Abstract
The p53 tumor suppressor gene is mutated in 50% of human cancers, resulting in more aggressive disease with greater resistance to chemotherapy and radiation therapy. Advances in gene therapy technologies offer a promising approach to restoring p53 function. We have developed polymeric nanoparticles (NPs), based on poly (lactic-co-glycolic acid), that provide sustained intracellular delivery of plasmid DNA, resulting in sustained gene expression without vector-associated toxicity. Our previous studies with p53 gene-loaded NPs (p53NPs) demonstrated sustained antiproliferative effects in cancer cells in vitro. The objective of this study was to evaluate the efficacy of p53NPs in vivo. Tumor xenografts in mice were established with human p53-null prostate cancer cells. Animals were treated with p53NPs by either local (intratumoral injection) or systemic (intravenous) administration. Controls included saline, p53 DNA alone, and control NPs. Mice treated with local injections of p53NPs demonstrated significant tumor inhibition and improved animal survival compared with controls. Tumor inhibition corresponded to sustained and greater p53 gene and protein expression in tumors treated with p53NPs than with p53 DNA alone. A single intravenous dose of p53NPs was successful in reducing tumor growth and improving animal survival, although not to the same extent as with local injections. Imaging studies showed that NPs accumulate in tumor tissue after intravenous injection; however, further improvement in tumor targeting efficiency of p53NPs may be needed for better outcome. In conclusion, the NP-mediated p53 gene therapy is effective in tumor growth inhibition. NPs may be developed as nonviral vectors for cancer and other genetic diseases.
Keywords: Biodegradable polymers, Nonviral vectors, Gene delivery, Cancers, Mutations
Introduction
The p53 tumor suppressor gene is the most commonly altered gene in human cancer (Vogelstein et al. 2000). It encodes for the transcription factor p53, which plays a central role in regulating cell cycle progression, senescence, differentiation, DNA repair, and apoptosis (Riley et al. 2008; Vousden and Lu 2002). In response to DNA damage or other stress signals, p53 activity is upregulated to initiate a cascade of biological events that ultimately results in prevention of tumor development (Vousden and Lu 2002). Mutations in p53 abrogate normal tumor suppressor functions, contributing to the survival and/or proliferation of abnormal cells with poorly differentiated phenotypes (Olivier et al. 2009; Carson and Lois 1995). Cancer cells containing mutant p53 are associated with more aggressive disease, increased resistance to chemotherapy and radiation therapy, and poor prognosis (Ecke et al. 2010; Hirshfield et al. 2010). As a result, there is great interest in therapeutic strategies aimed at restoring the function of p53 for the treatment of cancer (Brown et al. 2009; Bossi and Sacchi 2007).
Gene therapy is a promising approach to restoring p53 function through delivery of the wild-type p53 gene to cancer cells. A successful p53 gene delivery system must meet several criteria. The vector itself should be nontoxic and nonimmunogenic, allowing for multiple administrations if required. The p53 protein is potent but short-lived; therefore, a gene vector must provide sustained gene expression within tumors for the therapeutic effects to be long lasting. Interestingly, p53 exhibits potent bystander effects, so high levels of transfection may not be necessary for tumor inhibition, since transfected cells may affect the nontransfected portions of the tumor (Xu et al. 1997; Bouvet et al. 1998). Ideally, the gene vector would be delivered systemically and selectively target both primary and metastatic tumors.
We have developed polymeric biodegradable nanoparticles (NPs), based on poly(lactide-co-glycolide), with controlled release properties as nonviral vectors for gene therapy. Our studies have shown that these NPs are first internalized by cells via endocytosis and pinocytosis, then rapidly escape from endolysosomes into the cytoplasm (Panyam et al. 2002; Panyam and Labhasetwar 2003). As the NPs degrade, DNA is released into the cytosol over time, resulting in sustained gene expression (Prabha and Labhasetwar 2004a). In our previous study, cancer cells treated in vitro with p53 gene-loaded NPs (p53NPs) demonstrated prolonged p53 gene expression, greater antiproliferative activity, and less toxicity compared with p53 gene-loaded cationic liposomes (Prabha and Labhasetwar 2004a). The objective of this study was to evaluate NPs as vectors for p53 gene therapy in vivo for tumor inhibition. Based on previous in vitro findings, we hypothesized that the NPs would provide sustained gene transfection in tumor tissue, resulting in tumor growth inhibition and improved animal survival. To test this, we used a mouse xenograft model of human prostate cancer and evaluated the effects of NP-mediated gene therapy following local and systemic treatments.
Materials and methods
Plasmid DNA
The recombinant pCEP4 vector containing a cytomegalovirus-driven wild-type p53 (wt-human p53 cDNA) was kindly provided by Dr. Pi-Wang Cheng (Biochemistry and Molecular Biology, University of Nebraska Medical Center; Seki et al. 2002). The vector was transformed and propagated in DH5-alpha Escherichia coli (Invitrogen, Carlsbad, CA, USA). Plasmid was isolated using an Endofree Plasmid Giga Kit (Qiagen, Valencia, CA, USA) according to manufacturer’s protocol.
Formulation of NPs containing plasmid DNA
Poly(d,l-lactide-co-glycolide) (PLGA, copolymer ratio 50:50, inherent viscosity of 1.24 dL/g) was purchased from Durect Corporation, Pelham, AL, USA. Acetylated bovine serum albumin (Ac-BSA), polyvinyl alcohol (PVA, average molecular weight 30,000–70,000, 87–90% hydrolyzed), and Tris-acetate EDTA buffer (TE Buffer, pH 8.0) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). DNA-loaded NPs were formulated by a double-emulsion solvent evaporation technique (Prabha and Labhasetwar 2004b). In brief, an aqueous solution containing 1 mg of p53 plasmid DNA and 2 mg of Ac-BSA in 200 µL of TE buffer were emulsified into 1 mL of PLGA solution in chloroform (30 mg PLGA/mL) using a probe sonicator (XL 2015 Sonicator® ultrasonic processor, Misonix Inc., Farmingdale, NY, USA) at 55 W of energy output for 1 min over an ice bath. The Ac-BSA is nuclease free and provides stability to the primary emulsion, resulting in better encapsulation of DNA in NPs (Vasir et al. 2006). The primary emulsion was then emulsified into 6 ml of 2% w/v aqueous solution of PVA using sonication as above for 5 min to form a multiple (water-in-oil-in-water) emulsion. We have previously shown that sonication of the emulsion does not affect the integrity of the DNA or its transfection efficiency (Labhasetwar et al. 1998, 1999). The emulsion was stirred overnight at room temperature to evaporate chloroform. NPs were recovered by ultracentrifugation at 35,000 rpm (Beckman L80, Beckman Instruments, Inc., Palo Alto, CA, USA), washed three times with TE buffer to remove PVA and unentrapped DNA, resuspended in autoclaved water, and lyophilized. The washings following the recovery of NPs were saved to determine DNA encapsulation using an indirect method (Prabha and Labhasetwar 2004b). In brief, the DNA concentration in the washings was determined by UV absorbance at 260 nm using a spectrophotometer. Washings from control NPs (without DNA) were used to blank the spectrophotometer. The amount of DNA encapsulated in the NPs was determined by subtracting the amount in the washings from the amount used in the preparation. DNA encapsulation efficiency was approximately 60–80%, resulting in DNA loading of 2% w/w. NP size was determined using quasi-elastic light scattering (PSS/NICOMP 380/ZLS Particle Sizing Systems, Santa Barbara, CA, USA). The resulting NPs had an average hydrodynamic diameter of 280 nm (polydispersity index = 0.106); the diameter measured by transmission electron microscopy was ~100 nm (Panyam et al. 2002; Panyam and Labhasetwar 2003). Control and DNA-loaded NPs were similar in size.
