Abstract
Exercise intolerance is a cardinal symptom of right ventricular heart failure (RV HF) and skeletal muscle adaptations play a role in this limitation. We determined regional remodeling of muscle structure and mitochondrial function in a rat model of RV HF induced by monocrotaline injection (MCT; 60 mg·kg−1; n = 11). Serial sections of the plantaris were stained for fiber type, succinate dehydrogenase (SDH) activity and capillaries. Mitochondrial function was assessed in permeabilized fibers using respirometry, and isolated complex activity by blue native gel electrophoresis (BN PAGE). All measurements were compared with saline-injected control animals (CON; n = 12). Overall fiber cross-sectional area was smaller in MCT than CON: 1,843 ± 114 vs. 2,322 ± 120 μm2 (P = 0.009). Capillary-to-fiber ratio was lower in MCT in the oxidative plantaris region (1.65 ± 0.09 vs. 1.93 ± 0.07; P = 0.03), but not in the glycolytic region. SDH activity (P = 0.048) and maximal respiratory rate (P = 0.012) were each ∼15% lower in all fibers in MCT. ADP sensitivity was reduced in both skeletal muscle regions in MCT (P = 0.032), but normalized by rotenone. A 20% lower complex I/IV activity in MCT was confirmed by BN PAGE. MCT-treatment was associated with lower mitochondrial volume density (lower SDH activity), quality (lower complex I activity), and fewer capillaries per fiber area in oxidative skeletal muscle. These features are consistent with structural and functional remodeling of the determinants of oxygen supply potential and utilization that may contribute to exercise intolerance and reduced quality of life in patients with RV HF.
Keywords: bioenergetics, exercise, mitochondrial complex I, respirometry, monocrotaline
an increase in pulmonary arterial pressure increases the loading on the right ventricle of the heart. If sustained, pulmonary arterial hypertension (PAH) results in right ventricular hypertrophy and ultimately to right ventricular heart failure (RV HF), the major cause of death in sufferers of PAH (5, 21). Mortality rates of patients with PAH and RV HF are very high: 20 to 40% in the first 3 years after diagnosis (5). PAH and HF are characterized by a reduced tolerance to muscular exercise despite new therapies (2, 19, 21, 33). Interestingly, the reduction in the maximal oxygen uptake (V̇o2max) is a better predictor of mortality than the central hemodynamic deficit or other traditional risk factors (36). Consequently, correction of the central blood flow limitation by heart transplantation does not consistently resolve functional limitations in patients with HF (43), supporting the view that skeletal muscle remodeling plays an important role in exercise intolerance and mortality in many chronic disease states including PAH and RV HF (17, 33, 54).
A common observation in patients with LV (32) and RV (33) HF is muscle atrophy and weakness, that contributes to a loss of power generating capacity. This may be accompanied by a shift in muscle fiber type expression away from fatigue-resistant type I fibers, towards type II (33, 42, 44) and changes in calcium handling (37), which is consistent with a reduction in fatigue resistance in HF (37). Recently, patients with idiopathic PAH have been shown to have a lower proportion of type I fibers, reduced capillarity around those fibers, and lower aerobic enzyme activities in quadriceps biopsy samples, compared with control: changes that were correlated with the reduction in lactate threshold in whole body exercise in these patients (33). These changes have been variously attributed to systemic factors such as reduced blood flow, arterial hypoxemia, reduced habitual activity, nutritional deficits, systemic inflammation, or regional factors such as tissue hypoxia and reactive oxygen species (ROS) (17, 54), each of which are known to affect peripheral skeletal muscle structure and function (54). As the etiology of PAH is poorly understood the extrapolation of findings from LV failure to RV failure may not always be appropriate (18, 52).
That muscle atrophy appears to be greater in type II fibers (a feature also observed in other chronic diseases (16)) has lead to the suggestion that type I fibers are more resilient to systemic pathological insults (30). However, an alternative view is that atrophy in large, type II, fibers may be protective against developing an anoxic core (and, hence, cell damage) under conditions where local capillary blood flow (and O2 delivery) is reduced (24) or in hypoxia (55); whereas hypoxic/anoxic cell damage might be more prevalent following only small reductions in capillary contacts in highly oxidative, type I, fibers. The interaction between fiber size, capillarity and mitochondrial volume density is, therefore, far more complex than whole-muscle averages suggest (11). For example, type I fibers have fewer capillary contacts when surrounded by type IIx/b fibers, compared with those proximal to other type I fibers (55). Human locomotor muscles, such as the vastus lateralis, are not homogeneous (consisting of a superficial glycolytic, and a deep oxidative region (29)). Thus, the first aim of this study was to measure regional morphology and aerobic enzyme activity of individual muscle fibers within a mixed muscle in situ, from a rat model of RV HF and matched controls. We chose the plantaris muscle that is an important locomotor muscle in rats, and contains superficial glycolytic and a deep oxidative region. We aimed to determine whether skeletal muscle adaptations were related to fiber type per se (consistent with a systemic remodeling mechanism) as opposed to the muscle region within which the fibers were expressed (suggesting a local remodeling mechanism).
Pathological alterations in O2 delivery pathways are implicated in remodeling of O2 utilization by reduced mitochondrial expression (10, 32, 38). In health, muscle fiber maximal oxygen consumption can be assessed in situ using quantification of succinate dehydrogenase (SDH; complex II) activity (reflecting mitochondrial volume density; (47)). In conditions where the activity of mitochondrial electron transport chain complexes are selectively impaired, however, e.g., as demonstrated in failing cardiomyocytes where complex I activity is selectively inhibited (22), the relation between complex II activity and V̇o2max may be modulated. Such disturbances in cardiac bio-energetic function in HF are suggested to be an important contributor to progression of the disease (39). Therefore, the second aim of this study was to quantify SDH activity (as a measure of mitochondrial volume density) and the function of the electron transport chain of skeletal muscle mitochondria in RV HF. The latter was achieved by both high-resolution respirometry of permeabilized fibers and Blue-Native PAGE to assess the activity of isolated complexes of oxidative phosphorylation in RV HF and controls.
We wished to test the hypotheses that: 1) a reduction in skeletal muscle capillarity in RV HF is independent of muscle fiber type but proportional to muscle fiber area and mitochondrial volume density; 2) mitochondrial complex I activity is selectively reduced in skeletal muscle resulting in a reduction in both the sensitivity and the maximum rate of oxidative phosphorylation in RV HF; and 3) morphological adaptations supporting oxygen delivery are related to alterations in mitochondrial oxidative phosphorylation.
MATERIALS AND METHODS
Animals.
All protocols and procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986 and the U.S. Government Principles for the Utilization and Care of Vertebrate Animals, with the approval of the UK Home Office and the local ethics committee. Twenty-three male Wistar rats were divided into two groups: experimental rats were given a single intraperitoneal injection of monocrotaline (MCT, 60 mg·kg−1, n = 11) to induce PAH and subsequently RV hypertrophy and failure (4, 49), while control rats (CON, n = 12) received an equivalent volume of saline. Cardiovascular measures from a cohort of these animals are published elsewhere (3) and show significantly increased pulmonary pressure, RV dilatation and decreased ejection fraction in RV HF compared with control.
