SUMMARY
An indolent non-healing wound and insulin and/or insulin-like growth factor (IGF1) resistance are cardinal features of diabetes, inflammation and hypercortisolemia. Little is known about why these phenomena occur in so many contexts. Do the various triggers that induce insulin and/or IGF1 resistance and retard wound healing act through a common mechanism? Cultured dermal fibroblasts from rats and full-thickness excisional wounds were used as models to test the premise that reactive oxygen species (ROS) play a causal role in the development of IGF1 resistance and impaired wound healing under different but pathophysiologically relevant clinical settings, including diabetes, dexamethasone-induced hypercortisolemia and TNFα-induced inflammation. In normal fibroblasts, IGF1 initiated a strong degree of phosphorylation of insulin receptor substrate 1 (IRS1) (Tyr612) and Akt (Ser473), concomitantly with increased PI3K activity. This phenomenon seemed to be attenuated in fibroblasts that had phenotypic features of diabetes, inflammation or hypercortisolemia. Notably, these cells also exhibited an increase in the activity of the ROS–phospho-JNK (p-JNK)–p-IRS1 (Ser307) axis. The above-mentioned defects were reflected functionally by attenuation in IGF1-dependent stimulation of key fibroblast functions, including collagen synthesis and cell proliferation, migration and contraction. The effects of IGF1 on glucose disposal and cutaneous wound healing were also impaired in diabetic or hypercortisolemic rats. The ROS suppressors EUK-134 and α-lipoic acid, or small interfering RNA (siRNA)-mediated silencing of JNK expression, restored IGF1 sensitivity both in vitro and in vivo, and also ameliorated the impairment in IGF1-mediated wound responses during diabetes, inflammation and hypercortisolemia. Our data advance the notion that ROS constitute a convergence nexus for the development of IGF1 resistance and impaired wound healing under different but pathophysiologically relevant clinical settings, with a proof of concept for the beneficial effect of ROS suppressors.
INTRODUCTION
Wound healing is a dynamic biological process involving inflammation, re-epithelialization, angiogenesis and granulation-tissue formation. Growth factor receptors, as in the case of insulin-like growth factor-1 receptor (IGF1R), when bound by their ligands, initiate signaling cascades that culminate in the modulation of various cellular processes that are essential for wound healing, including cell proliferation, metabolic alterations, survival and differentiation (Werner and Roberts, 2003). Critical to the initiation and progression of these events are adaptor proteins, such as insulin receptor substrate (IRS) proteins, that serve as scaffolding proteins linking the activated receptors (e.g. IGF1R) to their downstream effectors, such as the phosphatidylinositol-3-kinase (PI3K) and RAS-Erk pathways (Boura-Halfon and Zick, 2009).
An IGF1-IGF1R signaling axis plays crucial and diverse roles in tissue development and function. It regulates cell cycle progression, apoptosis, immunity, inflammation and metabolism (Smith, 2010). Administration of IGF1 has been shown to improve insulin sensitivity, lower the risk of type 2 diabetes, and preserve β-cell mass and function (Rajpathak et al., 2009). Moreover, the IGF1 pathway also seems to protect against oxidative stress and the development of aging-related non-melanoma skin cancer (Lewis et al., 2010). To this end, it was proposed that altered IGF1 actions might contribute to the pathogenesis of a wide range of health conditions, including several common cancers (Jenkins and Bustin, 2004), osteoporosis (Zofkova, 2003), autoimmune diseases (Smith, 2010), insulin resistance (Rajpathak et al., 2009) and atherothrombosis (Conti et al., 2011).
A panoply of evidence supports the idea that IGF1 utilizes the same signaling intermediates as those seen within the insulin signaling cascade, which indicates that common mechanisms might function in IGF1 resistance and insulin resistance, with the latter phenomenon being extensively studied in the context of type 2 diabetes, Cushing syndrome and in vivo or in vitro exposure to supraphysiological concentrations of glucocorticoids (Severino et al., 2002; Frojdo et al., 2009). One important mechanism contributing to insulin resistance is the inhibition of IRS1 activation through phosphorylation at several inhibitory serine residues, including Ser307, Ser312 and Ser612 (Boura-Halfon and Zick, 2009). Oxidative stress and inflammation might serve as common mediators in promoting the IRS serine phosphorylation, possibly through a JNK-dependent pathway (Wellen and Hotamisligil, 2005).
Diabetes and hypercortisolemia represent major risk factors for the development of chronic non-healing wounds, with an annual cost of billions of dollars in the United States alone, often requiring many months of continuous treatment, with high recurrence rates. Although the exact pathophysiological mechanisms contributing to the aforementioned phenomenon are yet to be elucidated, it is thought that reduced responsiveness of cells at the wound site, especially fibroblasts, to the action of growth promoting polypeptides could contribute to delayed wound healing, at least when diabetes is also involved (Bitar, 1997; Eming et al., 2007; De Mattei et al., 2008). Similarly, enhanced reactive oxygen species (ROS) concentrations have also been shown to evoke disturbances in collagen biosynthesis, wound healing and IGF1 signaling (Siwik et al., 2001; Sienkiewicz et al., 2004; Schafer and Werner, 2008). There is evidence of increased ROS levels and decreased levels of expression and action of IGF1 in diabetic wounds (Bitar, 2000; Yu et al., 2007).
The current study examined the emerging premise that heightened state of oxidative stress (HSOS) constitutes a convergence nexus for the development of IGF1 resistance and impaired wound healing under different but pathophysiologically relevant clinical settings, i.e. diabetes, inflammation and hypercortisolemia. Moreover, the impact of the key ROS suppressors EUK-134 and α-lipoic acid (LA) on IGF1-mediated wound responses in the aforementioned conditions were also considered.
RESULTS
Rat fibroblasts with phenotypic features of diabetes and hypercortisolemia exhibit impaired IGF1-induced activation of PI3K-Akt signaling
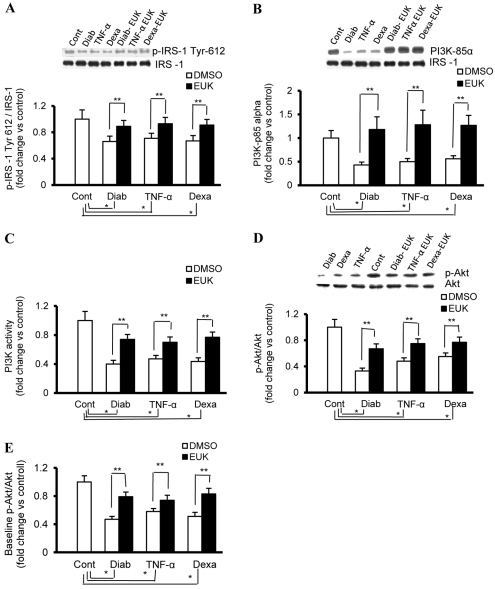
In fibroblasts, one of the major target cells of IGF1 during wound healing, we analyzed key intracellular molecules within the IGF1 signaling pathway using immunoprecipitation, western blotting and ELISA-based techniques. In control rat fibroblasts, 50 ng/ml IGF1 induced rapid and strong activation of IRS1, as evidenced by the phosphorylation of Tyr612, which is essential for IRS1 activation and to generate a docking site for the downstream PI3K (Fig. 1A). Consistent with the previous results, IGF1 also increased the activity of PI3K and promoted the phosphorylation of Akt at Ser473 in these cells (Fig. 1B–D). By contrast, this sequence of events was impaired in fibroblasts with phenotypic features of diabetes and hypercortisolemia (Fig. 1A–D). Because we were not able to detect baseline levels of p-Akt or p-JNK (please see below) reproducibly using western blotting, a fast activated cell-based (FAC) ELISA kit (Active Motif) was applied, with the resulting data documenting a significant decrease in the p-Akt:Akt ratio in the aforementioned disease-based models of fibroblasts (Fig. 1E).
Fig. 1.
Impaired IGF1 signaling in fibroblasts that have phenotypic features of diabetes, inflammation or hypercortisolemia. Fibroblasts with phenotypic characteristics of type 2 diabetes (Diab) and those of control fibroblasts treated chronically with either vehicle (Cont), TNFα (4 ng/ml every day for 4 days) or dexamethasone (Dexa; 20 ng/ml every other day for 8 days) were serum starved for 24 hours prior to treatment for 15 minutes with IGF1 (50 ng/ml). EUK-134 was applied concomitantly with Dexa or TNFα at 100 μM, a concentration that seems to have an optimum therapeutic benefit with a minimum detrimental effect on cell viability. (A,B) Whole-cell protein extracts from IGF1-treated cells were immunoprecipitated with IRS1 and probed with anti-p-IRS1 (Tyr612) (A) or anti-p85α (B) antibody. The membranes were stripped and reprobed with anti-total-IRS1 antibody. (C) PI3K activity in total protein extracts of IGF1-treated cells was determined using an ELISA-based assay (Echelon Biosciences). (D) Phosphorylated (p-Akt-Ser473) and total Akt in protein extracts of IGF1-treated cells were determined using a western-blotting-based technique. (E) Basal expression levels of Akt and its phosphorylated form (p-Akt-Ser473) in paraformaldehyde-fixed cells were quantified using FAC ELISA assays (Active Motif). Experiments were performed in triplicate on each of the cell lines used (n=4 for each). Results represent the means ± s.e.m. *Significantly different from corresponding control values at P≤0.05; **significantly different from corresponding DMSO-treated Diab, TNFα or Dexa values at P≤0.05.