Cell line
The PC-3 human prostate carcinoma cell line, which is p53 null, was purchased from American Type Culture Collection (ATCC, Rockville, MD, USA), cultured in RPMI 1640 (Life Technologies, Inc., Grand Island, NY, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum and 1:100 penicillin/streptomycin. Cells were maintained in a humidified incubator at 37°C and 5% CO2. Prior to use, cells were detached using Trypsin/EDTA (Life Technologies, Inc., Grand Island, NY, USA) at 37°C.
PC-3 prostate carcinoma mouse model
Male, athymic nude mice (nu/nu, 5–6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA, USA). Animal studies were performed in accordance with the Guide for Care and Use of Laboratory Animals (7th ed., Washington, DC, USA, National Academies Press 1996), and protocols were approved by the Institutional Animal Care and Use Committee. One million PC-3 cells were suspended in 200 µL of 50:50 phosphate buffered saline (PBS, Gibco) and Matrigel (Growth Factor Reduced, BD Biosciences, San Jose, CA, USA), and injected subcutaneously into the left flank of the mouse. Tumor size was measured every other day using digital calipers. Tumor volume was calculated using the formula, tumor volume (mm3) = 0.5 × length × width2. Animals were treated by either intratumoral injection when tumor volume reached ~100 mm3, or by intravenous injection when tumor volume reached ~300 mm3. We selected a larger tumor, with more mature vasculature, for intravenous injection to facilitate NP delivery to the tumor by the enhanced permeability and retention (EPR) effect. Treatment groups were as follows: (1) saline, (2) p53 plasmid DNA alone (p53DNA), (3) p53 gene-loaded NPs (p53NP), (4) control NPs without DNA, and (5) NPs containing a control plasmid, which does not encode for p53 [p53(−)NP]. A dose equivalent to 40 µg of DNA (approximately 2 mg of NPs) suspended in 50 µL of saline was administered for intratumoral injections. For intravenous injections, a dose equivalent to 200 µg of DNA (approximately 10 mg NPs) suspended in 200-µL saline was administered via the tail vein. A higher dose of NPs was used for intravenous injection considering the loss of the injected dose of NPs to other body compartments. Animals were monitored for tumor size and body weight every other day posttreatment. Animals were euthanized if tumors reached a size greater than 10% of body weight or if they had lost 20% or more of their original body weight.
Gene expression analysis
Total RNA was extracted from tumor tissues 3, 14, and 30 days after intratumoral treatment. Approximately 300 ng of total RNA was used for reverse transcription polymerase chain reaction (RT-PCR) using a GeneAmp PCR kit (Applied Biosystems, Foster City, CA, USA). All reverse transcription reactions were carried out for 20 min at 42°C, then 5 min at 99°C, finally 5 min at 4°C. The primers used for amplifying p53 gene fragment corresponding to residues 529–786 of p53 gene coding region were 5′-TCCACCAGGTCATCTACC-3′ (forward) and 5′-CTCTGAGCCGTTCATACACA-3′ (reverse). The primers used for amplifying β-actin were 5′-GTGGGGCGCCCCAGGCACCA-3′ (forward) and 5′-CTCCTTAATGTCACGCACGATTTC-3′ (reverse). PCR cycles were started with 94°C for 3 min, then performed for 27 cycles with 30 s of denaturation at 94°C, 40 s of annealing temperature at 55°C, and extension for 40 s at 72°C, next 3 min at 72°C, finally stopped at 4°C. The PCR products were analyzed by electrophoresis on a 1.5 % agarose gel.
Immunohistochemical staining for p53
The expression of p53 protein in tumor tissue was examined by immunohistochemistry. Sections (4 µm thick) were obtained from tumors treated with p53NP, p53DNA, and saline. The paraffin-embedded sections were deparaffinized in xylene, rehydrated in a decreasing ethanol series, and washed in distilled water. Samples were then incubated for 20 min with 0.3% hydrogen peroxide (H2O2) in methanol at room temperature to quench endogenous peroxidase activity. Antigen retrieval was performed in an antigen-retrieval fixative (citrate acid and citrate sodium buffer at pH 6.0) at 89°C for 10 min. After washing with PBS (pH 7.4), the samples were immersed in 1% BSA for 60 min to block nonspecific protein binding. Tissue sections were then incubated with primary antibody of anti-p53 monoclonal (Ab-1, Oncogene Research Products) at 4°C overnight. Bound antibody was detected with biotinylated IgG of secondary antibody and streptavidin-peroxidase complex (HRP Detection System, BD Pharmingen), using diaminobenzidine tetrahydrochloride as the substrate. Sections were counterstained with hematoxylin.
