Abstract
Human sepsis is characterized by a set of systemic reactions in response to intensive and massive infection that failed to be locally contained by the host. Currently, sepsis ranks among the top ten causes of mortality in the USA intensive care units 1. During sepsis there are two established haemodynamic phases that may overlap. The initial phase (hyperdynamic) is defined as a massive production of proinflammatory cytokines and reactive oxygen species by macrophages and neutrophils that affects vascular permeability (leading to hypotension), cardiac function and induces metabolic changes culminating in tissue necrosis and organ failure. Consequently, the most common cause of mortality is acute kidney injury. The second phase (hypodynamic) is an anti-inflammatory process involving altered monocyte antigen presentation, decreased lymphocyte proliferation and function and increased apoptosis. This state known as immunosuppression or immune depression sharply increases the risk of nocosomial infections and ultimately, death. The mechanisms of these pathophysiological processes are not well characterized. Because both phases of sepsis may cause irreversible and irreparable damage, it is essential to determine the immunological and physiological status of the patient. This is the main reason why many therapeutic drugs have failed. The same drug given at different stages of sepsis may be therapeutic or otherwise harmful or have no effect 2,3. To understand sepsis at various levels it is crucial to have a suitable and comprehensive animal model that reproduces the clinical course of the disease. It is important to characterize the pathophysiological mechanisms occurring during sepsis and control the model conditions for testing potential therapeutic agents.
To study the etiology of human sepsis researchers have developed different animal models. The most widely used clinical model is cecal ligation and puncture (CLP). The CLP model consists of the perforation of the cecum allowing the release of fecal material into the peritoneal cavity to generate an exacerbated immune response induced by polymicrobial infection. This model fulfills the human condition that is clinically relevant. As in humans, mice that undergo CLP with fluid resuscitation show the first (early) hyperdynamic phase that in time progresses to the second (late) hypodynamic phase. In addition, the cytokine profile is similar to that seen in human sepsis where there is increased lymphocyte apoptosis (reviewed in 4,5). Due to the multiple and overlapping mechanisms involved in sepsis, researchers need a suitable sepsis model of controlled severity in order to obtain consistent and reproducible results.
Keywords: Medicine, Issue 51, sepsis, systemic inflammation, infection, septic shock, animal model
Protocol
1. Cecal Ligation and Puncture as a Mouse Model for Human Sepsis
For this procedure C57BL/6 mice (7-9 weeks old) are used.
Anesthetize the mouse by injecting intraperitoneally a solution of 1:1 ketamine (75mg/kg) and xylazine (15mg/kg). As a reference, inject 30 μl of the 1:1 solution into a mouse weighing 20 grams. Alternatively, mice can be anesthetized with inhaled isoflurane using an anesthetic vaporizer.
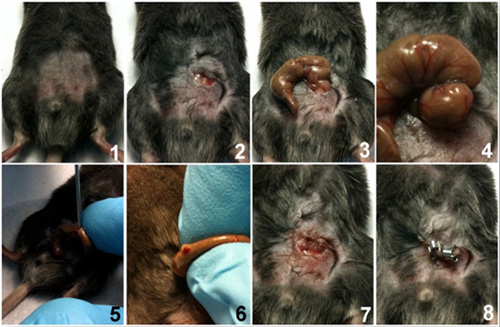
Shave the abdomen of the mouse and disinfect area by first applying betadine solution followed by wiping with a 70% alcohol swab (number 1 in the picture sequence below). Chlorhexidine can be used as an alternative antiseptic. Optional: a sterile drape may be used to maintain the area clean.
Under aseptic conditions, practice a 1 to 2 cm midline laparotomy and expose the cecum with adjoining intestine (2 and 3).
The cecum is tightly ligated with a 6.0 silk suture (6-0 PROLENE, 8680G; Ethicon) at its base below the ileo-cecal valve, and is perforated once or twice with a 19-gauge needle (4 and 5) on the same side of the cecum. Please note that the length of ligated cecum defined as the distance from the distal end of cecum to ligation point will determine the degree of severity. A distance of >1cm produces high grade sepsis while a distance of ≤1 cm produces mid-to-low grade sepsis. Also note that using the cecal perforation method shown here is different from the standard through and through puncture technique (introducing a needle through the cecum). Both methods reliably produce the same sepsis outcome. However, one disadvantage of the through and through technique is that it does not facilitate controlling the amount of feces extruded when the cecum is squeezed.
