Abstract
Smooth muscle cell (SMC) phenotypic modulation in atherosclerosis and in response to PDGF in vitro involves repression of differentiation marker genes and increases in SMC proliferation, migration, and matrix synthesis. However, SMCs within atherosclerotic plaques can also express a number of proinflammatory genes, and in cultured SMCs the inflammatory cytokine IL-1β represses SMC marker gene expression and induces inflammatory gene expression. Studies herein tested the hypothesis that IL-1β modulates SMC phenotype to a distinct inflammatory state relative to PDGF-DD. Genome-wide gene expression analysis of IL-1β- or PDGF-DD-treated SMCs revealed that although both stimuli repressed SMC differentiation marker gene expression, IL-1β distinctly induced expression of proinflammatory genes, while PDGF-DD primarily induced genes involved in cell proliferation. Promoters of inflammatory genes distinctly induced by IL-1β exhibited over-representation of NF-κB binding sites, and NF-κB inhibition in SMCs reduced IL-1β-induced upregulation of proinflammatory genes as well as repression of SMC differentiation marker genes. Interestingly, PDGF-DD-induced SMC marker gene repression was not NF-κB dependent. Finally, immunofluorescent staining of mouse atherosclerotic lesions revealed the presence of cells positive for the marker of an IL-1β-stimulated inflammatory SMC, chemokine (C-C motif) ligand 20 (CCL20), but not the PDGF-DD-induced gene, regulator of G protein signaling 17 (RGS17). Results demonstrate that IL-1β- but not PDGF-DD-induced phenotypic modulation of SMC is characterized by NF-κB-dependent activation of proinflammatory genes, suggesting the existence of a distinct inflammatory SMC phenotype. In addition, studies provide evidence for the possible utility of CCL20 and RGS17 as markers of inflammatory and proliferative state SMCs within atherosclerotic plaques in vivo.
Keywords: transcriptional profiling, atherosclerosis, vascular biology
atherosclerosis is a chronic disease of large arteries that involves formation of plaques composed of vascular and inflammatory cells, lipid, and extracellular matrix (45). The majority of the clinical complications of atherosclerosis arise through rupture of the plaque fibrous cap, exposing circulating blood to the highly thrombogenic necrotic core and precipitating occlusive thrombus formation (68). Plaques vulnerable to rupture are those with large necrotic cores and thin fibrous caps containing few smooth muscle cells (SMCs) and increased inflammatory cell content (16, 36). In the coronary circulation of the heart, rupture of vulnerable plaques can give rise to acute coronary syndromes such as unstable angina, myocardial infarction, and sudden cardiac death (68).
In normal, healthy arteries, SMCs are the contractile cells that play an important role in regulating vascular tone to control blood pressure and blood flow. To perform this specialized function, SMCs express a variety of cell-selective contractile and cytoskeletal proteins such as smooth muscle myosin heavy chain (SMMHC), smooth muscle 22 alpha (SM22α), smooth muscle alpha actin (SM α-actin), and h1-calponin (Cnn1), which in combination are expressed exclusively within differentiated SMCs of the vascular wall (57). SMCs are unique amongst muscle cells, however, in that they are not terminally differentiated and in response to altered environmental cues exhibit remarkable plasticity. This plasticity is evident during atherosclerosis as SMCs undergo a process termed phenotypic modulation (58). SMC phenotypic modulation is classically defined as a switch between a “contractile” to a “synthetic” state through repression of the SMC-selective contractile/cytoskeletal proteins that mark differentiated SMCs and concomitant increases in proliferation, migration, and matrix synthesis (11, 58, 64). Although these functional changes in SMCs contribute to increases in the overall size of atherosclerotic plaques, in advanced lesions they likely promote formation of a stable plaque through enhancing development of a thick fibrous cap (36, 53, 67).
Characterization of SMC phenotypic modulation as a shift from a contractile to a synthetic state is largely consistent with the effects of the classical mediator of SMC phenotypic modulation in vitro, platelet-derived growth factor (PDGF). PDGF-BB is a PDGF isoform found in atherosclerotic plaques that activates both PDGF receptor beta (PDGFR-β) and PDGF receptor alpha (PDGFR-α) (38, 65). In cultured SMCs, PDGF-BB represses expression of multiple SMC differentiation marker genes and coordinately increases SMC proliferation, migration, and matrix synthesis, consistent with SMC phenotypic modulation to a synthetic state (2, 4, 7, 26, 34). In addition, in vivo blockade of PDGF-BB or PDGFR-β reduces SMC migration into neointimal lesions after vascular injury and decreases SMC accumulation and fibrous cap formation within developing atherosclerotic plaques in mice (25, 37, 54, 66, 69). Recent studies have demonstrated that PDGF-DD, a newly discovered PDGF isoform that is a more selective agonist of the PDGFR-β, also promotes SMC phenotypic modulation through repressing SMC differentiation marker gene expression and increasing SMC proliferation and migration (5, 13, 38, 73). Genetic deletion of the PDGFR-β in cultured SMCs abrogated PDGF-DD- and PDGF-BB-induced repression of SMC differentiation marker genes, suggesting that these effects of PDGF on SMC phenotype are mediated via PDGFR-β activation (73). These results suggest that multiple PDGF isoforms acting at the PDGFR-β mediate SMC phenotypic modulation in a way that resembles the classically defined switch from a contractile to a synthetic state observed within atherosclerotic lesions in vivo (10, 64). It is unclear, however, if these effects of PDGF on SMC phenotype encompass the full scope of SMC phenotypic modulation in vitro or in vascular disease states in vivo.
Inflammation plays a critical role in the formation of atherosclerotic plaques and is also thought to be important in atherosclerotic plaque destabilization leading to rupture (41). Recent evidence in both humans and experimental animal models of atherosclerosis suggests that SMCs within atherosclerotic plaques can express a number of proinflammatory genes such as vascular cell adhesion molecule 1 (Vcam1) (56), intercellular adhesion molecule 1 (Icam1) (60, 61), chemokine (C-X-C motif) ligand 12 (Cxcl12) (1), chemokine (C-X3-C) motif ligand 1 (Cx3cl1) (44), and granulocyte macrophage colony stimulating factor (Gmcsf) (59). In vitro evidence demonstrates that production of VCAM1, ICAM1, and CX3CL1 by SMCs promotes adhesion of monocytes to SMCs as well as enhanced monocyte survival and activation (8, 48, 51), suggesting that expression of these proinflammatory genes by SMCs within plaques may contribute to formation of an inflammatory, unstable plaque phenotype (36). It is unclear, however, how or if inflammatory gene expression by plaque SMCs relates to the classical definition of SMC phenotypic modulation from a contractile to a synthetic state including repression of SMC differentiation marker genes and increased proliferation, migration, and matrix synthesis.
The inflammatory cytokine interleukin-1β (IL-1β) has recently been shown to repress expression of multiple SMC differentiation marker genes (14) and induce expression of proinflammatory genes such as Vcam1 (32), Icam1 (15), prostaglandin-endoperoxide synthase 2 (Ptgs2) (14), and chemokine (C-C) motif ligand 2 (Ccl2) (49) in cultured SMCs. These results suggest that IL-1 may promote SMC phenotypic modulation to an inflammatory state. However, it is unclear whether the effects of IL-1 are distinct relative to the classical mediator of SMC phenotypic modulation, PDGF, particularly since studies have shown that IL-1 can promote SMC proliferation (39) and migration (18, 80), and PDGF has been shown to promote expression of some proinflammatory genes such as Icam1 (52), Ccl2 (47), chemokine (C-X-C motif) ligand 1 (Cxcl1) (47), and Ptgs2 (63). Interestingly, in limited studies in which the effects of IL-1 and PDGF have been directly compared, IL-1 has been shown to promote greater expression of the inflammatory genes Icam1 (15), Nos2 (31), and Ccl2 (49); however, PDGF has been shown to induce greater expression of the proinflammatory gene Ptgs2 in SMCs (63). Reasons for these differences are unclear; however, a major limitation of these previous studies is they have focused on comparing the effects of IL-1 and PDGF on expression of one or a small number of genes in SMCs, and as a result it is unclear whether IL-1 and PDGF may have distinct effects on overall SMC phenotype at the level of genome-wide gene expression.
Studies within this manuscript have tested the hypothesis that IL-1β and PDGF-DD commonly alter SMC differentiation state through repression of SMC differentiation marker genes, but that IL-1β distinctly induces numerous proinflammatory genes in SMCs to modulate SMC phenotype to a distinct inflammatory state. Results using genome-wide analysis of gene expression have demonstrated that both IL-1β and PDGF-DD repress expression of multiple differentiation marker genes in cultured SMCs. However, IL-1β distinctly promotes expression of numerous proinflammatory genes, while PDGF-DD primarily induces expression of genes involved in cell cycle regulation. These effects of IL-1β to promote an inflammatory SMC phenotype are mediated at least in part by the transcription factor nuclear factor κB (NF-κB), which was critical for both IL-1β-induced repression of SMC marker genes and induction of inflammatory genes. Finally, results demonstrate that the IL-1β-induced proinflammatory factor chemokine (C-C motif) ligand 20 (CCL20) is expressed within murine atherosclerotic plaques by cells that are negative for the SMC differentiation marker SM α-actin as well as the PDGF-DD-induced gene regulator of G protein signaling 17 (RGS17), suggesting that a distinct inflammatory SMC phenotype may be present within atherosclerotic plaques in vivo.
METHODS
SMC culture.
SMCs were isolated from male Sprague-Dawley rats (Rattus norvegicus) weighing 150–175 g as described previously (23). Animal protocols used in this study were approved by the Animal Care and Use Committee at the University of Virginia.
Affymetrix GeneChip experiments.
Rat aortic SMCs were grown to confluence in 10% serum-containing DMEM/F12 media (Gibco, Carlsbad, CA) for 3 days, serum starved in serum-free media for an additional 3 days, and then treated with IL-1β (2.5 ng/ml; R&D Systems, Minneapolis, MN) or vehicle [0.1% bovine serum albumin (BSA) and 10 mM acetic acid], or PDGF-DD (30 ng/ml; Zymogenetics, Seattle, WA) or vehicle (0.01% BSA), for 24 h. After 24 h total RNA was harvested using TRIzol reagent (Invitrogen, Carlsbad, CA) and RNA was converted to cDNA, labeled, and hybridized to rat RAE230_2.0 Affymetrix GeneChips (n = 1) at the University of Virginia Biomolecular Research Facility.