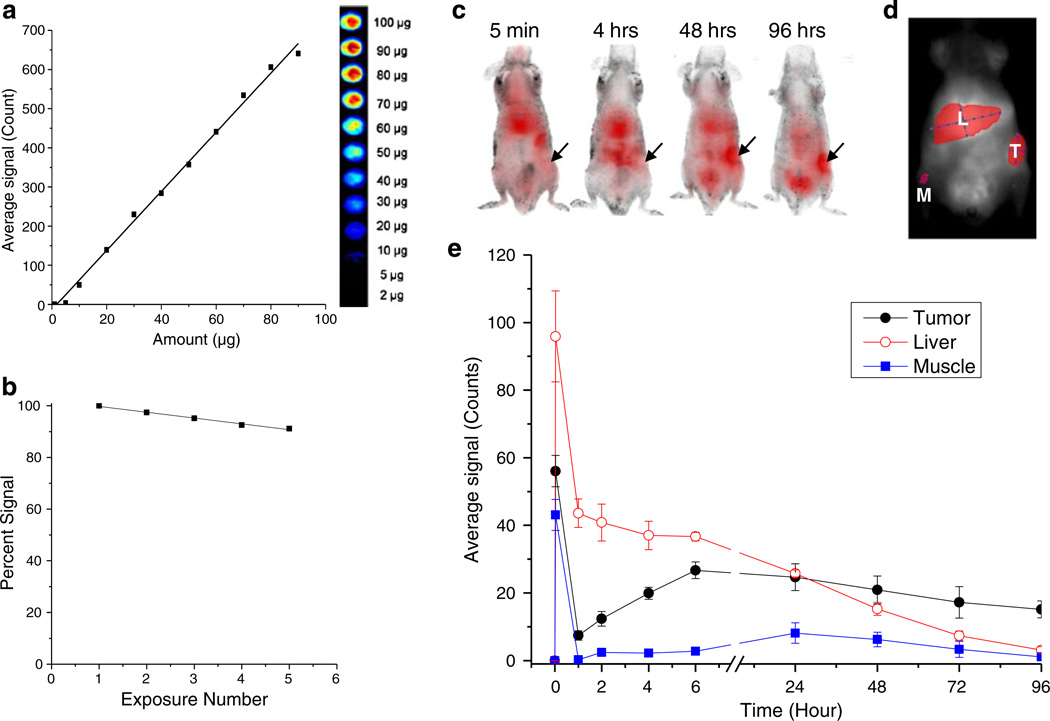
Imaging and evaluation of biodistribution of nanoparticles
The biodistribution of NPs was determined with the Cambridge Research and Instrumentation Maestro EX fluorescence imaging system (Woburn, MA, USA). These NPs were made as described above, with the addition of the near-infrared (NIR) dye SDB 5491 (HW Sands, Jupiter, FL, USA) to the PLGA solution (100 µg dye/3 mL PLGA solution) prior to emulsification in PVA. The resulting dye-loaded NPs were similar in size to the DNA loaded NPs, with an average hydrodynamic diameter of 285 (polydispersity index = 0.097). We have successfully used the above NIR dye in magnetic NP formulations to study their biodistribution in a breast tumor model (Foy et al. 2010). Initial studies were performed to optimize the dye loading and imaging protocols such that a linear correlation between the NIR signal and dye-loaded NPs was obtained. Different amounts of NPs in 100 µl of water were pipetted into white 96-well plates (Nunc Brand, Fisher Scientific, Pittsburg, PA, USA). The plate was imaged with the Maestro using the NIR filter set with auto-exposure. Regions of interest (ROIs) were created around each well and the average signal recorded. The average signal was plotted against the amount of NP. To determine the effects of photobleaching, a mouse was injected subcutaneously on the right lateral side with NPs and imaged with the Maestro five successive times using the blue and NIR filter set at exposure times of 900 and 1,800 ms, respectively. Data were normalized to the first image taken and plotted against the numbers of times exposed. To evaluate biodistribution of NPs, the dye-loaded NPs were injected intravenously in tumor-bearing mice, at a dose of 2.9 µg dye (3 mg NPs). The mice were imaged prior to injection and post-injection at different time points. The cubes (images) obtained were unmixed to obtain an image showing fluorescing regions. ROIs were created on anatomical locations corresponding to the liver, tumor, and muscle (as background) to obtain average signal counts.
