple drug and radiation doses such as those shown in Fig. 2A,B. CI computation takes into account the shape of the dose-response curve, the maximal effect, the intercept, and the IC50 values for drug at each dose of radiation and IC50 for radiation at each dose of drug (see Methods for more details). CI values can be highly variable at very low and very high drug concentrations (Zhao et al., 2004), which is what we observed also. CI values of above, below or equal to 1 indicate antagonistic, synergistic or additive effects, respectively. Our results show that Bouvardin synergizes with 1–2 Gy of radiation at many concentrations between 0.006 μM and 0.3 μM (Fig. 2).
Bouvardin enhances the effect of IR on H157 cells in clonogenic assays and xenografts
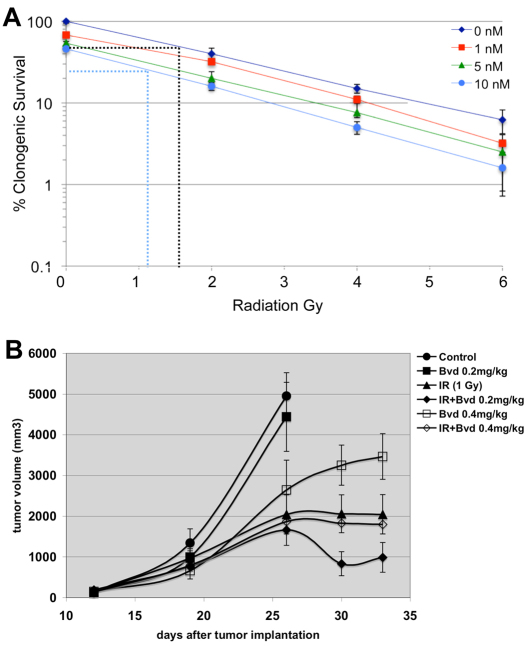
To assess the effect of Bouvardin, we used another measure of cell survival: clonogenic assays. We found that Bouvardin also increased the effect of radiation in reducing the clonogenicity of H157 cells (Fig. 3A). For example, IC50 for IR at 0 and 10 nM of Bouvardin, extrapolated from the graph in Fig. 3A, are approximately 1.5 Gy and 1.0 Gy (black and blue dotted lines, respectively).
Fig. 3.
Bouvardin enhances the effect of IR on H157 cells in clonogenic assays and xenografts. (A) Clonogenic assays were used to confirm the effects of Bouvardin in combination with radiation in H157 cells. Cells were plated in six-well plates at 1000 cells per well. 24 hours later, various doses (1, 5 and 10 nM) of Bouvardin were added, with media alone as a control. 24 hours after the addition of Bouvardin, the cells were irradiated at 0, 2, 4 or 6 Gy. Colonies were allowed to form for 2 weeks and then counted. The results are shown as a percentage of control (no drug, no IR). Dotted lines illustrate the extrapolation to get IC50 for IR at 0 and 10 nM of drug (see Results). Error bar = ±1 s.e.m. (B) The effect of Bouvardin (Bvd) and radiation on H157 xenografts in athymic mice. Bouvardin treatment began when tumors had reached approximately 200 mm3. Bouvardin was administered intraperitonealy twice a week, and radiation was administered at 24 hours after each drug administration. At day 26, animals in the control and drug-only groups had tumors that were too large and had to be sacrificed. Treatment continued for the other four groups for one more week. The difference in tumor volume between the combination-treated group and radiation-only group was statistically significant at day 30 (P=0.0387 on day 30; unpaired two-tailed t-test). The difference in tumor volume between the combination-treated group and radiation-only group remains on day 33 but is less significant (P=0.0967; unpaired two-tailed t-test). n=10 per group. The data points indicate mean tumor volumes ± 1 s.e.m.
The effectiveness of Bouvardin, IR and the combination of the two was next assessed in H157 tumor xenografts (Fig. 3B). We found that drug alone administered at 0.2 mg/kg body weight had little effect and 1 Gy of radiation alone showed partial tumor control. Importantly, the combination of drug and radiation showed improved control of tumor volume in a statistically significant manner (P=0.0387 on day 30; unpaired two-tailed t-test). Similar results were obtained in a repeat experiment (not shown). These data demonstrated a potential use for Bouvardin in cancer therapy, namely in combination with radiation. Bouvardin enhances the effect of radiation in Drosophila mutant larvae, human cancer cells and tumor xenografts in mice. The choice of drug concentration would be critical. At 0.4 mg/kg, the drug on its own showed tumor control but did not enhance the effect of radiation. Similar effects of higher drug dose were seen in two different experiments (only one shown here). We do not know the reason for this but note that drug concentration matters in how it combines with radiation in the cell-based results shown here (see Discussion).