Array normalization and processing were performed using the ExpressionFileCreator module of GenePattern (62). MAS5 was used for processing, and normalization was performed with median scaling. Significant differences were defined as fold changes greater or less than or equal to 2 and differences in signal intensity greater than or equal to 100. Microarray results were deposited in the Gene Expression Omnibus as accession number GSE31080.
Gene ontology analysis.
Gene ontology analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) to identify clusters of biological process gene ontology terms for each list of gene accession numbers (19, 27). Clusters with enrichment scores >3 were considered significant. Enrichment scores correspond to the −log10 of the geometric mean of the one-tail Fisher's exact test P values for each ontology term within the cluster, so enrichment scores >3 correspond to mean P values of <0.001 (19, 27). DAVID functional annotation clustering was used with medium stringency and program defaults such as similarity term overlap of 3, similarity threshold of 0.5, initial group membership of 3, final group membership of 3, and multiple linkage threshold of 0.5. The background gene list chosen was the prebuilt list of all genes present on the Affymetrix rat RAE230_2.0 GeneChip. Gene ontology cluster names were selected as the most representative gene ontology term within each cluster. Affymetrix Probeset Identifiers (IDs) were converted to Entrez official gene symbols for genes within each cluster using the DAVID Gene ID Conversion tool (19, 27). Some gene symbols may be repeated in the final gene list within each cluster due to the mapping of multiple Affymetrix Probeset IDs for those genes to the cluster.
Transcription factor binding site analysis.
DAVID was used to convert Affymetrix Probeset IDs into Entrez Gene IDs for the genes within each cluster. These Entrez Gene IDs were then converted to orthologous human Entrez Gene IDs using the Promoter Analysis Pipeline (12). Human Entrez Gene IDs, after removing any repeated IDs, were then used as input into Whole Genome rVISTA to identify overrepresented transcription factor binding sites (based on the Transfac Professional 9.2 library) that were also conserved between human and mouse sequences within 5,000 bp upstream of the transcription factor start site essentially as described (21, 50, 81).
Real-time reverse transcription PCR analysis.
Rat aortic SMCs were grown to confluence in serum-containing media for 3 days and then serum starved in serum-free media for an additional 3 days followed by treatment with IL-1β (2.5 ng/ml, R&D Systems) or vehicle (0.1% BSA), or PDGF-DD (30 ng/ml, Zymogenetics) or vehicle (water) for 24 h. After 24 h total RNA was harvested from SMCs using TRIzol reagent (Invitrogen) followed by cDNA synthesis using iScript cDNA synthesis kit (Bio-Rad). Real-time reverse transcription PCR (RT-PCR) was performed using iQ Sybergreen Supermix (Bio-Rad, Hercules, CA) and primers indicated in Table 1 and as described previously (42, 43).
Table 1.
Primers used in real-time RT-PCR analysis
| Primer | Sequence |
|---|---|
| P-SELECTIN FOR | AGCATACTCATGGAATAACTCAC |
| P-SELECTIN REV | GAGCGATTTCATTCTTGTTCTG |
| RGS17 FOR | CAAGGTTATCCATGATTCT |
| RGS17 REV | GATTGTTCAGAGGACTAG |
| CALPONIN FOR | TACTATAACTCTGCCTAGGGGC |
| CALPONIN REV | GCCTGATCTCCCCAAACTGT |
| CXCL6 FOR | AAGAACCAGAAGGATAATG |
| CXCL6 REV | TTATTTTCACTGCCCAAT |
| NOS2 FOR | AGTCACAGACATGGCTTGC |
| NOS2 REV | TGTTGTTGGGCTGGGAATAG |
| CCL20 FOR | TGGGTTTCACAACACAGATGG |
| CCL20 REV | CTTCTTGGTTCTTAGGCTGAGG |
| CXCL2 FOR | CTACACCACCTCCACACTG |
| CXCL2 REV | TGCCTTGAAAGCCCTCTG |
| CXCL5 FOR | CTGCTGCTGTTCACACTG |
| CXCL5 REV | TCCTTCTGGTTCTTCAACTTAG |
| CCL5 FOR | CACTCCCTGCTGCTTTGC |
| CCL5 REV | CACTTGGCGGTTCCTTCG |
| VCAM1 FOR | CAAGACAGGAGACATGGTGCTAAA |
| VCAM1 REV | TCAAGTGTTAAACTTCGCAACTGC |
Immunoblot analysis.
Whole cell lysates of cellular protein was harvested from SMCs at the indicated times using a modified RIPA buffer described previously (20) with the addition of protease and phosphatase inhibitors (Pierce, Rockford, IL). Protein concentration was determined using a BCA protein assay (Thermo Scientific, Rockford, IL) and equal total protein was run on SDS-PAGE gels followed by transfer to Immobilon PVDF membranes (Millipore, Billerica, MA). Blots were probed with antibodies to SMMHC (Biomedical Technologies, Stoughton, MA), SM α-actin (Sigma-Aldrich, St. Louis, MO), β-tubulin (Cell Signaling, Danvers, MA), VCAM1 (Santa Cruz Biotechnology, Santa Cruz, CA), Calponin-1 (Epitomics, Burlingame, CA), and SM22α (Abcam, Cambridge, MA). Densitometry was measured on digital images of scanned films using AlphaInnotech Imaging software (Cell Biosciences, Santa Clara, CA). Background correction was performed using the band intensity from the 10 lowest pixels within each region of interest followed by subsequent normalization of resulting band intensities to those of the GAPDH loading control.
NF-κB inhibition studies.
To block NF-κB activity, SMCs at ∼90% confluence were infected at a multiplicity of infection of 50 with an adenovirus containing a cytomegalovirus (CMV) promoter driving expression of a mutated form of IκBα termed the IκBα superrepressor (Ad-IκBαSR) (77) (Vector Biolabs, Philadelphia, PA) or a control empty adenovirus with only the CMV promoter (Gene Transfer Vector Core, University of Iowa). Twenty-four hours following adenoviral infection fresh serum-free medium was added to the cells, and the next day cells were treated with vehicle, IL-1β, or PDGF-DD for 24 h for mRNA analysis or 72 h for protein analysis as described above.
CCL20 ELISA.
Seventy-two hours after treatment of cultured SMCs, cell culture medium was removed and centrifuged at 250 g for 10 min to pellet any cellular material, and levels of CCL20 were determined in resulting supernatants using an ELISA assay designed to detect rat CCL20 (R&D Systems) according to the manufacturer's instructions.
NF-κB-luciferase activity assays.
Cultured SMCs were transfected with either NF-κB reporter constructs consisting of five NF-κB binding sites upstream of a luciferase reporter (5XNF-κB-luciferase) or the control vector, pCis-ck, which was without a promoter upstream of luciferase (both from Stratagene, Santa Clara, CA). SMCs were transfected in serum-free media at near confluence 3 days after plating. Three days later, SMCs were treated with IL-1β, PDGF-DD, or vehicle for 24 h, and cells were then lysed in Passive Lysis Buffer (Promega, Madison, WI), and luciferase activity was determined using the Promega Luciferase Assay System. Luminescence values reflecting luciferase activity from 5XNF-κB-luciferase-transfected cells were normalized to total protein determined by BCA protein assay (Thermo Scientific) and then by the values normalized the same way from identically treated cells transfected with the promoterless control vector.
Immunofluorescent staining of mouse brachiocephalic arteries.
For generating tissue sections for immunofluorescent staining of mouse atherosclerotic plaques, female Apoe−/− mice (Mus musculus, >98% C57BL/6J) fed a high fat (Western-type) diet (Harlan Teklad, Indianapolis, IN) containing 21% milk fat and 0.15% cholesterol for 28 wk beginning at 8 wk of age. As controls, 35 wk old female C57BL/6J mice fed a regular chow diet were used. At the time of death, mice were euthanized and perfused with 5 ml phosphate-buffered saline followed by 10 ml 4% paraformaldehyde. Brachiocephalic arteries were dissected and fixed for an additional 24 h in 4% paraformaldehyde prior to embedding in paraffin and sectioning at 5 μm thickness.
For immunofluorescent staining, sections were deparaffinized, and antigen retrieval was performed with Antigen Unmasking Solution (Vector Laboratories). The following primary and secondary antibodies were then added successively to the sections: goat anti-CCL20 (Santa Cruz) followed by donkey anti-goat Alexa-647 (Invitrogen), rabbit anti-RGS17 (Santa Cruz) followed by donkey anti-rabbit Alexa-555 (Invitrogen), and SM α-actin conjugated to FITC (Sigma-Aldrich). Slides were then stained with DAPI nuclear stain and coverslipped with ProlongGold (Invitrogen). As controls for the immunostaining, antibodies to CCL20 or RGS17 were replaced with nonimmune goat or rabbit IgGs (Vector Laboratories). Z-stack images of immunostained slides were acquired using a Zeiss LSM-700 confocal microscope (Carl Zeiss Microimaging, Thornwood, NY). Zen 2009 software (Carl Zeiss Microimaging) was used for processing of z-stack images including generating maximum intensity projections to integrate signals across the z-stack images and modifying brightness and contrast. Brightness and contrast of the signal for each channel were modified uniformly within each image and equivalently between staining with specific antibodies and with species-matched, nonimmune IgG controls.
Statistical analysis.
For two-group comparisons of three independent experiments each performed in triplicate, nested ANOVA was used (Minitab; Minitab, State College, PA). For two-group comparisons of data from three independent experiments with a single sample from each experiment, or if one or more missing values in any group precluded the use of nested ANOVA, two-sided unpaired Student's t-tests were used on the single or mean values from each experiment (SigmaStat; Systat Software, Chicago, IL).
RESULTS
IL-1β and PDGF-DD distinctly modulate SMC phenotype.