siRNA-mediated downregulation of JNK activity ameliorated the defect in PI3K-Akt-dependent signaling during diabetes and hypercortisolemia
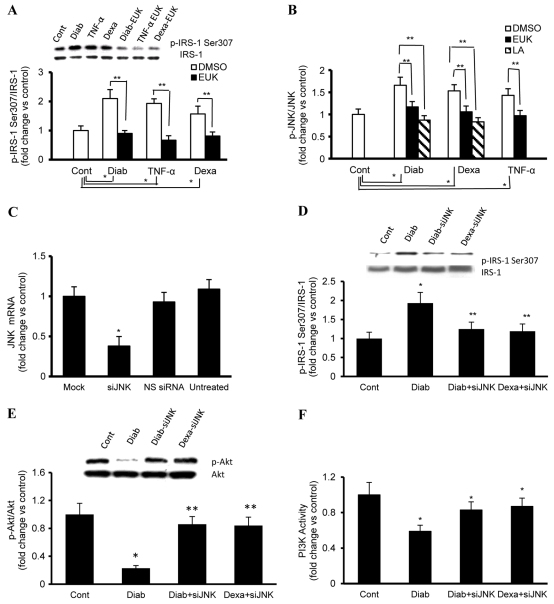
The impairment in the IRS1-PI3K-Akt signaling cascade described above in response to IGF1 prompted us to investigate the underlying mechanism of this phenomenon, and we initially focused on the phosphorylation of serine residues of IRS1, in particular p-Ser307. IRS1 p-Ser307 serves as a negative feedback regulator by ablating the ability of IRS1 to activate PI3K-dependent pathways (Tanti and Jager, 2009). Our data revealed that the level of IRS1 p-Ser307 was higher in fibroblasts with diabetic (2.1-fold) and hypercortisolemic (1.57-fold) phenotypes when compared with corresponding normal control values (Fig. 2A). Interestingly, enhanced phosphorylation of IRS1 Ser307 has also been observed in other conditions of insulin resistance, such as obesity (Tanti and Jager, 2009), pregnancy (Sevillano et al., 2007) or type 2 diabetes (Saltiel, 2001).
Fig. 2.
Genetic inhibition of JNK activity ameliorates the enhancement in p-IRS1 (Ser307) and the defect in PI3K-Akt-dependent signaling during diabetes and hypercortisolemia. (A) Whole-cell protein extracts of control (Cont), diabetic (Diab), dexamethasone (Dexa)- or TNFα-treated fibroblasts were immunoprecipitated with anti-IRS1 and probed with anti-p-IRS (Ser307). The membranes were stripped and reprobed with anti-total-IRS1 antibody. (B) Basal expression levels of JNK and its phosphorylated form (p-JNK) in paraformaldehyde-fixed fibroblasts were quantified using FAC ELISA assays (Active Motif). (C) Cells were transfected with 25 nM si-JNK, as indicated in the Methods, and the level of JNK mRNA was measured using TaqMan real-time PCR. The value of expression relative to that of mock controls (cells treated with transfection reagent only) was normalized against 18S rRNA. (D–F) Fibroblasts with phenotypic features of diabetes or hypercortisolemia were transfected with 25 nM si-JNK 48 hours before exposure to vehicle or IGF1 (50 ng), and cell lysates were collected and used for the assessment of (D) p-IRS1 (Ser307), (E) PI3K activity or (F) p-Akt:Akt ratio as described in the Methods. EUK-134 was applied at 100 μM, a concentration that seems to have an optimum therapeutic benefit with a minimum detrimental effect on cell viability. Experiments were performed in triplicate on each cell line used (n=4 for each). Results represent the means ± s.e.m. *Significantly different from corresponding Cont values at P≤0.05; **significantly different from corresponding DMSO/mock-treated Diab, TNFα or Dexa values at P≤0.05.
IRS1 contains numerous serine/threonine phosphorylation sites in amino acid sequence motifs, including Ser307, which is assessed in the present study. This amino acid is potentially recognized by different kinases, including the ROS-sensitive JNK (White, 2006). Accordingly, we measured the ratio of p-JNK:JNK as an indicator of the activity of this MAPK-kinase-based enzyme using a FAC ELISA kit (Active Motif) and found it to be enhanced in each of our models of IGF1 resistance (fold change vs control: diabetes 1.66, hypercortisolemic 1.53; Fig. 2B).
A gene silencing technique was used to evidence the participation of JNK in the development of IGF1 resistance during diabetes and hypercortisolemia. In this context, cultured fibroblasts were transfected with a silencing RNA [small interfering RNA (siRNA)] sequence directed against JNK mRNA, and the effectiveness of this strategy was evaluated using real-time PCR. A significant reduction in JNK at the mRNA level was demonstrated 24 hours following transfection, and this effect continued for up to 48 hours (Fig. 2C). As a control for the silencing specificity and for off-target effects, cells were also transfected with a commercially available non-silencing siRNA-like sequence (NS-siRNA), which does not recognize any eukaryotic sequence (Fig. 2C). The data derived from these studies revealed that silencing JNK in diabetic or hypercortisolemic fibroblasts resulted in a marked decrease in the level of IRS1 p-Ser307 (Fig. 2D) concomitantly with enhanced IGF1-mediated activation of PI3K-Akt-dependent signaling (Fig. 2E,F). These findings give credence to the notion that augmented JNK activity contributes significantly to the impairment in IGF1 actions during diabetes and hypercortisolemia.
Oxidative stress inhibits IGF-mediated PI3-Akt signaling but activates the JNK–p-IRS1 (Ser307) axis
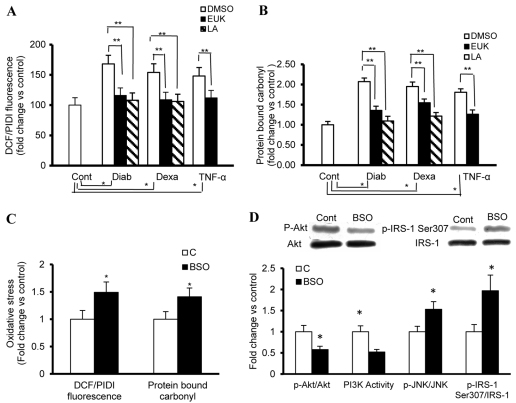
We next examined whether a common mechanism contributes to the JNK–p-IRS1 (Ser307)-induced impairment of IGF1 signaling during diabetes and hypercortisolemia. The initial focus was on ROS, which are by-products of mitochondrial respiration and enzymatic oxidases. These radicals have been shown to contribute to the development of insulin or IGF1 resistance in numerous cells and tissues, including muscles, liver, adipocytes and chondrocytes (Bitar et al., 2004; Bitar et al., 2005; Houstis et al., 2006; Hoehn et al., 2009). Our approach in addressing the above premise included: first, the confirmation that fibroblasts with phenotypic features of diabetes and hypercortisolemia exhibit high levels of ROS; second, the documentation that an induction of a state of chronic oxidative stress in normal fibroblasts might associate with both enhanced activity of p-IRS1 (Ser307) and a defect in IGF1-mediated activation of PI3K-Akt-dependent signaling; and, finally, the demonstration that ROS suppressors such as LA and EUK-134 ameliorate IGF1 resistance in diabetic or hypercortisolemic fibroblasts (see below). ROS levels in the current study were assessed by determining oxidation of the redox-sensitive dye DCF-DA. This probe is converted into a fluorescent product (DCF) on reaction with H2O2, hydroxyl radicals, nitric oxide or peroxynitrite (Myhre et al., 2003). The resulting ROS signal normalized to total cell number was markedly elevated in fibroblasts that had phenotypic features of diabetes and hypercortisolemia, compared with controls (Fig. 3A). Moreover, we also found that protein carbonyl levels, a marker of cumulative oxidative stress, were likewise increased in these disease states (Fig. 3B). The chronic model of oxidative stress was induced in cultured normal fibroblasts by perturbation of the glutathione redox cycle {cells were serially passaged while exposed to regular treatment with 10 μM L-buthionine-[S,R]-sulphoximine (BSO), an inhibitor of glutathione synthesis} (Kurz et al., 2004). The data derived from these studies recapitulated most of the abnormalities related to ROS (Fig. 3C) and IGF1 signaling (Fig. 3D) during diabetes and hypercortisolemia. Indeed, we have shown that cultured fibroblasts with a phenotypic feature of HSOS exhibit a marked elevation in the activity of the JNK–p-IRS (Ser307) axis (Fig. 3D). By contrast, IGF-mediated activation of PI3K-Akt-dependent signaling was suppressed in these cells (Fig. 3D).
Fig. 3.