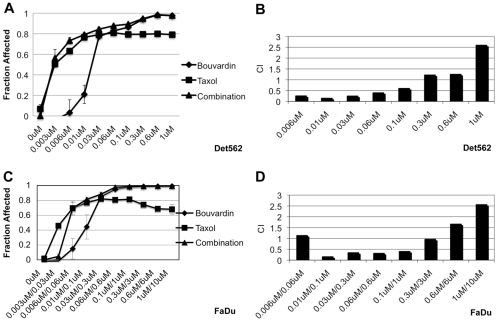
Bouvardin synergizes with taxol in human cancer cells
The therapeutic effect of radiation is thought to result from cell killing that follows radiation damage. Synergy between Bouvardin and radiation, we propose, is based, at least in part, on the ability of Bouvardin to prevent protein synthesis needed to recover from radiation-induced damage. If this was true, we might see synergy between Bouvardin and other agents that promote cell killing. To test this idea, we investigated whether Bouvardin also synergizes with a chemotherapeutic drug on two human HNC cell lines, Det562 and FaDu (Fig. 4). Det562 cells were more sensitive to taxol alone than are FaDu cells; this necessitates the use of lower taxol concentrations on the former. Nonetheless, Bouvardin showed synergy with taxol over a wide range of concentrations in both cell lines. In the same cell lines, Bouvardin showed a wide range of effects, synergistic to antagonistic, in combination with IR (supplementary material Fig. S3). Synergy was seen for up to 0.3 μM Bouvardin when combined with taxol (Fig. 3), but only at concentrations lower than 0.06 μM when combined with radiation (supplementary material Fig. S3).
Fig. 4.
Bouvardin shows synergy in combination with taxol in FaDu and Det562 HNC cell lines. CellTiter-Glo Luminescent Cell Viability Assays (Promega; G7570) were used to determine the effect of Bouvardin in combination with taxol on Det562 (A,B) and FaDu (C,D) cells. In A and B, the concentrations shown are those of each drug. For example, 0.1 μM=0.1 μM of Bouvardin and 0.1 μM of taxol. In C and D, the concentrations are those of Bouvardin and taxol, respectively. For example, 0.3 μM/3 μM means 0.3 μM of Bouvardin and 3 μM of taxol. (A,C) Fraction of cells killed or otherwise eliminated [Fraction affected (Fa)] at different doses of Bouvardin and taxol. (B,D) CI data for Det562 and FaDu cells are shown. CI below 1 indicates synergy. On Det652 cells, Bouvardin shows synergy in combination with taxol at doses ranging from 0.006 μM to 0.1 μM. On FaDu cells, Bouvardin shows synergy in combination with taxol at doses ranging from 0.01 μM to 0.3 μM for Bouvardin and 0.1 μM to 3 μM for taxol. Error bar is ±1 s.d.
DISCUSSION
We report here that three molecules with documented ability to inhibit translation elongation were identified in a screen for small molecules that enhance the effect of IR in Drosophila larvae. One of these also enhanced the effect of IR in human cancer cells and in tumor xenografts. In Drosophila, the combined effect of Bouvardin and IR was consistent with the combination being additive in p53 mutants and synergistic under some conditions in grp mutants. Drosophila p53 has an apoptotic role but not a cell cycle checkpoint role (Brodsky et al., 2000; Brodsky et al., 2004); p53-null mutants show reduced and delayed apoptosis compared with wild type. grp mutants, by contrast, show an apoptotic response that is comparable to that of wild type (Brodsky et al., 2004) but are deficient in compensatory proliferation to replace cells (Jaklevic and Su, 2004). Inhibition of protein synthesis by Bouvardin is expected to interfere with cellular growth that would be needed for compensatory proliferation and survival of irradiated larvae. We speculate that Bouvardin enhances radiation to a greater extent in grp mutants than in p53 mutants because the latter has reduced cell death and therefore reduced need to generate new cells. Additional experiments will be needed to show definitively that compensatory proliferation is really the basis for the effect of Bouvardin in Drosophila. But what is clear from these studies is that genotype differences can affect how Bouvardin and IR interact in the Drosophila model.
