Abstract
Mucus clearance is an important airway innate defense mechanism. Airway-targeted overexpression of the epithelial Na+ channel β-subunit [encoded by sodium channel nonvoltage gated 1, beta subunit (Scnn1b)] in mice [Scnn1b-transgenic (Tg) mice] increases transepithelial Na+ absorption and dehydrates the airway surface, which produces key features of human obstructive lung diseases, including mucus obstruction, inflammation, and air-space enlargement. Because the first Scnn1b-Tg mice were generated on a mixed background, the impact of genetic background on disease phenotype in Scnn1b-Tg mice is unknown. To explore this issue, congenic Scnn1b-Tg mice strains were generated on C57BL/6N, C3H/HeN, BALB/cJ, and FVB/NJ backgrounds. All strains exhibited a two- to threefold increase in tracheal epithelial Na+ absorption, and all developed airway mucus obstruction, inflammation, and air-space enlargement. However, there were striking differences in neonatal survival, ranging from 5 to 80% (FVB/NJ<BALB/cJ<C3H/HeN<C57BL/6N), which correlated with the incidence of upper airway mucus plugging and the levels of Muc5b in bronchoalveolar lavage. The strains also exhibited variable Clara cell necrotic degeneration in neonatal intrapulmonary airways and a variable incidence of pulmonary hemorrhage and lung atelectasis. The spontaneous occurrence of a high surviving BALB/cJ line, which exhibited delayed onset of Na+ hyperabsorption, provided evidence that: 1) air-space enlargement and postnatal death were only present when Na+ hyperabsorption occurred early, and 2) inflammation and mucus obstruction developed whenever Na+ hyperabsorption was expressed. In summary, the genetic context and timing of airway innate immune dysfunction critically determines lung disease phenotype. These mouse strains may be useful to identify key modifier genes and pathways.
Keywords: congenic mice, Scnn1b overexpression, airway epithelia Na+ absorption, airway surface dehydration, genetic background, modifier genes
airway-targeted overexpression of the murine amiloride-sensitive epithelial Na+ channel beta subunit [β-ENaC, encoded by the sodium channel nonvoltage gated 1, beta subunit (Scnn1b) gene] via the Clara cell secretory protein [encoded by the secretoglobin, family 1A, member 1 (Scgb1a1) gene] promoter leads to transepithelial Na+ hyperabsorption, which produces airway surface dehydration that impairs mucus clearance (27). The Scnn1b-transgenic (Tg) mouse phenotype, originally described on a mixed C3H/HeN:C57BL/6N background, recapitulates multiple aspects of human obstructive lung disease, including cystic fibrosis (CF), chronic bronchitis, and chronic obstructive pulmonary disease (COPD).
Phenotypically, Scnn1b overexpression increases neonatal mortality due to tracheal mucus plugging. Surviving mice exhibit lower airway mucus obstruction, inflammation, and distal air-space enlargement (27, 29). Airway inflammation in Scnn1b-Tg mice is characterized by: enlarged/highly vacuolated macrophages; chronic neutrophilia associated with elevated levels of the cytokines KC (keratinocyte-derived cytokine, Cxcl1), MIP-2 (macrophage inflammatory protein, Cxcl2), and TNF-α (tumor necrosis factor alpha, Tnf); transient (from 2 to 6 wk of age) eosinophilia with increased levels of IL-13 (interleukin 13, Il13) and eotaxin-1 (Ccl11); and increased bronchus-associated lymphoid tissue (BALT) (25), similar to that described in the lungs of COPD patients (16). Scnn1b-Tg mice also exhibit transient and spotty Clara cell necrosis in the intrapulmonary airways and a failure to clear deposited (27) or aspirated bacteria (26). Thus, within the limitations of mouse models for respiratory diseases, e.g., the absence of submucosal glands (9, 49) and cough (3), the Scnn1b-Tg mouse represents a unique tool to study the onset and development of obstructive lung disease (29) and to test novel pharmacological agents that may promote mucus clearance.