The cecum is then gently squeezed to extrude a small amount of feces from the perforation sites (6). The cecum is returned to the peritoneal cavity and the peritoneum is closed with 6.0 silk sutures. (7).
The skin is closed with Reflex 7mm clips (RS-9258, Roboz Surgical Instruments) or Michel wound clips (7 mm, RS-9270) (8).
Resuscitate mice by injecting subcutaneously 1 ml of pre-warmed 0.9% saline solution using a 25G needle. This fluid resuscitation measure will induce the hyperdynamic phase of sepsis. This critical step is described further in the discussion section.
Optional: Inject subcutaneously buprenorphine (0.05mg/ kg body weight) or tramadol (20mg/kg body weight) for post operative analgesia. Be advised that these opiates can suppress respiration and locomotion and these effects can be misinterpreted as signs of sepsis.
The animals are placed temporarily on a heating pad or alternatively returned immediately to a cage with exposure to an infrared heating lamp of 150W until they recover from the anesthesia. The recovery time is from 30 min to 1 hour.
Provide free access to food and water (hydrogel) placed on the bottom of the cage.
Mice are monitored every 12 hours for survival for one to two weeks or euthanized at different time points for analysis of different parameters.
As control for the experimental design, sham animals would follow the laparotomy technique without ligation and puncture. Six to 12 hours after the surgical procedure mice will become lethargic and develop fever, piloerection, diarrhea, huddling, and malaise; all the symptoms of sepsis. Mice with very severe sepsis can barely move prior to death and exhibit a dramatic decrease in body temperature. At this step mice should be euthanized to avoid prolonged pain and suffering.
2. Most Common Analyzed Parameters
To evaluate the outcome of the procedure, different parameters can be analyzed in organs, cell extracts or body fluids. Samples can be collected at different time points from 3 hours to one week after the surgical procedure.
Mice survival.
- Cytokines and chemokines in serum, peritoneal cavity and organ extracts.
- Interleukin-6: Produced and released by monocytes, dendritic cells, macrophages, B cells, T cells, granulocytes, mast cells and many more cell types. It plays an important role in acute phase reaction and inflammation.
- Tumor necrosis factor-α: Pleiotrophic cytokine that plays a central role in inflammation and apoptosis. Produced by monocytes, macrophages, neutrophils, dendritic cells and fibroblasts.
- nterleukin-1β: Produced by monocytes, NK cells, dendritic cells, B cells and T cells. Induces fever and acute phase protein synthesis.
- Interleukin 10: Promotes phagocytic uptake and Th2 responses but suppresses antigen presentation and Th1 proinflammatory responses.
- Interleukin 10: Promotes phagocytic uptake and Th2 responses but suppresses antigen presentation and Th1 proinflammatory responses.
- Monocyte chemotactic protein-1 (also known as CCL2): Recruits monocytes, memory T cells, and dendritic cells to sites of tissue injury and infection.
- KC (CXCL1): Produced by macrophages and endothelial cells. Mouse KC is a potent neutrophil attractant and activator.
- RANTES: A monocyte chemoattractant. It can chemoattract unstimulated CD4+/CD45RO+ memory T cells and stimulated CD4+ and CD8+ T cells with naïve and memory phenotypes.
- Interferon-γ: Secreted by Th1 cells, cytotoxic T cells, dendritic cells and natural killer (NK) cells. Increases antigen presentation and lytic activity in macrophages and suppresses Th2 cell activity. Promotes adhesion and binding required for leukocyte migration and promotes NK cell activity.
- High-mobility group B-1 protein: In monocytes is a nuclear factor kappa B activator by binding to the cellular receptor for advance glycation end products (RAGE), activating the release of proinflammatory mediators in the late phase of sepsis.
Myeloperoxidase determination in organs as a measure of neutrophil infiltration. Myeloperoxidase (MPO) is a peroxidase enzyme most abundantly present in neutrophil granulocytes. It is a lysosomal protein stored in azurophilic granules of the neutrophil. MPO has a heme pigment, which causes its green color in secretions rich in neutrophils, such as pus and some forms of mucus.