The interaction between Bouvardin and IR also varies from synergistic to antagonistic in different human cancer cell lines depending on experimental conditions. It is likely that underlying genetic differences among cell lines is a contributing factor. It is clear from our studies, however, that the interaction is synergistic in several cell lines and that Bouvardin can enhance the effect of IR in clonogenic assays and in xenograft models. We found that the concentration of the drug can affect the nature of its interaction with IR both in cells and in xenografts. A Bouvardin dose of 0.2 mg/kg had little effect on its own in the xenograft model but enhanced the effect of IR. A dose of 0.4 mg/kg, by contrast, had an effect on its own but did not enhance the effect of IR. If distributed homogeneously, 0.2 and 0.4 mg/kg would correspond to approximately 0.3 and 0.6 μM. In cell-based assays, 0.3 and 0.6 μM of drug acted, respectively, synergistically and additively/antagonistically with radiation, similar to the outcome in xenograft experiments (Fig. 2). More experiments will be needed to determine how well the cell culture results predict in vivo effects.
Bouvardin was found to synergize with another standard therapy, taxol, in cell-based assays. These results are in agreement with the finding that Didemnin B, another translation inhibitor that we found in the Drosophila screen, synergizes with doxorubicin in a murine leukemia model (Robert et al., 2009). These results are also in agreement with the finding that silvestrol, an inhibitor of translation initiation, synergizes with doxorubicin in the same murine model of cancer (Bordeleau et al., 2008). Collectively, these results suggest that translation inhibitors as a class of molecules might show clinical efficacy in combination with other anti-cancer therapies.
Increased translation capacity was recently shown to predict resistance to inhibitors of the mTOR-PI3K pathway, consistent with the idea that protein translation is integral to cancer biology (Ilic et al., 2011). Yet, protein translation remains under-utilized as a target for cancer therapy compared with other cellular processes. Indeed, current cancer therapies target every aspect of cancer cell growth and division, from growth factors at the cell surface, transcriptional activation that follows growth factor signaling, and DNA replication and mitosis that follow increased growth. The exception seems to be protein translation. One reason for this omission might be a bias that translation inhibitors lack specificity to target cancer cells and are therefore too toxic for use. Data show otherwise. For example, the median lethal dose (LD50) in mice of the translation inhibitors that we found are within the range shown by FDA-approved chemotherapy agents. LD50 for Bouvardin is 12.4 mg/kg; LD50 for vincristine, cisplatin and doxorubicin are 1.3, 6.6 and 12.5 mg/kg, respectively (www.chemcas.com). Moreover, multiple studies have now found that increased translation contributes to oncogenesis and that reducing translation can specifically inhibit the growth of cancer cells (Dolma et al., 2003; Gandin et al., 2008; Silvera et al., 2009). The reason for specificity seems to be that rapidly proliferating cancer cells rely on increased protein synthesis such that even partial inhibition can severely disrupt the growth of the former (Silvera et al., 2010). In isogenic primary human fibroblasts transformed with various combinations of oncogenes, the degree of oncogenic transformation inversely correlated with IC50 for Bouvardin (Dolma et al., 2003). In other words, the more cancerous the cells are, the more sensitive they are to Bouvardin. In another example, oncogenic MYC exerts its effect via increased translation, and reduction of translation by mutations in ribosome components reduces oncogenicity (Ruggero et al., 2004). Translation efficiency is also targeted by PI3K signaling and modulated by mutation in tumor suppressors PTEN and TSC. Despite numerous indications that translation control is important for cancer, there are no FDA-approved anti-cancer agents that target the ribosome. This situation might change soon, however. Two inhibitors of translation elongation, Aplidin and homoharringtonine, are being assessed in multiple clinical trials.
Our screen uncovered three inhibitors of protein synthesis, which led us to propose that inhibition of protein synthesis is the mechanism by which Bouvardin increases the effect of IR and taxol. We note, however, that natural products of >700 Da could have additional targets or additional mechanisms by which they act. In fact, an analog of Bouvardin, RA700, from a related plant has been shown to alter the interaction between F-actin and phalloidin, a bicyclic heptapeptide, in vitro, with an IC50 of 0.2 μM (Fujiwara et al., 2004). Thus, it remains possible that Bouvardin has at least two activities and that the ability of Bouvardin to increase the effect of IR relies on more than one of these. Three lines of data suggest strongly, however, that the anti-translation effect of Bouvardin is key. These are: the IC50 for Bouvardin in cells (Figs 2–4; supplementary material Figs S2, S3) and in in vitro translation reactions (supplementary material Fig. S1) are in a similar, nM, range; Bouvardin and mutations in Minute genes both increase the effect of radiation on Drosophila larvae; and another translation inhibitor, Streptovitacin A, behaves similarly to Bouvardin in the same cell line (supplementary material Fig. S4).