Oxidative stress inhibits IGF-mediated PI3-Akt signaling but activates the JNK–p-IRS1 (Ser307) axis. (A,B) Fibroblasts from a rat model of type 2 diabetes (Diab) and control fibroblasts treated chronically with either vehicle (Cont), TNFα (4 ng/ml every day for 4 days) or dexamethasone (Dexa; 20 ng/ml every other day for 8 days) were used for the evaluation of the status of oxidative stress, as represented by the level of ROS and protein-bound carbonyls. (A) ROS generation of cells cultured in 96-well plates was measured using the fluorescence probes DCF-DA (5 μM). Values are expressed as DCF fluorescence after 1 hour of incubation normalized to total cell number derived by propidium iodide (PI) fluorescence in the presence of 160 μM digitonin (PIDI). (B) Protein-bound carbonyl levels in total cell extracts were determined using an ELISA-based technique. (C,D) Fibroblasts with phenotypic features of HSOS induced by BSO (10 μM) were used for the determination of the level of ROS and protein-bound carbonyls, and key elements of IGF1 signaling. (C) ROS and protein-bound carbonyl levels were measured as described above. (D) Whole-cell protein extracts were used for the assessment of the ratio of p-IRS1 (Ser307):IRS1 (immunoprecipitated with anti-IRS1 and probed with anti-p-IRS1 (Ser307), stripped and reprobed with ant-IRS1), the ratio of p-Akt (Ser473):Akt (western blotting) and PI3K activity (ELISA). In addition, the ratio of p-JNK:JNK in paraformaldehyde-fixed cells was quantified using FAC ELISA assays (Active Motif). EUK-134 or LA was applied at 100 μM (EUK) or 500 μM (LA), concentrations that seem to have an optimum therapeutic benefit with a minimum detrimental effect on cell viability. Experiments were performed in triplicate on each cell lines used (n=4 for each). *Significantly different from corresponding Cont values at P≤0.05; **significantly different from corresponding DMSO-treated Diab, TNFα or Dexa values at P≤0.05.
Diabetes- or hypercortisolemia-induced attenuation of IGF1 signaling alters wound-related fibroblast functions
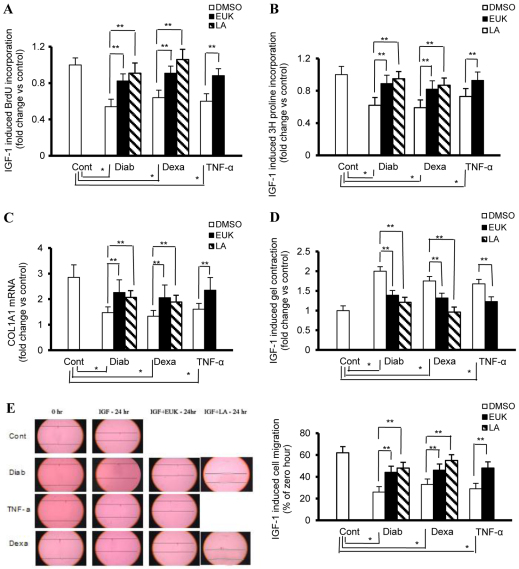
To investigate the mechanistic basis underlying the contribution of oxidative-stress-induced IGF1 resistance to impaired tissue repair mechanism during diabetes and hypercortisolemia, we used cultured dermal fibroblasts exhibiting the aforementioned pathogenetic features and studied key indices that are essential for wound healing, including collagen production and cell proliferation, migration and contraction. A BrdU cell proliferation assay revealed that treatment of control fibroblasts with IGF1 (50 ng/ml) for 24 hours caused an ∼fivefold increase in BrdU incorporation compared with the medium-only control (Fig. 4A). This action of IGF1 in inducing DNA synthesis was reduced in diabetic or hypercortisolemic fibroblasts by about 46 and 36%, respectively (Fig. 4A).
Fig. 4.
Key fibroblast functions that are essential for wound healing are altered as a function of diabetes, low-grade inflammation and hypercortisolemia. Fibroblast from a rat model of type 2 diabetes (Diab) and control fibroblasts treated chronically with either vehicle (Cont), TNFα (4 ng/ml every day for 4 days) or dexamethasone (Dexa; 20 ng/ml every other day for 8 days) were serum starved for 24 hours prior to treatment with IGF1 (50 ng/ml) and used for the assessment of collagen synthesis and cell proliferation, contraction and migration. EUK-134 and LA were used at concentrations of 100 μM and 500 μM, respectively. (A) BrdU incorporation into DNA in cells cultured in 96-well plates was used as an indicator for the determination of the rate of cell proliferation. (B) Radiolabelled proline uptake by cells cultured in 24-well plates was used as an indicator for the measurement of the rate of collagen synthesis. (C) TaqMan real-time PCR conducted on cDNA of cells cultured in six-well plates was used in the determination of the rate of COL1A1 expression. (D) The weight of floating collagen gel matrix of cells cultured in 24-well plates was used for the quantification of the rate of cell contraction. (E) An in vitro scratch wound assay of cells cultured in six-well plates was used to assess the rate of cell migration. Experiments were performed in triplicate for each of the four cell lines (n=4 for each). Results represent the mean ± s.e.m. *Significantly different from corresponding Cont values at P≤0.05; **significantly different from corresponding DMSO-treated Diab, TNFα or Dexa values at P≤0.05.
A radiolabelled proline uptake assay was used to study the impact of IGF1 on collagen synthesis in fibroblasts of different models of oxidative-stress-induced IGF1 resistance. The data revealed that, in control fibroblasts, IGF1 increased collagen synthesis by about 63%, a phenomenon that was markedly impaired in fibroblasts with diabetic (↓ 38%) or hypercortisolemic (↓ 41%) phenotypes (Fig. 4B). Consistent with these results, a TaqMan real-time PCR demonstrated that the increase in COL1A1 mRNA expression by IGF1 was also suppressed in these cells [Fig. 4C; diabetic (Diab) ↓ 43%, hypercortisolemia (Dexa) ↓ 53%].
We next examined the ability of IGF1 to reduce a floating collagen gel matrix, an indicator of cell contraction, with the resulting data confirming that the weights of the gels were higher in diabetic (twofold) and hypercortisolemic (1.47-fold) fibroblasts, as compared with corresponding control values (Fig. 4D). Finally, we assessed the ability of fibroblasts to migrate in response to IGF1 in each of our models of oxidative-stress-induced IGF1 resistance. In this context, a linear scratch was made in a fibroblast monolayer reaching confluence using a pipette tip, and fibroblast migration into the wounded area in the presence or absence of IGF1 was monitored over 24 hours. IGF1-induced migration in dermal rat fibroblasts was markedly reduced as a function of diabetes (↓ 58%) and hypercortisolemia (↓ 47%) (Fig. 4E).
TNFα in cultured fibroblast initiates cellular responses that mimics those produced by diabetes and dexamethasone-induced hypercortisolemia
The above data clearly indicated that HSOS, IGF1 resistance and in vitro impaired wound healing are characteristic features of diabetes and dexamethasone-induced hypercortisolemia. Dexamethasone signals through a nuclear hormone receptor and is known for its anti-inflammatory effect. Interestingly, TNFα, a proinflammatory cytokine exerting an effect through a cytokine membrane receptor, has also been shown to be associated with insulin resistance under various clinical and experimental settings (McCall et al., 1992; Hotamisligil et al., 1995). Accordingly, we decided to test the premise that TNFα, which has a distinct and very different signaling pathway from that of dexamethasone, might mimic the various cellular responses induced by hypercortisolemia. The current finding supports this proposition because it showed evidence for IGF1 resistance (Fig. 1A–E), HSOS (Fig. 3A,B) and impaired in vitro wound healing (Fig. 4A–E) in control fibroblasts exposed chronically to TNFα.
ROS suppressors ameliorate oxidative-stress-induced IGF1 resistance and impaired wound healing in diseased fibroblasts
To assess whether a cause and effect relationship exists between ROS and impaired wound healing or IGF1 resistance, we used selected ROS suppressors: the small antioxidant molecules LA and EUK-134. EUK-134 is derived from a compound with SOD activity that has been modified to obtain a strong catalase activity and it diffuses freely through the plasma membrane. By contrast, LA exhibits dual effects in which it scavenges ROS and enhances the expression of endogenous antioxidant enzymes (Smith et al., 2004). The data related to these studies clearly demonstrated that these antioxidants were able to lessen the HSOS (Fig. 3A,B) and to correct the defect in IGF1 signaling (Fig. 1A–E, Fig. 2A,B) in fibroblasts with diabetic, inflammatory or hypercortisolemic phenotypes. Moreover, in the aforementioned disease states, this treatment also ameliorated the impairment in key fibroblast functions that are essential for wound healing (Fig. 4A–E), including cell proliferation (Fig. 4A; EUK-134/LA vs DMSO % increase: Diab 43/52, Dexa 30/47, TNFα 27/N.D.), collagen synthesis (EUK-134/LA vs DMSO % increase: Fig. 4B, Diab 52/68, Dexa 42/65, TNFα 47/N.D.; Fig. 4C, Diab 54/41, Dexa 55/42, TNFα, 46/ND), cell contraction (Fig. 4D; EUK-134/LA vs DMSO % decrease: Diab 30/39, Dexa 25/45, TNFα, 27/N.D.) and cell migration (Fig. 4E; EUK-134/LA vs DMSO % increase: Diab 69/84, Dexa 39/66, TNFα 65/N.D.).
Effects of IGF1 diminution on glucose disposal and cutaneous wound healing during diabetes and hypercortisolemia
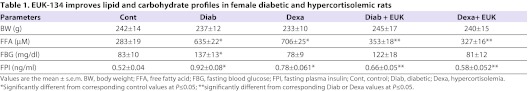
This study was intended to extend the above-described observations from cellular levels to in vivo models of excisional wounds and IGF1 resistance. Initial data confirmed that diabetic or hypercortisolemic animals exhibited a marked increase in fasting plasma insulin, free fatty acid and glucose [only in Goto Kakizaki (GK) rats, a model for non-obese type 2 diabetes] levels when compared with corresponding control values (Table 1). As for the GK rats, we have established previously that these animals exhibited hepatic and skeletal muscle insulin resistance in connection with increased key markers of oxidative stress (Bitar et al., 2004; Bitar et al., 2005), features that were mimicked in animals with hypercortisolemia (our unpublished observations).