Bacteria load in organs and blood stream, measure as the number of colony forming units per milliliter.
3. Representative Results
The CLP procedure was initially performed in the C57BL/6 mouse strain. We tested several parameters to modulate the severity of sepsis by altering the length of the ligation and thickness of the needle as shown in Figures 1 and 2. Among these two factors, the length of the ligation seems to be more effective than the thickness of the needle to alter percent survival. As shown in Figure 1, increasing the length of the ligation by more than 1cm provokes an increase in mortality of 100% compared with mice having a ligation of ≤1cm. Increasing the thickness of the needle also decreased the percent survival from 100% (using a 22G needle) to 55% (using a 19G needle) with two punctures. We also tested the effect of CLP on C57BL/6 and 129SvJ mice to determine whether different mouse strains display similar or distinct susceptibility to CLP-induced sepsis. Figure 3 shows that under the same conditions, 129SvJ mice were more susceptible to infection than C57BL/6, indicated by an increased percent mortality.
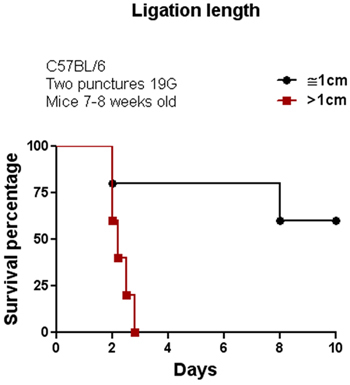 Figure 1. Effect of ligation length of the cecum in animal survival. CLP was performed in C57BL/6 mice using two different lengths of cecal ligation. This single parameter dramatically affects animal survival since all of the animals in the group with a ligation area longer than 1 cm died in less than 3 days when compared with a group of animals having a ligation area of 1 cm approx. (n=8).
Figure 1. Effect of ligation length of the cecum in animal survival. CLP was performed in C57BL/6 mice using two different lengths of cecal ligation. This single parameter dramatically affects animal survival since all of the animals in the group with a ligation area longer than 1 cm died in less than 3 days when compared with a group of animals having a ligation area of 1 cm approx. (n=8).
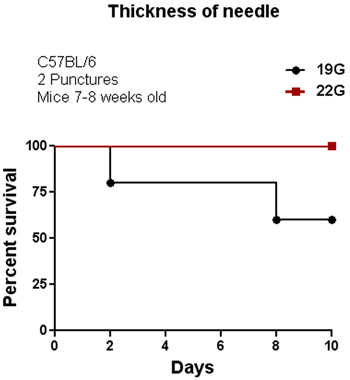 Figure 2. Influence of needle thickness on survival. CLP was performed in C57BL/6 mice using two different needle sizes, 19G and 22G. Mice with the cecum perforated using a 19G needle showed a 55-60% survival. In contrast, mice with the cecum perforated using a 22G needle showed 100% survival although they experienced typical symptoms of inflammation during the first 3-4 days; which disappeared after 4 days (n=8).
Figure 2. Influence of needle thickness on survival. CLP was performed in C57BL/6 mice using two different needle sizes, 19G and 22G. Mice with the cecum perforated using a 19G needle showed a 55-60% survival. In contrast, mice with the cecum perforated using a 22G needle showed 100% survival although they experienced typical symptoms of inflammation during the first 3-4 days; which disappeared after 4 days (n=8).
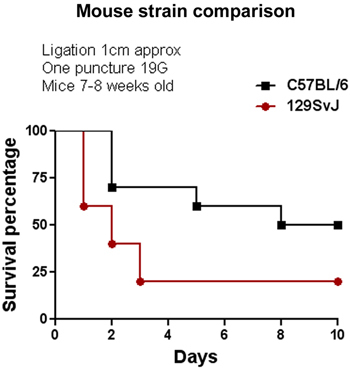 Figure 3. Comparison of sepsis susceptibility between two different mouse strains. The CLP model was performed in 129SvJ and C57BL/6 under the same conditions. At the end of the evaluation period the percent survival of the 129SvJ strain was 25% less than in the C57BL/6 strain, indicating a higher susceptibility to inflammation induced by polymicrobial infection. (n=6).
