SUMMARY
Ovarian cysts affect women of all ages and decrease fertility. In particular, polycystic ovarian syndrome (PCOS), in which multiple follicular cysts develop, affects 5–10% of women of reproductive age and can result in infertility. Current non-invasive treatments for PCOS can resolve cysts and restore fertility, but unresponsive patients must undergo severe ovarian wedge resection and resort to in vitro fertilization. PCOS is related to the deregulation of leutinizing hormone (LH) signaling at various levels of the hypothalamic-pituitary-ovarian axis and resultant hyperproduction of androgens. Because insulin resistance and compensatory hyperinsulinemia are observed in 50–70% of individuals with PCOS, deregulated insulin signaling in the ovary is considered an important factor in the disease. Here we report that aged mice specifically lacking the PKBβ (also known as Akt2) isoform that is crucial for insulin signaling develop increased testosterone levels and ovarian cysts, both of which are also observed in insulin-resistant PCOS patients. Young PKBβ knockout mice were used to model PCOS by treatment with LH and exhibited a cyst area that was threefold greater than in controls, but without hyperinsulinemia. Thus, loss of PKBβ might predispose mice to ovarian cysts independently of hyperactive insulin signaling. Targeted therapeutic augmentation of specific PKBβ signaling could therefore provide a new avenue for the treatment and management of ovarian cysts.
INTRODUCTION
Ovarian cysts are the most common reproductive abnormality in women of all ages (for a review, see Goodarzi et al., 2011). Simple cysts have been poorly studied because they usually result in only minor discomfort, are generally slow growing and benign, and can resolve without treatment. Large simple cysts, which are commonly detected in older women, for whom maintaining fertility is often not an issue, can be dealt with by uni- or bilateral oophorectomy surgery. By contrast, polycystic ovarian syndrome (PCOS), characterized by multiple small cysts in the ovary, is observed from puberty, affects 5–10% of women of reproductive age and can result in infertility. PCOS can be resolved in some cases by lifestyle changes, weight loss and by treatments ranging from insulin-sensitizing drugs to hormone supplementation. In particularly refractory cases and when continued fertility is an issue, ovarian wedge resection might be necessary and in vitro fertilization performed.
PCOS has been associated with deregulation of leutinizing hormone (LH) signaling, which can occur at various levels of the hypothalamic-pituitary-ovarian (HPO) axis and stimulate hyperproduction of androgens (Goodarzi et al., 2011). Deregulation can occur through hyperstimulation of the pituitary by the hypothalamus, leading to increased LH release, or commonly by increased sensitivity of ovarian LH-sensitive thecal cells to LH. In both cases, abnormal LH receptor (LHR) signaling within the LH-responsive ovarian thecal cells results in an increased steroidogenic response and androgen production (Strauss, 2003). Genetic manipulation of LHR signaling in mice or modeling PCOS by administration of exogenous LH or androgens results in ovarian cyst development, as well as the hallmark of increased testosterone production (Familiari, 1985; Bogovich, 1987; Huhtaniemi et al., 2006; Manneras et al., 2007). This is also observed in PCOS patients, who display abnormalities in thecal steroidogenesis (Nelson et al., 1999).
Deregulation of the canonical thecal steroidogenic pathway, from LHR activation through protein kinase A (PKA) and the cAMP response element-binding protein (CREB) transcription factor, seems to be central to cyst development (Johnson and Sen, 1989; Tremblay et al., 2002; Towns et al., 2005; Towns and Menon, 2005). However, a contribution of non-classical cAMP-independent signaling, such as from protein kinase C (PKC), mitogen-activated protein kinases [MAPKs; also known as extracellular-signal-regulated kinases (ERKs)] and phosphoinositol-3-kinase–protein-kinase-B (PI3K-PKB; note that PKB is also known as Akt), seems probable in PCOS because defects in these signaling pathways are known to affect LH-mediated androgenesis (Manna et al., 2006; Diamanti-Kandarakis et al., 2008). In particular, several further observations point to an involvement of insulin receptor (InsR) signaling in PCOS. Defects in InsR phosphorylation (Dunaif et al., 1995) and further genetic lesions in this pathway, affecting InsR, PKBβ (also known as Akt2) and downstream glycogen synthase kinase 3β (GSK3β), are found in individuals with PCOS (George et al., 2004; Tan et al., 2007; Goodarzi et al., 2008; Mukherjee et al., 2009). Insulin can act as a ‘co-gonadotrophin’ and exogenous administration of LH in rodent models of PCOS can exacerbate PCOS cyst formation (Poretsky et al., 1992). As many as 50–70% of PCOS patients display insulin resistance (IR) and compensatory hyperinsulinemia (Diamanti-Kandarakis et al., 2008). Furthermore, up to 60% of PCOS patients are obese, which is the most common factor leading to IR and can result in decreased InsR expression and post-receptor dysfunction in downstream kinase activation (Mlinar et al., 2007; Vrbikova and Hainer, 2009).