Table 1.
EUK-134 improves lipid and carbohydrate profiles in female diabetic and hypercortisolemic rats
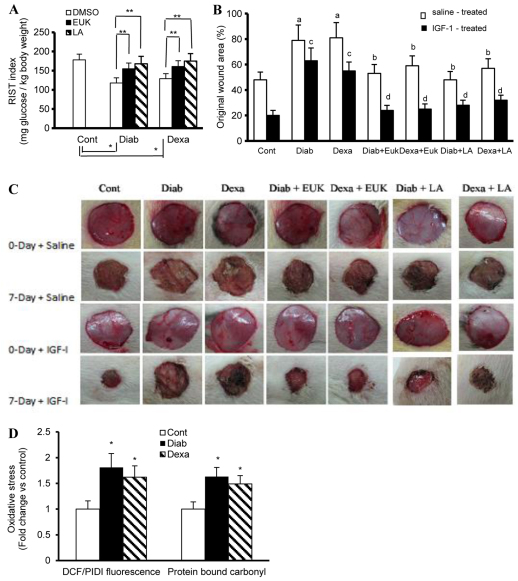
Next, the rapid insulin sensitivity test (RIST) was used to assess IGF1 sensitivity (e.g. total amount of glucose, mg/kg body weight needed to maintain euglycemia following IGF1 infusion), whereas the rate of healing was evaluated using a 7-day-old full-thickness dermal wound. Our data revealed that IGF1 sensitivity was markedly reduced as a function of diabetes (↓ 34%) and hypercortisolemia (28%) (Fig. 5A). This impairment in IGF1 systemic action in the aforementioned disease states was significantly improved following EUK-134 and LA treatment (Fig. 5A; EUK-134/LA vs vehicle % increase: Diab 31/42, Dexa 25/36). As for our in vivo wound healing studies, we showed that the 7-day diabetic or hypercortisolemic wounds were larger than corresponding control values (Fig. 5B,C). IGF1-based therapy involving IGF1 and IGFBP1 at a ratio of 5:1 accelerated the closure of cutaneous wounds by about 41% in control animals (Fig. 5B,C). This favorable effect of IGF1 on the healing process was eliminated in diabetic or hypercortisolemic animals (Fig. 5B,C). More interestingly, pretreatment with EUK-134 restored the sensitivity of diabetic or hypercortisolemic wounds to the action of IGF1 (Fig. 5B,C). A similar finding was also observed using the other well-known antioxidant, LA. Consistent with these in vivo results, we also confirmed that cells isolated from 7-day-old diabetic or hypercortisolemic wounds showed a characteristic feature of HSOS, as exemplified by the increased levels of ROS and protein-bound carbonyls (Fig. 5D). To this end, our data support the notion that HSOS during diabetes and hypercortisolemia contributes, at least in part, to the impairment in wound tissue sensitivity to the action of IGF1.
Fig. 5.
Effects of IGF1 on glucose disposal and cutaneous wound healing in control (Cont), diabetic (Diab) and hypercortisolemic (Dexa) animals. (A) IGF1 sensitivity as indicated by the rate of glucose metabolism was evaluated in Cont, Diab (GK rats, a genetic model of type II diabetes) and Dexa (Wistar rats receiving 5 μg Dexa/kg/day, subcutaneously) animals using the RIST with IGF1 (200 μg/kg) infused for 5 minutes instead of insulin. (B,C) Full-thickness excisional wounds induced in Cont, Diab and Dexa animals were subjected to IGF1 and IGFBP1 pluronic acid gel-based therapy. Wound closure was studied at 7 days following wound initiation. (C) Photographs of the macroscopic kinetic analysis of wound sites were taken. (B) Quantitation of data from C, demonstrating the proportion of wound remaining open relative to the initial wound area at the 7-day time point following injury. EUK-134 (12.5 mg/kg) or LA (50 mg/kg) were administered by i.p. injection every other day for 30 days prior to wound induction and this form of therapy continued during the course of wound healing. Similarly, animals used in the IGF1 sensitivity studies were also subjected for 30 days to EUK-134 and LA treatment. (D) Cells isolated from the 7-day diabetic or hypercortisolemic wounds were assessed for oxidative stress using fluorescence- and ELISA-based technique. Results are expressed as the mean ± s.e.m. of six to eight animals/group. *Significantly different from corresponding Cont values at P≤0.05; **significantly different from corresponding DMSO-treated Diab and Dexa animals at P≤0.05; asignificantly different from corresponding Cont saline-treated values at P≤0.05; bsignificantly different from corresponding saline-treated Diab or Dexa values at P≤0.05; csignificantly different from corresponding control IGF1-treated values at P≤0.05; dsignificantly different from corresponding IGF1-treated Diab or Dexa values at P≤0.5.
Overall, our data could lead to a number of predictions: firstly, a clinical condition associated with IGF1 resistance and impaired wound healing might also exhibit evidence of increased ROS levels, and secondly, diseases that elicit HSOS (e.g. diabetes, hypercortisolemia, inflammation) are likely to cause IGF1 resistance and impaired wound healing. Obviously, further studies need to be conducted in this regard.
DISCUSSION
An indolent non-healing wound constitutes a major health problem that predisposes individuals to infection, long-term morbidity and mortality, especially in those in high-risk groups, including the elderly and those with diabetes or hypercortisolemia (Bitar, 1998; Williams and Harding, 2003; Khanolkar et al., 2008; Milne, 2008). Accordingly, there is an urgent need firstly to understand the underlying mechanisms of this phenomenon and secondly to develop an effective strategy to accelerate the processes of tissue repair under various conditions of impaired wound healing. In this connection, wound healing and tumor growth share many common features (Dvorak, 1986) and it would thus be of value to take advantage of the emerging information of growth promoting signaling pathways in cancer cells (e.g. PI3K-Akt-GSK pathway) for the benefit of understanding and treating impaired wound healing during diabetes and hypercortisolemia.
The current study showed that IGF1-induced activation of the PI3K-Akt pathway was attenuated in fibroblasts that had phenotypic features of diabetes or hypercortisolemia. By contrast, an enhancement in the activity of the ROS–JNK–p-IRS1 (Ser307) axis was evident in these disease states. The in vitro and in vivo functional relevance of IGF1 resistance was illuminated by our findings documenting that IGF1-induced collagen synthesis, cell proliferation and in vitro repair of a scratch wound was reduced in fibroblasts exhibiting diabetic or hypercortisolemic phenotypes. Interestingly, the ability of this growth-promoting polypeptide to stimulate cutaneous ulcer healing was also impaired as a function of diabetes or hypercortisolemia. Most of the aforementioned abnormalities were ameliorated following the institution of antioxidant or Akt mimetic therapy.
Oxidative stress is involved in the development of endothelial dysfunction, insulin resistance and impaired wound healing (Bitar et al., 2004; Houstis et al., 2006; Sen and Roy, 2008). IGF1R and its ligand are expressed during the course of wound healing and are implicated in re-epithelialization and granulation-tissue formation in skin wounds (Werner and Roberts, 2003; Beckert et al., 2007). In addition, IGF1R, like the insulin receptor, utilizes the PI3K–Akt–GSK-3β-dependent pathway (Werner and Roberts, 2003; Beckert et al., 2007). Accordingly, we examined the premise that ROS constitute a common convergence nexus in the induction of IGF1 resistance and possibly impaired wound healing (see below) under different but pathophysiologically relevant clinical settings, i.e. diabetes and TNFα- or dexamethasone-induced inflammation and hypercortisolemia, respectively. In this context, control fibroblasts exposed chronically to dexamethasone exhibited HSOS (e.g. ↑ ROS, ↑ protein-bound carbonyls) in addition to becoming IGF1 resistant, as evidenced by an impaired ability of IGF1 to stimulate the PI3K-Akt-dependent pathway. Intriguingly, in addition to elevating ROS in control fibroblasts, TNFα, which is known to have a quite distinct cellular response pathway from that of dexamethasone, also led to IGF1 resistance. Analogous data were obtained using cultured fibroblasts retrieved from GK diabetic rats. Collectively, these data are consistent with the notion that ROS might be a key feature in the aforementioned three models of IGF1 resistance. Indeed, a genome-wide gene expression analysis of cultured fibroblasts with phenotypic features of diabetes, inflammation or hypercortisolemia revealed that a substantial fraction of the overlapping expressed genes among these cell lines are related to the biology of ROS (our unpublished observations). The current findings are somewhat relevant to previously reported results showing disturbances in IGF1R signaling in cultured human dermal fibroblasts exposed chronically to the oxidative-stress-inducing agent tert-butyl hydroperoxide (Sienkiewicz et al., 2004). These oxidative-stress-based abnormalities are not unique to cultured fibroblasts: sublethal doses of hydrogen peroxide attenuated the neuroprotective effect of IGF1 in cultured cerebellar granule neurons (Zhong and Lee, 2007). Moreover, the ability of IGF1 to stimulate PI3K-Akt signaling is markedly suppressed in tert-butyl-hydroperoxide-treated human cultured chondrocytes (Yin et al., 2009). It is worth noting that 3T3-L1 adipocytes and l6 myotubes exposed to various stress-related insults also develop resistance to the action of insulin (Houstis et al., 2006; Hoehn et al., 2009).