All rats were kept at room temperature and in a 12-h light-dark cycle. Food and water were given ad libitum. MCT animals were killed (23 ± 1 days after MCT injection) by cervical dislocation following stunning when they expressed clinical signs of HF including weight loss, tachypnea, lethargy, cold extremities and piloerection. These have been shown to correlate with decompensated RV dysfunction in this model (3, 19). CON animals were kept for the same period of time as MCT and killed on corresponding days. Animals were initially weighed and all hindlimb muscles (i.e., soleus, gastrocnemius and plantaris), heart, lungs and liver were dissected from the surrounding tissue, blotted dry and weighed individually. The left plantaris muscle was used for sectioning, while the right plantaris muscle was used for respirometry and blue native electrophoresis (BN PAGE). The gastrocnemius and soleus muscles were used to determine the muscle water content.
Histology.
The left plantaris muscle was pinned on cork, slightly stretched above its slack length to minimize bias in the determination of fiber cross-sectional area and capillary parameters, frozen in isopentane submerged in liquid nitrogen and then stored at −80°C until later analysis. Serial 10-μm sections from the middle of the plantaris muscle were cut on a cryostat at −20°C and mounted on polylysine-coated slides. There are two distinct regions of the plantaris muscle: a deep region near the tendon with more oxidative type I and IIa fibers and more extensive capillary network (deep, or oxidative region), and a superficial region containing predominantly glycolytic, type IIx and IIb fibers and a spare capillary network (Fig. 1 and (55)). Whole sections were stained for SDH-activity, myofibrillar ATPase activity and capillaries, after which they were mounted in glycerin-gelatin. SDH activity was determined immediately after sectioning, whereas other sections were stored at −80°C until later analysis. All analyses were made on the two distinct plantaris regions.
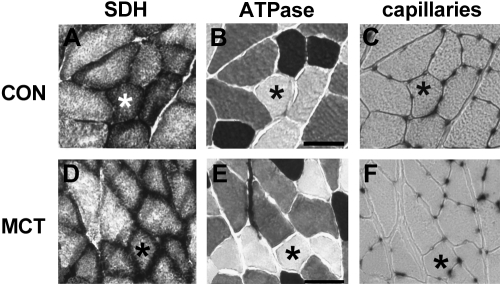
Fig. 1.
Serial sections of plantaris muscle from saline-treated rats (Con; top) and monocrotalone-injected rats (MCT; bottom) stained for succinate dehydrogenase (SDH; complex II) activity (A and D), fiber types (B and E), and capillaries (C and F). *Same fiber in each section and condition. Scale bar = 50 μm.
Succinate dehydrogenase (SDH) activity.
The SDH activity in individual muscle fibers in histological sections was determined as described previously (55). Sections were incubated at 37°C in the dark for 20 min in a medium consisting of 37 mM sodium phosphate buffer pH 7.6, containing 74 mM sodium succinate and 0.4 mM tetranitroblue tetrazolium (TNBT). The reaction was stopped in 0.01 M hydrogen chloride.
Myosin ATPase.
Serial sections were stained for myosin ATPase to classify fibers as type I (oxidative), IIa (intermediate) and IIx/b (glycolytic) as described previously (55). In short, sections were preincubated for 10 min in 0.1 M acetic acid and 0.1 M potassium chloride (pH 4.50), set with acetic acid, rinsed in 20 mM glycine and 20 mM calcium chloride (pH 9.4) and subsequently incubated for 25–30 min in 40 mM glycine, 20 mM calcium chloride and 2.5 mM ATP (pH 9.4). Sections were rinsed briefly with 1% (w/v) calcium chloride, left in 2% (w/v) cobalt chloride for 3 min, washed with distilled water and colored in 2% (w/v) ammonium sulfide and washed in distilled water. Type I fibers were stained dark, type IIa fibers stained white, while type IIx/b fibers were stained grey (Fig. 1).
Capillarisation.
Capillaries were visualized by alkaline phosphatase staining as described previously (55). Sections were fixed in chloroform-acetone (1:1) at 4°C, rinsed briefly in distilled water and subsequently stained for 1 h in 0.01% (w/v) nitro-blue-tetrazolium (NBT) and 0.002% (w/v) 5-bromo-4-chloro-3-indolyl phosphate p-toluidine salt in 6.9 mM magnesium sulfate and 27.5 mM sodium borate buffer (pH 9.2). Sections were then rinsed in distilled water and fixed in 4% (w/v) formaldehyde for 1 h.
Image analysis.
Black and white photographs of serial sections for stained fiber type, SDH activity and capillaries were taken on a Leica DMRE (Newcastle-upon-Tyne, UK) using a Deltapix Infinity X Digital Camera (Maalov, Denmark) at 20 × magnification and analyzed in ImageJ (NIH, Bethesda, USA). The staining intensity of SDH activity was determined by measuring the optical density of the final reaction product (47). Seventy-five to 100 fibers were measured in each muscle region and averaged per fiber type.
Mitochondrial respiration.
The deep and superficial regions of the right plantaris muscle were dissected, and fiber bundles (∼12 mg) were separated using sharp forceps in respiration solution B (Oroboros Instruments, Innsbrück, Austria), consisting of 2.8 mM CaK2EGTA, 7.2 mM K2EGTA, 5.8 mM ATP, 6.6 mM magnesium chloride, 20 mM taurine, 15 mM phosphocreatine, 20 mM imidazole, 0.5 mM DTT and 50 mM MES (pH 7.1). Muscle samples in solution B plus 10% (w/v) fatty acid free BSA and 30% (v/v) DMSO were quickly frozen in liquid nitrogen and stored at −80°C until analysis within one mo (25).
For analysis, muscle samples were quickly thawed by immersion in respiration solution B at room temperature, washed and permeabilized in saponin (50 μg.ml−1 in solution B) for 30 min at 4°C. Fibers were subsequently washed in mitochondrial respiration solution (MiR05, Oroboros Instruments, Innsbrück, Austria), containing 0.5 mM EGTA, 3 mM magnesium chloride, 60 mM K-lactobionate, 20 mM taurine, 10 mM potassium dihydrogen phosphate, 20 mM HEPES, 110 mM sucrose and 1 g.L−1 fatty acid free BSA (pH 7.1). Fiber bundles were then weighed before adding to a 2 mL chamber in a high-resolution respirometer (Oxygraph-2k; Oroboros Instruments, Innsbrück, Austria) and incubated with MiR05 at 37°C. To avoid oxygen diffusion limitation, oxygen concentration was increased to ∼400 μM by adding pure oxygen and was kept above 270 μM throughout the experiment.
Basal respiration was assessed by adding the Krebs cycle intermediates sodium glutamate (10 mM), sodium malate (2 mM) and sodium pyruvate (5 mM). Maximal ADP-stimulated respiration was measured in 2.5 mM ADP. The integrity of the outer-mitochondrial membrane was tested by adding 10 μM cytochrome c; any samples with a >15% increase in respiratory rate was excluded from further analysis. Maximal respiration, with simultaneous input of electrons through complex I and II, was measured following the addition of 10 mM succinate. Maximal uncoupled respiration was measured after stepwise addition of 0.01 μM carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP). Subsequently, complex I was blocked by rotenone (0.5 μM). Finally, antimycin A was added to inhibit complex III and measure residual oxygen consumption (non-mitochondrial respiration), which was subtracted from all values. All measurements were performed in duplicate, simultaneously and subsequently averaged. Values were normalized to wet fiber mass and reported as pmol O2.s−1.mg−1. The flux control ratio for ADP was calculated as the maximal ADP-stimulated oxygen flux normalized to maximal respiration. Between experiments, the chambers were washed with 100% ethanol for 30 min and thoroughly rinsed out with water.