There are three isoforms of PKB – PKBα (also known as Akt1), PKBβ and PKBγ (also known as Akt3) – and these display both redundant and specific functions, of which PKBβ has specific and crucial functions in metabolism and insulin signaling (Dummler and Hemmings, 2007). Indeed, PKBβ knockout (KO) mice progressively develop a diabetes-like phenotype, displaying peripheral IR and compensatory hyperinsulinemia (Cho et al., 2001a; Garofalo et al., 2003; Dummler et al., 2006). We report that aged female PKBβ KO mice develop severe ovarian cysts. Consistent with an involvement of LH signaling in the formation of these cysts, we observed hyperplasia of the thecal-interstitium in these KO mice, which increased with age and correlated with the severity and size of the cysts. Because targeted therapeutic augmentation of specific PKBβ activities could provide a new avenue for the treatment and management of ovarian cysts, we set out to plot more precisely the involvement of PKBβ in cyst development, by analysis of cysts from aged PKBβ KO mice and subjecting young PKBβ KO mice to a PCOS model.
RESULTS
Specific ablation of the PKBβ isoform leads to the development of severe ovarian cysts in aged mice
Examination of aged female mice with distended abdomens led to the discovery that mice lacking PKBβ, but not wild-type (WT) mice, develop severe ovarian cysts (Fig. 1A,B). Cysts developed in almost 80% of PKBβ KO mice, were generally filled with serous fluid, and bilateral presentation was observed in 50% of cases (Table 1). Cysts were absent in WT mice or were present as small follicular cysts with a unilateral distribution (Fig. 1B). Larger cysts were also observed in older PKBβ KO mice than were in younger KO mice, suggesting an increase in cyst size with age. Ovaries of PKBα KO mice, the other major PKB isoform found in the ovary, were similar to WT mice, i.e. were small with a unilateral presentation of follicular cysts (Fig. 1B). This suggests that severe cyst development was due to specific loss of the PKBβ isoform.
Fig. 1.
Specific loss of PKBβ in aged mice results in the development of severe ovarian cysts with an increase in the thecal-interstitial cell population. (A) 91- to 120-week-old WT (i) and PKBβ KO (ii) mice with distended abdomens. (B) Cystic ovaries isolated from WT (i) and PKBα KO (iii) mice show no atresia or small ovarian cyst formation, whereas PKBβ KO mice show severe ovarian cyst formation. (C) Increased stromal accumulation in 91-week-old (early; i, iii) and 120-week-old (late; ii, iv) aged PKBβ KO mice, shown by H&E staining (i, ii). This accumulation reflects increased thecal-interstitial hyperplasia, as indicated by positive vimentin immunohistochemical staining (iii, iv). 40× magnification.
Table 1.
Overview of ovarian cyst incidence and characteristics from aged 90- to 120-week-old female WT, PKB KO and PKBβ KO mice
Ovarian cysts in aged PKBβ KO mice are characterized by thecal-interstitial hyperplasia
To identify histological abnormalities that might precede cyst development, cysts isolated from PKBβ KO mice were examined by hematoxylin and eosin (H&E) and immunohistochemical staining. H&E staining showed a severe reduction or lack of corpus luteum in aged ovaries, which also stained negative for the granulosa cell marker anti-mullerian inhibiting substance (supplementary material Fig. S1A,B). This suggests a cessation of estrous cycling. Hyperplasia of spindle-like stromal cells that increased with ovarian cyst size was observed in PKBβ KO mice. Positive staining for vimentin identified these cells as part of the thecal-interstitial cell population (Fig. 1C).
Ovarian cysts in aged PKBβ KO mice show increased steroidogenic capacity
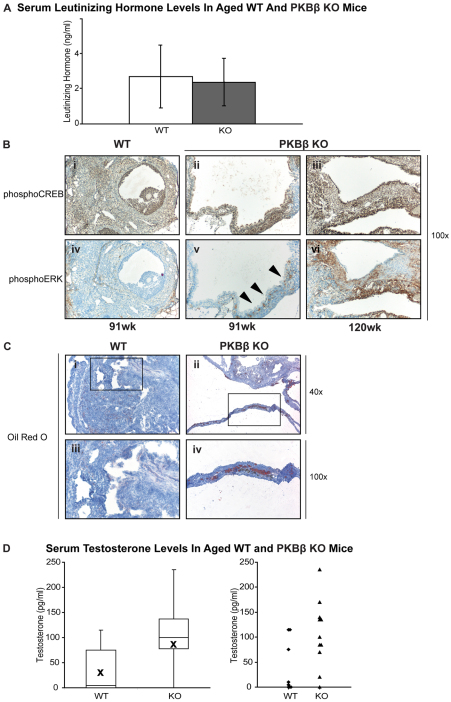
The potential for active steroidogenic signaling by thecal-interstitial cells in cystic ovaries was indicated by hyperplasia in cystic ovaries of the LH-responsive thecal-interstitial cells, which are responsible for ovarian steroidogenesis, and absence of granulosa cells, which are important for controlling LH levels via negative feedback signaling on the pituitary (Massicotte et al., 1984; Couzinet, 1993). Circulating LH was found in serum with no significant difference in hormone levels between WT and KO animals (Fig. 2A). LH activation of LHR is crucial for steroidogenesis, by triggering the phosphorylation of CREB transcription factor and ERK kinase (Salvador et al., 2002); this activation mediates intracellular signaling pathways and the transcription of enzymes that regulate uptake of cholesterol and its enzymatic conversion to C-19 androgens. Cystic ovaries from PKBβ KO mice displayed activating phosphorylation of CREB and ERK, with strong ERK activation commonly observed in cells adjacent to the cystic lumen (Fig. 2B). By contrast, WT ovaries, although displaying CREB activation, were devoid of ERK. Consistent with the activation of ERK in PKBβ KO cysts, lipids were observed in cells surrounding the cystic lumen of PKBβ KO mice but were absent in WT mice, indicating functional uptake of cholesterol for steroidogenesis in PKBβ KO mice only (Fig. 2B). To determine whether the hyperplastic thecal-interstitial cell population observed in ovarian cysts from PKBβ KO mice is steroidogenically active and producing bioactive androgens, serum testosterone levels were measured in WT and PKBβ KO mice. Whereas WT showed generally low testosterone levels, aged PKBβ KO mice consistently showed a twofold higher content (Fig. 2C).