The above-described findings raised a number of issues that necessitate further investigation, as discussed below.
What downstream processes translate elevated ROS levels into IGF1 resistance?
ROS seem to alter various intracellular processes involving phospholipase C, Foxo, p53, MAPK and other proteins in a cell-type-specific manner. One prediction to consider in the current study is that ROS induce IGF1 resistance via a JNK–p-IRS1 (Ser307)-mediated mechanism. In this vein, oxidative stress is known to activate JNK and this MAPK-based molecule seems to phosphorylate the Ser307 residue of IRS1 (Aguirre et al., 2000; Hirosumi et al., 2002). The latter molecule, through its interference with PI3K recruitment, inhibits the PI3K-Akt-dependent pathway. Contrastingly, suppression of JNK activity through genetic knockout or an inhibitory peptide improves insulin sensitivity in mice and in cultured cells (Bennett et al., 2003; Lee et al., 2003; Tuncman et al., 2006). Our studies documented that JNK activity and the levels of IRS1 p-Ser307 were elevated in each of the three models of IGF1 resistance. To this end, it is not unreasonable to suggest that the impairment in IGF1 signaling during diabetes (GK fibroblasts), inflammation (TNFα-treated fibroblasts) and hypercortisolemia (dexamethasone-treated fibroblasts) might stem from ROS-induced activation of the JNK–p-IRS1 (Ser307) axis. A documentation regarding this cause and effect relationship between ROS and IGF1 resistance is best illuminated by the marked improvement in IGF1 sensitivity following the administration of the ROS suppressors EUK-134 and LA. Moreover, we also showed that a chronic model of oxidative stress induced in cultured fibroblasts by perturbation of the glutathione redox cycle [cells were serially passaged while being exposed to regular treatment with 10μM BSO, an inhibitor of glutathione synthesis (Kurz et al., 2004)] recapitulated most of the abnormalities seen in IGF1 signaling during diabetes, inflammation and hypercortisolemia.
What is the functional relevance of the ROS-mediated attenuation in fibroblast IGF1 signaling during diabetes, inflammation and hypercortisolemia?
This notion was examined by assessing key fibroblast functions that are essential for wound healing, namely collagen synthesis and cell proliferation, migration and contraction. Some of these cellular processes have been shown to respond to IGF1 and seem to be regulated by the PI3K–Akt–GSK-3β network (Rolfe et al., 2007; Bujor et al., 2008; Cain and Ridley, 2009). Our data disclosed that, in control fibroblasts, IGF1 promoted collagen deposition and enhanced cell proliferation and contraction. Moreover, using the scratch wound assay for in vitro repair, we were able to demonstrate that IGF1 induced wound closure in these cells via a PI3K-dependent mechanism (here we used PI-103, a selective class I PI3K inhibitor). Interestingly, the responses of most of these wound-related processes to IGF1 were impaired in fibroblast of type 2 diabetes and in control fibroblast treated chronically with dexamethasone or TNFα. These findings harmonize well with recently published data confirming that blockade of Akt using pharmacological inhibitors, siRNA and a dominant-negative Akt mutant led to inhibition of basal collagen production in cultured dermal fibroblasts (Bujor et al., 2008). Similarly, a skin derived from Akt−/− mice exhibited impaired matrix organization with reduced amounts of collagen (Chen et al., 2005), a characteristic feature that is not dissimilar from those seen during diabetes. A pharmacological strategy (e.g. EUK-134) intended to suppress ROS partially restored wound IGF1-mediated responses. To this end, we believe that our data point to the possibility that ROS, by inducing IGF1 resistance, contribute at least in part to the impaired fibroblast functions during diabetes, inflammation and hypercortisolemia. Consistent with this concept, we have shown that, as with IGF1 singling, a chronic model of oxidative stress induced in cultured fibroblasts by tert-butyl hydroperoxide or BSO recapitulated most of the abnormalities seen in fibroblast functions in each of the three models of IGF1 resistance (data not shown).
What are the mechanisms involved in the induction of HSOS in diabetic, inflammatory and hypercortisolemic fibroblasts?
In this regard, we have shown recently that the elevation in ROS levels during diabetes stems from a marked increase in the activity and/or expression of NADPH oxidase, an impaired mitochondrial electron transport chain and a defect in the Nrf2 signaling pathway (Bitar and Al-Mulla, 2011). Further studies dealing with the above issue are currently under consideration in our laboratory.
We sought next to extend the in-vitro-based cellular observations to in vivo models of IGF1 resistance and impaired wound healing typified in the current study by type 2 diabetic or hypercortisolemic animals. Application of IGF1 in combination with IGFBP1 at a molar ratio of 5:1 to dorsal excisional wound of control animals significantly enhanced ulcer healing when compared with corresponding vehicle-treated values, a finding that harmonizes with previously reported results using the same method of application (Jyung et al., 1994). This beneficial effect of IGF1 in accelerating the healing process was not evident in type 2 diabetic or hypercortisolemic animals. Similarly, IGF1-induced glucose metabolism, assessed by the RIST protocol, was also attenuated in these disease states. We reasoned that, as in cultured fibroblasts, the in vivo impairment in IGF1 actions during diabetes and hypercortisolemia might result partially from an augmented ROS–p-IRS1 (Ser307) axis. Indeed, the current data documenting that the ROS suppressors EUK-134 and LA restored wound IGF1 sensitivity and accelerated cutaneous wound healing in the aforementioned disease states strongly support this hypothesis. A further credence for the above finding is the recent data showing that a synthetic uric acid analog with free radical scavenging properties accelerated the healing process in mice (Chigurupati et al., 2010). Obviously, more in-depth studies should be conducted into the interrelationship between the IGF1 signaling pathway and the ROS–JNK–p-IRS1 (Ser307) axis in diabetic, inflammatory and hypercortisolemic wounds.
Overall, the data described in this manuscript support the concept that HSOS induces IGF1 resistance both in vitro (reflected by an attenuation in the PI3K-Akt signaling pathway) and in vivo (reflected by an impairment in glucose disposal), and that this phenomenon might contribute, at least in part, to impaired wound healing under numerous clinical settings, including diabetes, inflammation and hypercortisolemia. This proposition gains further credibility when viewed in the context of previous reports showing that the PI3K-Akt pathway is overactivated in sclerodermic fibroblasts as well as in other chemically or genetically related fibrotic disorders (Xu et al., 2009). It also identifies the PI3K-Akt cascade as a future therapeutic target for the treatment of non-healing or chronic skin wounds, and of fibro-proliferative related disorders.
METHODS
Primary dermal fibroblast isolation, cell culture and drug treatment
Primary dermal fibroblasts were obtained from the dorsal skin of female Goto Kakizaki (GK; age 12–15 months) rats, a model for non-obese type 2 diabetes, and their Wistar control counterparts. After sterilization in povidone solution, rat skin was washed in sterile water and rinsed in 70% ethanol in phosphate-buffered saline (PBS). Epidermis and dermis were separated following overnight incubation in 0.25% trypsine/EDTA at 4°C. Dermis was cut into small pieces and incubated in Dulbecco’s modified Eagle medium (DMEM; Invitrogen) containing collagenase (250 U/ml; Sigma) for 30 minutes at 37°C in 5% CO2 with constant agitation. The sections were triturated vigorously to release fibroblasts, which were collected by centrifugation. The cell pellet was washed twice with PBS, resuspended in complete medium [DMEM supplemented with 10% fetal calf serum, penicillin (100 U/ml), streptomycin (100μg/ml), 2 mM L-glutamine and 10 mM HEPES] and then cultured under a standard conditions.
A hypercortisolemic state was mimicked by exposing control fibroblasts to dexamethasone (Dexa; Sigma) administered at 20 ng/ml every other day for a duration of 8 days. Similarly, the state of low-grade inflammation in fibroblasts was recapitulated experimentally by exposing these cells to TNFα (4 ng/ml every day for 4 days). EUK-134 (Cayman) and LA (Sigma) were most effective in cultured fibroblasts at 100 μM and 500 μM, respectively: doses that seem to have a minimum effect on cell viability as determined by the WST-based technique (Roche Diagnostics). The concentration of IGF1 (50 ng/ml; Peprotech) was determined by a prior dose-response experiment and was somewhat similar to that used previously in fibroblasts and other cells (Chetty et al., 2006; Izumi et al., 2006; Yin et al., 2009).
IGF1 signaling studies
Levels and/or activities of key intracellular molecules in the IGF1 signaling cascade [IRS1, p-IRS1 (Tyr612), PI3K-p85α, p-IRS1 (Ser-307), PI3K, Akt, p-Akt, JNK and p-JNK] were assessed using western blotting alone or in combination with immunoprecipitation (Bitar et al., 2005). Briefly, cells were lysed with RIPA buffer supplemented with a protease/phosphatase inhibitor cocktail. Proteins or IRS1-based immunoprecipitants were then separated using 7.5–15% SDS-PAGE, transferred to nitrocellulose membrane and incubated overnight at 4°C with primary antibodies [IRS1, p-IRS1 (Tyr612), p-IRS1 (Ser307), PI3K-p85α, Akt, p-Akt; all from Cell Signaling). After incubation with secondary antibodies, proteins were detected by enhanced chemiluminescence.