Statistical analysis
All numerical data were expressed as the average of the values obtained, with error bars representing the standard error of mean. Tumor growth curves were analyzed by calculating the area under the curve from day 0 to the day on which animals began to die (day 17 in the intratumoral injection experiment, and day 13 in the intravenous experiment). The area-under-the curve provided estimates of percentage inhibition in tumor growth compared to control groups over the time course of the study, rather than at a single time point (Dings et al. 2010). We performed Student’s t test (without correction for multiple hypothesis tests) to determine differences between treatment and control data sets. Analysis of the Kaplan–Meier survival curve was conducted by a log-rank statistical test. We considered p≤0.05 to be statistically significant.
Results
Local administration of p53NPs
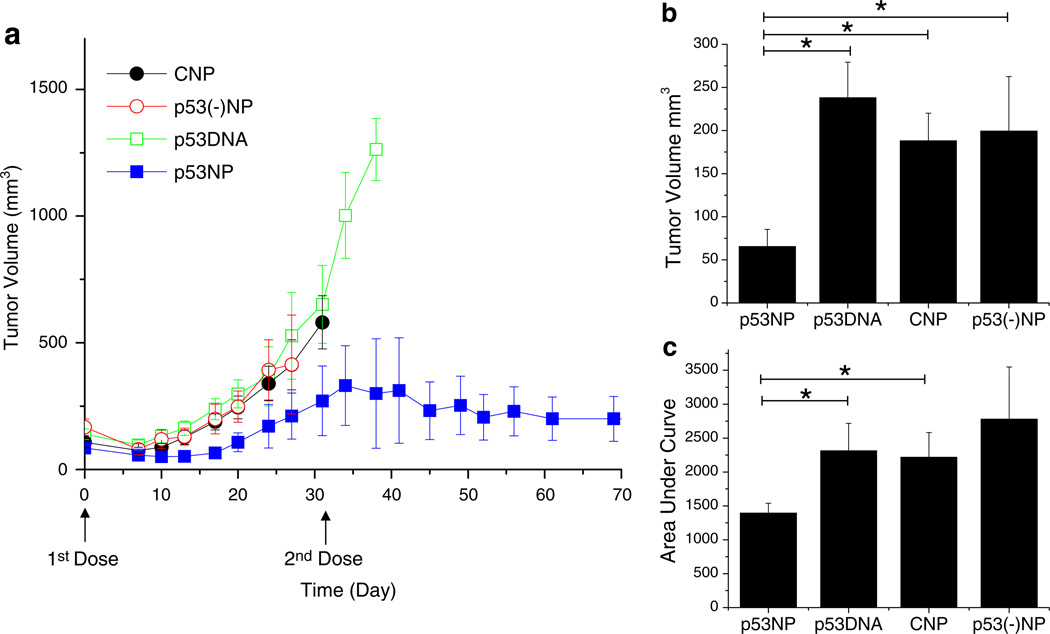
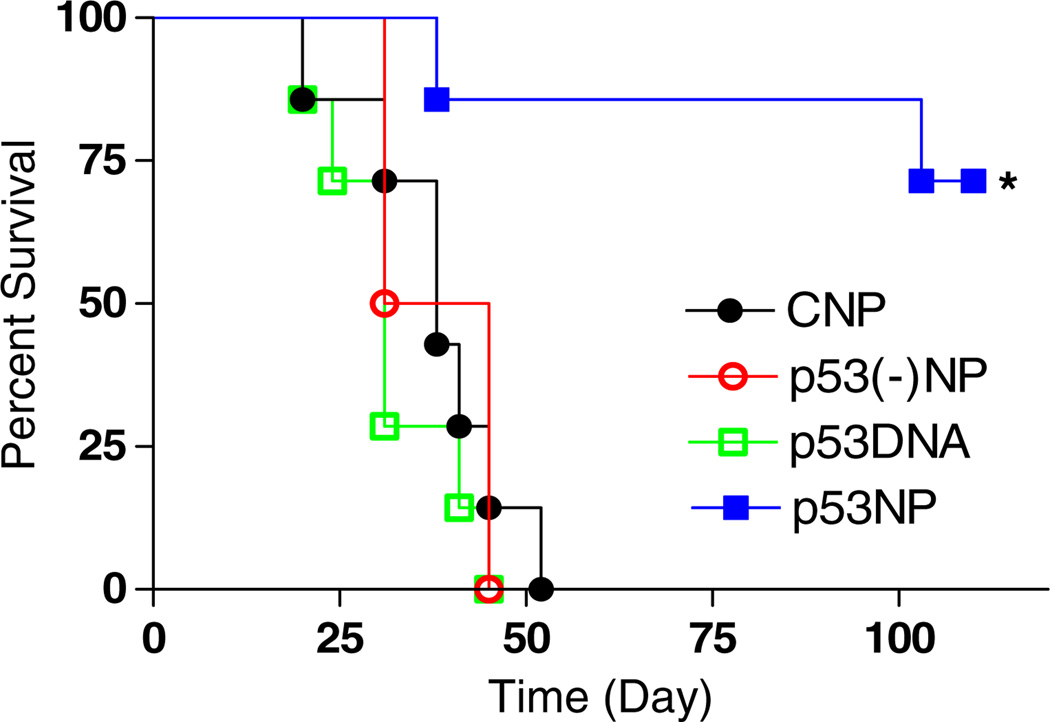
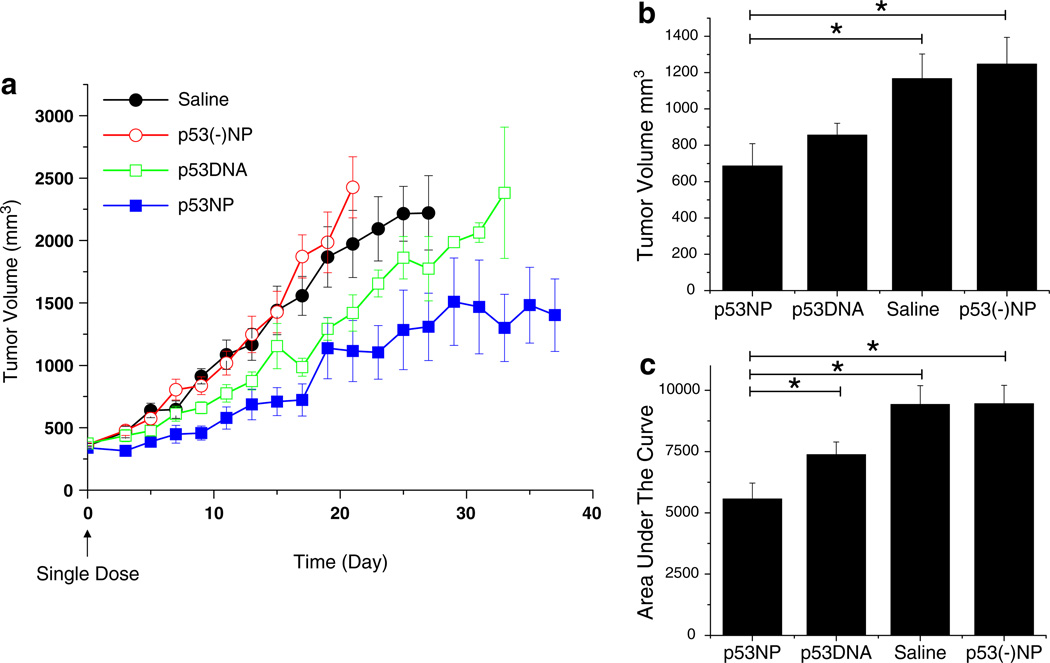
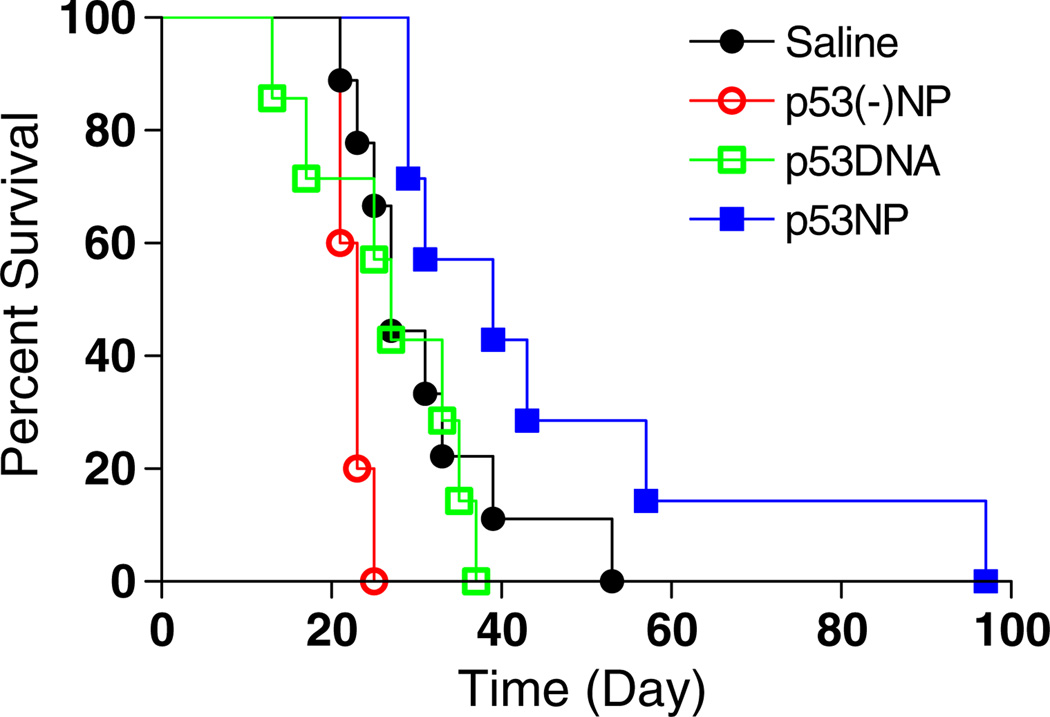
Intratumoral injection of p53NPs resulted in tumor growth inhibition (Fig. 1). All groups showed an initial reduction in tumor volume, likely due to trauma induced by the injection itself. In the p53NP group, tumor inhibition was sustained for approximately 20 days, after which tumors began to grow again, albeit more slowly than controls. A second dose of p53NPs, administered at day 32, restored tumor growth inhibition for the duration of the study. Second doses were not administered in the control groups at this point, since nearly half the animals had died. Survival rates among the control groups were similar, with a median survival of 38 days (Fig. 2). In the p53NP group, all animals survived to receive the second dose on day 32, and all but one animal survived to 100 days.