Fig. 2.
Aged PKBβ KO mice show active LH signaling in ovarian cysts, which display increased steroidogenic signaling and lipid accumulation, resulting in increased serum testosterone. (A) WT and PKBβ KO mice exhibit circulating serum LH, with no significant difference in hormone levels. (B) PKBβ KO ovarian cysts display both active CREB (i–iii) and ERK (iv–vi) signaling, which are required for steroidogenesis. ERK is located at the cystic lumen (arrowheads), and increases with the severity of the cysts and age of the KO mice (ii, iii, v, vi) but is absent in WT mice (i, iv). Magnification: 100×. (C) PKBβ ovarian cysts (ii, iv) display increased lipid accumulation adjacent to the cystic lumen, a prerequisite for conversion to steroids; this is absent in WT mice (i, iii). Magnifications: 40× and 100×. (D) Consistent with increased active steroidogenesis, PKBβ KO mice show increased serum testosterone levels compared with WT mice.
Young PKBβ-ablated mice display normal steroidogenic and reproductive functions
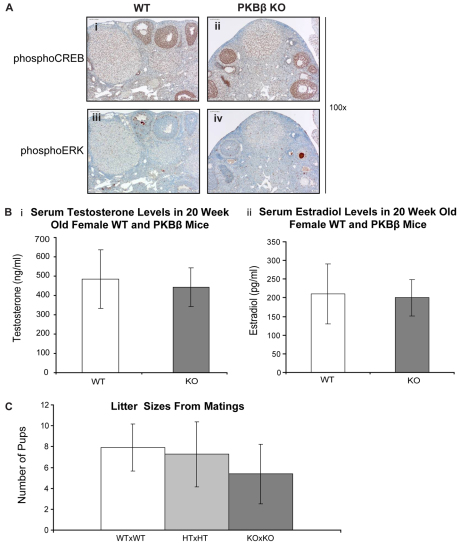
Because steroidogenesis was found to be abnormal in cysts from aged PKBβ KO mice, steroidogenesis and reproduction in young (4- to 8-week-old) PKBβ KO mice were also examined. Neither function was compromised by loss of PKBβ (Fig. 3). There was no difference in the activation of steroidogenic signaling as shown by phosphorylation of CREB and ERK between WT and PKBβ KO ovaries from young animals (Fig. 3A). The thecal-interstitial population showed low to moderate activation of both proteins compared with aged cystic ovaries, and testosterone and estrodiol serum levels were equivalent in young WT and PKBβ KO mice (Fig. 3B). Finally, reproduction, as assessed by litter sizes, was not significantly different between young WT and PKBβ KO animals (Fig. 3C). Thus, compensatory mechanisms in the HPO axis seem to be sufficient to maintain normal ovarian function in young PKBβ KO mice.
Fig. 3.
Loss of PKBβ has no significant impact on normal ovarian steroidogenic signaling or reproductive function in young WT or PKBβ KO mice. (A) Steroidogenic signaling through CREB (i, ii) and ERK (iii, iv) in WT (i, iii) and PKBβ KO (ii, iv) mice. (B) Circulating serum hormone levels of testosterone (i) and estrodiol (ii) in WT and PKBβ KO animals. (C) PKBβ KO mice are fertile and produce litter sizes similar to those of WT animals. HT, heterozygous.
Induction of PCOS via tonic LH stimulation in young PKBβ KO mice increases the severity of polycystic ovaries
Given that aged KO mice showed severe cystic development and enhanced activation of androgenic steroidogenesis in the thecal-interstitium, compared with WT animals, but young KO mice showed no such dysfunctions, it was possible to design an experiment using LH treatment of young mice to plot more precisely the importance of PKBβ in the chain of events leading to ovarian steroidogenesis and cyst development. Tonic LH treatment hyperstimulates LH signaling, mimics the PCOS setting, produces features of PCOS pathology, including increased steroidogenic signaling and testosterone production, and results in cyst formation (Poretsky et al., 1992). To counter a possible effect on LH level of an increase in negative feedback to the pituitary (Poretsky et al., 1992), LH stimulation was also administered in the presence of a gonadotrophin-releasing hormone antagonist (GnRHAnt).