Protein extraction and assessment of PI3K class IA activity
PI3K activity was measured using PI3K ELISA (Echelon Biosciences). This kit was used in connection with anti-p85 PI3K antibody, and it measures class IA PI3K activity as a conversion of PtdIns(4,5)P2 into PtdIns(3,4,5)P3. Briefly, cells were lysed using buffer A containing 1% NP40 and protease inhibitors, incubated on ice for 30 minutes and then centrifuged at 14,000 g. Following the step of immunoprecipitation of the supernatants with anti-p85 PI3K antibody and protein A/G-agarose beads, the kinase reaction was carried out according to the specifications provided by the manufacturers.
FAC ELISA assay
The levels of total and phosphorylated Akt and JNK were determined using FAC ELISA assays (Active Motif) according to the manufacturer’s protocol. Briefly, fibroblasts derived from various experimental groups were fixed at room temperature in 3.7% paraformaldehyde in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 and 1.4 mM KH2PO4) for 20 minutes. After washing and quenching the endogenous peroxidase activity with 1% hydrogen peroxide, the cells were blocked for 1 hour and then incubated with anti-p-Ser473-specific Akt or anti-p-Thr-183/Tyr-185-specific JNK primary antibody. The same procedure was repeated using antibodies against total Akt and JNK. After a washing step, HRP-conjugated secondary antibody was added for 1 hour at room temperature. Subsequently, the chemiluminescence was measured using a GloMax luminometer (Promega). After chemiluminescence readings were recorded, the number of cells in each well was quantified by crystal violet staining, determining absorbance at 595 nm. The cell density per well was used to normalize chemiluminescence readings and the basal phosphorylation status in each cell line was calculated by dividing the active phospho-protein-specific antibody with that of total-protein-specific antibody. The final data related to the various cell lines were expressed as a fold of change vs control (e.g. normal fibroblast cell line).
mRNA real-time PCR
Total RNA from cells or frozen wound tissues was extracted using Trizol reagent (Invitrogen) and the integrity of the RNA was verified by gel electrophoresis. Total RNA was reverse transcribed and then amplified using TaqMan Assay on Demand (Applied Biosystems) in a 25 μl reaction volume containing two unlabeled primers and a 6-carboxyfluorescein-labeled TaqMan MGB probe and a Master Mix. Amplified sequences were assessed using the ABI 7500 Prism Sequence Detection system machine. GAPDH or 18S was used for normalizing between individual samples. The results were expressed as mRNA levels corrected for 18S or GAPDH in each sample.
siRNA transfection
Expression of JNK was inhibited by siRNA oligonucleotides. The sequences were designed and synthesized by Qiagen. The best silencing efficiency was obtained by incubating 2.0×105 cells/well in a six-well plate with complexes formed by 5 nM siRNA (1 μl) and 9 μl of HiPerfect transfection reagent (Qiagen) dissolved in 90 μl medium, according to the manufacturer’s instructions. The transfection was achieved by adding 0.9 ml of medium to the seeded cells followed by 100 μl of siRNA/HiPerfect complex. 24 hours later, 1 ml of fresh medium was added; 48 hours after transfection, the cells were exposed to either vehicle or IGF1. Knock-out efficiency was verified by real-time PCR and ELISA-based assay.
Assessment of key indices related to HSOS
ROS generation in cultured fibroblasts were evaluated using dichlorofluorescein-diacetate (DCF-DA; Molecular Probes), a probe that is oxidized to the fluorescent product DCF on exposure to hydrogen peroxide, peroxynitrite, hydroxyl radicals or nitric oxide. Its concentration serves as an indicator of the overall degree of intracellular oxidative stress (Barja, 2002). Cells seeded in 96-well plates were incubated for 30 minutes at 37°C in the dark in media containing 5 μM of DCF-DA. The plates were then washed twice with Krebs Ringer Buffer (KR) to eliminate the excess DCF-DA. Formation of ROS was detected by measuring the fluorescence absorbance (485-nm excitation and 530-nm emission) by a plate reader (Promega, E9032). Subtracted background values were obtained from wells containing DCF-DA without cells. All the values of ROS were normalized to the total number of cells using a propidium-iodide-based assay. Protein-bound carbonyl levels in fibroblasts, a marker of cumulative oxidative stress, were determined using a previously published procedure (Winterbourn and Buss, 1999).
Chronic model of oxidative stress
To induce a state of heightened oxidative stress, cultured dermal fibroblasts were exposed for four passages to a non-toxic concentration (10 μM) of BSO, an inhibitor of glutathione synthesis (Griffith and Meister, 1979). In a preliminary study, we found that this dose of BSO is capable of increasing the intracellular levels of ROS, assessed using DCF-DA, with no or a minimum effect on cell viability. BSO was added to the culture medium 48 hours after seeding and then every 2–3 days at the time of feeding.
Assessment of key fibroblast functions essential for wound healing
The proliferation and collagen synthesis of cultured fibroblasts were determined using, respectively, the 5-bromo-2′-deoxyuridine (BrdU) incorporation into DNA and a radiolabelled proline uptake assay. For cell proliferation, fibroblasts were seeded onto 96-microtiter plates at a concentration of 1.5×104 and allowed to adhere overnight in DMEM supplemented with 10% FCS. After arrest by incubation in DMEM supplemented with 0.5% FCS for 24 hours, cells were exposed to IGF1 (50 ng/ml) in DMEM containing 10 μM BrdU (BrdUrd). Incorporation of BrdUrd into DNA was estimated using a BrdU labeling and detection kit (Roche Applied Science) according to the manufacturer’s instructions. Similarly, for collagen synthesis, confluent fibroblast monolayer was prepared in a 24-well plate and cultured overnight in media supplemented with 10 mM HEPES, 0.1% serum, 2 mM L-proline and 50 μg/ml ascorbic acid. Thereafter, the media was replaced with a fresh media containing 5 μCi/ml 3H L-proline (New England Nuclear) and IGF1 (50 ng/ml), and the incubation continued for 24 hours. Synthesis of collagen and non-collagen protein was expressed, respectively, as collagenase-soluble and collagenase-insoluble count per minute. A correction factor of 5.4 for non-collagen protein was used to adjust for the relative abundance of proline and hydroxyproline in collagen.
For in vitro wounding (migration) experiments, cultured fibroblasts were grown in six-well plates until they reached confluence. Medium was removed, and cells were rinsed and then cultured for 24 hours in serum-free medium plus 0.1% BSA. The monolayer was artificially injured by scratching across the plate with a pipette tip, washed to remove detached cells and then cultured in serum-free medium in the presence of mitomyocin C (10 μg/ml) to prevent cell proliferation. After 24 hours, images of the scratched area under various experimental conditions were photographed. Scratch wound area was measured and the percentage of wound closure was measured according to the following formula: [1 – (current wound size/initial wound size)] × 100. Finally, fibroblast contraction assay was evaluated according to previously published procedure (Liang et al., 2007).
Animal models and treatment
All animal procedures were performed in accordance with the NIH Guidance for the Care and Use of Laboratory Animals. The current study used the GK and dexamethasone-treated rats as models for diabetes and hypercortisolemia, respectively. Detailed information regarding the GK rats as an animal model for non-obese type II diabetes has already been described in our previous publications (Bitar et al., 2004; Bitar et al., 2005). Dexamethasone was administered subcutaneously at a dose of 2.5 μg/kg body weight in the morning (8:00 AM) and in the evening (8:00 PM) for a duration of 4 weeks before wounding, and this form of therapy continued during the course of healing. Preliminary studies involving a concentration-dependent curve revealed that the aforementioned dose of dexamethasone chosen was effective in inducing IGF1 resistance and also in impairing the healing process without a significant effect on body weight. Weight- and age-matched female Wistar rats (Kuwait University breeding colony) served as the corresponding controls. All of the animals were maintained under standard conditions with a 12-hour on/off light cycle, commercial diet and water ad libitum. GK rats destined for wounding were initially matched with regards to body weight (e.g. 230–250 g), and plasma levels of glucose, free fatty acids and insulin. These indices are commonly used to reflect the severity of the diabetic state.
Animals used for IGF1 sensitivity (n=6/group) and wound healing (n=8) studies were partitioned into five study groups: control, diabetic, hypercortisolemic, diabetic + EUK-134 and hypercortisolemic + EUK-134. EUK-134 was administered for a duration of 4 weeks before wound induction and administration continued during the course of healing. Preclinical studies have shown that intraperitoneal (i.p.) administration of EUK-134 prevented HSOS, attenuated kainate-induced neuropathology and protected against paraquat pneumotoxicity in rat models at a dose of 10 mg/kg body weight (Rong et al., 1999; Shopova et al., 2009). EUK-134 at a dose of 12.5 mg/kg body weight every other day seems to be most effective in our wound healing studies. We also used LA, an ROS scavenger/antioxidant enzyme inducer, at a dose of 50 mg/kg body weight/day (Bitar et al., 2005; Bitar et al., 2010).
TRANSLATIONAL IMPACT.
Clinical issue
Non-healing wounds and insulin resistance constitute cardinal features of diabetes, inflammation and hypercortisolemia. Approximately 15% of all individuals with diabetes will at some time have a non-healing wound despite insulin treatment and a meticulously controlled diet, and impaired wound healing contributes to more in-patient hospital days than any other complication of diabetes. Moreover, impaired wound healing has been judged as a factor in 81% of lower extremity amputations in individuals with diabetes and other chronic diseases. Thus, there is an urgent need first to understand the underlying mechanisms of impaired wound healing and second to develop an effective strategy to accelerate the processes of tissue repair under various pathological conditions.