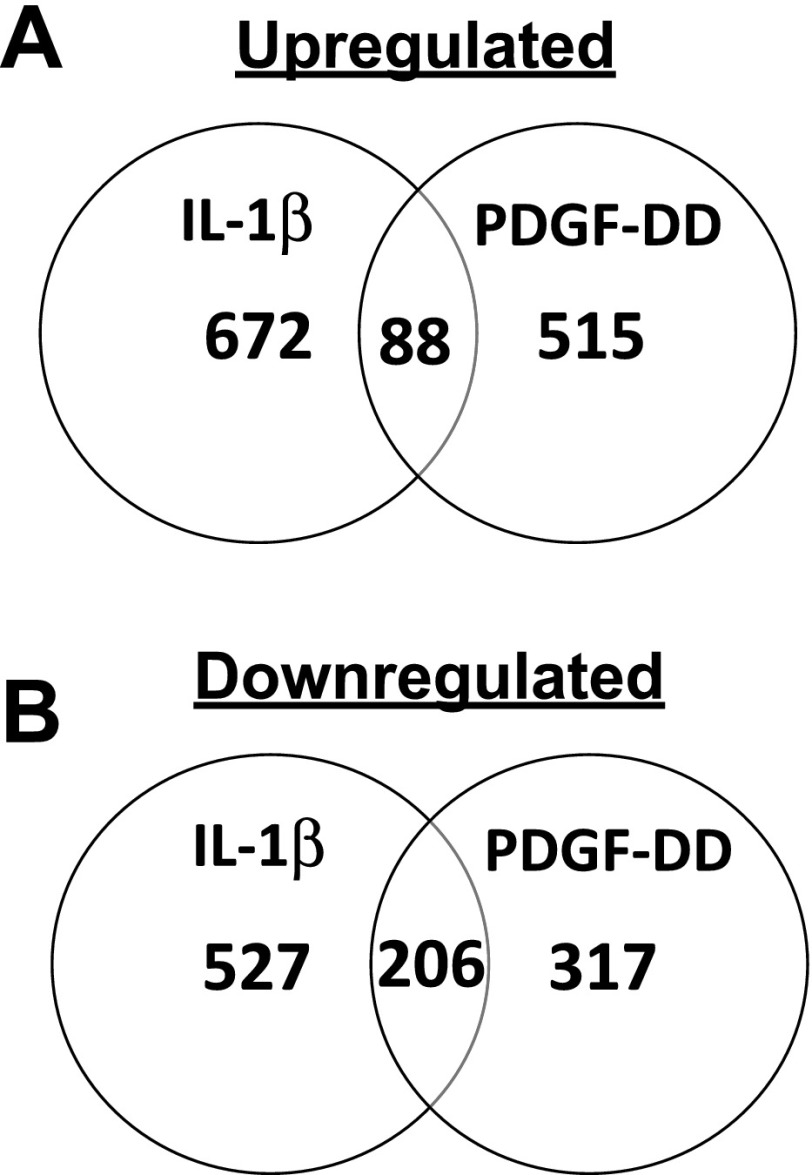
To determine whether IL-1β distinctly modulates SMCs relative to the classical mediator of SMC phenotypic switching, PDGF, genome-wide gene expression analysis was performed on cultured SMCs. Since the PDGFR-β mediates the effects of PDGF to promote SMC accumulation within atherosclerotic plaques in vivo (66), we chose to treat SMCs with PDGF-DD, which is expressed in mouse atherosclerotic lesions and is the PDGF family member that is the most selective agonist of the PDGFR-β (5, 38, 73). Cultured rat aortic SMCs were treated with IL-1β or PDGF-DD for 24 h, and isolated RNA was subjected to genome-wide gene expression analysis using Affymetrix GeneChips to determine whether global gene expression patterns may differ in response to these cytokines. Results revealed that IL-1β and PDGF-DD induced distinct differences in global gene expression, as evidenced by the large number of genes uniquely induced by IL-1β and PDGF-DD (Fig. 1). IL-1β treatment distinctly increased expression of 672 genes relative to its vehicle control, while PDGF-DD distinctly induced 515 genes, with only 88 genes commonly increased by either treatment (representing 7% of the total genes induced by either treatment) (Fig. 1A and Supplemental Tables I–III).1 Among the genes downregulated relative to vehicle controls, IL-1β distinctly decreased expression of 527 genes and PDGF-DD distinctly decreased expression of 317 genes, while 206 genes were downregulated by both treatments (representing 20% of the total genes repressed by either treatment) (Fig. 1B and Supplemental Tables IV–VI). Consistent with previous results (14, 73), both IL-1β and PDGF-DD repressed expression of multiple SMC differentiation marker genes including smooth muscle alpha actin (SM α-actin/Acta2), smoothelin (Smtn), smooth muscle myosin heavy chain (SMMHC/Myh11), and Cnn1 (Supplemental Table VI). These results demonstrate that although both cytokines profoundly altered the differentiation state of the SMCs through repression of multiple SMC differentiation marker genes, there were marked differences in gene expression patterns induced by these factors.
Fig. 1.
IL-1β and PDGF-DD distinctly modulate SMC phenotype by global gene expression analysis. Venn diagrams demonstrating numbers of significantly upregulated (A) and downregulated (B) genes in smooth muscle cells (SMCs) after IL-1β or PDGF-DD treatment relative to vehicle controls.
IL-1β distinctly induces inflammatory gene expression in SMCs.
To determine the potential functional roles of the 672 genes distinctly induced by IL-1β, gene ontology analysis was performed to classify the genes into functional categories using DAVID (19, 27). Interestingly, the genes distinctly induced by IL-1β mapped to numerous gene ontology clusters related to the control of inflammation including inflammatory response, response to lipopolysaccharide, adaptive immune response, and antigen processing and presentation (Table 2 and Supplemental Table VII). In contrast, genes distinctly induced by PDGF-DD mapped to gene ontology clusters related primarily to control of cell growth and proliferation including cell cycle, DNA repair, and chromosome localization (Table 3 and Supplemental Table VIII), suggesting that PDGF-DD promotes a primarily proliferative SMC phenotype. In examining genes repressed by IL-1β and PDGF-DD in SMCs, there were no gene ontology clusters significantly overrepresented in genes commonly downregulated by IL-1β and PDGF-DD or distinctly repressed by IL-1β. However, the gene ontology cluster antigen processing and presentation was significantly overrepresented amongst genes distinctly downregulated by PDGF-DD (Supplemental Table IX).
Table 2.
Gene ontology clustering of distinctly IL-1β-induced genes in SMCs
| Gene Ontology Cluster | Enrichment Score |
|---|---|
| Inflammatory response | 11.3 |
| Response to lipopolysaccharide | 6.8 |
| Adaptive immune response | 4.9 |
| Antigen processing and presentation | 4.5 |
| Positive regulation of inflammatory response | 3.8 |
| Response to hormone stimulus | 3.5 |
| Regulation of leukocyte activation | 3.3 |
| Leukocyte homeostasis | 3.2 |
| Regulation of cytokine production | 3.2 |
| Defense response to bacterium | 3.0 |
SMC, smooth muscle cell.
Table 3.
Gene ontology clustering of distinctly PDGF-DD-induced genes in SMCs
| Gene Ontology Cluster | Enrichment Score |
|---|---|
| Cell cycle | 16.7 |
| DNA repair | 12.5 |
| Chromosome localization | 5.5 |
| Microtubule cytoskeleton organization | 5.0 |
| Regulation of cell cycle | 4.6 |
| DNA duplex unwinding | 4.1 |
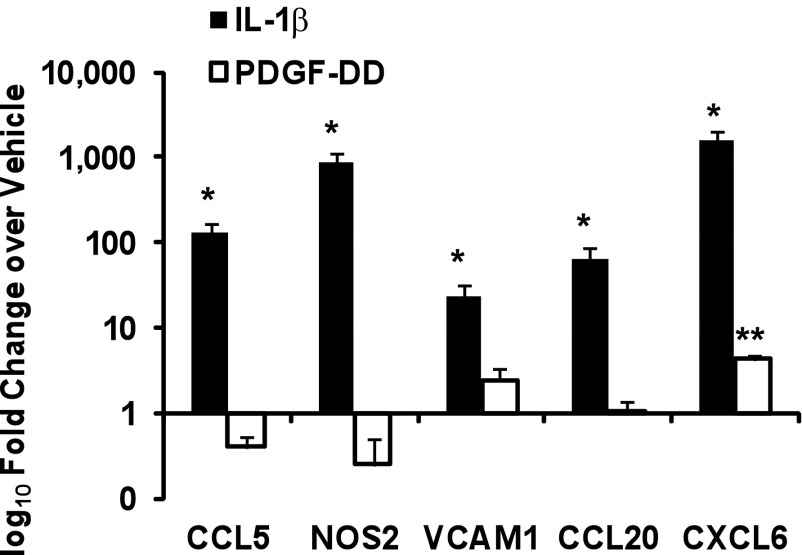
To further examine genes distinctly induced by IL-1β and PDGF-DD in the microarray analysis, real-time RT-PCR was performed on IL-1β- and PDGF-DD-stimulated SMCs, which demonstrated that IL-1β significantly induced expression of the proinflammatory genes Vcam1 (24-fold), Ccl20 (64-fold), Nos2 (870-fold), chemokine (C-C motif) ligand 5 (Ccl5) (129-fold), and chemokine (C-X-C motif) ligand 6 (Cxcl6) (1,634-fold) (Fig. 2). PDGF-DD on the other hand had little effect on expression of these genes with the exception of a slight induction of Cxcl6 (Fig. 2). Taken together, these results suggest that IL-1β modulates SMC phenotype to an inflammatory state that is distinct from phenotypic modulation induced by PDGF-DD.
Fig. 2.
IL-1β distinctly induces expression of multiple proinflammatory genes in SMCs. Real-time RT-PCR analysis of proinflammatory genes in SMCs 24 h after treatment with IL-1β (2.5 ng/ml) or PDGF-DD (30 ng/ml). Data represent means ± SE of mRNA levels normalized to 18s rRNA and the vehicle treatment group from 3 independent experiments (*P < 0.001 vs. vehicle by nested ANOVA; **P < 0.05 vs. vehicle by Student's t-test).
NF-κB mediates IL-1β-induced upregulation of multiple inflammatory genes in SMCs.