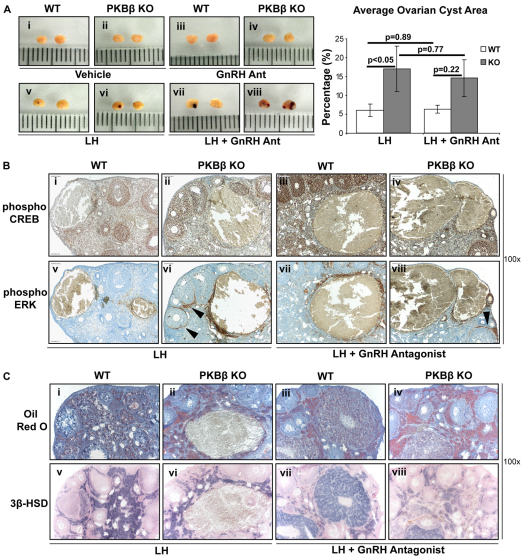
Consistent with previous reports (Poretsky et al., 1992), administration of LH with or without GnRHAnt led to the development of hemorrhagic follicular cysts in young WT mice, whereas treatment with either the dosing vehicle or GnRHAnt alone did not (Fig. 4A). By marked contrast, treatment of young PKBβ KO mice with LH alone or with GnRHAnt led to an approximate threefold increase in cystic area in the ovaries, compared with treated WT mice; cyst development was not affected by the vehicle or GnRHAnt treatment alone (Fig. 4A). CREB was activated and supported steroidogenesis in the ovaries of both young WT and PKBβ KO mice treated with LH (Fig. 4Bi–iv), and activated ERK was strongly expressed in the thecal cells adjacent to the cystic lumen (Fig. 4Bv–viii), reminiscent of the pattern in the cysts of aged PKBβ KO mice. In the young mice, ERK activation was particularly strong around cysts in PKBβ KO ovaries and, unlike in WT ovaries, ERK activation was also strong in thecal cells surrounding large follicles (Fig. 4Bvi and viii, arrows). Such CREB and ERK activity could support increased androgen steroidogenesis, follicular degeneration and cyst development, thus causing the increase in ovarian cyst area in young PKBβ-null ovaries. Consistent with this, 3β-hydroxysteroid dehydrogenase (3β-HSD) staining revealed active steroidogenesis in the thecal-interstitial population of all cystic ovaries (Fig. 4Cv–viii). Lipid accumulation was higher in this steroidogenic population of PKBβ KO ovaries than in WT (Fig. 4Ci–iv), indicating increased cholesterol uptake in young PKBβ KO mice that could support androgen production in an environment of ERK activation.
Fig. 4.
Induction of PCOS via tonic LH administration increases the severity of ovarian cysts observed in PKBβ KO ovaries, with cyst formation being associated with ERK activation and lipid accumulation in steroidogenically active ovaries. (A) PKBβ KO ovaries showed an approximately threefold increase in ovarian cyst area in LH-treated ovaries (vi, viii) compared with WT (v, vii), independent of administration of a GnRHAnt. Treatment of WT and PKBβ KO mice with vehicle (i, ii) or GnRHAnt (iii, iv) alone had no effect on cyst formation. Values are shown in the graph on the right. (B) Steroidogenic signaling was active and seen in ovaries from both WT (i, iii, v, vii) and PKBβ KO (ii, iv, vi, viii) mice treated with LH. ERK, however, was also strongly active in thecal cells, which accumulated and lined large follicles predominantly in PKBβ KO ovaries (arrowheads). (C) Increased lipid accumulation in ovaries treated with LH was also observed in PKBβ KO mice (ii, iv) in areas with active androgen steroidogenesis [indicated by active 3β-HSD staining (v–viii)] compared with WT (i, iii). All magnifications for IHC are 100×.
As described above, insulin can act as a ‘co-gonadotrophin’ that can increase the severity of cysts, and PKBβ KO mice develop a progressive diabetes-like syndrome with hyperinsulinemia. Importantly, despite the marked difference in PCOS-like symptoms, young PKBβ KO mice did not display hyperinsulinemia under the random-fed conditions of the experiment, with no significant difference observed in circulating insulin levels between young WT and PKBβ KO animals (supplementary material Fig. S2). This lack of hyperinsulinemia is probably due to the young age and genetic background, because female PKBβ KO mice on a genetic background displaying a more severe IR phenotype have a weaker IR phenotype than male mice and only mild differences in circulating insulin levels up to 24 weeks of age (Garofalo et al., 2003). Thus, the high prevalence of cysts in young mice following PKBβ ablation does not seem to be the result of hyperstimulation of ovarian InsR.
DISCUSSION
The PKB kinases are active in diverse physiological functions and have been shown to be important for the activity of various hormones. PCOS is associated with deregulation of LH signaling at various levels of the HPO axis and the resulting hyperproduction of androgens (Goodarzi et al., 2011). Because IR and compensatory hyperinsulinemia are observed in 50–70% of PCOS patients (Diamanti-Kandarakis et al., 2008) and the β-isoform of PKB is crucial for insulin signaling (Cho et al., 2001b; Garofalo et al., 2003; Dummler et al., 2006), this indicates that deregulated activity of this isoform might be an important factor in this disease. Although PKB has been shown to be activated upon acute LH stimulation in the thecal-interstitium (Lima et al., 2006), its subsequent contribution to androgenesis and PCOS pathology has remained unclear.
Here, we report that aged PKBβ KO mice develop ovarian cysts associated with enhanced thecal steroidogenesis and hyperinsulinemia. This effect was PKBβ-isoform specific. However, in a young mouse model of PCOS, the ovaries of young animals lacking PKBβ and treated with LH displayed enhanced steroidogenesis and developed a cystic area that was threefold greater than WT controls, but with no indication of hyperinsulinemia. Thus, the increased severity of cyst formation in young PKBβ-ablated mice was not due to ‘co-gonadotrophin’ stimulatory effects of hyperinsulinemia (Poretsky et al., 1992), and loss of PKBβ seems to predispose mice to ovarian cysts independent of hyperactive insulin signaling.