Blue native gel electrophoresis.
Blue Native gel electrophoresis (BN PAGE) was used to assess the activity of complex I and IV of the mitochondrial electron transport chain (53, 57). Mitochondria were isolated in 4 CON and 6 MCT rats by homogenizing the deep and superficial regions of the plantaris muscle in homogenization buffer consisting of 440 mM sucrose, 20 mM imidazole/hydrogen chloride, 1 mM EDTA (pH 7.0) and a protease inhibitor cocktail tablet (Roche, Welwyn Garden City, UK). Samples were then centrifuged at 20,000 g for 20 min at 4°C. The pellet was re-suspended in 100 μl homogenization buffer and frozen at −80°C. On the day of analysis, membranes were solubilized using 2% (w/v) dodecyl β-d-maltoside in solubilisation buffer (1 mM EDTA, 2 mM aminocaproic acid, 50 mM imidazole/ hydrogen chloride and 50 mM sodium chloride) for 30 min. After centrifugation (15,000 g for 20 min at 4°C), glycerol and 5% (w/v) Coomassie blue G-250 dye (both 25 μl) was added to 200 μl of the supernatant. Protein concentration was measured in samples without Coomassie blue using the Bradford method. Twenty μg of mitochondrial protein was loaded onto 6–12% gradient acrylamide gels and run as described before (53). Complex I activity was assessed by staining the gel for ∼30 min in incubation medium A (2 mM Tris/HCl buffer, pH 7.4, containing 0.1 mg·ml−1 NADH and 2.5 mg·ml−1 NBT). Complex IV activity was assessed by staining the gel for 16 h in 9.0 ml incubation medium B (50 mM phosphate buffer, pH 7.4, containing 5 mg DAB, 20 μg·ml−1 catalase, 10 mg cytochrome c and 750 mg sucrose). Blots of in-gel-complex-activity were photographed using ChemiDoc™ XRS+ System (Bio-Rad, Hemel Hempstead, UK) and analyzed using Quantity One 1-D Analysis Software.
Statistical analysis.
Independent t-tests were used to compare means between the two groups. Repeated measures ANOVA was used to test for significant differences in capillarity, SDH activity and fiber size between conditions (CON and MCT), muscle regions (deep and superficial) and fiber types (type I, IIa and IIx/b). Repeated measures ANOVA were also used to detect significant differences in oxygen consumption between conditions and muscle regions. Differences were considered significant at P < 0.05. Values are mean ± SEM.
RESULTS
Right ventricular heart failure.
As expected, intraperitoneal injection of 60 mg·kg−1 monocrotaline (MCT) induced right ventricular hypertrophy and, subsequently, clinical signs of RV HF (Table 1; (3)). At 23 ± 1 days, the RV mass (both absolute and normalized to body mass) was significantly greater in MCT compared with CON (Table 1; P < 0.001). Body mass averaged 200 ± 2 g at the start of the experiment, but MCT-treated rats grew less rapidly than saline controls (P < 0.001 from day 7). The body mass of the MCT rats declined from ∼day 20 to the point at which they were killed (−5.8 ± 3% at 23 ± 1 days). The body mass increased in CON over the same period (+2.4 ± 1.9%; interaction effect P < 0.001). As such, at the end of the study MCT rats weighed significantly less than CON (P < 0.001). The loss of body mass in the final ∼3 days, a lower liver mass (P < 0.001), and a higher lung mass confirmed other extra-cardiac features of PAH and RV HF (Table 1).
Table 1.
Pathophysiological characteristics of control and monocrotaline-treated animals
| Control | Monocrotaline | P | |
|---|---|---|---|
| n | 12 | 11 | |
| Mass | |||
| Body, g | 317 ± 6 | 260 ± 3 | <0.001 |
| Heart, g | 1.27 ± 0.03 | 1.54 ± 0.07 | 0.001 |
| Right ventricle, mg | 190 ± 40 | 380 ± 20 | <0.001 |
| Left ventricle, mg | 498 ± 30 | 469 ± 26 | 0.487 |
| Lung, g | 1.77 ± 0.38 | 2.84 ± 0.11 | <0.001 |
| Liver, g | 13.7 ± 0.08 | 10.3 ± 0.4 | <0.001 |
| Plantaris mass, mg | 264 ± 7 | 231 ± 4 | 0.001 |
| Mass/body mass, mg/g | |||
| Heart | 4.02 ± 0.08 | 5.95 ± 0.25 | <0.001 |
| Right ventricle | 0.68 ± 0.04 | 1.49 ± 0.10 | <0.001 |
| Left ventricle | 1.57 ± 0.08 | 1.82 ± 0.10 | 0.066 |
| Lung | 5.6 ± 0.2 | 11.1 ± 0.5 | <0.001 |
| Liver | 43.1 ± 01.0 | 39.9 ± 1.4 | 0.058 |
| Plantaris | 0.834 ± 0.21 | 0.889 ± 0.20 | 0.072 |
Muscle morphology and fiber type distribution.
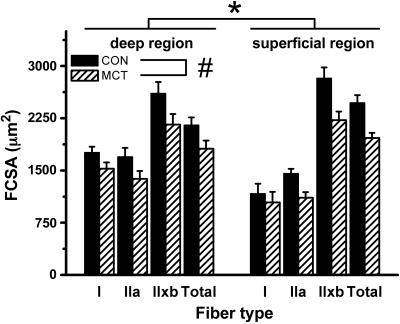
Hindlimb muscle mass was 13% greater in CON than MCT (plantaris: 264 ± 7 mg vs. 231 ± 4 mg; P = 0.001; Table 1). Histological sections of the plantaris muscle in CON and in MCT are shown in Fig. 1. The mean fiber cross-sectional area (FCSA) in the deep (oxidative) and superficial (glycolytic) regions of the plantaris muscle is shown in Fig. 2. The FCSA in MCT was 13% lower in type I and 20% lower in type II fibers compared with CON in both regions (P = 0.016), but this difference tended to be greater in the superficial region in MCT (interaction effect: P = 0.087). No differences between conditions were observed in the water content of the hindlimb muscles: 75.1 ± 0.2 vs. 75.2 ± 0.2% in CON vs. MCT respectively (P = 0.835).
Fig. 2.
Fiber cross-sectional area (FCSA) in the deep (oxidative) and superficial (glycolytic) region of the plantaris muscle in saline-treated (CON; n = 11) and monocrotaline-injected (MCT; n = 12) animals. *P < 0.05 between regions; #P < 0.05 between conditions. ′Total′ is the weighted average for all fibers. Type I fibers were only isolated in the superficial region in 4 and 5 rats (in CON and MCT, respectively).