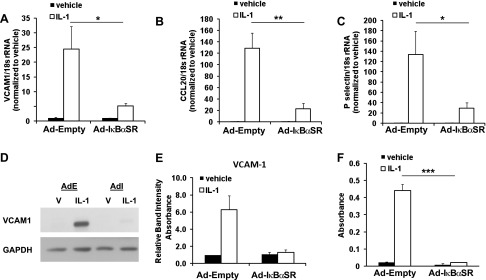
To determine potential mechanisms by which IL-1β distinctly induces expression of proinflammatory genes in SMCs, we took an unbiased approach to analyze the promoter regions of the genes from the top gene ontology group (inflammatory response, see Table 2) for overrepresentation of transcription factor binding sites from the Transfac database using Whole Genome rVISTA (21). Interestingly, these results demonstrated an overrepresentation of three NF-κB binding sites corresponding to a general binding site for NF-κB (NFKAPPAB), a binding site for the p65 subunit of NF-κB (NFKAPPAB65), and a binding site for the p50 subunit of NF-κB (NFKAPPAB50) within the promoter regions of genes within the inflammatory response gene ontology cluster (Table 4). Although less significant, there was also an overrepresentation of the interferon stimulated response element (ISRE) within these gene promoters (Table 4). In contrast, genes from the top gene ontology group distinctly induced by PDGF-DD (cell cycle, Table 3) primarily exhibited overrepresentation of binding sites related to the E2F family of transcription factors that play important roles in regulating the cell cycle (Table 5) (3, 24). The marked overrepresentation of multiple NF-κB binding sites within promoter regions of distinctly IL-1β-induced genes suggests that NF-κB may play a major role in the regulation of IL-1β-induced inflammatory gene expression in SMCs. To determine whether NF-κB functionally mediates IL-1β-dependent regulation of the expression of these genes, we utilized an adenovirus overexpressing a mutant form of IκB alpha that prevents its phosphorylation and subsequent NF-κB activation [termed IκB alpha superrepressor (IκBαSR)] (77). In the presence of the IκBαSR, IL-1β-induced upregulation of the mRNA levels of proinflammatory genes Vcam1, Ccl20, and P-selectin in SMCs was partially abrogated (Fig. 3, A–C). To determine whether these effects of NF-κB inhibition also occur at the protein level, Western blot analysis of cellular VCAM1 levels and secreted CCL20 was performed. Results demonstrate that IL-1β-induced upregulation of protein levels of VCAM1 were nearly totally abrogated in the presence of IκBαSR (Fig. 3D) by Western blot analysis, consistent with previous results (32). Additionally, IL-1β-induced release of CCL20 by SMCs into the cell culture media was significantly reduced with NF-κB inhibition as determined by ELISA (Fig. 3E). These results demonstrate that NF-κB plays an important role in promoting expression of multiple proinflammatory genes induced by IL-1β in SMCs.
Table 4.
Overrepresented and conserved transcription factor binding sites of inflammatory genes selectively induced by IL-1β in SMCs
| Transcription Factor Binding Site | Number of Hits in Submitted Regions | Total Number of Hits on Genome | P Value | Annotated Genes With Hits in Promoter Region |
|---|---|---|---|---|
| NFKAPPAB65 | 23 | 2,232 | 3.5 × 10−10 | Ccl20, Ccl5, Cd74, Cxcl2, Cxcl6, Il1r1, Il6, Jak2, Map2k3, Nfkbiz, Pdgfra, Ptges, Timp1, Vcam1, Vcan |
| NFKAPPAB50 | 16 | 2,308 | 2.3 × 10−5 | Ccl20, Cd74, Cxcl2, Cxcl6, Il1r1, Map2k3, Nfkbiz, Pdgfra, Tgfb1, Vcam1, Vcan |
| NFKAPPAB | 68 | 23,956 | 2.7 × 10−3 | Arg1, Bmp6, Ccl20, Ccl5, Cd74, Clu, Cxcl2, Cxcl6, Elk3, Gch1, Hif1A, Il1r1, Il6, Jak2, Lyn, Map2k3, Nfkbiz, Nos2, Pdgfra, Pkm2, Rbpj, Selp, Tgfb1, Timp1, Vcam1, Vcan |
| ISRE | 5 | 485 | 3.0 × 10−3 | Bmp6, Il6, Rbpj, Selp |
Table 5.
Overrepresented and conserved transcription factor binding sites of cell cycle-related genes distinctly induced by PDGF-DD in SMCs
| Transcription Factor Binding Site | Number of Hits in Submitted Regions | Total Number of Hits on Genome | P Value | Annotated Genes With Hits in Promoter Region |
|---|---|---|---|---|
| E2F4DP2 | 31 | 4,525 | 5.5 × 10−8 | Asf1b, Aspm, Aurka, Ccdc99, Ccne1, Ccnf, Cdc25a, Cdca3, Chek1, E2f1, Espl1, Mcm2, Mcm6, Mki67, Psmc6, Racgap1, Rad51c, Rassf1 |
| E2F4DP1 | 14 | 1,292 | 3.1 × 10−6 | Cdc25a, Cdca3, Chek1, E2f1, Espl1, Mcm6, Rad51c |
| E2F1DP1RB | 14 | 1,419 | 4.5 × 10−6 | Cdc25a, Chek1, E2f1, Espl1, Mcm6, Mki67, Rad51c |
| ACAAT | 4 | 273 | 3.1 × 10−3 | Aspm, Nusap1, Spc25, Ube2c |
| E2F1DP2 | 99 | 34,433 | 4.5 × 10−3 | Asf1b, Aspm, Aurka, Ccdc99, Ccne1, Ccnf, Cdc25a, Cdc25b, Cdca3, Cdca5, Chek1, Dscc1, E2f1, Ercc1, Espl1, Fbxo5, Inhba, Kif18a, Mcm2, Mcm6, Mki67, Nasp, Npm1, Pole, Psmc6, Racgap1, Rad51, Rad5 1c, Rasa1, Rassf1, Ruvbl1, Sass6, Ska3, Spc25, Suv39 h1, Timeless, Ube2c |
Fig. 3.
NF-κB mediates IL-1β-induced upregulation of multiple inflammatory genes in SMCs. Real-time RT-PCR analysis of VCAM1 (A), CCL20 (B), and P-selectin (C) mRNA levels 24 h after IL-1β (5 ng/ml) treatment of cultured SMCs infected with adenoviral IκBα superrepressor (Ad-IκBαSR) or control empty adenovirus (Ad-Empty). Data represent means ± SE of mRNA levels normalized to 18s rRNA and to each vehicle from 3 independent experiments performed in triplicate (*P < 0.001 and **P < 0.05 by nested ANOVA). D: representative Western blots of VCAM1 protein levels from SMC lysates after treatment with IL-1β or vehicle in cells infected with empty (Ad-E) or IκBαSR-overexpressing (AdI) adenoviruses. E: densitometry of VCAM1 band intensity relative to GAPDH by Western blot analysis as in D from 3 independent experiments. F: absorbance measures from analysis of CCL20 levels by ELISA in cell culture media 72 h after treatment with vehicle or IL-1β in cells infected with empty or IκBαSR-overexpressing adenoviruses (***P < 0.001 vs. vehicle by Student's t-test). Data represent means ± SE from 3 independent experiments.
NF-κB mediates IL-1β- but not PDGF-induced repression of SMC marker genes.
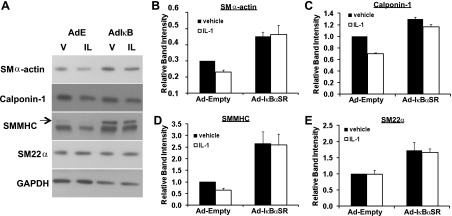
NF-κB has been shown previously to mediate repression of basal levels of SMC marker genes in cultured SMCs (72); however, whether NF-κB mediates SMC marker gene repression in response to a physiological stimulus is unknown. We tested whether NF-κB mediates SMC marker gene repression by IL-1β in SMCs in addition to regulating inflammatory gene expression. Results in Fig. 4 demonstrate that IL-1β-induced repression of the mRNAs encoding the SMC marker genes SM α-actin, SM22α, and calponin-1 was nearly completely abrogated in SMCs expressing the NF-κB inhibitor IκBαSR. Consistent with these findings, IL-1β also induced reductions in SMMHC, SM α-actin, and calponin-1 protein levels (albeit changes were modest over the 72 h time course of these experiments since these proteins are long lived), and decreases were nearly completely abrogated by NF-κB inhibition (Fig. 5). Interestingly, in contrast to other SMC differentiation marker genes, IL-1β strongly repressed SM22α mRNA levels (Fig. 4) but did not repress SM22α protein expression. This observation is in contrast to our previous results with PDGF-BB treatment (17) and suggests that there is something unique about IL-1β and SM22α protein expression including the possibility that IL-1β is somehow selectively increasing the stability of SM22α protein but not other SMC marker gene proteins. Taken together, the preceding results demonstrate that NF-κB plays an important role in promoting IL-1β-induced repression of multiple SMC differentiation marker genes, in addition to its role in promoting expression of multiple proinflammatory genes (see Fig. 3).
Fig. 4.
IL-1β-induced repression of the mRNA levels of multiple SMC marker genes is NF-κB dependent. Real-time RT-PCR analysis of SM α-actin (A), SM22α (B), and calponin-1 (C) mRNA levels 24 h after IL-1β (5 ng/ml) treatment of cultured SMCs infected with adenoviral IκBα superrepressor (Ad-IκBαSR) or control empty adenovirus (Ad-Empty). Data represent means ± SE of 3 independent experiments each performed in triplicate (*P < 0.001 vs. vehicle by nested ANOVA; **P < 0.05 by Student's t-test; n = 3).
Fig. 5.
IL-1β-induced repression of smooth muscle myosin heavy chain (SMMHC) and smooth muscle (SM) α-actin protein levels are NF-κB dependent. A: representative images of Western blot analysis of multiple smooth muscle cell marker genes from cell lysates taken after 72 h of treatment with IL-1β (IL, 5 ng/ml) or vehicle (V) in cells infected with empty adenovirus (AdE) or IκBαSR-expressing adenovirus (AdIκB). GAPDH represents the loading control. Arrow, SM1 isoform of SMMHC (lower band is nonmuscle MHC). Densitometric quantitation of band intensity for SM α-actin (B), calponin-1 (C), SMMHC (D), and SM22α (E) normalized to GAPDH levels. All values were then normalized to the Ad-Empty vehicle control group. Data represent means ± SE from 3 independent experiments.