Numerous studies have linked ovarian cyst development to increased LHR signaling and subsequent testosterone biosynthesis in the thecal-interstitium of the ovary (Chang, 2007). Postmenopausal women display follicular exhaustion that results in decreased conversion of testosterone to estrogen and an increase in LH due to loss of negative feedback to the pituitary (Choi et al., 2007). An involvement of LHR deregulation in cyst development is suggested by mouse models that have disrupted LH expression. Mice overexpressing LH, similar to PKBβ knockout mice, display bilateral ovary involvement, thecal hyperplasia, increased testosterone levels and cyst development; cyst development is also found in LHR KO mice (Danilovich and Ram Sairam, 2006; Huhtaniemi et al., 2006). However, circulating LH was detected in aged mice in this present study, with no significant difference between WT and PKBβ KO mice. Thus, in this setting, only the specific loss of PKBβ led to severe cyst development, with cyst development being independent of deregulated LHR activation owing to differences in LH levels.
Our results suggest that the single consistent consequence of the loss of specific PKBβ functions in aged ovaries that could contribute to cyst development is exacerbated androgenic signaling. At least in part, the effect of the ablation of PKBβ seems to be equivalent to the loss of functions controlling ERK activation and lipid accumulation, which leads to increased testosterone production. The identification in vivo of activated ERK, which was correlated with severe cyst formation in aged mice, and its high expression specifically in thecal cells adjacent to cysts in young mice in the PCOS model, suggests that inhibitors of ERK currently in clinical trials might have applications in treating ovarian cyst development in PCOS. However, it has been reported that ERK signaling is lost in thecal cells derived from ovaries of PCOS patients (Nelson-Degrave et al., 2005). This could reflect a difference between the PCOS mouse model and the greater complexity of PCOS in humans, or a difference between thecal cell signaling in the ovarian environment and that in isolated PCOS thecal cells in vitro.
It remains to be seen whether as-yet-undefined PKBβ substrates in the ovary control hyper-androgenic production and that loss of this signaling promotes PCOS, or alternatively whether it is inappropriate compensatory signaling by other PKB isoforms, in particular PKBα, which is markedly expressed in the ovary, that promotes pro-androgenic signaling. Opposing functions of PKBα and PKBβ have been reported in various tissues (Heron-Milhavet et al., 2006; Yun et al., 2008), as have isoform-specific interactions in the ovary (Nechamen et al., 2007). Solving this question will be crucial to the potential targeting of PKB signaling therapeutically in PCOS.
In conclusion, the results of this study highlight for the first time in vivo a newly identified and specific effect of the loss of PKBβ on the development of ovarian cysts in the environment of pathogenically increased LHR signaling, and demonstrate in vivo that activation of ERK in thecal cells is strongly associated with lipid accumulation and cyst development (as depicted in Fig. 5). These results spotlight ERK inhibition and effectors downstream of PKBβ that display loss of function in an environment of increased LH in ovarian thecal cells as potential targets in the treatment and management of ovarian cysts and PCOS.
Fig. 5.
Model outlining the pro-androgenic contributions of PKBβ loss to LH-driven pathogenic cyst formation. In normal physiology, the negative feedback along the HPO axis ensures that steroidogenic signaling is tightly controlled. In pathogenic scenarios of ovarian cyst formation, such as the formation of simple cysts in aged mice or cyst formation in PCOS, a lack of PKBβ combined with increased LH and activated LHR signaling results in increased ERK1/2 activation, lipid accumulation and testosterone production, leading to granulosa cell death and cyst formation.
METHODS
Reagents
Human LH (Lutophin) was obtained from Provet (Lyssach, Switzerland). GnRHAnt was generously provided by Jean Rivier (The Salk Institute, San Diego, CA).
Mice
The PKBα and PKBβ mutant mice, as described previously (Yang et al., 2003; Dummler et al., 2006), were housed in groups with 12-hour dark-light cycles and with access to food and water ad libitum, in accordance with the Swiss Animal Welfare laws. Matched WT and KO female mice aged 21–28 days were housed together to promote synchronous estrous cycling. PCOS induction experiments were commenced at ∼28 days. All procedures were conducted with the appropriate approval of the Swiss authorities.
Tissue preparation for histology
For histological analysis, organs were dissected and either immediately gently snap-frozen in OCT compound in a 2-methylbutane bath in dry ice or fixed in 4% paraformaldehyde (PFA)-phosphate buffered saline (PBS). Tissues placed in 4% PFA-PBS were allowed to fix overnight (∼18 hours) at 4°C. Tissues were then subjected to washes with PBS, 50% ethanol (EtOH)/PBS and 70% EtOH/PBS before being processed and embedded in paraffin using the Medite TPC15 Paraffin Processing Unit (Medite, Winter Garden, FL). Histological staining and immunohistochemistry (IHC) were performed on 12-μm frozen or 4-μm paraffin tissue sections, cut using a HM560H cryostat or M355S microtome (Thermo Scientific, Fremont, CA).