The fiber type distribution was different between muscle region, with the deep regions containing a greater percentage of oxidative type I and a smaller proportion of type IIx/b fibers than the superficial region in both CON and MCT (P < 0.001). The overall fiber type distribution, however, was similar between CON and MCT (% type I fibers in the deep region: 14.2 ± 1.3 vs. 15.4 ± 1.2% respectively; and in the superficial region: 0.9 ± 0.4 vs. 1.4 ± 0.7% respectively, P = 0.330).
Capillarisation.
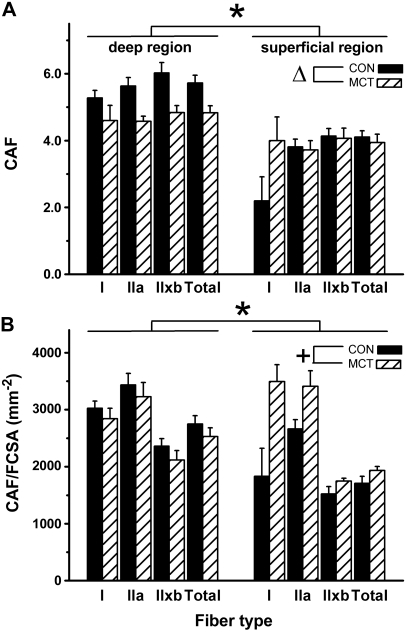
Capillaries around a fiber (CAF) and capillary-to-fiber (C:F) ratio were determined for different fiber types and muscle regions from serial sections stained for fiber type (Fig. 1B and 1E) and capillaries (Fig. 1C and 1F). In CON, CAF was higher in all fiber types in the deep compared with the superficial region of the plantaris (Fig. 3A, P < 0.001). In addition, because there were more type I fibers in deep region (P < 0.001), the type I and IIa fibers within that region had a higher CAF than the same fiber types found in the superficial region. Similarly, there were more capillaries around type IIx/b fibers within the deep region than the superficial region (Fig. 3A, P < 0.001). In MCT, the C:F ratio was lower than in CON in the deep region of the muscle (2.37 ± 0.08 vs. 1.85 ± 0.09), but not in the superficial region (1.64 ± 0.11 vs. 1.46 ± 0.11; interaction effect: P = 0.024). CAF was 16% lower in MCT than CON for all fiber types in the deep region, but CAF was the same in MCT and CON in the superficial region (Fig. 3A). Because muscle fiber area was smaller in both regions in MCT, CAF/FCSA (Fig. 3B) was ∼8% lower in the deep region in MCT compared with CON, but 13% greater in the superficial region in MCT (interaction effect: P = 0.14).
Fig. 3.
Capillaries around the fiber (CAF; A) and normalized to fiber size (FCSA; B) in the deep (oxidative) and superficial (glycolytic) region of the plantaris muscle in saline-treated (CON; n = 11) and monocrotaline-injected (MCT; n = 12) animals. *P < 0.05 between regions; ΔP < 0.05 between conditions only in deep region; +interaction effect between region and condition: P = 0.14. ′Total′ is the weighted average for all fibers. Type I fibers were only isolated in the superficial region in 4 and 5 rats (in CON and MCT, respectively).
Skeletal muscle mitochondria and control of oxidative phosphorylation.
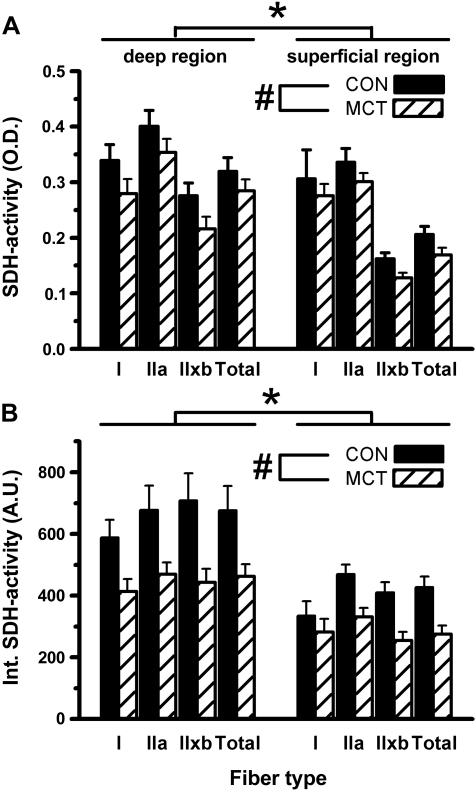
In CON, SDH activity (Fig. 1A) was lower in type IIx/b fibers compared with type I and IIa fibers (Fig. 4; P = 0.03). Consequently, the total SDH activity was greater in the deep region compared with the superficial region (P = 0.001). In MCT, SDH activity (Fig. 1D) was lower compared with CON in both muscle regions (−15%; P = 0.048), and in all fiber types (Fig. 4A). The integrated SDH activity (which accounts for differences in FCSA and is related to the total volume of mitochondria; Fig. 4B; (47)) was significantly lower in MCT than in CON (P = 0.011): −25% in the deep compared with −36% in the superficial region (interaction effect: P = 0.054). This trend was largely attributed to a larger reduction in FCSA in type IIx/b (−38%) compared with type I fibers (−24%) in MCT compared with CON.
Fig. 4.
Optical density of SDH activity staining (A) and spatially integrated SDH activity [FCSA × SDH activity, in arbitrary units (au); B] in the deep (oxidative) and superficial (glycolytic) region of the plantaris muscle in saline-treated (CON; n = 11) and monocrotaline-injected (MCT; n = 12) animals. *P < 0.05 between regions; #P < 0.05 between conditions. ′Total′ is the weighted average for all fibers. Type I fibers were only isolated in the superficial region in 4 and 5 rats (in CON and MCT, respectively).