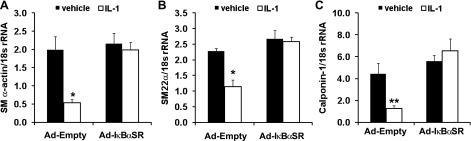
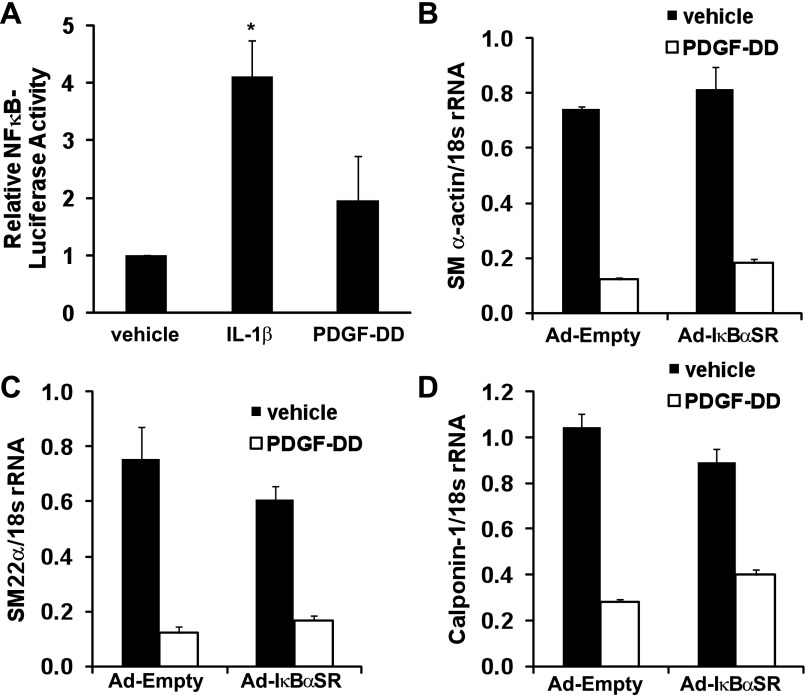
To determine if NF-κB is a common mediator of SMC marker gene repression in response to PDGF as well as IL-1β, we first tested whether PDGF and IL-1β induce NF-κB activity in SMCs. As shown in Fig. 6A, IL-1β significantly increased NF-κB activity more than fourfold (P < 0.001), while PDGF-DD treatment did not (1.7-fold, P = 0.10). Consistent with these results, whereas overexpression of IκBαSR abolished IL-1β-induced suppression of SMC marker genes (Fig. 4), overexpression of IκBαSR did not alter PDGF-DD-induced repression of SMC marker genes SM α-actin, SM22α, or calponin-1 (Fig. 6, B–D). Taken together, these results demonstrate that NF-κB activation is critical to IL-1β-induced upregulation of multiple proinflammatory genes as well as repression of smooth muscle marker genes and at least in part mediates the distinct effects of IL-1β on SMC phenotype relative to PDGF-DD.
Fig. 6.
PDGF-DD does not significantly induce NF-κB activity in SMCs and PDGF-DD-induced repression of SMC marker genes is NF-κB-independent. A: luciferase activity in cell lysates from cultured SMCs transfected with 5XNF-κB-luciferase reporter constructs or control promoterless constructs and treated with IL-1β (2.5 ng/ml) or PDGF-DD (30 ng/ml) for 24 h. Data represent means ± SE of 5XNF-κB-luciferase activity normalized to promoterless control and total cellular protein followed by normalization to the vehicle group from 3 independent experiments (*P < 0.001 vs. vehicle by nested ANOVA). Real-time RT-PCR analysis of SM α-actin (B), SM22α (C), and calponin-1 (D) mRNA levels 24 h after PDGF-DD treatment (30 ng/ml) of cultured SMCs infected with empty adenovirus (Ad-Empty) or IκBαSR-expressing adenovirus (Ad-IκBαSR). Data represent means ± SE of 3 independent experiments in triplicate.
A subset of SM α-actin-negative cells within atherosclerotic plaques express inflammatory but not proliferative phenotypic markers.
To determine whether a distinct inflammatory SMC phenotype may exist within atherosclerotic lesions in vivo, results of genome-wide gene expression changes from IL-1β and PDGF-DD stimulation of SMCs were compared with identify optimal markers of an IL-1β-induced inflammatory phenotype and PDGF-induced proliferative phenotype. To identify an optimal marker of an inflammatory state SMC, genes significantly induced by IL-1β were sorted based on the ratio of the IL-1β to PDGF-DD fold changes over their respective vehicle controls. The genes at the top of the list are thus those with the largest induction by IL-1β relative to changes evoked by PDGF-DD. From this list, as seen in Table 6, Ccl20 was the gene with the highest IL-1β/PDGF-DD fold-change ratio (1,193-fold greater induction) that is present in C57BL/6J mice [as opposed to phospholipase A2 group IIA (55)] and for which antibodies are available for immunostaining of mouse tissues. In addition, CCL20 has recently been shown to play a proinflammatory role in promoting macrophage chemotaxis, and inactivation of its receptor, CCR6, reduces atherosclerotic plaque size and macrophage content (76). To identify a gene highly induced by PDGF-DD that is repressed or unchanged with IL-1β, PDGF-DD-induced genes were sorted based on the ratio of the PDGF-DD to IL-1β fold changes over their respective vehicle controls (Table 7). RGS17 was selected from this list as the gene with the top PDGF-DD/IL-1β fold change ratio (14.3-fold greater induction) that has been shown to play a role in promoting cell proliferation (29).
Table 6.
Top 10 genes with highest ratio of IL-1β to PDGF-DD fold changes over vehicle
| Description | IL-1β Fold Change | PDGF-DD Fold Change | IL-1β/PDGF-DD Fold Change Ratio |
|---|---|---|---|
| Phospholipase A2, group IIA (platelets, synovial fluid), Pla2 g2a | 4,147 | 0.13 | 31,327 |
| Orosomucoid 1, Orm1 | 248 | 0.06 | 4,260 |
| Chemokine (C-C motif) ligand 20, Ccl20 | 58 | 0.05 | 1,193 |
| Colony stimulating factor 2 (granulocyte-macrophage), Csf2 | 214 | 0.18 | 1,175 |
| Mesenchyme homeobox 1 (predicted), Meox1_predicted | 134 | 0.19 | 710 |
| Argininosuccinate synthetase 1, Ass1 | 651 | 1.15 | 565 |
| Kallmann syndrome 1, Kal1 | 408 | 0.76 | 541 |
| Ankyrin repeat and SOCS box-containing protein 12, Asb12 | 39 | 0.09 | 435 |
| Complement factor B | 49 | 0.12 | 423 |
| Colony stimulating factor 3 (granulocyte), Csf3 | 134 | 0.38 | 355 |
Table 7.
Top 10 genes with highest ratio of PDGF-DD to IL-1β fold changes over vehicle
| Description | IL-1β Fold Change | PDGF-DD Fold Change | PDGF-DD/IL-1β Fold Change Ratio |
|---|---|---|---|
| Cytidine deaminase (predicted), Cda_predicted | 0.58 | 41.8 | 72.0 |
| Transcribed locus, — | 0.17 | 3.1 | 18.7 |
| Regulator of G protein signaling 17 (predicted), Rgs17_predicted | 0.33 | 4.7 | 14.3 |
| Cell division cycle associated 2, Cdca2 | 0.19 | 2.4 | 13.1 |
| G patch domain containing 4, Gpatch 4 | 0.22 | 2.8 | 12.7 |
| Transcribed locus, — | 0.79 | 9.6 | 12.2 |
| Heparan sulfate (glucosamine) 3-O-sulfotransferase 1, Hs3 st1 | 1.24 | 15.1 | 12.2 |
| Aquaporin 1, Aqp1 | 0.26 | 3.2 | 12.0 |
| Ttk protein kinase (predicted), Ttk_predicted | 0.25 | 2.9 | 11.5 |
| Similar to palladin; CGI-151 protein, LOC364558 | 1.70 | 19.1 | 11.2 |
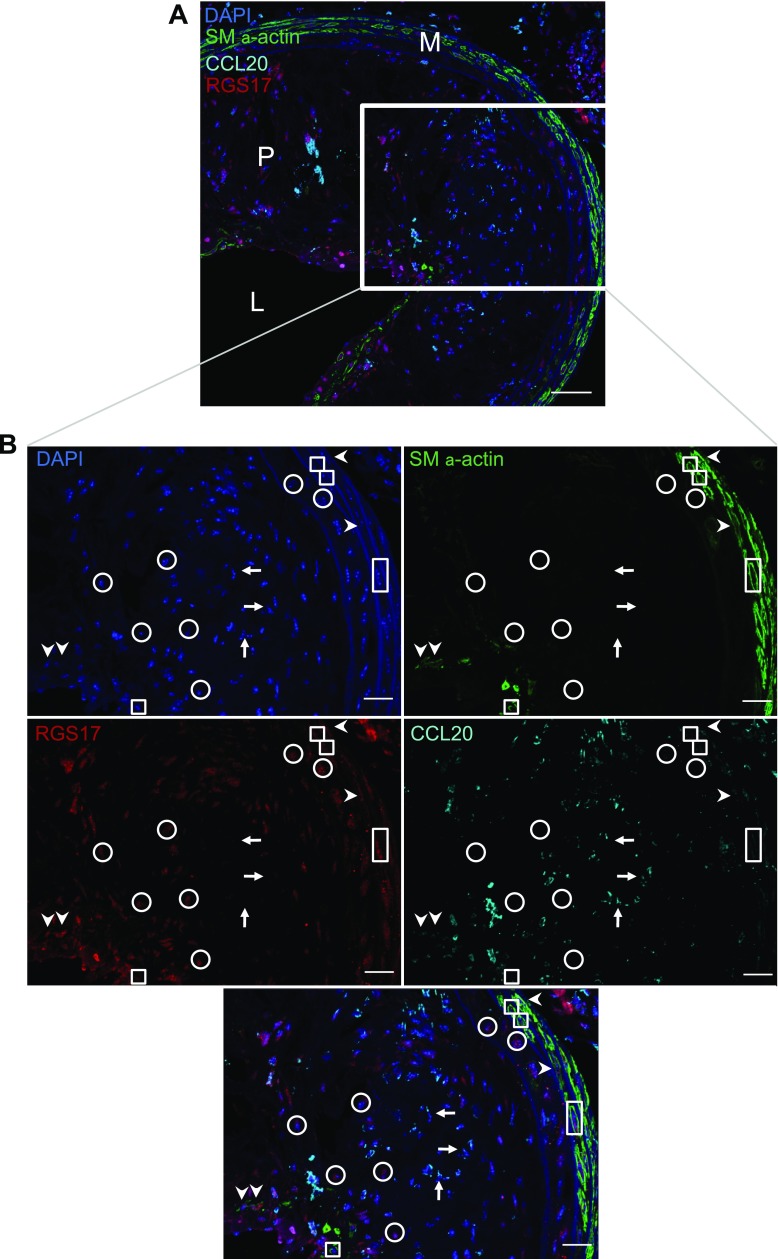
Having utilized our microarray data to identify markers of an IL-1β-induced inflammatory phenotype and PDGF-DD-induced proliferative SMC phenotype, we performed immunofluorescent staining and high-resolution z-stack confocal microscopy to determine whether these markers may allow us to identify distinct inflammatory state SMCs within atherosclerotic plaques in vivo. Immunofluorescent staining was performed for CCL20, RGS17, SM α-actin, and DAPI (to identify cell nuclei) in both normal and atherosclerotic arteries. In nonatherosclerotic brachiocephalic arteries of 35 wk old C57BL/6J mice, most medial SMC were positive for SM α-actin but negative for CCL20 and RGS17, although a small number of medial cells were positive for both SM α-actin and CCL20 (data not shown). Within atherosclerotic brachiocephalic arteries of high-fat diet-fed Apoe−/− mice, however, there were numerous cells positive for CCL20 and/or RGS17 within the plaque (Fig. 7 and data not shown). Careful analysis of the pattern of staining for CCL20 and RGS17 in individual cells within these atherosclerotic vessels provided several interesting observations. First, there were a small fraction of SM α-actin-positive cells, presumably SMC or SMC derivatives, that were positive for both CCL20 and RGS17 (arrowheads in Fig. 7B), suggesting that at least some SMC show a mixed phenotype intermediate between that observed in cultured SMC treated with IL-1β or PDGF-DD alone. This phenotype may reflect cells exposed simultaneously to both “classes” of agents that induce SMC phenotypic switching and suggests that at least in some cells one phenotype is not clearly dominant. Second, we found a few cells that were SM α-actin+ RGS17+ but CCL20−, presumably reflecting SMCs that have modulated to a proliferative state, similar to