Bouvardin does not exhibit successful tumor control in combination with vincristine and cisplatin in an advanced murine leukemia model (Adwankar et al., 1984). Vincristine is a microtubule depolymerizing agent that interferes with mitosis, much like taxol, which interferes with mitosis by preventing microtubule dynamics. Taxol, however, shows synergy with Bouvardin in human HNC cells (this study). These results indicate that the behavior of drug combinations is complex and does not obey simple rules. A further complication is indicated by the fact that three translation inhibitors behaved differently in NCI-60 cell lines, where the most sensitive and most resistant grouping of cell lines for Bouvardin, Didemnin B and Streptovitacin A include common as well as different lines. Such behavior is no different than that of, for instance, different microtubule poisons, and serves to illustrate the complexity of cancer as a disease and challenges of drug development as a field.
METHODS
Drosophila assays
Wild-type flies were of the Sevelin stock. Mutants have been described before: p535A-1–4 results from targeted deletion of the gene (Rong et al., 2002), and is maintained and used as homozygotes. grp1 (Chk1 mutants) results from a p-element insertion and is a genetic null (Fogarty et al., 1997). Homozygotes of the latter were identified by the lack of GFP marker on the balancer chromosome. Minute mutants were of the genotypes y[1] w[*]; P[w[+mC]=lacW]RpS131/CyO and P[lacW]RpL36G0471 w67c23/FM7a. Because Minute mutants were in the yw or w background, y1w1 (Bloomington stock #1495) was used as ‘wild-type’ control in these experiments.
Feeding-stage third instar larvae were irradiated as previously described (Jaklevic et al., 2006). Briefly, 120-hour-old larvae were rinsed to remove food and passed through sizing sieves to obtain animals of uniform size. Larvae were placed in a Petri dish and irradiated using a TORREX X-ray generator, set at 115 kV and 5 mA (producing 2.4 Rads/second or 1.44 Gy/minute). Irradiated larvae were then cultured on cornmeal-agar media (Jaklevic et al., 2006) containing drug or DMSO carrier. In all experiments using human cells, cells in 96-well plates were irradiated with an RS2000 Biological Irradiator (Rad Source Technologies) delivering 1 Gy/minute.
Cell lines
HNC and NSCLC cell lines were kindly provided by David Raben and Paul Bunn (University of Colorado Cancer Center, CO). H157 cells were maintained in RPMI (Roswell Park Memorial Institute) medium with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT), and Detroit562 and FaDu were maintained in Dulbecco’s modified Eagle medium (DMEM) with heat-inactivated 10% FBS. These cell lines were maintained in a humidified incubator with 5% CO2. Cell lines were authenticated by DNA fingerprinting during the course of the experiments, at the Genomics Shared Resource core facility, University of Colorado Cancer Center.
Cell growth assays
The growth inhibitory effects of Bouvardin with IR were evaluated using an MTT assay (Carmichael et al., 1988). In the MTT assay, 1000–2000 viable cells were plated in 100 μl of growth medium in 96-well plates (Corning, Ithaca, NY). Following an overnight incubation, drug was added in varying concentrations and the plates were irradiated on the same day (co-treatment) or 24 hours later (pre-treatment) and incubated for 6–7 days. The tetrazolium salt was added at a concentration of 0.4 mg/ml to each well following the 6- to 7-day treatment. The plates were incubated with the salt for 4 hours at 37°C. At 4 hours, the medium was aspirated off, leaving the dark blue formazan product at the bottom of the wells. The reduced MTT product was solubilized by adding 100 ml of 0.2 N HCl in 75% isopropanol, 23% MilliQ water to each well. Thorough mixing was done using a Titertek multichannel pipetman. The absorbency of each well was measured using an automated plate reader (Molecular Devices, Sunnyvale, CA). All experiments were done in triplicate.
The growth inhibitory effects of Bouvardin with taxol were evaluated using the CellTiter-Glo Luminescent Cell Viability Assay (Promega; G7570). The CellTiter-Glo Reagent lyses cells and generates a luminescent signal proportional to the amount of ATP present. In this assay, 4000 viable cells were plated in 100 μl of growth medium in 96-well plates (Corning). Following an overnight incubation, both drugs were added in varying concentrations and incubated for 6 days. 100 μl of CellTiter-Glo Reagent was added to each well. Plates were incubated with mixing at room temperature for 30 minutes. Luminescence of each well was measured using an automated plate reader.
The use of Bouvardin and derivatives in combination therapy and standard agents (radiation and chemotherapeutics) is covered in the International Patent Application number PCT/US2011/063192.