Fig. 1.
Tumor growth inhibition after local treatment with p53NP. a Tumor growth after direct intratumoral injection of p53NP (n = 7), p53DNA (n = 7), CNP (n = 7), and p53(−)NP (n = 6). A second dose of p53NP was administered on day 32. b Tumor volume at day 17 (first animal death) shows significant reduction in tumor volume in the p53NP group compared with p53DNA, CNP, and p53(−)NP controls. c Area under the tumor growth curve by day 17 demonstrates overall reduction in tumor growth in the p53NP group compared with controls. Statistical analysis performed using Student’s t test; *p≤0.05
Fig. 2.
Animal survival after local treatment. Kaplan–Meier plot showing significant improvement in animal survival after intratumoral injection of p53NPs compared with that with controls (*p<0.05 by log-rank test)
In vivo expression of p53
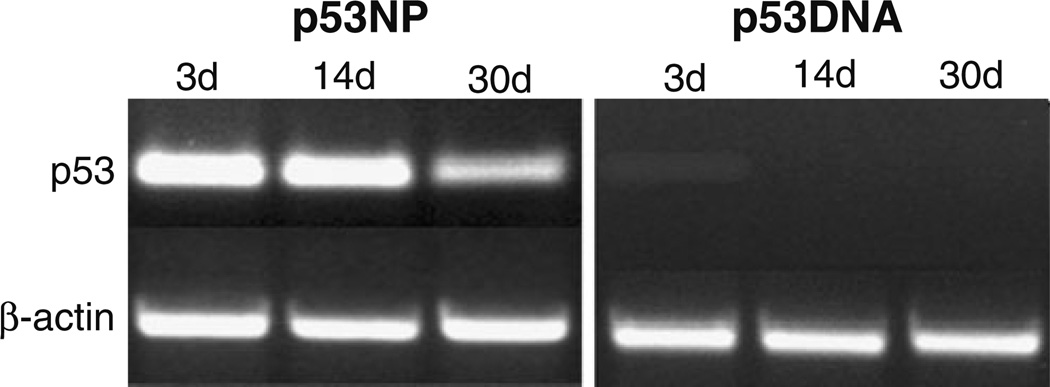
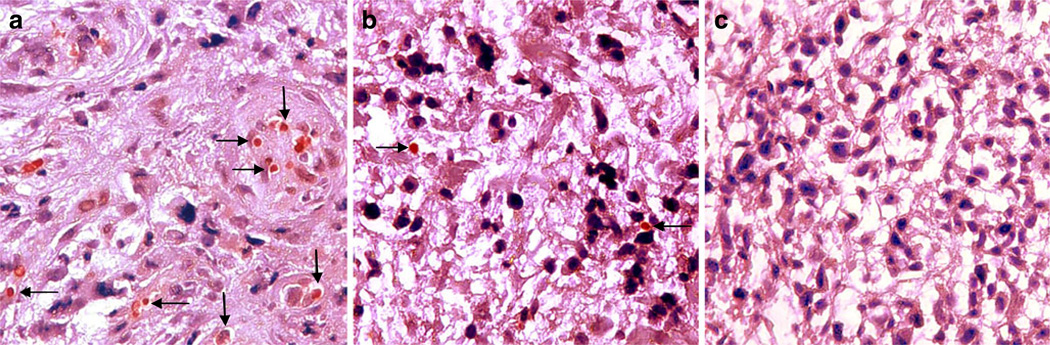
NPs induced expression of the p53 gene in tumor cells, which was detected by RT-PCR up to 30 days posttreatment (Fig. 3). In comparison, tumors treated with p53 plasmid DNA demonstrated a relatively lower level of p53 expression 3 days post-injection, and no expression after 14 and 30 days. In the p53NP group, there appeared to be a reduction in p53 expression between 14 and 30 days posttreatment, which corresponds to the time frame in which the rate of tumor growth increased. Gene expression results were consistent with immunohistochemistry results for p53 protein expression. Qualitatively, a greater number of p53-positive cells were observed in tumors treated with p53NPs (Fig. 4a) compared to p53DNA after 14 days (Fig. 4b). No p53 protein was found in saline controls (Fig. 4c).
Fig. 3.
Intratumoral expression of p53. p53 transcript levels in the tumor tissues treated with p53NPs and p53DNA at 3, 14, and 30 days posttreatment
Fig. 4.
p53 protein expression. Immunohistochemical analysis of p53 expression in tumors treated with p53NPs (a), p53DNA (b), and saline (c) after 14 days. Arrows indicate positive staining of tumor cell nucleus for p53. Original magnification, ×400
NP localization after systemic administration
After demonstrating that NPs could induce p53 expression in tumor cells, reduce tumor growth, and improve animal survival using intratumoral injections, we sought to evaluate their efficacy via systemic administration. First, using optimized imaging techniques, we evaluated if NPs localize to tumors after intravenous injection. Imaging protocols were established that provided a linear correlation between the NIR signal and NIR-dye loaded NPs (Fig. 5a). It was also demonstrated that dye-loaded NPs provide a stable NIR signal when injected in vivo and imaged repeatedly (Fig. 5b). Therefore the same animal could be imaged at successive time points without photobleaching of the dye. Initially after intravenous injection, a high NIR signal was observed in the liver. After several hours, the relative signal increased in the tumor and remained there for more than 4 days (Fig. 5c–e). The presence of the NIR signal in the tumor indicated that NPs are retained in the tumor after intravenous injection and may therefore be able to deliver the p53 gene to tumor cells when administered systemically.
Fig. 5.