that of cultured SMC treated with PDGF-DD (boxes in Fig. 7B). Third, we found a number of cells within the plaque that were negative for SM α-actin but were CCL20+ RGS17− (arrows in Fig. 7B) or CCL20− RGS17+ (circles in Fig. 7B). These cells may represent SMCs within the plaque that have profoundly modulated their phenotype to an inflammatory state (CCL20+ RGS17−) or a proliferative state (CCL20− RGS17+) as characterized by complete loss of expression of SM α-actin but distinct induction of CCL20 or RGS17, respectively. However, it is also possible that these cells may not be of SMC origin (see discussion), although observations that these cells were positive only for CCL20 or RGS17 and not both markers suggest that they do represent distinct phenotypes. Taken together, these results indicate the CCL20 and RGS17 may have utility in identifying distinct SMC phenotypes within lesions in vivo, although SMC lineage tracing methods will need to be used in conjunction with these markers to clearly establish that distinct inflammatory state SMCs exist within atherosclerotic lesions in vivo.
Fig. 7.
A subset of SM α-actin-negative cells within atherosclerotic plaques express an inflammatory but not proliferative phenotype marker. A: merged image from confocal microscopy of immunofluorescent staining for CCL20 (cyan), RGS17 (red), SM α-actin (green), and nuclei using DAPI (blue) of an atherosclerotic brachiocephalic artery from an Apoe−/− mouse fed a high-fat, Western-type diet for 28 wk (scale bar = 50 μm; M, media; P, plaque; L, lumen). B: single channel images from confocal microscopy at higher magnification on the boxed region in A showing the staining for each antigen separately along with the merged image on the far right (scale bars = 25 μm). Arrows indicate SM α-actin− CCL20+ RGS17− cells, boxes indicate SM α-actin+ CCL20− RGS17+ cells, circles indicate SM α-actin− CCL20− RGS17+ cells, and arrowheads indicate SM α-actin+ CCL20+ RGS17+ cells. Images are representative of brachiocephalic arteries from 3 mice.
DISCUSSION
Studies reported in this article have utilized an unbiased analysis of genome-wide gene expression changes in cultured SMCs to demonstrate that although both IL-1β and PDGF-DD promote repression of SMC marker genes, IL-1β distinctly promotes expression of numerous proinflammatory genes, while PDGF-DD primarily induces expression of genes involved in regulating cell proliferation. In addition, inhibition of NF-κB activity in SMCs significantly reduced IL-1β-induced expression of multiple proinflammatory genes and repression of SMC differentiation marker genes. Since PDGF-DD-induced repression of SMC marker genes was not NF-κB dependent, these results suggest that NF-κB is an important mediator of the effects of IL-1β and promotes a distinct inflammatory phenotype. Finally, results demonstrate that a population of SM α-actin-negative cells within mouse atherosclerotic plaques express either an IL-1β-induced marker, CCL20, or a PDGF-DD-induced marker, RGS17, suggesting that these markers may have utility in identifying SMCs that have modulated their phenotype to distinct inflammatory and proliferative states within atherosclerotic plaques in vivo.
Our results provide novel evidence that SMCs in vitro can modulate their phenotype to distinct inflammatory and proliferative states in response to differing environmental cues. Recent studies have demonstrated considerable diversity in a variety of myeloid-derived cell populations in vitro and within atherosclerotic plaques in vivo (40, 46). For example, distinct Ly6chi and Ly6clo populations of monocytes have been found both in the circulation and within atherosclerotic lesions (70, 71), and macrophages of M1, M2, and Mox phenotypes as well as T lymphocytes of Th1, Th2, and Th17 subtypes are found in atherosclerotic plaques (22, 33, 35, 82). Each of these inflammatory cell subtypes are thought to have unique functional roles and thus impact atherogenesis in distinct ways (40). Our results suggest that SMCs may similarly exhibit phenotypic and functional diversity in response to differing environmental cues. One key difference between the distinct phenotypic states in SMCs observed here versus inflammatory cell subpopulations identified to date is that SMC phenotypic modulation involves repression of the gene products that mark the differentiated state of the cell (58). This alteration in SMC differentiation state complicates efforts to identify phenotypically modulated SMCs within atherosclerotic plaques in vivo since it is possible that at least some SMC may show complete loss of markers required to identify them. Indeed, our laboratory has previously shown that a subpopulation of SMC within advanced atherosclerotic lesions of Apoe−/− mice lacks detectable expression of endogenous SMC markers but can be identified using a mutant SM22α promoter-reporter transgene resistant to suppression during phenotypic switching (75). As such, future studies to determine whether distinct SMC phenotypes exist within atherosclerotic lesions in vivo will require the use rigorous SMC-specific lineage tracing systems such as mice with SMC-specific Cre expression combined with a stable Cre-induced reporter system (74). SMC lineage tracing will permit identification of SMCs regardless of their differentiation state and can be combined with immunostaining for the markers of distinct SMC phenotypes that we have identified, CCL20 and RGS17, to determine whether distinct inflammatory and proliferative SMC phenotypes, respectively, may exist within atherosclerotic lesions in vivo.
Results reported in this article suggest that IL-1β promotes formation of a distinct inflammatory SMC phenotype in vitro that involves induction of numerous proinflammatory genes including a variety of chemokines and adhesion molecules that promote chemotaxis and adherence of inflammatory cells, respectively (76, 78, 79). These results suggest that in atherosclerosis IL-1 stimulation of SMCs would promote inflammatory responses and enhance plaque recruitment and activation of macrophages. Interestingly, antibody-mediated inhibition of IL-1β has recently been shown to reduce macrophage content within murine atherosclerotic plaques (6), and inactivation of the endogenous antagonist of the IL1R1, interleukin-1 receptor antagonist, increased plaque macrophage content in advanced lesions of atheroprone mice (28). Although it is unclear whether these effects of IL-1 on atherosclerosis occur through direct action on SMCs, these results are consistent with our in vitro studies demonstrating that IL-1 promotes an inflammatory SMC phenotype including release of multiple chemokines that promote monocyte/macrophage chemotaxis such as CCL20 (76) and CCL2 (78) and induction of monocyte/macrophage adhesion molecules such as VCAM1 (79). Future studies involving SMC-specific inactivation of the IL1R1 in atheroprone mice will be necessary to determine whether the effects of IL-1 on SMCs alter atherosclerotic plaque development and features of plaque instability such as enhanced lesional macrophage content (16).