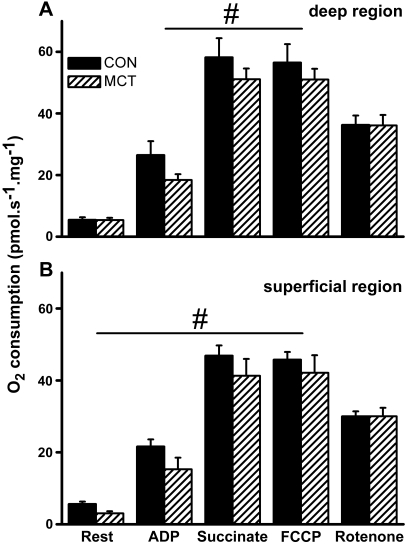
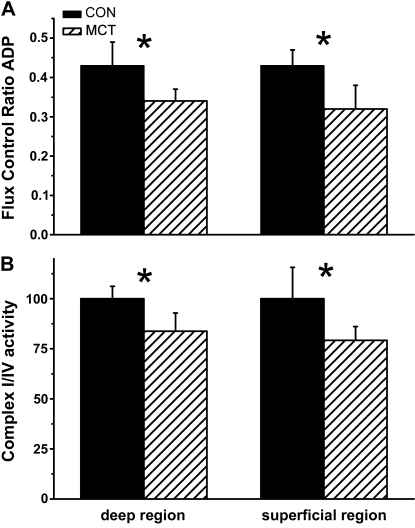
Consistent with these histological measurements, we observed a higher basal oxygen consumption in saponin-permeabilized fiber bundles taken from the deep region compared with the superficial region of the plantaris muscle (P = 0.031); a region-difference that was maintained under all respiratory conditions (Fig. 5A and B). Oxygen consumption was significantly lower in MCT compared with CON in the basal state (only in the glycolytic region; P = 0.017), after adding ADP (P = 0.02), and after adding the complex II substrate, succinate (P = 0.012; Fig. 5A and B). Respiratory rate before and after uncoupling by FCCP were not different in either condition. Despite normalizing for differences in maximal respiration, the flux control ratio for ADP remained significantly lower in MCT than CON (P = 0.032; Fig. 6A). The oxygen consumption after complex I inhibition by rotenone, however, was similar between MCT and CON (P = 0.357; Fig. 5A and 5B), suggesting a reduced complex I activity in MCT. This was confirmed in separate samples by the absence of a respiratory response to ADP after adding rotenone. Similar results were obtained from the soleus muscle (n = 10 CON and n = 5 MCT). To test whether mitochondrial complex I dysfunction was dependent on the disease severity, a lower dose of MCT of 30 mg·kg−1 was used to induce compensated RV hypertrophy (HYP; n = 6). We observed a similar complex I dysfunction in HYP skeletal muscle (flux control ratio for ADP was 0.332 ± 0.055 vs. 0.432 ± 0.056 in CON; P = 0.032), but without a concomitant reduction in maximally uncoupled oxygen consumption (62.3 ± 11.3 vs. 56.5 ± 6.0 pmol·s−1·mg−1 for the oxidative region in HYP vs. CON respectively).
Fig. 5.
Oxygen consumption from permeabilized fibers from the deep, oxidative (A) and superficial, glycolytic region (B) of the plantaris muscle saline-treated (Con) and monocrotaline-injected (MCT) rats, after adding subsequently glutamate, malate, and pyruvate (rest), 2.5 mM ADP (ADP), 10 mM succinate (succinate), a respiratory-chain uncoupler (FCCP), and a complex I inhibitor (rotenone). #P < 0.05 between conditions.
Fig. 6.
A: flux control ratio for ADP is the O2 consumption rate during ADP-stimulated respiration, normalized to maximal oxygen consumption. B: activity of complex I isolated by Blue-Native gel electrophoresis, normalized to complex IV activity. Values of optical density were set to 100% of control. *P < 0.05 compared with control within the same region.
A reduction in complex I activity in MCT was confirmed from isolated complex I and IV activity by BN PAGE. We observed an 18% lower activity of complex I relative to complex IV in MCT compared with CON in both the deep and superficial regions of the plantaris muscle (Fig. 6B): 2.59 ± 0.41 vs. 2.05 ± 0.18 arbitrary units (A.U.) in the deep region and 4.13 ± 0.25 vs. 3.46 ± 0.38 A.U. in the superficial region of CON and MCT muscles respectively (P = 0.004).
DISCUSSION
The data from the present study provide three novel findings about the determinants of skeletal muscle oxygen delivery and utilization following MCT-induced RV HF. The first was that skeletal muscle capillary remodeling was not dependent on fiber type expression. Contrary to current suggestions, skeletal muscle capillary loss and the consequent reduction in capillary-to-fiber-area ratio in MCT was restricted to muscle regions with a high oxidative capacity, but was not dependent on the type of muscle fiber around which capillaries were expressed. This suggests that local, rather than systemic, factors are responsible for skeletal muscle capillary remodeling in MCT animals. The second key finding was that the reduction in SDH activity (reflecting a lower mitochondrial volume density) and maximal oxygen consumption in MCT was not consequent to increased type II fiber expression as typically proposed (4, 44). We were able to demonstrate this because SDH activity was lower in MCT before any shift in fiber type occurred. Lastly, the remaining mitochondria in MCT were qualitatively impaired in that they showed a lower respiratory sensitivity to ADP. The complex I dysfunction in MCT that this suggests was corroborated by the normalization of respiratory function by rotenone blockade, and, for the first time, in a ∼20% reduction in isolated complex I activity. The observation that these reductions in mitochondrial function occurred in muscle regions without capillary remodeling (e.g., in the superficial region) indicates that mitochondrial dysfunction in MCT is independent of oxygen supply. Together, these data suggest that RV HF is accompanied by structural and functional adaptations in the peak potential for O2 delivery and in the maximal O2 utilization in skeletal muscle; the resultant bio-energetic disturbances of which are likely to contribute to exercise intolerance, quality of life and disease progression.
Muscle size in chronic heart failure.
We observed a decline in body mass in the MCT rats towards the end of the disease progression and that skeletal muscle fiber cross-sectional area was lower in rats with RV HF than controls (49); findings consistent with the fiber atrophy observed in patients with PAH (33) and chronic heart (32, 44) and lung failure (16, 17). Moreover, our results showed a preferential loss of fast twitch, glycolytic, muscle fiber area in RV HF.
The underlying reasons for the more-pronounced reduction in size of the glycolytic fibers are not well understood. Li and co-workers (30) previously reported a preferential atrophy of glycolytic fibers in a mouse model of HF, which has also been observed in patients with PAH (33), sepsis (35) and chronic obstructive pulmonary disease (16). Chronic hypoxia (commonly observed in PAH patients (2) and in this model, where arterial O2 saturation can be less than 80% (27)), however, results in a similar decline in FCSA in all fiber types and not a preferential loss in glycolytic fibers (55), leaving hypoxia per se an unlikely explanation. A more pronounced activation of the ubiquitin-proteasome system and protein breakdown have been suggested to occur in glycolytic compared with oxidative muscle groups (7). Moreover, apoptosis is involved in muscle atrophy in patients with chronic HF (1) and in the MCT model (50), and is known to be more pronounced in glycolytic than in oxidative muscles (31). Not only are the catabolic signaling pathways more stimulated, an increased inhibition of muscle protein synthesis suggests an impeded regenerative potential in glycolytic compared with oxidative muscles (48). In that respect, pulmonary TNF-α over-expression (which is elevated in MCT (38)) results in skeletal muscle wasting through an impaired muscle regeneration (28) but also suppresses peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) and aerobic enzyme activity (46). Consequently, a misbalance in anabolic and catabolic pathways, such as induced by systemic inflammation, may help explain the observed reduction in muscle mass and FCSA in the present data.
Capillarisation.
We demonstrated a capillary loss in the deep, oxidative, region of the plantaris in MCT without a change in the number of capillaries around fibers in the superficial, glycolytic muscle region. However, these regional effects were unrelated to fiber type per se, as capillary contacts were lost in all fiber types in the deep region in MCT. This novel finding is significant as it demonstrates a clear within-muscle, regional effect of RV HF on the skeletal muscle capillary network; an effect isolated to muscle regions that normally express the greatest capillaries. Interestingly, a reduction in capillarity was previously observed in the mixed plantaris muscle in LV HF, but not in the homogenous oxidative soleus muscle; despite a greater overall capillarisation in the soleus (56). Together this is consistent with the suggestion that the balance between capillary growth and remodeling is highly dependent on the regional interplay of fiber size, metabolic activity and local blood flow, and not on fiber type per se.