Biodistribution of nanoparticles after intravenous delivery. a Correlation of the amount of NIR dye-loaded NPs with average signal count. b In vivo photobleaching of NIR dye loaded-NPs. c Images from one animal at various time points post-intravenous injection with NPs containing an NIR dye. The arrows indicate the location of tumor; red indicates relative NIR signal from the NPs. d The signal intensity of the NPs in the tumor (T), liver (L), and muscle (M) was determined from ROIs drawn over the area of each organ/tissue. e Quantification of NIR signal in tumor, liver, and muscle (background) over time (n = 3 animals). NIR signal accumulates in the tumor several hours after NP injection and is retained for several days
Intravenous administration of p53NPs
Intravenous administration of p53NPs was well-tolerated by the animals, and no signs of toxicity were observed. A single dose of p53NPs resulted in a reduction in tumor growth compared to all control groups (Fig. 6). Saline-treated and p53(−)NP-treated animals exhibited similar tumor growth kinetics, indicating that there were no nonspecific effects of the NPs. Treatment with plasmid p53 DNA alone caused a 20% decrease in tumor growth compared to saline controls, whereas mice treated with p53NPs demonstrated a 41% reduction in tumor growth compared to saline controls (based on area-under-the curve values). Unlike results from the use of p53NPs, treatment with p53DNA failed to improve the survival of animals (Fig. 7). The median survival time for animals treated with either saline or p53DNA was 27 days, and for those treated with p53NP, the median time was 39 days. Most animals (six of eight) treated with p53DNA alone were euthanized because of severe weight loss, despite reductions in tumor growth. We observed that weight loss often corresponded to gross evidence of metastatic progression, such as enlarged lymph nodes or tumor invasion into the peritoneal cavity, which was confirmed by autopsy. In p53NP-treated animals, only one of the seven animals was euthanized for severe weight loss. The remaining animals were eventually euthanized when tumors grew to 10% body weight, but there were no gross signs of metastatic disease. In addition to affecting tumor growth rates, p53NP treatment may affect the metastatic potential of the tumor.
Fig. 6.
Tumor growth inhibition after intravenous treatment. a Tumor growth after a single intravenous injection of p53NP (n = 7), p53DNA (n = 8), p53(−)NP (n = 5), and saline (n = 9). b Tumor volume at day 13 (first animal death, p53DNA group) shows significant reduction in tumor volume in the p53NP group compared to saline and p53(−)NP groups. c Area under the tumor growth curve by day 13 demonstrates overall reduction in tumor growth in the p53NP group compared with controls. Statistical analysis performed using Student’s t test, *p≤0.05
Fig. 7.
Animal survival after intravenous treatment. Kaplan–Meier plot shows improvement in animal survival after intravenous injection with p53NPs compared with controls. Log-rank test of p53NP and respective control groups yield the following p values: p53NP vs. p53DNA, p = 0.02; p53NP vs. p53(−)NP, p = 0.0003; p53NP vs. saline, p = 0.06
Discussion
Gene therapy holds great promise for the treatment of cancer through the delivery of genes that suppress and/or reverse tumor growth and disease progression. However, this strategy requires the development of safe, stable gene delivery systems that, ideally, can be delivered systemically to target tumor cells. We report on a nonviral, polymeric NP-based gene vector for delivery of a prominent tumor suppressor gene, p53. We demonstrated that this vector is nontoxic and can be delivered locally or systemically to induce functional expression of p53 in tumor cells, resulting in tumor growth inhibition and improved animal survival. Our previous studies have shown that NPs act as an intracellular depot, resulting in sustained DNA delivery and gene expression (Prabha and Labhasetwar 2004a). In this study, we show sustained p53 mRNA levels and enhanced p53 protein expression in tumor tissue with direct intratumoral injection, resulting in significant tumor growth inhibition and increased survival of treated animals as compared to controls. There were no nonspecific effects of control NPs or NPs with control DNA (p53(−)NPs), indicating that the vector alone is not toxic to cells and that the efficacy of the p53NPs is attributable to delivery of the p53 gene.
Treatment of tumors by direct intratumoral injection is a clinically relevant technique (Pisters et al. 2004) and also provided a simple model to initially evaluate the efficacy of the p53NPs in vivo. It is interesting to note in our study that the second dose of p53NPs was more effective in sustaining tumor growth inhibition than the first dose (Fig. 1). This difference in efficacy may be attributed to the difference in the way we injected p53NPs in the tumor. The first dose was injected directly through the top of the tumor as a single injection, whereas the second dose was injected through the base of the tumor at three different locations. This change in injection strategy was due to the effects observed after the first injection. Direct injection through the top of the tumor resulted in regression of tumor tissue in the area of the injection, but the periphery of the tumor was not affected and continued to grow to form a tumor similar in shape and size to the pre-injection tumor. Injection of p53NPs in multiple locations at the base of the tumor caused more uniform tumor inhibition. This difference in response could be due to the distribution of NPs in the tumor mass, with the second approach perhaps resulting in better distribution of the vector than the direct single injection through the top of the tumor. These observations suggest that the uniform distribution of NPs into the tumor mass is critical for better efficacy.
Levels of p53 mRNA corresponded to tumor growth, such that a decline in intratumoral p53 gene expression occurred at the same time that tumor growth increased after the first intratumoral injection (Fig. 3). Whether the decrease in p53 expression from 14 to 30 days in the tumor is a cause or effect of tumor growth cannot be distinguished and may be related to the manner in which the first dose was administered. A decrease in p53 expression may certainly cause tumor growth, but growth of untransfected cells in the periphery of the tumor may also result in a lower ratio of p53 mRNA to β-actin mRNA in the tumor. Therefore, dose administration to the tumor should be optimized to maintain therapeutic levels of p53 gene expression throughout the tumor to achieve a uniform response.
A single-dose intravenous injection of p53NPs also demonstrated inhibition of tumor growth and prolonged animal survival compared with controls, but the magnitude of the effect was less compared with the effects of direct intratumoral injection. This difference may be attributable in part to the difference in the tumor size at the time of treatment; however, we suspect that the loss of NPs to other body compartments after intravenous injection is also a factor. The primary mechanism by which unmodified NPs localize in the tumor is via the EPR effect; however, this effect could be quite inefficient in localizing NPs selectively to tumor. In this regard, NPs could be modified with different ligands that target the tumor vasculature, such as RGD peptide (Danhier et al. 2009) and/or target receptors overexpressed in cancer cells such as transferrin and folate (Yu et al. 2010). The optical imaging method developed to study the biodistribution of NPs in vivo could be used to optimize tumor targeting efficiency of different formulations of NPs for tumor-targeted delivery. Our current imaging system is limited in its ability to accurately measure NIR signal in deep organs because signal is lost due to scatter and absorbance with depth into tissue. To get a more accurate representation of the amount of NPs in the various body compartments, ex vivo imaging of organs posttreatment may be required. Despite this limitation, the relative NIR signal in the tumor in vivo over time is useful for determining if targeting techniques are effective in improving NP accumulation in the tumor.