Histological staining
For H&E staining, sections were deparaffinized and stained according to the standard protocols using reagents purchased from Sigma (St Louis, MO). Histochemical staining for 3β-HSD activity was carried out according to a modified protocol of Klinefelter et al. (Klinefelter et al., 1987). Briefly, 12-μm ovarian sections were cut on poly-L-lysine-coated glass slides (Menzel-Gläser, Braunschweig, Germany) and covered with a staining solution prepared by mixing equal volumes of solution A: nitroblue tetrazolium (NBT; #N6639, Sigma, St Louis, MO) and dehydroepiandosterone (DHEA; #D1629, LKT Laboratories, St Paul, MN) in PBS pH 7.4, with solution B: β-nicotinamide adenine dinucleotide (β-NAD; #N7004, Sigma, St Louis, MO) in PBS pH 7.4. Final concentrations were 0.25 mM NBT, 1.5 mM β-NAD, 0.2 mM DHEA. Tissue slides were stained for 90 minutes at 37°C and fixed in 10% formalin in PBS with 5% sucrose, pH 7.4 at 4°C for 5 minutes. Slides were then rinsed in distilled water and counterstained for 5 minutes with Nuclear Fast Red (#H-3403, Vector Laboratories, Burlingame, CA). After rinsing again with distilled water, the sections were mounted and photographed under the microscope. Staining of lipids with Oil Red O was performed using the propolene glycol (PG) method: 12-μm fresh frozen sections were cut and air-dried at room temperature (RT) before being fixed at 4°C in 10% formalin for 5 minutes. Sections were then rinsed three times in double distilled water (ddH2O), allowed to air dry at RT and then placed in 100% PG for 5 minutes before staining for 15 minutes with 0.5% (w/v) Oil Red O solution in PG pre-warmed to 60°C. Oil Red O solution was prepared by dissolving Oil Red O in PG at 90°C, filtering and allowing to stand at RT overnight. Staining was differentiated in 85% PG for 5 minutes and then rinsed twice with ddH2O. Slides were then counterstained with Gill’s Haematoxylin (#GHS216, Sigma, St Louis, MO) for 15 seconds, rinsed three times in tap water, soaked in ddH2O for 5 minutes and mounted.
Immunohistochemistry
Sections of 4 μm were cut from paraformaldehyde-fixed, paraffin-embedded tissues and stained using the Ventana Discovery automated immunostainer (Ventana Medical Systems, Tucson, AZ). IHC was performed with or without cell conditioning using buffers CC1 or CC2, blocked with 5% normal donkey, goat or sheep serum for 1 hour. Primary antibodies diluted in Ventana antibody diluent were then applied and allowed to incubate for 1–12 hours at 25°C. Primary antibodies and dilutions used were vimentin (#V2009; 1:100; Biomedia, Foster City, CA), Muellerin inhibiting substance (MIS, sc-6886; 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA), pCREB S133 (#9198; 1:100), pERK1/2 T202Y204 (#4370; 1:125; both Cell Signaling Technologies, Danvers, MA). After washing, sections were incubated with biotinylated donkey anti-mouse (#715-067-003) or anti-rabbit (711-067-003) secondary antibodies (Jackson ImmunoResearch, West Grove, PA) for 32 minutes at 37°C, before detection with HRP/DAB or UltraMap conjugates and counterstaining with hematoxylin (all Ventana Medical Systems, Tucson, AZ). Photomicrographs were taken on a Nikon Eclipse E600 microscope (Nikon, Milville, NY).
Serum hormone measurement
Blood samples were collected by sublingual vein puncture into Microvette CB300 tubes (Sarstedt, Nümbrecht, Germany) and serum was separated by centrifugation at 5000 g for 10 minutes. To account for the daily variation in hormone levels, a sample was taken in the morning and a second sample 6 hours later. Aliquots (50 μl) of each sample were then pooled and concentrated to 50μl by diethyl ether hormone extraction as described previously (Wijayagunawardane et al., 2003). Concentrated hormone samples were then used in commercial enzyme-linked immunosorbent assay (EIA) kits (DRG Instruments GmbH, Marburg, Germany) to measure serum testosterone levels, according to the manufacturer’s instructions. For ultrasensitive insulin (Mercodia, Uppsala, Sweden) and LH (Endocrine Technologies, Newark, CA) ELISAs, 25 μl of unconcentrated serum was used directly according to the manufacturer’s instructions.
PCOS induction and cyst measurement in mice
Mice were subjected to the standard protocol for LH-induced PCOS by injection of 0.05 IU/g human LH twice daily, with or without GnRHAnt for 21 days as described previously (Bogovich and Richards, 1982; Bogovich, 1987). At day 21, mice were sacrificed and samples collected for further analysis. Formation of hemorrhagic cysts, observed in mice treated with LH with or without GnRHAnt, was quantified by sectioning through the ovaries and measuring every 100 μm the percentage of the total ovary area occupied by hemorrhagic cysts using ImageAccess Enterprise v10 software (Imagic Bildverarbeitung, Glattbrugg, Switzerland).
TRANSLATIONAL IMPACT.
Clinical issue
Ovarian cyst formation and polycystic ovarian syndrome (PCOS) are the most prevalent ovarian abnormalities, with significant social and economic impacts resulting from management and the often demanding invasive treatment. In addition, PCOS is the most common cause of female infertility and is associated with increased risks of other pathologies, such as cardiovascular disease and metabolic disorders. The increasing prevalence of obesity and insulin resistance, as well as a later average age of pregnancy, in the female population are associated with an increased incidence of PCOS and its associated pathologies. Notably, defects in the insulin signaling pathway are found in individuals with PCOS, and there is some indication that this pathway is involved in the pathology of the disease. However, whether targeting the insulin signaling pathway could provide a new treatment avenue for PCOS has not been fully explored.
Results
Here, the authors show that specific deletion of PKBβ (also known as Akt2) – a kinase downstream of the insulin receptor – in mice predisposes animals to the formation of severe ovarian cysts that seem to increase in size with age. Furthermore, induction of ovarian cysts by chronic administration of leutinizing hormone (a model of PCOS) results in more severe cyst formation in PKBβ knockout mice than in wild-type mice. Ovarian cysts in aged PKBβ knockout mice and in PCOS-induced mice display increased androgen production, lipid accumulation and activation of ERK (a kinase required for steroidogenesis), and the mice had increased circulating testosterone levels.