Figure 3. Comparison of sepsis susceptibility between two different mouse strains. The CLP model was performed in 129SvJ and C57BL/6 under the same conditions. At the end of the evaluation period the percent survival of the 129SvJ strain was 25% less than in the C57BL/6 strain, indicating a higher susceptibility to inflammation induced by polymicrobial infection. (n=6).
Discussion
Here we show in detail how to perform the CLP model in mice and modulate the grade of severity.
Compared to other animal models of sepsis, CLP can be performed in any mouse strain of different age and sex. It is a relatively easy and inexpensive surgical procedure. In this model, the grade of severity has a direct impact on the percentage of survival. The length of the ligation, the thickness of the needle and the number of punctures are parameters that can be controlled to modulate the severity/mortality of CLP. Consequently, to obtain dramatic changes in CLP severity, the user can change the length of the ligation while slight modification in severity can be introduced by adjusting the thickness of the needle.
Other important parameters that could introduce a substantial variability in the CLP results are the mouse strain 6,7, sex8 and age of the animal9. In this regard we show that different mouse strains display distinct susceptibilities to the procedure when performed under the same conditions. Indeed, differences in plasma cytokine levels and tissue damage have been detected between different mouse strains10-12. Inclusion of fluid resuscitation and analgesic administration are factors that can introduce significant variability in the CLP model. For instance, fluid resuscitation increases survival rate possibly by diminishing the production of early pro-inflammatory cytokines11, 13, 14 thus masking the effects of potential therapeutic interventions. In contrast, narcotic analgesics can suppress respiration and locomotion in the animal and provoke an irreversible effect unrelated to sepsis. Their use, therefore, can influence survival rate and immune cell function15.
In conclusion, when performing the CLP model all of these conditions and the technical parameters must be carefully considered in order to obtain reproducible and consistent results. Therefore, a deep knowledge and mastering of the procedure is absolutely needed to perform the CLP model accurately.
Disclosures
Animal experiments were performed in accordance with the National Institute of Health Guide for Care and Use of Laboratory Animals, and approved by the animal care and use committee at Temple University.
Acknowledgments
This work was supported by a grant from the Pennsylvania Department of Health. Dr. Miguel Garcia Toscano was a postdoctoral Fellow at Temple University, funded by the Alfonso Martin Escudero Foundation during this study. We want to thank Iliya Yordanov and Kevin Kotredes for the making of the video.
References
- Angus DC. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. Am J Respir Crit Care Med. 2002;166:1510–1514. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat Rev Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godshall CJ, Scott MJ, Peyton JC, Gardner SA, Cheadle WG. Genetic background determines susceptibility during murine septic peritonitis. J Surg Res. 2002;102:45–49. doi: 10.1006/jsre.2001.6319. [DOI] [PubMed] [Google Scholar]
- De Maio A, Torres MB, Reeves RH. Genetic determinants influencing the response to injury, inflammation, and sepsis. Shock. 2005;23:11–17. doi: 10.1097/01.shk.0000144134.03598.c5. [DOI] [PubMed] [Google Scholar]
- Diodato MD, Knoferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14:162–169. doi: 10.1006/cyto.2001.0861. [DOI] [PubMed] [Google Scholar]
- Turnbull IR. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–313. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- Miyaji T. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620–1631. doi: 10.1046/j.1523-1755.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- Torres MB, De Maio A. An exaggerated inflammatory response after CLP correlates with a negative outcome. J Surg Res. 2005;125:88–93. doi: 10.1016/j.jss.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Doi K. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA. Fluid resuscitation attenuates early cytokine mRNA expression after peritonitis. J Trauma. 1996;41:622–627. doi: 10.1097/00005373-199610000-00005. [DOI] [PubMed] [Google Scholar]
- Zanotti-Cavazzoni SL. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Med. 2009;35:748–754. doi: 10.1007/s00134-008-1360-9. [DOI] [PubMed] [Google Scholar]
- Hugunin KM, Fry C, Shuster K, Nemzek JA. Effects of tramadol and buprenorphine on select immunologic factors in a cecal ligation and puncture model. Shock. 2010;34:250–260. doi: 10.1097/shk.0b013e3181cdc412. [DOI] [PubMed] [Google Scholar]