Our results indicate that capillary remodeling in RV HF is regulated by local, autocrine factors, which is influenced by the metabolic surrounding of the fiber, rather than fiber type (40, 55). The etiology of capillary remodeling in RV HF is however, unknown. While a systemic hypoxemia (that is typically present in MCT (27)) may be involved in altering the local expression of VEGF, its involvement in regionalized capillary loss in MCT seems unlikely (55, 56). More likely is that systemic inflammation, increased ROS production, reduced microvascular blood flow (49) and shear stress are all important factors in capillary remodeling in RV HF. Therefore, the region-specific capillary remodeling in the present data suggests that muscle regions with more capillaries (where oxidative, type I fibers are expressed) show greater capillary plasticity than regions with fewer capillaries (40). Interestingly, the capillary network in the superficial, glycolytic region of the plantaris muscle was well preserved in MCT, whereas there was a large reduction in mitochondrial volume (integrated SDH activity) in this region. Thus, there appears to be a delicate regional interplay between the requirement for O2 delivery (oxidative capacity) and the peak potential for O2 supply (reflected in capillarity) that is resolved differentially in different muscle regions. Overall, therefore, the present data show that structural remodeling of capillaries in skeletal muscle in RV HF is more detrimental to muscle regions with high oxidative capacity, the mechanism for which is currently unknown.
Skeletal muscle mitochondria and control of oxidative phosphorylation.
A reduction in SDH activity in situ (reflecting mitochondrial volume density) was observed in MCT-treated animals, and is consistent with the overall reduction in oxidative enzyme activity in patients with PAH (33) and chronic HF (32). In the present study SDH activity was ∼15% lower in MCT than CON in all muscle fiber types. This difference was independent of muscle region and occurred without a change in fiber type expression. These novel findings reveal that mitochondrial remodeling in RV HF occurs more rapidly than the shift in fiber type (10). Others, using a similar model, have seen small (∼5%) shifts in myosin heavy chain expression of the EDL and soleus away from type I (49). Such small changes may have been beyond the resolution of our in situ method of fiber type determination, which cannot fully account for hybrid expression of different myosin heavy chains. Despite this limitation, the reduction in SDH activity was seen even in fast twitch type IIx/b fibers, which suggests that a change in fiber type itself was not the cause of the reduction in SDH activity and capillary contacts in MCT (11). Nevertheless, while remodeling of fiber size and mitochondrial expression might be subject to distinct cellular pathways (10, 11), the combination of fiber atrophy and reduced mitochondrial volume density may provide a mechanism to help protect against the development of low intracellular PO2 or even an anoxic core in skeletal muscle in RV HF. A lower level of the mitochondrial transcription factor PGC-1α is thought to be a potential modulator of mitochondrial biogenesis in chronic diseases such as HF (14, 15), which can be mediated through increased TNF-α (46) and NF-κB (38). This suggests, therefore, that not only an increased breakdown, but also an impaired mitochondrial synthesis contribute to a loss of mitochondria in chronic heart failure.
The low oxidative capacity that we observed in MCT was mirrored in a low mitochondrial respiratory sensitivity to ADP. Blocking complex I in permeabilized fibers by rotenone normalized the respiratory sensitivity between MCT and CON, which is consistent with a mitochondrial complex I dysfunction in RV HF. A similar complex I dysfunction has been suggested in cardiac muscle in chronic LV HF (22), following reperfusion injury (26), in critically ill septic patients (6) and in neurons from Parkinson's patients (20). For the first time we have identified that intact skeletal muscle mitochondria in RV HF have a lower ADP-sensitivity, which is associated with a ∼20% lower activity of complex I in isolation (relative to complex IV activity). These data suggest that mitochondrial dysfunction can be observed peripheral to the primary disease site in RV HF, and is consistent with a role for systemic inflammation (as seen in sepsis (6)), ROS and/or reactive nitrogen species (RNS) in modulating complex I activity (13). Indeed, Remels et al. (38) have recently demonstrated a link between TNF-α and the regulation of mitochondrial biogenesis and ADP sensitivity using a similar methodology. Hypoxia (present in this model (27)) and oxidative stress have each been implicated in causing or fixing a change in the conformational state of complex I from the A (active) to the D (de-active) state (13). From a teleological standpoint, this mechanism is protective in reducing complex I ROS production in hypoxia and hence limiting free-radical damage. It may be of note therefore that, in humans, HF is associated with a high degree of contractile protein oxidation (8, 51). Interestingly, mitochondrial complex I dysfunction was also evident in the glycolytic, superficial regions of the plantaris muscle, where the capillary network was well preserved. Therefore, these adaptations in mitochondrial function, which act to limit muscle oxygen consumption, appear to be independent of capillary remodeling per se.
To test whether mitochondrial complex I dysfunction was dependent on the disease severity, a lower dose of MCT of 30 mg·kg−1 was used to induce compensated RV hypertrophy (HYP). We observed a similar complex I dysfunction in skeletal muscle in HYP, but without a concomitant reduction in maximally (uncoupled) oxygen consumption. Therefore, complex I dysfunction might precede the reduction in skeletal muscle mitochondrial volume density observed in RV HF, perhaps via induction of mitochondrial apoptosis, alterations with mitochondrial fission/fusion and/or defects in the supermolecular assembly (39) in more severe RV HF (15).
Whether complex I dysfunction is manifest in skeletal muscle of patients with PAH is currently unknown. However, complex I deactivation (coupled with reductions in oxygen transport and mitochondrial volume density) may provide a profound limitation to energy provision (9), especially under conditions of high ATP demand such as during exercise (2). This mitochondrial dysfunction therefore might, at least in part, underlie the strong relationship between reduction in exercise tolerance and mortality in left and right ventricular heart failure, but also other chronic diseases (6, 36).
Clinical implications for exercise tolerance and mortality in chronic HF.
Together with the observed contractile protein dysfunction in patients with CHF (51), the present data contributes to our understanding of exercise intolerance in patients with heart failure. The reductions in the number of capillaries around each fiber may limit the potential for oxygen delivery and metabolite removal in skeletal muscle in HF, especially during exercise. These potential effects appear to be minimized in the glycolytic muscle region in our MCT model by a lower FCSA and oxidative enzyme activity. In oxidative muscle regions, however, capillarity was also severely reduced. A reduction in SDH activity, fiber size and capillary density could, in part, explain the lower V̇o2max observed in patients with chronic HF (36, 43, 45), and the poor relation between V̇o2max and ejection fraction in these patients (45, 51). In addition, a reduction in skeletal muscle mitochondrial volume density would slow the rate at which oxidative phosphorylation rises to meet the energy demands of exercise (V̇o2max kinetics (2, 41)). These dynamics are expected to be further slowed by the reduced ADP sensitivity of the remaining mitochondria: a greater ADP accumulation being required to obtain a given drive for oxidative phosphorylation in chronic HF muscle. As such, the present data may help to explain why relatively small reductions in V̇o2max or mitochondrial enzyme expression are accompanied by a severe slowing of V̇o2 kinetics in patients with PAH (2) and chronic HF - a process that has a close relation to limiting the activities of daily living and to mortality (41).