The ability to provide sustained gene transfection without vector-associated toxicity is a significant advantage over other gene vectors. Viral vectors are very efficient at cellular targeting and gene transfection and have been studied for p53 gene delivery; however, serious safety issues, such as toxicity, insertional mutagenesis, and immunogenicity (especially with multiple administrations), often limit their use to local injections rather than systemic delivery (Pisters et al. 2004; Cristofanilli et al. 2006; Heilbronn and Weger 2010). As a result, clinical trials of adenoviral vector-mediated p53 gene therapy have been conducted for cancers in which intratumoral injections were feasible (Zhang et al. 2009; Weill et al. 2000). Non-viral vectors offer a safer alternative for gene therapy, but often result in transient gene delivery. Many nonviral vectors, such as liposomes, lipoplexes, and cationic polyplexes, are limited in their ability to provide controlled release of DNA and may require multiple doses per week to achieve efficacious results (Seki et al. 2002; Ditto et al. 2009). These vectors also have cytotoxicity issues, since they gain entry into cells through interaction or fusion with the plasma membrane, which can lead to nonspecific cell lysis (Ditto et al. 2009). NPs may therefore provide an improved therapeutic window compared to other gene vectors, especially for systemic delivery, because of sustained gene expression (requiring less frequent dosing) and biocompatibility of the polymer system.
Although multiple genes are involved in carcinogenesis, p53 is the most widely studied tumor suppressor gene, since the majority of cancers demonstrate mutations in the gene itself or other defects in the p53 pathway. In this study, we investigated a gene therapy approach to restore p53 function in a p53-deficient prostate cancer model. Given the prevalence of p53 mutations, our NP-mediated p53 delivery system could be applicable to a wide range of cancers, including those of the breast, colon, lung, and head and neck (Vogelstein et al. 2000). p53 is involved in a number of mechanisms relating to cancer progression and treatment. Activation of p53 typically occurs in response to DNA damage, cellular stress (including oncogene activation), and chemotherapeutic agents and often results in cell cycle arrest and/or apoptosis. Mutations in p53 not only contribute to tumor progression by allowing aberrant cells to grow, but also contribute to drug resistance since the efficacy of many chemotherapy drugs rely on p53-mediated apoptosis (Lowe et al. 1993). Gene therapy with p53 could therefore be useful in suppressing tumor growth directly, as our study showed, and also for sensitizing tumors to chemotherapy and radiation therapy (Xu et al. 2001).
Another role of p53, although less well-characterized, is its involvement in angiogenesis. Upregulation of wild-type p53 in tumors has been related to upregulation of antiangiogenic proteins, such as thrombospondin-1 (Liu et al. 1999) and downregulation of angiogenic proteins, such as vascular endothelial growth factor (Bouvet et al. 1998). As a result, tumor inhibition may occur via reduction of the tumor’s blood supply.
The growing body of research in the area of cancer stem cells suggests that p53 is involved in their regulation (Jerry et al. 2008). Many researchers have identified subsets of cells within tumors that have stem cell-like properties (i.e., cancer stem cells) that are responsible for driving tumor growth, metastasis, and recurrence of disease (Dalerba et al. 2007). A recent study demonstrated that loss of p53 favored symmetric divisions (i.e., self-renewal) of cancer stem cells, contributing to tumor growth (Cicalese et al. 2009). Thus, there are a number of possible mechanisms by which p53 gene therapy may be beneficial. In our study, intratumoral injections caused tumors to stop growing, but the tumors did not completely regress. It may be possible that the cells in the tumor underwent cell cycle arrest rather than apoptosis, that inhibition of blood supply prevented tumor growth without destroying the entire tumor, or that the stem cell population within the tumor was reduced but other, less tumorigenic cells remained. It may also be possible that with intratumoral injections, the tumor cells died and were replaced by scar tissue. Further study of the mechanisms involved in NP-mediated p53 gene delivery will be important to understanding the basis of tumor inhibition and the impact on disease progression. A better mechanistic understanding will also be useful in identifying synergies with other cancer treatment modalities and optimizing targeting strategies.
Most cancer-related deaths are caused by metastasis, not by the primary tumor. The metastatic potential of cancers has been correlated to mutations in the p53 tumor suppressor gene (Wang et al. 1993). Therefore, effective delivery of functional p53 that prevents metastatic progression from the primary tumor and/or treats the metastases may be the key to improving survival in cancer patients. In this study, we demonstrated improved animal survival upon treatment with p53NPs by both intratumoral and intravenous injection. We hypothesize that this improvement in survival is related to decreased metastasis. Animals treated with p53 DNA alone demonstrated some degree of tumor inhibition (Fig. 6); however, many animals experienced severe weight loss, which was correlated with gross evidence of metastasis, such as enlarged lymph nodes and invasion into the peritoneal cavity. In most p53NP-treated animals, these signs of metastatic disease were not observed. The ability to increase and/or prolong expression of p53 in the tumor with NPs may serve to decrease the metastatic potential of the tumor cells by some of the mechanisms described above, such as arresting growth, reducing angiogenesis, and/or altering the phenotypic characteristics of the tumor cells.
Conclusions
We have developed a nonviral, polymeric NP-based gene vector that is effective in sustaining p53 expression in tumors and may be beneficial in the treatment of cancer. Both local and systemic administration of p53NPs led to a reduction in tumor growth and improved animal survival. The methods and results of this study provide a foundation for further optimization of a promising gene therapy system.
Acknowledgments
The study reported here is funded by grant 1R01 EB 003975 from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (to VL). Part of the animal studies was carried out at Nebraska Medical Center under the Nebraska Research Initiative Funds (to VL). IA is a predoctoral student in Cleveland Clinic’s Molecular Medicine Ph.D. Program, which is funded by the “Med into Grad” initiative of the Howard Hughes Medical Institute. IA is also supported by grant 1F31CA150566-01 from the National Cancer Institute of the National Institutes of Health.
Footnotes
Conflict of interest Authors declare no conflict.