Implications and future directions
These findings confirm a role for dysregulated PKBβ signaling in ovarian cyst formation in mice, and might provide a novel avenue for the development of new PCOS therapies in humans. In particular, they provide support for further studies into the effects of ERK inhibitors (currently in clinical trials for other indications) and/or the identification of other targets that control excess androgen production downstream of PKBβ. These findings also indicate that ovarian cyst formation or PCOS might be a side effect of non-specific PKB inhibitors that are currently in development for the treatment of other disorders.
Supplementary Material
Acknowledgments
The Friedrich Miescher Institute is part of the Novartis Research Foundation. The authors would like to acknowledge the support of the FMI histology facility, and Patrick King for editing the manuscript.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
AUTHOR CONTRIBUTIONS
All work was performed in the lab of B.A.H., who also provided theoretical input into the design and execution of this study. D.H. managed the mouse colonies, experimental licenses and proof read the manuscript. D.H. and D.F.R. performed aged necropsies and sampling. All other work and the assembly of the manuscript was performed by D.F.R.
FUNDING
This work was supported by the Swiss Bridge Foundation.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.008136/-/DC1
REFERENCES
- Bogovich K. (1987). Induction of follicular cysts in rat ovaries by prolonged administration of human chorionic gonadotropin. Adv. Exp. Med. Biol. 219, 659–663 [DOI] [PubMed] [Google Scholar]
- Bogovich K., Richards J. S. (1982). Androgen biosynthesis in developing ovarian follicles: evidence that luteinizing hormone regulates thecal 17 alpha-hydroxylase and C17-20-lyase activities. Endocrinology 111, 1201–1208 [DOI] [PubMed] [Google Scholar]
- Chang R. J. (2007). The reproductive phenotype in polycystic ovary syndrome. Nat. Clin. Pract. Endocrinol. Metab. 3, 688–695 [DOI] [PubMed] [Google Scholar]
- Cho H., Thorvaldsen J. L., Chu Q., Feng F., Birnbaum M. J. (2001a). Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276, 38349–38352 [DOI] [PubMed] [Google Scholar]
- Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B., 3rd, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001b). Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292, 1728–1731 [DOI] [PubMed] [Google Scholar]
- Choi J. H., Wong A. S., Huang H. F., Leung P. C. (2007). Gonadotropins and ovarian cancer. Endocr. Rev. 28, 440–461 [DOI] [PubMed] [Google Scholar]
- Couzinet B. S. G. (1993). The control of gonadotrophin secretion by ovarian steroids. Hum. Reprod. 8, 97–101 [DOI] [PubMed] [Google Scholar]
- Danilovich N., Ram Sairam M. (2006). Recent female mouse models displaying advanced reproductive aging. Exp. Gerontol. 41, 117–122 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Argyrakopoulou G., Economou F., Kandaraki E., Koutsilieris M. (2008). Defects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome (PCOS). J. Steroid Biochem. Mol. Biol. 109, 242–246 [DOI] [PubMed] [Google Scholar]
- Dummler B., Hemmings B. A. (2007). Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 35, 231–235 [DOI] [PubMed] [Google Scholar]
- Dummler B., Tschopp O., Hynx D., Yang Z. Z., Dirnhofer S., Hemmings B. A. (2006). Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol. Cell. Biol. 26, 8042–8051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunaif A., Xia J., Book C. B., Schenker E., Tang Z. (1995). Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J. Clin. Invest. 96, 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familiari G. T. V., Motta P. M. (1985). Morphological studies of polycystic mouse ovaries induced by dehydroepiandrosterone. Cell Tissue Res. 240, 519–528 [DOI] [PubMed] [Google Scholar]
- Garofalo R. S., Orena S. J., Rafidi K., Torchia A. J., Stock J. L., Hildebrandt A. L., Coskran T., Black S. C., Brees D. J., Wicks J. R., et al. (2003). Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J. Clin. Invest. 112, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S., Rochford J. J., Wolfrum C., Gray S. L., Schinner S., Wilson J. C., Soos M. A., Murgatroyd P. R., Williams R. M., Acerini C. L., et al. (2004). A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science 304, 1325–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi M. O., Jones M. R., Chen Y. D., Azziz R. (2008). First evidence of genetic association between AKT2 and polycystic ovary syndrome. Diabetes Care 31, 2284–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi M. O., Dumesic D. A., Chazenbalk G., Azziz R. (2011). Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 7, 219–231 [DOI] [PubMed] [Google Scholar]
- Heron-Milhavet L., Franckhauser C., Rana V., Berthenet C., Fisher D., Hemmings B. A., Fernandez A., Lamb N. J. C. (2006). Only Akt1 is required for proliferation, while Akt2 promotes cell cycle exit through p21 binding. Mol. Cell. Biol. 26, 8267–8280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I., Ahtiainen P., Pakarainen T., Rulli S. B., Zhang F. P., Poutanen M. (2006). Genetically modified mouse models in studies of luteinising hormone action. Mol. Cell. Endocrinol. 252, 126–135 [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Sen M. (1989). Increased activity of ovarian thecal cytochrome P450(17 alpha) in the hamster induced by endogenous LH. Acta Endocrinol. 121, 374–382 [DOI] [PubMed] [Google Scholar]