Our results suggest that the transcription factor NF-κB plays a critical role in mediating the effects of IL-1β to modulate SMC phenotype to a distinct inflammatory state. To identify major mediators of the effects of IL-1β on SMC phenotype, we utilized an unbiased approach that demonstrated that NF-κB binding sites were markedly overrepresented within the promoters of the inflammatory genes distinctly induced by IL-1β in the genome-wide expression analysis. Additionally, studies of NF-κB inhibition in SMCs revealed that NF-κB plays an important role in mediating IL-1β-dependent induction of several of these genes. Although previous studies have demonstrated that NF-κB mediates induction of several proinflammatory genes in SMCs including VCAM-1 and CCL20 (9, 32), our unbiased analysis of transcription factor binding sites combined with NF-κB inhibition studies in cultured SMCs provides novel evidence that NF-κB is a major regulator of the marked and distinct inflammatory gene expression by IL-1. In addition, results reported here demonstrate novel roles for NF-κB in promoting expression of proinflammatory genes such as P-selectin in SMCs. Moreover, our results demonstrate that NF-κB also plays a critical role in IL-1-induced repression of SMC marker genes. Tang et al. (72) recently demonstrated that the NF-κB subunit p65 negatively regulates basal levels of SMC marker genes by binding to and inhibiting the transactivating function of the transcription factor myocardin. However, these experiments involved overexpression of exogenous p65 and/or knockdown of p65 in unstimulated cells, so it is unclear whether NF-κB regulates SMC differentiation marker gene repression in response to a physiological stimulus (72). Our studies provide novel evidence that NF-κB plays an important role in SMC marker gene repression in response to IL-1β. Taken together, results demonstrate that NF-κB represents a critical mediator of overall SMC phenotypic modulation to an inflammatory state in response to IL-1β due to its ability to coordinately promote inflammatory gene induction and repress SMC marker gene expression. Interestingly, PDGF-DD did not significantly increase NF-κB activity in SMCs and PDGF-DD-induced phenotypic modulation of SMCs was NF-κB-independent (Fig. 6). Indeed, as opposed to our observation of overrepresentation of NF-κB binding sites in distinctly IL-1-induced genes, genes distinctly induced by PDGF-DD exhibited overrepresentation of binding sites for E2F family members (Table 5), which are known regulators of cell proliferation (3, 24). As such, it is interesting to postulate that it may be feasible to therapeutically target NF-κB to selectively inhibit transition of SMC to an inflammatory state while retaining a PDGF-induced proliferative SMC phenotype that may contribute to plaque stabilization through enhancing SMC accumulation within plaques and formation of a protective fibrous cap (37, 66).
Although IL-1β and PDGF-DD induce distinct cohorts of inflammation- and proliferation-related genes in SMCs, both factors repress expression of multiple SMC differentiation marker genes. Results reported here demonstrate that NF-κB plays an important role in IL-1β-induced, but not PDGF-DD-induced, repression of SMC differentiation marker genes. Previous studies have demonstrated that PDGF-DD represses SMC differentiation marker genes through MEK-ERK-mediated activation of Elk-1 as well as induction of the transcription factor KLF4 (73). Like PDGF-DD, IL-1β has been shown previously to activate the ERK signaling pathway in SMCs (30), and both IL-1β and PDGF-DD induced expression of KLF4 in the microarray analysis (Supplemental Table III). Hence, future studies will be necessary to determine whether in addition to activation of NF-κB, IL-1β may also activate ERK-Elk-1 and/or KLF4-dependent mechanisms to repress SMC marker gene expression.
Results reported in this manuscript demonstrate that the inflammatory cytokine interleukin-1β promotes formation of an inflammatory phenotype in cultured SMCs that is distinct from SMC phenotypic modulation in response to the growth factor PDGF-DD. In addition, we have provided evidence that NF-κB is a critical regulator of IL-1β-induced SMC phenotypic modulation to an inflammatory state but does not play an important role in PDGF-DD-induced SMC phenotypic modulation. These results clearly challenge the definition of SMC phenotypic modulation as a two-state model of contractile- and synthetic-state SMCs, and suggest that SMCs may be capable of undergoing phenotypic modulation from a contractile state to multiple distinct phenotypic states depending on the environmental cues present. In addition, results lay the foundation for determining whether distinct SMC phenotypic states are present within atherosclerotic lesions in vivo and suggest the importance of future studies to further explore the potential diversity of SMC phenotypic modulation in vitro and in atherosclerosis in vivo, as well as the functional importance of these different SMC phenotypes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1 HL-38854, P01 HL-19242, R01 HL-57353, and R01 HL-087867 to G. K. Owens, American Heart Association predoctoral fellowships to M. R. Alexander (0615326U) and M. Murgai (11PRE7750030), and Medical Scientist Training Program Training Grant (5T32GM007267-26) to M. R. Alexander.
DISCLOSURES
G. K. Owens is a shareholder and Chief Scientific Officer of Nanomedical Systems Incorporated, which develops stent technology unrelated to these studies, and has received research funding from AstraZeneca Pharmaceutical, Inc. for studies separate from those reported in this manuscript.
AUTHOR CONTRIBUTIONS
Author contributions: M.R.A. and G.K.O. conception and design of research; M.R.A., M.M., and C.W.M. performed experiments; M.R.A. analyzed data; M.R.A., M.M., and G.K.O. interpreted results of experiments; M.R.A. prepared figures; M.R.A. drafted manuscript; M.R.A., M.M., C.W.M., and G.K.O. edited and revised manuscript; M.R.A., M.M., C.W.M., and G.K.O. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank John Sanders, Laura Shankman, Rupanda Tripathi, Dominique Rose, and Mary McCanna for excellent technical assistance and Paul Manser and Jae Lee for helpful advice on statistics.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libby P, Luster AD. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ Res 86: 131–138, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb 11: 1223–1230, 1991. [DOI] [PubMed] [Google Scholar]
- 3. Attwooll C, Denchi EL, Helin K. The E2F family: specific functions and overlapping interests. EMBO J 23: 4709–4716, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banai S, Wolf Y, Golomb G, Pearle A, Waltenberger J, Fishbein I, Schneider A, Gazit A, Perez L, Huber R, Lazarovichi G, Rabinovich L, Levitzki A, Gertz SD. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation 97: 1960–1969, 1998. [DOI] [PubMed] [Google Scholar]
- 5. Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol 3: 512–516, 2001. [DOI] [PubMed] [Google Scholar]
- 6. Bhaskar V, Yin J, Mirza AM, Phan D, Vanegas S, Issafras H, Michelson K, Hunter JJ, Kantak SS. Monoclonal antibodies targeting IL-1 beta reduce biomarkers of atherosclerosis in vitro and inhibit atherosclerotic plaque formation in apolipoprotein E-deficient mice. Atherosclerosis 216: 313–320, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Blank RS, Owens GK. Platelet-derived growth factor regulates actin isoform expression and growth state in cultured rat aortic smooth muscle cells. J Cell Physiol 142: 635–642, 1990. [DOI] [PubMed] [Google Scholar]
- 8. Cai Q, Lanting L, Natarajan R. Interaction of monocytes with vascular smooth muscle cells regulates monocyte survival and differentiation through distinct pathways. Arterioscler Thromb Vasc Biol 24: 2263–2270, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Calvayrac O, Rodríguez-Calvo R, Alonso J, Orbe J, Martín-Ventura JL, Guadall A, Gentile M, Juan-Babot O, Egido J, Beloqui O, Paramo JA, Rodríguez C, Martínez-González J. CCL20 is increased in hypercholesterolemic subjects and is upregulated by LDL in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 31: 2733–2741, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Campbell JH, Campbell GR. The role of smooth muscle cells in atherosclerosis. Curr Opin Lipidol 5: 323–330, 1994. [DOI] [PubMed] [Google Scholar]
- 11. Campbell JH, Campbell GR. The role of smooth muscle cells in atherosclerosis. Curr Opin Lipidol 5: 323–330, 1994. [DOI] [PubMed] [Google Scholar]
- 12. Chang LW, Nagarajan R, Magee JA, Milbrandt J, Stormo GD. A systematic model to predict transcriptional regulatory mechanisms based on overrepresentation of transcription factor binding profiles. Genome Res 16: 405–413, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen J, Han Y, Lin C, Zhen Y, Song X, Teng S, Chen C, Chen Y, Zhang Y, Hui R. PDGF-D contributes to neointimal hyperplasia in rat model of vessel injury. Biochem Biophys Res Commun 329: 976–983, 2005. [DOI] [PubMed] [Google Scholar]
- 14. Clement N, Gueguen M, Glorian M, Blaise R, Andreani M, Brou C, Bausero P, Limon I. Notch3 and IL-1beta exert opposing effects on a vascular smooth muscle cell inflammatory pathway in which NF-kappaB drives crosstalk. J Cell Sci 120: 3352–3361, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Couffinhal T, Duplaa C, Moreau C, Lamaziere JM, Bonnet J. Regulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in human vascular smooth muscle cells. Circ Res 74: 225–234, 1994. [DOI] [PubMed] [Google Scholar]
- 16. Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J 69: 377–381, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deaton RA, Gan Q, Owens GK. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol 296: H1027–H1037, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delbosc S, Glorian M, Le Port AS, Bereziat G, Andreani M, Limon I. The benefit of docosahexanoic acid on the migration of vascular smooth muscle cells is partially dependent on notch regulation of MMP-2/-9. Am J Pathol 172: 1430–1440, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4: 3, 2003. [PubMed] [Google Scholar]
- 20. Faure S, Lee MA, Keller T, ten Dijke P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development 127: 2917–2931, 2000. [DOI] [PubMed] [Google Scholar]
- 21. Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucl Acids Res 32: W273–W279, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, Zhang Y, Chen W, Zhang L. A Critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol 185: 5820–5827, 2010. [DOI] [PubMed] [Google Scholar]
- 23. Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res 62: 749–756, 1988. [DOI] [PubMed] [Google Scholar]
- 24. Giangrande PH, Zhang J, Tanner A, Eckhart AD, Rempel RE, Andrechek ER, Layzer JM, Keys JR, Hagen PO, Nevins JR, Koch WJ, Sullenger BA. Distinct roles of E2F proteins in vascular smooth muscle cell proliferation and intimal hyperplasia. Proc Natl Acad Sci USA 104: 12988–12993, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hart CE, Kraiss LW, Vergel S, Gilbertson D, Kenagy R, Kirkman T, Crandall DL, Tickle S, Finney H, Yarranton G, Clowes AW. PDGF beta receptor blockade inhibits intimal hyperplasia in the baboon. Circulation 99: 564–569, 1999. [DOI] [PubMed] [Google Scholar]