CI computation
This is summarized from Zhao et al. (Zhao et al., 2004). Synergism cannot be invoked simply because a combination of two agents results in a larger effect than the sum of the effects of the individual agents. Unless all the dose-responses are perfectly linear, this will always be the case. Synergism can only be proven by measuring dose-responses with each individual agent and with the combination. Quantitative measure of synergy in terms of CIs were then calculated using CalcuSyn software (Biosoft), which uses the method of Chou and Talalay (Chou and Talalay, 1983). This method first computes drug-induced effect using the Hill equation:
where E is the measured effect; C is the drug concentration; Emax is the full range of drug effect, usually at or near 100%; IC50 is the drug concentration producing the median effect of 50%; and n is the curve shape parameter describing the steepness of the concentration-effect relationship. The Chou and Talalay method then linearizes the Hill equation by logarithmic transformation as:
where fu is the fraction of cells left unaffected after drug exposure; fa is the fraction of cells affected by the exposure; C is the drug concentration used; Cm is the concentration to achieve the median effect; and n is the curve shape parameter. Cm and n are equivalent to IC50 and n, respectively, in the Hill equation. The values of n (obtained from the slope), nlog(Cm) (obtained from the absolute value of the intercept) and, therefore, Cm, are obtained by plotting log(fu−1 – 1) versus log(C). These numbers are then used to compute the CI according to the formula: CI=(D)1/(Dx)1+(D)2/(Dx)2 (Chou and Talalay, 1983). (Dx)1 and (Dx)2 are the doses required to achieve a given effect level for each treatment, i.e. a specified value of Fa. (D)1 and (D)2 are the doses of each treatment in a given combination that gives the same Fa.
We note that CI values are highly variable at very low and very high drug concentrations. This has been described before (Zhao et al., 2004). CI value calculation includes the linearization of a concentration-effect curve by logarithmic transformation, which exaggerates errors at very low or very high drug effects (<10% and >90% Fa). Put another way, at very low doses, the effect of a single agent is not above background; it is therefore hard to increase it significantly. At very high doses, an agent on its own has a very high Fa; it is therefore hard to increase it significantly. Under these conditions, the addition of the second agent makes very little improvement to the outcome, thus appearing to antagonize with very high CI values.
Standard deviation
S.d. for expected fraction survival for co-treatment of drug and radiation is computed according to the formula for the s.d. of a product of two normally distributed variables. For two normally distributed variables with means m1 and m2 and s.d. of s1 and s2, the product will have mean m1m2 and the s.d.=square root of (m12s22 + m22s12 + s12s22) [page 140 of Menzel (Menzel, 1960)]. Fractional survival after genotoxin treatment shows a normal (Gaussian) distribution around a mean [figure 4 of Jaklevic et al. (Jaklevic et al., 2006) and our unpublished observations].
Clonogenic assays
The survival of cells treated with Bouvardin and IR was measured by performing standard clonogenic assays. Approximately 1000 cells were added per well in 2 ml of media in six-well plates. Following an overnight incubation, varying concentrations of drug were added to the wells. After 24 hours, the plates were irradiated at varying doses and incubated for ∼2 weeks or until colonies were large enough to count. The colonies were then stained with crystal violet (1% in methanol), rinsed 3× with water, and colonies with greater than 50 cells were counted using a dissection microscope. Fraction survival was calculated as a percentage of the control (no drug, no IR).
Xenograft assays
Female nude athymic mice that were 6- to 8-weeks old were purchased from Harlan Laboratories and housed in a pathogen-free facility approved by the American Association for the Accreditation of Laboratory Animal Care and met all current regulations and standards of the US Department of Agriculture, the US Department of Health and Human Services, and the National Institutes of Health. Animal procedures were carried out in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the University of Colorado. Cancer cells were grown to 80% confluence and harvested by trypsinization. Trypsin was neutralized with complete medium containing FBS. Cells were washed 3× with RPMI (no FBS) and resuspended in unsupplemented RPMI to a concentration of 2×106 per 100 μl. Cells were mixed 1:2 with Matrigel (BD Biosciences #354234) and 1×106 cells/mouse (100 μl) were injected subcutaneously into the rear flanks of athymic nude mice. Mice bearing tumors with a volume of ∼200 mm3 were randomly assigned to treatment groups of eight to ten animals each (control, drug alone, radiation alone or the combination). Each week, animals received drug diluted in saline (or saline carrier) by intraperitoneal injection on Mondays and Wednesdays, and a 2 Gy radiation dose on Tuesdays and Thursdays was delivered by X-ray irradiation. Mice were anesthetized with ketamine/xylazine before radiation and positioned under a lead shield such that only the tumor-bearing leg was exposed. Mice were treated for 3 weeks, and tumor growth was measured until volume exceeded 2 cm3, when animals were euthanized. Tumor volume was measured on Tuesdays and Fridays. Tumor volume was calculated using the formula V=(a2×b)/2, where a and b are the smallest and largest tumor diameters, respectively, as determined using calipers.