Mammalian cells and tissues with phenotypic features of diabetes, low-grade chronic inflammation or hypercortisolemia often exhibit high levels of reactive oxygen species (ROS), leading to a heightened state of oxidative stress (HSOS). ROS can act as signaling molecules, and also cause damage to proteins, lipids and nucleic acids. ROS have previously been shown to be involved in the development of insulin resistance, as well as to disrupt the process of tissue repair. Growth-promoting polypeptides, including insulin and IGF1, are also involved in tissue repair mechanisms. Notably, evidence exists supporting the concept that IGF1 uses the same intermediates as those of the insulin signaling cascade, suggesting that common mechanisms function in IGF1 resistance and insulin resistance.
Results
Here, the authors explore whether ROS plays a crucial role in connecting IGF1 resistance with impaired wound healing using experimental systems representing pathological conditions that are associated with HSOS. They confirm that HSOS and the attenuation in IGF1-induced activation of the canonical phosphatidylinositol-3-kinase (PI3K)-Akt pathway are characteristic features of diabetic, inflammatory and hypercortisolemic fibroblasts. This IGF1 resistance is associated with impairments in key fibroblast functions that are essential for wound healing, including collagen synthesis, cell proliferation, cell migration and cell contraction in vitro. These impairments are minimized by inhibiting the activity of ROS using antioxidant treatment. In vivo, the capacity of IGF1 treatment to stimulate cutaneous ulcer healing was diminished in rats with diabetes or hypercortisolemia, compared with control rats without disease. In line with in vitro data, the capacity of IGF1 to reduce signs of disease and accelerate wound healing was increased when animals were pretreated with antioxidants.
Implications and future directions
The finding that ROS are involved in both IGF1 resistance and impaired wound healing during diabetes and hypercortisolemia has crucial implications for oxidative stress biology. The molecular defects uncovered in this work open up new avenues for therapeutic interventions that might aid in the treatment of non-healing wounds. Future work with human subjects should provide more in-depth evidence-based support for considering ROS inhibitors as pharmacological strategies that aim to reduce the incidence of amputation during chronic diabetes.
In vivo IGF1 sensitivity studies
IGF1 sensitivity in control, diabetic or hypercortisolemic animals was determined using the RIST protocol with IGF1 (200 μg/kg body weight) being infused instead of insulin (Xie et al., 1996; Sadri and Lautt, 2000). The RIST index is the amount of glucose per kg body weight required to maintain euglycemia following a bolus of insulin (50 mU/kg body weight).
Wound model, drug treatment and macroscopic evaluation
Animals derived from various experimental groups were anesthetized by i.p. injection of 90 mg ketamine + 10 mg xylazine/kg body weight, and their back skin was shaved, depilated with Nair and cleaned with 70% alcohol. Six bilateral full-thickness excisional wounds (8 mm in diameter) that were equidistant from the midline were created on the dorsorostral back skin. Wounds were separated by a minimum of 1 cm of uninjured skin. The IGF1 therapeutic regimen included a combination of IGF1 and IGFBP1 (5 μg IGF1 and 1.5 μg IGFBP1), which were applied every other day to the wound in a vehicle of pluronic acid in PBS solution (300 mg/ml, 250 μl total volume per wound). The aforementioned doses of IGF1 and IGFBP1 have previously been shown to be optimum in promoting the skin tissue repair mechanism in rats (Jyung et al., 1994). Wounds were photographed at 0 and 7 days after wounding using a Sony D-9 digital camera. The wound area was analyzed using Adobe PhotoShop (version 7.0; Adobe Systems) and the percentage of wound closure was derived by the following formula: [1 – (current wound size/initial wound size)] × 100. The 7-day period post-wounding is a crucial time for healing (Pierce et al., 1988) and shows significant activity in response to IGF1 (Jyung et al., 1994; Beckert et al., 2007).
Isolation of cells from wounds
Cells from 7-day-old wounded tissues were isolated as described (Wilson et al., 2002) and plated in 60-mm culture dishes or microtiter plates. Key parameters related to oxidative stress, including ROS and protein carbonyls, were assessed as outlined above.
Statistical analysis
Data are expressed as the mean ± s.e.m. One-way analysis of variance with Bonferroni post hoc validation or the Mann-Whitney test was used to compare data derived from various experimental groups. A level of P≤0.05 was considered to be significant.
Acknowledgments
We are indebted to Sabine Werner (Institute of Cell Biology, ETH Zurich, Switzerland) for critical reading of the manuscript. The authors also thank Anees Fatima Beema, Jucy Gabriel and Waleed Al-Ali for their excellent technical support.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
Both authors contributed to the design and execution of the experiments, and M.S.B. wrote the manuscript.
FUNDING
This work was supported by the Kuwait Foundation for the Advancement of Sciences (KFAS) [grant number 2002-130-206].
REFERENCES
- Aguirre V., Uchida T., Yenush L., Davis R., White M. F. (2000). The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307). J. Biol. Chem. 275, 9047–9054 [DOI] [PubMed] [Google Scholar]
- Barja G. (2002). Rate of generation of oxidative stress-related damage and animal longevity. Free Radic. Biol. Med. 33, 1167–1172 [DOI] [PubMed] [Google Scholar]
- Beckert S., Haack S., Hierlemann H., Farrahi F., Mayer P., Konigsrainer A., Coerper S. (2007). Stimulation of steroid-suppressed cutaneous healing by repeated topical application of IGF-I: different mechanisms of action based upon the mode of IGF-I delivery. J. Surg. Res. 139, 217–221 [DOI] [PubMed] [Google Scholar]
- Bennett B. L., Satoh Y., Lewis A. J. (2003). JNK: a new therapeutic target for diabetes. Curr. Opin. Pharmacol. 3, 420–425 [DOI] [PubMed] [Google Scholar]
- Bitar M. S. (1997). Insulin-like growth factor-1 reverses diabetes-induced wound healing impairment in rats. Horm. Metab. Res. 29, 383–386 [DOI] [PubMed] [Google Scholar]
- Bitar M. S. (1998). Glucocorticoid dynamics and impaired wound healing in diabetes mellitus. Am. J. Pathol. 152, 547–554 [PMC free article] [PubMed] [Google Scholar]
- Bitar M. S. (2000). Insulin and glucocorticoid-dependent suppression of the IGF-I system in diabetic wounds. Surgery 127, 687–695 [DOI] [PubMed] [Google Scholar]
- Bitar M. S., Al-Mulla F. (2011). A defect in Nrf2 signaling constitutes a mechanism for cellular stress sensitivity in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 301, E1119–E1129 [DOI] [PubMed] [Google Scholar]
- Bitar M. S., Wahid S., Pilcher C. W., Al-Saleh E., Al-Mulla F. (2004). Alpha-lipoic acid mitigates insulin resistance in Goto-Kakizaki rats. Horm. Metab. Res. 36, 542–549 [DOI] [PubMed] [Google Scholar]
- Bitar M. S., Al-Saleh E., Al-Mulla F. (2005). Oxidative stress-mediated alterations in glucose dynamics in a genetic animal model of type II diabetes. Life Sci. 77, 2552–2573 [DOI] [PubMed] [Google Scholar]
- Boura-Halfon S., Zick Y. (2009). Serine kinases of insulin receptor substrate proteins. Vitam. Horm. 80, 313–349 [DOI] [PubMed] [Google Scholar]
- Bujor A. M., Pannu J., Bu S., Smith E. A., Muise-Helmericks R. C., Trojanowska M. (2008). Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J. Invest. Dermatol. 128, 1906–1914 [DOI] [PubMed] [Google Scholar]
- Cain R. J., Ridley A. J. (2009). Phosphoinositide 3-kinases in cell migration. Biol. Cell 101, 13–29 [DOI] [PubMed] [Google Scholar]
- Chen J., Kasper M., Heck T., Nakagawa K., Humpert P. M., Bai L., Wu G., Zhang Y., Luther T., Andrassy M., et al. (2005). Tissue factor as a link between wounding and tissue repair. Diabetes 54, 2143–2154 [DOI] [PubMed] [Google Scholar]
- Chetty A., Cao G. J., Nielsen H. C. (2006). Insulin-like Growth Factor-I signaling mechanisms, type I collagen and alpha smooth muscle actin in human fetal lung fibroblasts. Pediatr. Res. 60, 389–394 [DOI] [PubMed] [Google Scholar]
- Chigurupati S., Mughal M. R., Chan S. L., Arumugam T. V., Baharani A., Tang S. C., Yu Q. S., Holloway H. W., Wheeler R., Poosala S., et al. (2010). A synthetic uric acid analog accelerates cutaneous wound healing in mice. PLoS ONE 5, e10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti E., Musumeci M. B., De Giusti M., Dito E., Mastromarino V., Autore C., Volpe M. (2011). IGF-1 and atherothrombosis: relevance to pathophysiology and therapy. Clin. Sci. (Lond.) 120, 377–402 [DOI] [PubMed] [Google Scholar]
- De Mattei M., Ongaro A., Magaldi S., Gemmati D., Legnaro A., Palazzo A., Masieri F., Pellati A., Catozzi L., Caruso A., et al. (2008). Time- and dose-dependent effects of chronic wound fluid on human adult dermal fibroblasts. Dermatol. Surg. 34, 347–356 [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. (1986). Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 [DOI] [PubMed] [Google Scholar]