Study limitations.
Simonini et al. (42) have shown that inactivity does not underlie the skeletal muscle adaptations in humans with chronic HF. However, we cannot rule out that reduced physical activity contributed to the skeletal muscle structural remodeling observed here. The present study investigated regional differences within a single muscle (the plantaris), thereby attempting to minimize any muscular activity bias. In fact, physical activity levels recorded by telemetry were similar in a different group of MCT-treated animals (n = 9) and CON (n = 9) up to 22 days postinjection (Stones and White, unpublished observations). Nevertheless, the loss in body mass and clinical signs (cold extremities, piloerection) observed in MCT-treated animals during the final ∼3 days of the experiment would be expected to accompany a reduction in physical activity (and caloric intake), the effects of which cannot be discounted in contributing to our observations of skeletal muscle remodeling. Similarly, we did not monitor FCSA over time, or in weight-matched animals, and therefore we cannot know whether the low FCSA in MCT was consequent to a ′pure′ catabolic state (i.e., during the loss in body mass in the final few days of the experiment), or through a continuous disbalance in anabolic and catabolic states (54) throughout the development of PAH and RV HF: an effect that may have been exacerbated by the relatively young age of the animals.
The half life of the MCT pyrrole has been suggested to be <4s in vivo, and the dose used here (60 mg.kg−1) results in concentrations of ∼2 nmol.g−1 24 h after injection (12). The absence of LV compensation or failure also suggests that MCT does not influence vascular function in the systemic circulation. Nevertheless, MCT pyrrole administration at far higher concentrations (>100 μM) may cause a direct, noncompetitive inhibition of complex I in isolated mitochondria in vitro (34) through oxidation of complex I cysteine thiols (13, 23). Therefore, in the present study, the lack of a systemic effect on blood pressure and LV function, the half life of the toxin and the low resultant concentration of MCT each suggests that MCT administration did not have a direct influence on peripheral skeletal muscle after ∼23 days.
Conclusion
These data demonstrate for the first time that the major structural adaptations in the pathways determining oxygen delivery and utilization in skeletal muscle in right ventricular heart failure are muscle region dependent. The combined effects of remodeling of capillaries and fiber area in RV HF were more detrimental to sustained energetic function in the oxidative regions of the muscle, and implicate a local mechanism capillary remodeling. Mitochondrial loss however, was observed throughout skeletal muscle in RV HF and occurred independently of changes in fiber type. This notion was supported in the novel demonstration that the sensitivity of ADP-stimulated respiration was reduced by ∼20% in permeabilized fibers in RV HF, which is similar to the reduction in activity of isolated mitochondrial complex I. Therefore, both mitochondrial quantity and quality were reduced in skeletal muscle in RV HF. These results implicate a systemic influence, such as systemic inflammation and/or ROS, on mitochondrial function in RV HF. Overall, these data reveal structural and functional alterations in skeletal muscle that may contribute to exercise intolerance and reduced quality of life in chronic RV HF.
GRANTS
The study was in part supported by the British Heart Foundation (PG/08/027/24774) and an Emma and Leslie Reid endowed Ph.D. studentship (to D. Benoist). R. C. I. Wüst is supported by the Biotechnology and Biological Sciences Research Council (BB/F019521/1).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.C.I.W., R.S., C.P., E.W., and H.B.R. conception and design of research; R.C.I.W., D.S.M., R.S., D.B., P.A.R., and J.P.B. performed experiments; R.C.I.W., D.S.M., R.S., D.B., P.A.R., and H.B.R. analyzed data; R.C.I.W., R.S., D.B., P.A.R., J.P.B., C.P., E.W., and H.B.R. interpreted results of experiments; R.C.I.W. prepared figures; R.C.I.W. and H.B.R. drafted manuscript; R.C.I.W., D.S.M., R.S., D.B., J.P.B., C.P., E.W., and H.B.R. edited and revised manuscript; R.C.I.W., D.S.M., R.S., D.B., P.A.R., J.P.B., C.P., E.W., and H.B.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the help of Claire Windle (supported by a Welcome Trust vacation studentship) for help in the BN PAGE.
Present address of D. Benoist: Inserm U1045 Centre de Recherche Cardio-Thoracique, Université Bordeaux Segalen, 33076 Bordeaux, France.
REFERENCES
- 1. Adams V, Jiang H, Yu J, Mobius-Winkler S, Fiehn E, Linke A, Weigl C, Schuler G, Hambrecht R. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol 33: 959–965, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Barbosa PB, Ferreira EM, Arakaki JS, Takara LS, Moura J, Nascimento RB, Nery LE, Neder JA. Kinetics of skeletal muscle O2 delivery and utilization at the onset of heavy-intensity exercise in pulmonary arterial hypertension. Eur J Appl Physiol 111: 1851–1861, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Benoist D, Stones R, Drinkhill M, Bernus O, White E. Arrhythmogenic substrate in hearts of rats with monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 300: H2230–H2237, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bernocchi P, Cargnoni A, Vescovo G, Dalla Libera L, Parrinello G, Boraso A, Ceconi C, Ferrari R. Skeletal muscle abnormalities in rats with experimentally induced heart hypertrophy and failure. Basic Res Cardiol 98: 114–123, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135: 794–804, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Carvalho RF, Castan EP, Coelho CA, Lopes FS, Almeida FL, Michelin A, de Souza RW, Araujo JP, Jr, Cicogna AC, Dal Pai-Silva M. Heart failure increases atrogin-1 and MuRF1 gene expression in skeletal muscle with fiber type-specific atrophy. J Mol Histol 41: 81–87, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Dalla Libera L, Ravara B, Gobbo V, Betto DD, Germinario E, Angelini A, Evangelista S, Vescovo G. Skeletal muscle proteins oxidation in chronic right heart failure in rats: can different beta-blockers prevent it to the same degree? Int J Cardiol 143: 192–199, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Davey GP, Canevari L, Clark JB. Threshold effects in synaptosomal and nonsynaptic mitochondria from hippocampal CA1 and paramedian neocortex brain regions. J Neurochem 69: 2564–2570, 1997 [DOI] [PubMed] [Google Scholar]
- 10. De Sousa E, Veksler V, Bigard X, Mateo P, Ventura-Clapier R. Heart failure affects mitochondrial but not myofibrillar intrinsic properties of skeletal muscle. Circulation 102: 1847–1853, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Delp MD, Duan C, Mattson JP, Musch TI. Changes in skeletal muscle biochemistry and histology relative to fiber type in rats with heart failure. J Appl Physiol 83: 1291–1299, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Estep JE, Lame MW, Morin D, Jones AD, Wilson DW, Segall HJ. [14C]monocrotaline kinetics and metabolism in the rat. Drug Metab Dispos 19: 135–139, 1991 [PubMed] [Google Scholar]