TRANSLATIONAL IMPACT.
Clinical issue
Radiation and taxanes are primary treatment choices for multiple types of cancer. A common problem remains the re-growth and recurrence of tumors that are not completely eradicated by cancer therapy. Compounds that can prevent the re-growth of cancers have the potential for use in combination therapies with standard agents for improved tumor control.
Results
A previously published screen for inhibitors of tissue maintenance after irradiation in Drosophila larvae identified three inhibitors of protein translation. The authors show here that one of these agents, Bouvardin, enhances the effect of radiation on Drosophila larvae in a genotype-specific manner, and enhances the effect of radiation on human cancer cells and xenografts in mice. Bouvardin also enhances the effect of taxol on human cancer cells.
Implications and future directions
Of the essential cellular processes that can be targeted by cancer therapy, translation has been under-exploited. This study provides evidence that inhibition of translation with Bouvardin can enhance the effect of radiation and taxol in several preclinical models, suggesting that targeting this function in tumor cells has the potential to improve existing cancer therapies.
Supplementary Material
Acknowledgments
We thank the Bloomington stock center for Drosophila stocks and the National Cancer Institute Developmental Therapeutic Program for the compounds, Chienyem Adaobi Nweke for help with Drosophila experiments, and Paul A. Bunn for lung cancer cell lines.
Footnotes
COMPETING INTERESTS
A patent has been filed based on this work (PCT/US2011/063192).
AUTHOR CONTRIBUTIONS
M.G. performed experiments, wrote sections of the paper and edited the manuscript; B.F. designed and performed experiments and edited the manuscript; D.Z., A.E., P.Y., S.S. and T.D. performed experiments; D.C.C. designed and performed experiments; D.R. conceived and designed experiments; T.T.S. conceived and designed experiments, performed experiments and wrote the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.008722/-/DC1
REFERENCES
- Adwankar M. K., Khandalekar D. D., Chitnis M. P. (1984). Combination chemotherapy of early and advanced murine P388 leukaemia with bouvardin, cis-diamminedichloroplatinum and vincristine. Oncology 41, 370–373 [DOI] [PubMed] [Google Scholar]
- Ahuja D., Vera M. D., SirDeshpande B. V., Morimoto H., Williams P. G., Joullie M. M., Toogood P. L. (2000). Inhibition of protein synthesis by didemnin B: how EF-1alpha mediates inhibition of translocation. Biochemistry 39, 4339–4346 [DOI] [PubMed] [Google Scholar]
- Bordeleau M. E., Robert F., Gerard B., Lindqvist L., Chen S. M., Wendel H. G., Brem B., Greger H., Lowe S. W., Porco J. A., Jr, et al. (2008). Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J. Clin. Invest. 118, 2651–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscia R. E., Korbut T., Holden S. A., Ara G., Teicher B. A. (1993). Interaction of topoisomerase I inhibitors with radiation in cis-diamminedichloroplatinum(II)-sensitive and -resistant cells in vitro and in the FSAIIC fibrosarcoma in vivo. Int. J. Cancer 53, 118–123 [DOI] [PubMed] [Google Scholar]
- Brodsky M. H., Nordstrom W., Tsang G., Kwan E., Rubin G. M., Abrams J. M. (2000). Drosophila p53 binds a damage response element at the reaper locus. Cell 101, 103–113 [DOI] [PubMed] [Google Scholar]
- Brodsky M. H., Weinert B. T., Tsang G., Rong Y. S., McGinnis N. M., Golic K. G., Rio D. C., Rubin G. M. (2004). Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol. Cell. Biol. 24, 1219–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J., Mitchell J. B., DeGraff W. G., Gamson J., Gazdar A. F., Johnson B. E., Glatstein E., Minna J. D. (1988). Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer 57, 540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastagner P., Merlin J. L., Marchal C., Hoffstetter S., Barberi-Heyob M., Vassal G., Duprez A. (2000). In vivo potentiation of radiation response by topotecan in human rhabdomyosarcoma xenografted into nude mice. Clin. Cancer Res. 6, 3327–3333 [PubMed] [Google Scholar]