Contributor Information
Blanka Sharma, Department of Biomedical Engineering, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Wenxue Ma, Department of Biomedical Engineering, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Isaac Morris Adjei, Department of Biomedical Engineering, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Jayanth Panyam, Department of Biomedical Engineering, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Sanja Dimitrijevic, Department of Biomedical Engineering, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Vinod Labhasetwar, Email: labhasv@ccf.org, Department of Biomedical Engineering, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, OH 44195, USA; Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
References
- Bossi G, Sacchi A. Restoration of wild-type p53 function in human cancer: relevance for tumor therapy. Head Neck. 2007;29(3):272–284. doi: 10.1002/hed.20529. [DOI] [PubMed] [Google Scholar]
- Bouvet M, Ellis LM, Nishizaki M, Fujiwara T, Liu W, Bucana CD, et al. Adenovirus-mediated wild-type p53 gene transfer downregulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res. 1998;58(11):2288–2292. [PubMed] [Google Scholar]
- Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9(12):862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- Carson DA, Lois A. Cancer progression and p53. Lancet. 1995;346(8981):1009–1011. doi: 10.1016/s0140-6736(95)91693-8. [DOI] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138(6):1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Krishnamurthy S, Guerra L, Broglio K, Arun B, Booser DJ, et al. A nonreplicating adenoviral vector that contains the wild-type p53 transgene combined with chemotherapy for primary breast cancer: safety, efficacy, and biologic activity of a novel gene therapy approach. Cancer. 2006;107(5):935–944. doi: 10.1002/cncr.22080. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, et al. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release. 2009;140(2):166–173. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Dings RP, Van Laar ES, Loren M, Webber J, Zhang Y, Waters SJ, et al. Inhibiting tumor growth by targeting tumor vasculature with galectin-1 antagonist anginex conjugated to the cytotoxic acylfulvene, 6-hydroxylpropylacylfulvene. Bioconjug Chem. 2010;21(1):20–27. doi: 10.1021/bc900287y. [DOI] [PubMed] [Google Scholar]
- Ditto AJ, Shah PN, Yun YH. Non-viral gene delivery using nanoparticles. Expert Opin Drug Deliv. 2009;6(11):1149–1160. doi: 10.1517/17425240903241796. [DOI] [PubMed] [Google Scholar]
- Ecke TH, Schlechte HH, Schiemenz K, Sachs MD, Lenk SV, Rudolph BD, et al. TP53 gene mutations in prostate cancer progression. Anticancer Res. 2010;30(5):1579–1586. [PubMed] [Google Scholar]
- Foy SP, Manthe RL, Foy ST, Dimitrijevic S, Krishnamurthy N, Labhasetwar V. Optical imaging and magnetic field targeting of magnetic nanoparticles in tumors. ACS Nano. 2010;4(9):5217–5224. doi: 10.1021/nn101427t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. 2010;197:143–170. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- Hirshfield KM, Rebbeck TR, Levine AJ. Germline mutations and polymorphisms in the origins of cancers in women. J Oncol. 2010;2010:297671. doi: 10.1155/2010/297671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerry DJ, Tao L, Yan H. Regulation of cancer stem cells by p53. Breast Cancer Res. 2008;10(4):304. doi: 10.1186/bcr2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhasetwar V, Bonadio J, Goldstein S, Chen W, Levy RJ. A DNA controlled-release coating for gene transfer: transfection in skeletal and cardiac muscle. J Pharm Sci. 1998;87(11):1347–1350. doi: 10.1021/js980077+. [DOI] [PubMed] [Google Scholar]
- Labhasetwar V, Bonadio J, Goldstein S, Levy RJ. Gene transfection using biodegradable nanospheres: results in tissue culture and a rat osteotomy model. Colloids and Surfaces B: Biointerfaces. 1999;16:281–290. [Google Scholar]
- Liu Y, Thor A, Shtivelman E, Cao Y, Tu G, Heath TD, et al. Systemic gene delivery expands the repertoire of effective antiangiogenic agents. J Biol Chem. 1999;274(19):13338–13344. doi: 10.1074/jbc.274.19.13338. [DOI] [PubMed] [Google Scholar]
- Lowe SW, Ruley HE, Jacks T, Housman DE. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Olivier M, Petitjean A, Marcel V, Petre A, Mounawar M, Plymoth A, et al. Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 2009;16(1):1–12. doi: 10.1038/cgt.2008.69. [DOI] [PubMed] [Google Scholar]
- Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(d, l-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharm Res. 2003;20(2):212–220. doi: 10.1023/a:1022219003551. [DOI] [PubMed] [Google Scholar]
- Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(d, l-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16(10):1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- Pisters LL, Pettaway CA, Troncoso P, McDonnell TJ, Stephens LC, Wood CG, et al. Evidence that transfer of functional p53 protein results in increased apoptosis in prostate cancer. Clin Cancer Res. 2004;10(8):2587–2593. doi: 10.1158/1078-0432.ccr-03-0388. [DOI] [PubMed] [Google Scholar]
- Prabha S, Labhasetwar V. Nanoparticle-mediated wild-type p53 gene delivery results in sustained antiproliferative activity in breast cancer cells. Mol Pharmaceutics. 2004a;1(3):211–219. doi: 10.1021/mp049970+. [DOI] [PubMed] [Google Scholar]
- Prabha S, Labhasetwar V. Critical determinants in PLGA/PLA nanoparticle-mediated gene expression. Pharm Res. 2004b;21(2):354–364. doi: 10.1023/b:pham.0000016250.56402.99. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9(5):402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Seki M, Iwakawa J, Cheng H, Cheng PW. p53 and PTEN/MMAC1/TEP1 gene therapy of human prostate PC-3 carcinoma xenograft, using transferrin-facilitated lipofection gene delivery strategy. Hum Gene Ther. 2002;13(6):761–773. doi: 10.1089/104303402317322311. [DOI] [PubMed] [Google Scholar]
- Vasir J, Labhasetwar V. Biodegradable Nanoparticles. In: Friedmann T, Rossi J, editors. Gene Transfer: Delivery and Expression of DNA and RNA, A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Laboratory Press; 2006. [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2(8):594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wang NP, To H, Lee WH, Lee EY. Tumor suppressor activity of RB and p53 genes in human breast carcinoma cells. Oncogene. 1993;8(2):279–288. [PubMed] [Google Scholar]
- Weill D, Mack M, Roth J, Swisher S, Proksch S, Merritt J, et al. Adenoviral-mediated p53 gene transfer to non-small cell lung cancer through endobronchial injection. Chest. 2000;118(4):966–970. doi: 10.1378/chest.118.4.966. [DOI] [PubMed] [Google Scholar]
- Xu M, Kumar D, Srinivas S, Detolla LJ, Yu SF, Stass SA, et al. Parenteral gene therapy with p53 inhibits human breast tumors in vivo through a bystander mechanism without evidence of toxicity. Hum Gene Ther. 1997;8(2):177–185. doi: 10.1089/hum.1997.8.2-177. [DOI] [PubMed] [Google Scholar]
- Xu L, Pirollo KF, Chang EH. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo/radiotherapy. J Control Release. 2001;74(1–3):115–128. doi: 10.1016/s0168-3659(01)00324-8. [DOI] [PubMed] [Google Scholar]
- Yu B, Tai HC, Xue W, Lee LJ, Lee RJ. Receptor-targeted nanocarriers for therapeutic delivery to cancer. Mol Membr Biol. 2010;27(7):286–298. doi: 10.3109/09687688.2010.521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li Y, Li L, Zhang Y, Gao N, Zhang Z, et al. Phase I study of repeated intraepithelial delivery of adenoviral p53 in patients with dysplastic oral leukoplakia. J Oral Maxillofac Surg. 2009;67(5):1074–1082. doi: 10.1016/j.joms.2008.06.079. [DOI] [PubMed] [Google Scholar]