- Klinefelter G. R., Hall P. F., Ewing L. L. (1987). Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol. Reprod. 36, 769–783 [DOI] [PubMed] [Google Scholar]
- Lima M. H., Souza L. C., Caperuto L. C., Bevilacqua E., Gasparetti A. L., Zanuto R., Saad M. J., Carvalho C. R. (2006). Up-regulation of the phosphatidylinositol 3-kinase/protein kinase B pathway in the ovary of rats by chronic treatment with hCG and insulin. J. Endocrinol. 190, 451–459 [DOI] [PubMed] [Google Scholar]
- Manna P. R., Chandrala S. P., Jo Y., Stocco D. M. (2006). cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation. J. Mol. Endocrinol. 37, 81–95 [DOI] [PubMed] [Google Scholar]
- Manneras L., Cajander S., Holmang A., Seleskovic Z., Lystig T., Lonn M., Stener-Victorin E. (2007). A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 148, 3781–3791 [DOI] [PubMed] [Google Scholar]
- Massicotte J., Lagacé L., Godbout M., Labrie F. (1984). Modulation of rat pituitary gonadotrophin secretion by porcine granulosa cell ‘inhibin’, LH releasing hormone and sex steroids in rat anterior pituitary cells in culture. J. Endocrinol. 100, 133–140 [DOI] [PubMed] [Google Scholar]
- Mlinar B., Marc J., Janez A., Pfeifer M. (2007). Molecular mechanisms of insulin resistance and associated diseases. Clin. Chim. Acta 375, 20–35 [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Shaikh N., Khavale S., Shinde G., Meherji P., Shah N., Maitra A. (2009). Genetic variation in exon 17 of INSR is associated with insulin resistance and hyperandrogenemia among lean Indian women with polycystic ovary syndrome. Eur. J. Endocrinol. 160, 855–862 [DOI] [PubMed] [Google Scholar]
- Nechamen C. A., Thomas R. M., Dias J. A. (2007). APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol. Cell. Endocrinol. 260–262, 93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson V. L., Legro R. S., Strauss J. F., 3rd, McAllister J. M. (1999). Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol. Endocrinol. 13, 946–957 [DOI] [PubMed] [Google Scholar]
- Nelson-Degrave V. L., Wickenheisser J. K., Hendricks K. L., Asano T., Fujishiro M., Legro R. S., Kimball S. R., Strauss J. F., 3rd, McAllister J. M. (2005). Alterations in mitogen-activated protein kinase kinase and extracellular regulated kinase signaling in theca cells contribute to excessive androgen production in polycystic ovary syndrome. Mol. Endocrinol. 19, 379–390 [DOI] [PubMed] [Google Scholar]
- Poretsky L., Clemons J., Bogovich K. (1992). Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metabolism 41, 903–910 [DOI] [PubMed] [Google Scholar]
- Salvador L. M., Maizels E., Hales D. B., Miyamoto E., Yamamoto H., Hunzicker-Dunn M. (2002). Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology 143, 2986–2994 [DOI] [PubMed] [Google Scholar]
- Strauss J. F., 3rd (2003). Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome. Ann. N. Y. Acad. Sci. 997, 42–48 [DOI] [PubMed] [Google Scholar]
- Tan K., Kimber W. A., Luan J., Soos M. A., Semple R. K., Wareham N. J., O’Rahilly S., Barroso I. (2007). Analysis of genetic variation in Akt2/PKB-beta in severe insulin resistance, lipodystrophy, type 2 diabetes, and related metabolic phenotypes. Diabetes 56, 714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towns R., Menon K. M. (2005). The role of cyclic AMP response element binding protein in transactivation of scavenger receptor class B type I promoter in transfected cells and in primary cultures of rat theca-interstitial cells. Mol. Cell. Endocrinol. 245, 23–30 [DOI] [PubMed] [Google Scholar]
- Towns R., Azhar S., Peegel H., Menon K. (2005). LH/hCG-stimulated androgen production and selective HDL-cholesterol transport are inhibited by a dominant-negative CREB construct in primary cultures of rat theca-interstitial cells. Endocrine 27, 269–277 [DOI] [PubMed] [Google Scholar]
- Tremblay J. J., Hamel F., Viger R. S. (2002). Protein kinase A-dependent cooperation between GATA and CCAAT/enhancer-binding protein transcription factors regulates steroidogenic acute regulatory protein promoter activity. Endocrinology 143, 3935–3945 [DOI] [PubMed] [Google Scholar]
- Vrbikova J., Hainer V. (2009). Obesity and polycystic ovary syndrome. Obes. Facts 2, 26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayagunawardane M. P., Gabler C., Killian G., Miyamoto A. (2003). Tumor necrosis factor alpha in the bovine oviduct during the estrous cycle: messenger RNA expression and effect on secretion of prostaglandins, endothelin-1, and angiotensin II. Biol. Reprod. 69, 1341–1346 [DOI] [PubMed] [Google Scholar]
- Yang Z. Z., Tschopp O., Hemmings-Mieszczak M., Feng J., Brodbeck D., Perentes E., Hemmings B. A. (2003). Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 278, 32124–32131 [DOI] [PubMed] [Google Scholar]
- Yun S. J., Kim E. K., Tucker D. F., Kim C. D., Birnbaum M. J., Bae S. S. (2008). Isoform-specific regulation of adipocyte differentiation by Akt/protein kinase Balpha. Biochem. Biophys. Res. Commun. 371, 138–143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.