- Chou T. C., Talalay P. (1983). Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol. Sci. 4, 450–454 [Google Scholar]
- Chou T. C., Talalay P. (1987). Applications of the median-effect principle for the assessment of low-dose risk of carcinogens and for the quantitation of synergism and antagonism of chemotherapeutic agents. In Bristol-Myers Symposium series: New Avenues in Developmental Cancer Chemotherapy (ed. Harrap K. R., Connors T. A.) New York: Academic Press [Google Scholar]
- Ciuffreda L., Di Sanza C., Incani U. C., Milella M. (2010). The mTOR pathway: a new target in cancer therapy. Curr. Cancer Drug Targets 10, 484–495 [DOI] [PubMed] [Google Scholar]
- Dancey J. (2010). mTOR signaling and drug development in cancer. Nat. Rev. Clin. Oncol. 7, 209–219 [DOI] [PubMed] [Google Scholar]
- Dederick M. M., Nevinny H. B., Hall T. C., Potee K. G. (1963). Preliminary report on human toxicity study of streptovitacin A. Cancer Chemother. Rep. 27, 81–86 [PubMed] [Google Scholar]
- Delta B. G., Pinkel D., Magtibay L., Hubbard J. (1961). Streptovitacin A in children with cancer. Cancer Chemother. Rep. 11, 45–49 [PubMed] [Google Scholar]
- Dolma S., Lessnick S. L., Hahn W. C., Stockwell B. R. (2003). Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 [DOI] [PubMed] [Google Scholar]
- Edwards A., Gladstone M., Yoon P., Raben D., Frederick B., Su T. T. (2011). Combinatorial effect of maytansinol and radiation in Drosophila and human cancer cells. Dis. Model Mech. 4, 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicetti L., Colombo B., Baglioni C. (1966). Inhibition of protein synthesis in reticulocytes by antibiotics. II. The site of action of cycloheximide, streptovitacin A and pactamycin. Biochim. Biophys. Acta 119, 120–129 [PubMed] [Google Scholar]
- Field J. B. (1963). Clinical evaluation of streptovitacin A. Cancer Chemother Rep. 31, 53–59 [PubMed] [Google Scholar]
- Fogarty P., Campbell S. D., Abu-Shumays R., Phalle B. S., Yu K. R., Uy G. L., Goldberg M. L., Sullivan W. (1997). The Drosophila grapes gene is related to checkpoint gene chk1/rad27 and is required for late syncytial division fidelity. Curr. Biol. 7, 418–426 [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Saito S. Y., Hitotsuyanagi Y., Takeya K., Ohizumi Y. (2004). RA-VII, a cyclic depsipeptide, changes the conformational structure of actin to cause G2 arrest by the inhibition of cytokinesis. Cancer Lett. 209, 223–229 [DOI] [PubMed] [Google Scholar]
- Gandin V., Miluzio A., Barbieri A. M., Beugnet A., Kiyokawa H., Marchisio P. C., Biffo S. (2008). Eukaryotic initiation factor 6 is rate-limiting in translation, growth and transformation. Nature 455, 684–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geran R. I., Greenberg N. H., Macdonald M. M., Schumacher A. M., Abbott B. J. (1972). Protocols for screening chemical agents and natural products against animal tumors and other biological systems (3rd Edition). Cancer Chemother. Rep. 3, 1–103 [Google Scholar]
- Hall E., Giaccia A. J. (2005). Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams & Wilkins [Google Scholar]
- Hennequin C., Giocanti N., Balosso J., Favaudon V. (1994). Interaction of ionizing radiation with the topoisomerase I poison camptothecin in growing V-79 and HeLa cells. Cancer Res. 54, 1720–1728 [PubMed] [Google Scholar]
- Ilic N., Utermark T., Widlund H. R., Roberts T. M. (2011). PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc. Natl. Acad. Sci. USA 108, E699–E708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklevic B., Uyetake L., Lemstra W., Chang J., Leary W., Edwards A., Vidwans S., Sibon O., Tin Su T. (2006). Contribution of growth and cell cycle checkpoints to radiation survival in Drosophila. Genetics 174, 1963–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklevic B. R., Su T. T. (2004). Relative contribution of DNA repair, cell cycle checkpoints, and cell death to survival after DNA damage in Drosophila larvae. Curr. Biol. 14, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolad S. D., Hoffmann J. J., Torrance S. J., Wiedhopf R. M., Cole J. R., Arora S. K., Bates R. B., Gargiulo R. L., Kriek G. R. (1977). Bouvardin and deoxybouvardin, antitumor cyclic hexapeptides from Bouvardia ternifolia (Rubiaceae). J. Am. Chem. Soc. 99, 8040–8044 [DOI] [PubMed] [Google Scholar]