- 13. Galkin A, Abramov AY, Frakich N, Duchen MR, Moncada S. Lack of oxygen deactivates mitochondrial complex I: implications for ischemic injury? J Biol Chem 284: 36055–36061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 551: 491–501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garnier A, Fortin D, Zoll J, N′Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J 19: 43–52, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Gosker HR, Kubat B, Schaart G, van der Vusse GJ, Wouters EF, Schols AM. Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease. Eur Respir J 22: 280–285, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr 71: 1033–1047, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117: 1717–1731, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 120: 42–49, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4: 600–609, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 85: 357–363, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Jha N, Jurma O, Lalli G, Liu Y, Pettus EH, Greenamyre JT, Liu RM, Forman HJ, Andersen JK. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson's disease. J Biol Chem 275: 26096–26101, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Kindig CA, Musch TI, Basaraba RJ, Poole DC. Impaired capillary hemodynamics in skeletal muscle of rats in chronic heart failure. J Appl Physiol 87: 652–660, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kuznetsov AV, Kunz WS, Saks V, Usson Y, Mazat JP, Letellier T, Gellerich FN, Margreiter R. Cryopreservation of mitochondria and mitochondrial function in cardiac and skeletal muscle fibers. Anal Biochem 319: 296–303, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Mark W, Steurer W, Saks V, Usson Y, Margreiter R, Gnaiger E. Mitochondrial defects and heterogeneous cytochrome c release after cardiac cold ischemia and reperfusion. Am J Physiol Heart Circ Physiol 286: H1633–H1641, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Lai YL, Thacker AA, Diana JN. Hypoxemia and elevated tachykinins in rat monocrotaline pneumotoxicity. Lung 174: 195–203, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Langen RC, Schols AM, Kelders MC, van der Velden JL, Wouters EF, Janssen-Heininger YM. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol 35: 689–696, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Lexell J, Henriksson-Larsen K, Sjostrom M. Distribution of different fibre types in human skeletal muscles. 2. A study of cross-sections of whole m vastus lateralis. Acta Physiol Scand 117: 115–122, 1983 [DOI] [PubMed] [Google Scholar]
- 30. Li P, Waters RE, Redfern SI, Zhang M, Mao L, Annex BH, Yan Z. Oxidative phenotype protects myofibers from pathological insults induced by chronic heart failure in mice. Am J Pathol 170: 599–608, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Libera LD, Zennaro R, Sandri M, Ambrosio GB, Vescovo G. Apoptosis and atrophy in rat slow skeletal muscles in chronic heart failure. Am J Physiol Cell Physiol 277: C982–C986, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Lipkin DP, Jones DA, Round JM, Poole-Wilson PA. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol 18: 187–195, 1988 [DOI] [PubMed] [Google Scholar]
- 33. Mainguy V, Maltais F, Saey D, Gagnon P, Martel S, Simon M, Provencher S. Peripheral muscle dysfunction in idiopathic pulmonary arterial hypertension. Thorax 65: 113–117, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Mingatto FE, Dorta DJ, dos Santos AB, Carvalho I, da Silva CH, da Silva VB, Uyemura SA, dos Santos AC, Curti C. Dehydromonocrotaline inhibits mitochondrial complex I. A potential mechanism accounting for hepatotoxicity of monocrotaline. Toxicon 50: 724–730, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Minnaard R, Drost MR, Wagenmakers AJ, van Kranenburg GP, Kuipers H, Hesselink MK. Skeletal muscle wasting and contractile performance in septic rats. Muscle Nerve 31: 339–348, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Perreault CL, Gonzalez-Serratos H, Litwin SE, Sun X, Franzini-Armstrong C, Morgan JP. Alterations in contractility and intracellular Ca2+ transients in isolated bundles of skeletal muscle fibers from rats with chronic heart failure. Circ Res 73: 405–412, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Remels AH, Gosker HR, Schrauwen P, Hommelberg PP, Sliwinski P, Polkey M, Galdiz J, Wouters EF, Langen RC, Schols AM. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J 24: 5052–5062, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, Sabbah HN, Hoppel CL. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res 80: 30–39, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roudier E, Gineste C, Wazna A, Dehghan K, Desplanches D, Birot O. Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. J Physiol 588: 4579–4591, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schalcher C, Rickli H, Brehm M, Weilenmann D, Oechslin E, Kiowski W, Brunner-La Rocca HP. Prolonged oxygen uptake kinetics during low-intensity exercise are related to poor prognosis in patients with mild-to-moderate congestive heart failure. Chest 124: 580–586, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Simonini A, Long CS, Dudley GA, Yue P, McElhinny J, Massie BM. Heart failure in rats causes changes in skeletal muscle morphology and gene expression that are not explained by reduced activity. Circ Res 79: 128–136, 1996 [DOI] [PubMed] [Google Scholar]
- 43. Stratton JR, Kemp GJ, Daly RC, Yacoub M, Rajagopalan B. Effects of cardiac transplantation on bioenergetic abnormalities of skeletal muscle in congestive heart failure. Circulation 89: 1624–1631, 1994 [DOI] [PubMed] [Google Scholar]
- 44. Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81: 518–527, 1990 [DOI] [PubMed] [Google Scholar]
- 45. Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol 55: 1037–1042, 1985 [DOI] [PubMed] [Google Scholar]
- 46. Tang K, Wagner PD, Breen EC. TNF-alpha-mediated reduction in PGC-1alpha may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol 222: 320–327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van der Laarse WJ, Diegenbach PC, Elzinga G. Maximum rate of oxygen consumption and quantitative histochemistry of succinate dehydrogenase in single muscle fibres of Xenopus laevis. J Muscle Res Cell Motil 10: 221–228, 1989 [DOI] [PubMed] [Google Scholar]
- 48. Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol Cell Physiol 262: C1513–C1519, 1992 [DOI] [PubMed] [Google Scholar]
- 49. Vescovo G, Ceconi C, Bernocchi P, Ferrari R, Carraro U, Ambrosio GB, Libera LD. Skeletal muscle myosin heavy chain expression in rats with monocrotaline-induced cardiac hypertrophy and failure. Relation to blood flow and degree of muscle atrophy. Cardiovasc Res 39: 233–241, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Vescovo G, Dalla Libera L. Skeletal muscle apoptosis in experimental heart failure: the only link between inflammation and skeletal muscle wastage? Curr Opin Clin Nutr Metab Care 9: 416–422, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Vescovo G, Ravara B, Dalla Libera L. Skeletal muscle myofibrillar protein oxidation and exercise capacity in heart failure. Basic Res Cardiol 103: 285–290, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Wittig I, Braun HP, Schagger H. Blue native PAGE. Nat Protoc 1: 418–428, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Wüst RCI, Degens H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis 2: 289–300, 2007 [PMC free article] [PubMed] [Google Scholar]
- 55. Wüst RCI, Jaspers RT, van Heijst AF, Hopman MT, Hoofd LJ, van der Laarse WJ, Degens H. Region-specific adaptations in determinants of rat skeletal muscle oxygenation to chronic hypoxia. Am J Physiol Heart Circ Physiol 297: H364–H374, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Xu L, Poole DC, Musch TI. Effect of heart failure on muscle capillary geometry: implications for O2 exchange. Med Sci Sports Exerc 30: 1230–1237, 1998 [DOI] [PubMed] [Google Scholar]
- 57. Yan LJ, Forster MJ. Resolving mitochondrial protein complexes using nongradient blue native polyacrylamide gel electrophoresis. Anal Biochem 389: 143–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]