- Eming S. A., Krieg T., Davidson J. M. (2007). Inflammation in wound repair: molecular and cellular mechanisms. J. Invest. Dermatol. 127, 514–525 [DOI] [PubMed] [Google Scholar]
- Frojdo S., Vidal H., Pirola L. (2009). Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochim. Biophys. Acta. 1792, 83–92 [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. (1979). Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 254, 7558–7560 [PubMed] [Google Scholar]
- Hirosumi J., Tuncman G., Chang L., Gorgun C. Z., Uysal K. T., Maeda K., Karin M., Hotamisligil G. S. (2002). A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 [DOI] [PubMed] [Google Scholar]
- Hoehn K. L., Salmon A. B., Hohnen-Behrens C., Turner N., Hoy A. J., Maghzal G. J., Stocker R., Van Remmen H., Kraegen E. W., Cooney G. J., et al. (2009). Insulin resistance is a cellular antioxidant defense mechanism. Proc. Natl. Acad. Sci. USA 106, 17787–17792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. (1995). Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N., Rosen E. D., Lander E. S. (2006). Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 [DOI] [PubMed] [Google Scholar]
- Izumi K., Kurosaka D., Iwata T., Oguchi Y., Tanaka Y., Mashima Y., Tsubota K. (2006). Involvement of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in corneal fibroblasts during corneal wound healing. Invest. Ophthalmol. Vis. Sci. 47, 591–598 [DOI] [PubMed] [Google Scholar]
- Jenkins P. J., Bustin S. A. (2004). Evidence for a link between IGF-I and cancer. Eur. J. Endocrinol. 151 Suppl 1, S17–S22 [DOI] [PubMed] [Google Scholar]
- Jyung R. W., Mustoe J. A., Busby W. H., Clemmons D. R. (1994). Increased wound-breaking strength induced by insulin-like growth factor I in combination with insulin-like growth factor binding protein-1. Surgery 115, 233–239 [PubMed] [Google Scholar]
- Khanolkar M. P., Bain S. C., Stephens J. W. (2008). The diabetic foot. QJM 101, 685–695 [DOI] [PubMed] [Google Scholar]
- Kurz D. J., Decary S., Hong Y., Trivier E., Akhmedov A., Erusalimsky J. D. (2004). Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J. Cell Sci. 117, 2417–2426 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Giraud J., Davis R. J., White M. F. (2003). c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 278, 2896–2902 [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Travers J. B., Somani A. K., Spandau D. F. (2010). The IGF-1/IGF-1R signaling axis in the skin: a new role for the dermis in aging-associated skin cancer. Oncogene 29, 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C. C., Park A. Y., Guan J. L. (2007). In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2, 329–333 [DOI] [PubMed] [Google Scholar]
- McCall J. L., Tuckey J. A., Parry B. R. (1992). Serum tumour necrosis factor alpha and insulin resistance in gastrointestinal cancer. Br. J. Surg. 79, 1361–1363 [DOI] [PubMed] [Google Scholar]
- Milne C. T. (2008). Wound healing in older adults: unique factors should guide treatment. Adv. Nurse Pract. 16, 53–56 [PubMed] [Google Scholar]
- Myhre O., Andersen J. M., Aarnes H., Fonnum F. (2003). Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem. Pharmacol. 65, 1575–1582 [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Deuel T. F. (1988). Transforming growth factor beta induces increased directed cellular migration and tissue repair in rats. Prog. Clin. Biol. Res. 266, 93–102 [PubMed] [Google Scholar]
- Rajpathak S. N., Gunter M. J., Wylie-Rosett J., Ho G. Y., Kaplan R. C., Muzumdar R., Rohan T. E., Strickler H. D. (2009). The role of insulin-like growth factor-I and its binding proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab. Res. Rev. 25, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe K. J., Cambrey A. D., Richardson J., Irvine L. M., Grobbelaar A. O., Linge C. (2007). Dermal fibroblasts derived from fetal and postnatal humans exhibit distinct responses to insulin like growth factors. BMC Dev. Biol. 7, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y., Doctrow S. R., Tocco G., Baudry M. (1999). EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc. Natl. Acad. Sci. USA 96, 9897–9902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri P., Lautt W. W. (2000). Glucose disposal by insulin, but not IGF-1, is dependent on the hepatic parasympathetic nerves. Can. J. Physiol. Pharmacol. 78, 807–812 [PubMed] [Google Scholar]
- Saltiel A. R. (2001). New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104, 517–529 [DOI] [PubMed] [Google Scholar]
- Schafer M., Werner S. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 9, 628–638 [DOI] [PubMed] [Google Scholar]
- Sen C. K., Roy S. (2008). Redox signals in wound healing. Biochim. Biophys. Acta 1780, 1348–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino C., Brizzi P., Solinas A., Secchi G., Maioli M., Tonolo G. (2002). Low-dose dexamethasone in the rat: a model to study insulin resistance. Am. J. Physiol. Endocrinol. Metab. 283, E367–E373 [DOI] [PubMed] [Google Scholar]
- Sevillano J., de Castro J., Bocos C., Herrera E., Ramos M. P. (2007). Role of insulin receptor substrate-1 serine 307 phosphorylation and adiponectin in adipose tissue insulin resistance in late pregnancy. Endocrinology 148, 5933–5942 [DOI] [PubMed] [Google Scholar]
- Shopova V. L., Dancheva V. Y., Salovsky P. T., Stoyanova A. M. (2009). Protective effects of a superoxide dismutase/catalase mimetic compound against paraquat pneumotoxicity in rat lung. Respirology 14, 504–510 [DOI] [PubMed] [Google Scholar]
- Sienkiewicz P., Palka M., Palka J. (2004). Oxidative stress induces IGF-I receptor signaling disturbances in cultured human dermal fibroblasts. A possible mechanism for collagen biosynthesis inhibition. Cell Mol. Biol. Lett. 9, 643–650 [PubMed] [Google Scholar]
- Siwik D. A., Pagano P. J., Colucci W. S. (2001). Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 280, C53–C60 [DOI] [PubMed] [Google Scholar]
- Smith T. J. (2010). Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol. Rev. 62, 199–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. R., Shenvi S. V., Widlansky M., Suh J. H., Hagen T. M. (2004). Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 11, 1135–1146 [DOI] [PubMed] [Google Scholar]
- Tanti J. F., Jager J. (2009). Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr. Opin. Pharmacol. 9, 753–762 [DOI] [PubMed] [Google Scholar]
- Tuncman G., Hirosumi J., Solinas G., Chang L., Karin M., Hotamisligil G. S. (2006). Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 103, 10741–10746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen K. E., Hotamisligil G. S. (2005). Inflammation, stress, and diabetes. J. Clin. Invest. 115, 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H., Roberts C. T., Jr (2003). The IGFI receptor gene: a molecular target for disrupted transcription factors. Genes Chromosomes Cancer 36, 113–120 [DOI] [PubMed] [Google Scholar]
- White M. F. (2006). Regulating insulin signaling and beta-cell function through IRS proteins. Can. J. Physiol. Pharmacol. 84, 725–737 [DOI] [PubMed] [Google Scholar]
- Williams D. T., Harding K. (2003). Healing responses of skin and muscle in critical illness. Crit. Care Med. 31, S547–S557 [DOI] [PubMed] [Google Scholar]
- Wilson L., Fathke C., Isik F. (2002). Tissue dispersion and flow cytometry for the cellular analysis of wound healing. Biotechniques 32, 548–551 [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C., Buss I. H. (1999). Protein carbonyl measurement by enzyme-linked immunosorbent assay. Methods Enzymol. 300, 106–111 [DOI] [PubMed] [Google Scholar]
- Xie H., Zhu L., Zhang Y. L., Legare D. J., Lautt W. W. (1996). Insulin sensitivity tested with a modified euglycemic technique in cats and rats. J. Pharmacol. Toxicol. Methods 35, 77–82 [DOI] [PubMed] [Google Scholar]
- Xu S. W., Liu S., Eastwood M., Sonnylal S., Denton C. P., Abraham D. J., Leask A. (2009). Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS ONE 4, e7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W., Park J. I., Loeser R. F. (2009). Oxidative stress inhibits insulin-like growth factor-I induction of chondrocyte proteoglycan synthesis through differential regulation of phosphatidylinositol 3-kinase-Akt and MEK-ERK MAPK signaling pathways. J. Biol. Chem. 284, 31972–31981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D. H., Mace K. A., Hansen S. L., Boudreau N., Young D. M. (2007). Effects of decreased insulin-like growth factor-1 stimulation on hypoxia inducible factor 1-alpha protein synthesis and function during cutaneous repair in diabetic mice. Wound Repair Regen. 15, 628–635 [DOI] [PubMed] [Google Scholar]
- Zhong J., Lee W. H. (2007). Hydrogen peroxide attenuates insulin-like growth factor-1 neuroprotective effect, prevented by minocycline. Neurochem. Int. 51, 398–404 [DOI] [PubMed] [Google Scholar]
- Zofkova I. (2003). Pathophysiological and clinical importance of insulin-like growth factor-I with respect to bone metabolism. Physiol. Res. 52, 657–679 [PubMed] [Google Scholar]