- Kohara H., Tabata M., Kiura K., Ueoka H., Kawata K., Chikamori M., Aoe K., Chikamori K., Matsushita A., Harada M. (2002). Synergistic effects of topoisomerase I inhibitor, 7-ethyl-10-hydroxycamptothecin, and irradiation in a cisplatin-resistant human small cell lung cancer cell line. Clin. Cancer Res. 8, 287–292 [PubMed] [Google Scholar]
- Lamond J. P., Mehta M. P., Boothman D. A. (1996a). The potential of topoisomerase I inhibitors in the treatment of CNS malignancies: report of a synergistic effect between topotecan and radiation. J. Neurooncol. 30, 1–6 [DOI] [PubMed] [Google Scholar]
- Lamond J. P., Wang M., Kinsella T. J., Boothman D. A. (1996b). Concentration and timing dependence of lethality enhancement between topotecan, a topoisomerase I inhibitor, and ionizing radiation. Int. J. Radiat. Oncol. Biol. Phys. 36, 361–368 [DOI] [PubMed] [Google Scholar]
- Lamond J. P., Wang M., Kinsella T. J., Boothman D. A. (1996c). Radiation lethality enhancement with 9-aminocamptothecin: comparison to other topoisomerase I inhibitors. Int. J. Radiat. Oncol. Biol. Phys. 36, 369–376 [DOI] [PubMed] [Google Scholar]
- Lee M., Draoui M., Zia F., Gazdar A., Oie H., Bepler G., Bellot F., Tarr C., Kris R., Moody T. W. (1992). Epidermal growth factor receptor monoclonal antibodies inhibit the growth of lung cancer cell lines. J. Natl. Cancer Inst. Monogr. 13, 117–123 [PubMed] [Google Scholar]
- Marchesini R., Colombo A., Caserini C., Perego P., Supino R., Capranico G., Tronconi M., Zunino F. (1996). Interaction of ionizing radiation with topotecan in two human tumor cell lines. Int. J. Cancer 66, 342–346 [DOI] [PubMed] [Google Scholar]
- Menzel D. H. (1960). Fundamental formulas for physics. Nineola, NY: Dover Publications, Inc [Google Scholar]
- Robert F., Carrier M., Rawe S., Chen S., Lowe S., Pelletier J. (2009). Altering chemosensitivity by modulating translation elongation. PLoS ONE 4, e5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y. S., Titen S. W., Xie H. B., Golic M. M., Bastiani M., Bandyopadhyay P., Olivera B. M., Brodsky M., Rubin G. M., Golic K. G. (2002). Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 16, 1568–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero D., Montanaro L., Ma L., Xu W., Londei P., Cordon-Cardo C., Pandolfi P. P. (2004). The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat. Med. 10, 484–486 [DOI] [PubMed] [Google Scholar]
- Silvera D., Arju R., Darvishian F., Levine P. H., Zolfaghari L., Goldberg J., Hochman T., Formenti S. C., Schneider R. J. (2009). Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat. Cell. Biol. 11, 903–908 [DOI] [PubMed] [Google Scholar]
- Silvera D., Formenti S. C., Schneider R. J. (2010). Translational control in cancer. Nat. Rev. Cancer 10, 254–266 [DOI] [PubMed] [Google Scholar]
- SirDeshpande B. V., Toogood P. L. (1995). Mechanism of protein synthesis inhibition by didemnin B in vitro. Biochemistry 34, 9177–9184 [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Orlicky D. J., Deaven L. L., Rall L. B., Kissane R. J. (1978). Effects of Bouvardin (NSC 259968), a cyclic hexapeptide from Bouvardia ternifolia, on the progression capacity of cultured Chinese hamster. Cancer Res. 38, 4415–4421 [PubMed] [Google Scholar]
- Zalacain M., Zaera E., Vazquez D., Jimenez A. (1982). The mode of action of the antitumor drug bouvardin, an inhibitor of protein synthesis in eukaryotic cells. FEBS Lett. 148, 95–97 [DOI] [PubMed] [Google Scholar]
- Zhao L., Wientjes M. G., Au J. L. (2004). Evaluation of combination chemotherapy: integration of nonlinear regression, curve shift, isobologram, and combination index analyses. Clin. Cancer Res. 10, 7994–8004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.