- 26. Holycross BJ, Blank RS, Thompson MM, Peach MJ, Owens GK. Platelet-derived growth factor-BB-induced suppression of smooth muscle cell differentiation. Circ Res 71: 1525–1532, 1992. [DOI] [PubMed] [Google Scholar]
- 27. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 4: 44–57, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, Kusuhara M, Iwakura Y, Ohsuzu F. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 24: 1068–1073, 2004. [DOI] [PubMed] [Google Scholar]
- 29. James MA, Lu Y, Liu Y, Vikis HG, You M. RGS17, an overexpressed gene in human lung and prostate cancer, induces tumor cell proliferation through the cyclic AMP-PKA-CREB pathway. Cancer Res 69: 2108–2116, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jiang B, Brecher P, Cohen RA. Persistent activation of nuclear factor-kappaB by interleukin-1beta and subsequent inducible NO synthase expression requires extracellular signal-regulated kinase. Arterioscler Thromb Vasc Biol 21: 1915–1920, 2001. [DOI] [PubMed] [Google Scholar]
- 31. Jiang B, Xu S, Brecher P, Cohen RA. Growth factors enhance interleukin-1beta-induced persistent activation of nuclear factor-kappaB in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 22: 1811–1816, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-kappaB activation by ERK differentially regulates interleukin-1beta-induced gene expression. J Biol Chem 279: 1323–1329, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 107: 737–746, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW. Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation 96: 3555–3560, 1997. [DOI] [PubMed] [Google Scholar]
- 35. Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, Dussiot M, Levillain O, Graff-Dubois S, Nicoletti A, Caligiuri G. Macrophage plasticity in experimental atherosclerosis. PLoS One 5: e8852, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, Finn AV, Virmani R. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 16: 285–292, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Kozaki K, Kaminski WE, Tang J, Hollenbach S, Lindahl P, Sullivan C, Yu JC, Abe K, Martin PJ, Ross R, Betsholtz C, Giese NA, Raines EW. Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice. Am J Pathol 161: 1395–1407, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, Burgess CE, Fernandes E, Deegler LL, Rittman B, Shimkets J, Shimkets RA, Rothberg JM, Lichenstein HS. PDGF-D, a new protease-activated growth factor. Nat Cell Biol 3: 517–521, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Libby P, Warner SJ, Friedman GB. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest 81: 487–498, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Libby P, Nahrendorf M, Pittet MJ, Swirski FK. Diversity of denizens of the atherosclerotic plaque: not all monocytes are created equal. Circulation 117: 3168–3170, 2008. [DOI] [PubMed] [Google Scholar]
- 41. Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 54: 2129–2138, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 280: 9719–9727, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Liu Y, Sinha S, Owens G. A Transforming growth factor-beta control element required for SM alpha-actin expression in vivo also partially mediates GKLF-dependent transcriptional repression. J Biol Chem 278: 48004–48011, 2003. [DOI] [PubMed] [Google Scholar]
- 44. Lucas AD, Bursill C, Guzik TJ, Sadowski J, Channon KM, Greaves DR. Smooth muscle cells in human atherosclerotic plaques express the fractalkine receptor CX3CR1 and undergo chemotaxis to the CX3C chemokine fractalkine (CX3CL1). Circulation 108: 2498–2504, 2003. [DOI] [PubMed] [Google Scholar]
- 45. Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol 29: 1419–1423, 2009. [DOI] [PubMed] [Google Scholar]
- 47. Marmur JD, Poon M, Rossikhina M, Taubman MB. Induction of PDGF-responsive genes in vascular smooth muscle. Implications for the early response to vessel injury. Circulation 86: III53–III60, 1992. [PubMed] [Google Scholar]
- 48. Marx N, Neumann FJ, Zohlnhofer D, Dickfeld T, Fischer A, Heimerl S, Schomig A. Enhancement of monocyte procoagulant activity by adhesion on vascular smooth muscle cells and intercellular adhesion molecule-1-transfected Chinese hamster ovary cells. Circulation 98: 906–911, 1998. [DOI] [PubMed] [Google Scholar]
- 49. Massberg S, Vogt F, Dickfeld T, Brand K, Page S, Gawaz M. Activated platelets trigger an inflammatory response and enhance migration of aortic smooth muscle cells. Thromb Res 110: 187–194, 2003. [DOI] [PubMed] [Google Scholar]
- 50. Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, Voss N, Stegmaier P, Lewicki-Potapov B, Saxel H, Kel AE, Wingender E. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucl Acids Res 34: D108–D110, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meng L, Park J, Cai Q, Lanting L, Reddy MA, Natarajan R. Diabetic conditions promote binding of monocytes to vascular smooth muscle cells and their subsequent differentiation. Am J Physiol Heart Circ Physiol 298: H736–H745, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morisaki N, Takahashi K, Shiina R, Zenibayashi M, Otabe M, Yoshida S, Saito Y. Platelet-derived growth factor is a potent stimulator of expression of intercellular adhesion molecule-1 in human arterial smooth muscle cells. Biochem Biophys Res Commun 200: 612–618, 1994. [DOI] [PubMed] [Google Scholar]
- 53. Newby AC, Zaltsman AB. Fibrous cap formation or destruction – the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res 41: 345–360, 1999. [PubMed] [Google Scholar]
- 54. Noiseux N, Boucher CH, Cartier R, Sirois MG. Bolus endovascular PDGFR-beta antisense treatment suppressed intimal hyperplasia in a rat carotid injury model. Circulation 102: 1330–1336, 2000. [DOI] [PubMed] [Google Scholar]
- 55. Novelli MR, Wasan H, Rosewell I, Bee J, Tomlinson IP, Wright NA, Bodmer WF. Tumor burden and clonality in multiple intestinal neoplasia mouse/normal mouse aggregation chimeras. Proc Natl Acad Sci USA 96: 12553–12558, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. O'Brien KD, Allen MD, McDonald TO, Chait A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K, Benjamin CD. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest 92: 945–951, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Owens GK. Role of alterations in the differentiated state of vascular SMC in atherogenesis. In: Atherosclerosis and Coronary Artery Disease, edited by Ross R, Fuster V, Topol E. New York: Raven Press, 1995. [Google Scholar]
- 58. Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. [DOI] [PubMed] [Google Scholar]
- 59. Plenz G, Koenig C, Severs NJ, Robenek H. Smooth muscle cells express granulocyte-macrophage colony-stimulating factor in the undiseased and atherosclerotic human coronary artery. Arterioscler Thromb Vasc Biol 17: 2489–2499, 1997. [DOI] [PubMed] [Google Scholar]
- 60. Poston RN, Haskard DO, Coucher JR, Gall NP, Johnson-Tidey RR. Expression of intercellular adhesion molecule-1 in atherosclerotic plaques. Am J Pathol 140: 665–673, 1992. [PMC free article] [PubMed] [Google Scholar]
- 61. Printseva OY, Peclo MM, Gown AM. Various cell types in human atherosclerotic lesions express ICAM-1. Further immunocytochemical and immunochemical studies employing monoclonal antibody 10F3. Am J Pathol 140: 889–896, 1992. [PMC free article] [PubMed] [Google Scholar]
- 62. Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 20. Nat Genet 38: 500–501, 2006. [DOI] [PubMed] [Google Scholar]
- 63. Rimarachin JA, Jacobson JA, Szabo P, Maclouf J, Creminon C, Weksler BB. Regulation of cyclooxygenase-2 expression in aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 14: 1021–1031, 1994. [DOI] [PubMed] [Google Scholar]
- 64. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362: 801–809, 1993. [DOI] [PubMed] [Google Scholar]
- 65. Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasahara M, Malden LT, Masuko H, Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science 248: 1009–1012, 1990. [DOI] [PubMed] [Google Scholar]
- 66. Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, Nishikawa S, Nishikawa SI, Kita T. Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation 103: 2955–2960, 2001. [DOI] [PubMed] [Google Scholar]
- 67. Schwartz SM, Virmani R, Rosenfeld ME. The good smooth muscle cells in atherosclerosis. Curr Atheroscler Rep 2: 422–429, 2000. [DOI] [PubMed] [Google Scholar]
- 68. Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol 41: S15–S22, 2003. [DOI] [PubMed] [Google Scholar]
- 69. Sirois MG, Simons M, Edelman ER. Antisense oligonucleotide inhibition of PDGFR-beta receptor subunit expression directs suppression of intimal thickening. Circulation 95: 669–676, 1997. [DOI] [PubMed] [Google Scholar]
- 70. Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6C monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 117: 195–205, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117: 185–194, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tang RH, Zheng XL, Callis TE, Stansfield WE, He J, Baldwin AS, Wang DZ, Selzman CH. Myocardin inhibits cellular proliferation by inhibiting NF-kappaB(p65)-dependent cell cycle progression. Proc Natl Acad Sci USA 105: 3362–3367, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thomas JA, Deaton RA, Hastings NE, Shang Y, Moehle CW, Eriksson U, Topouzis S, Wamhoff BR, Blackman BR, Owens GK. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am J Physiol Heart Circ Physiol 296: H442–H452, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wamhoff B, Sinha S, Owens G. Conditional mouse models to study developmental and pathophysiological gene function in muscle. In: Conditional Mutagenesis: An Approach to Disease Models, edited by Feil R, Metzger D. Berlin, Heidelberg: Springer, 2007, p. 441–468. [DOI] [PubMed] [Google Scholar]
- 75. Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res 95: 981–988, 2004. [DOI] [PubMed] [Google Scholar]
- 76. Wan W, Lim JK, Lionakis MS, Rivollier A, McDermott DH, Kelsall BL, Farber JM, Murphy PM. Genetic deletion of chemokine receptor ccr6 decreases atherogenesis in ApoE-deficient mice/novelty and significance. Circ Res 109: 374–381, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang CY, Mayo MW, Baldwin AS. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappa B. Science 274: 784–787, 1996. [DOI] [PubMed] [Google Scholar]
- 78. Wang JM, Sica A, Peri G, Walter S, Padura IM, Libby P, Ceska M, Lindley I, Colotta F, Mantovani A. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb 11: 1166–1174, 1991. [DOI] [PubMed] [Google Scholar]
- 79. Wang X, Feuerstein GZ, Gu JL, Lysko PG, Yue TL. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 115: 89–98, 1995. [DOI] [PubMed] [Google Scholar]
- 80. Yamamoto M, Aoyagi M, Fukai N, Matsushima Y, Yamamoto K. Differences in cellular responses to mitogens in arterial smooth muscle cells derived from patients with Moyamoya disease. Stroke 29: 1188–1193, 1998. [DOI] [PubMed] [Google Scholar]
- 81. Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, Insel PA. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc Natl Acad Sci USA 102: 8561–8566, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest 101: 1717–1725, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.