The original characterization of β-ENaC transgene expression on a mixed strain background revealed substantial variability in the severity of the phenotype, suggesting genetic contributions to disease severity (27). Several studies utilizing inbred mouse strains have identified genetic contributions to pulmonary phenotypes in response to various challenges or injuries. For example, significant differences between mouse strains have been reported after ovalbumin (6, 41, 48), ozone (21, 32, 36), or hyperoxia (47) challenge, and after Streptococcal lung infection (17) or irritant exposures (24, 31, 46). Other studies revealed strain-specific differences (40, 42–44) and quantitative trait loci controlling various aspects of pulmonary function (1, 34, 50). Subsequent analyses identified candidate genes for respiratory function in mice, e.g., superoxide dismutase 3 and ectonucleotide pyrophosphatase 2 associations with dead space volume (12). Recently, mouse models of asthma have been used to identify potentially important mechanistic links between G protein-coupled receptors and asthma pathophysiology (20). Our previous studies on the effect of IL-4Rα (25) and MyD88 (26) genetic deletion in Scnn1b-Tg mice also indicate that specific aspects of lung pathology in this model are significantly modulated by deletion of specific genes.
Recently published gene modifier studies in humans also highlight the potential role of genetic background to affect the severity of the pulmonary phenotype when mucus clearance is impaired. For example, polymorphisms near APIP, an apoptosis regulator potentially important for resolution of mucus cell metaplasia, contributed to the variance in CF lung disease severity in a large cohort of CF subjects (51). Genetic associations between severity of lung disease and polymorphisms in a major secreted mucin, MUC5AC, have also been recently reported (15).
To assess the influence of genetic background in determining the response to dehydration-induced impairment in mucus clearance, we characterized the pulmonary phenotypes in four strains of congenic Scnn1b-Tg mice, using a variety of traditional histological and bronchoalveolar lavage (BAL) measurements of disease pathology and inflammation, novel assays of mucin load, and techniques specifically designed to characterize the novel phenotypes exhibited by some of the congenic strains, such as pulmonary hemorrhage, pulmonary hypertension, and atelectasis.
MATERIAL AND METHODS
Development of mouse strains.
All mice were housed in individually ventilated micro-isolator cages in a specific pathogen-free facility maintained at the University of North Carolina (UNC) at Chapel Hill on a 12-h day/night cycle. They were fed a regular chow diet and given water ad libitum. The original Scnn1b-Tg mice were derived on a mixed background (C3H/HeN:C57BL/6N) (27). Founder line 6608 was utilized for this study. Congenic strains were developed by crossing hemizygous Scnn1b-Tg mice (Scnn1b-Tg+/−) to inbred C57BL/6N (Taconic, C57BL6/NTac, Hudson, NY), C3H/HeN (Taconic, C3H/HeNTac), BALB/cJ (stock #000651; Jackson Laboratories, Bar Harbor ME), and FVB/NJ (stock #001800, Jackson Laboratories) mice. Mice were genotyped as described (27). Multiple breeders and litters per breeder were utilized for all studies to minimize founder and litter order effects. All animal procedures were approved by the Institutional Animal Care and Use Committee of the UNC at Chapel Hill and performed according to the principles outlined by the Animal Welfare and the National Institutes of Health guidelines for the care and use of animals in biomedical research.
Phenotyping.
All strains, except FVB, were backcrossed nine generations before analyses. A completely congenic FVB strain could not be generated due to extremely poor survival (this strain only reached N4 in 2 yr of active breeding). In all cases, wild-type (WT) littermates, i.e., not carrying the Scnn1b-Tg, were used as controls. BAL, differential cell counts, histopathology scoring, Western blot analysis of secreted mucins (25), quantification of airway epithelial cell necrosis (29), and Ussing chamber measurements of airway bioelectric properties (14) were conducted as previously described. For Ussing chamber studies, amiloride was used at 10−4 M, forskolin at 10−5 M, UTP at 10−4 M, and bumetanide at 10−4 M. Based on our previous findings describing the development of lung pathology in mixed background Scnn1b-Tg mice (29), phenotypes were studied at four designated time points: postnatal day (PND) 3–5, early-neonatal; PND 10, late-neonatal; 4 wk, juvenile; and 8 wk, adult.
Echocardiograms.
Nonsedated mouse pups were gently taped to a warmed mouse board. Warmed ultrasound gel was applied, the transducer placed over the rib cage, and echocardiograms performed according to previously described methods (2).
Immunohistochemistry.
Immunohistochemical detection of native and transgenic Scnn1b was performed on 10% neutral buffered formalin (NBF) immersion-fixed, paraffin-embedded lung sections using a rabbit polyclonal antibody (1:400 dilution; StressMarq Biosciences, Victoria, Canada). Normal rabbit IgG was used as an isotype-matched negative control. Secondary antibody was horseradish peroxidase (HRP)-conjugated anti-rabbit (1:400 donkey; Jackson Immunoresearch, West Grove, PA). Immunohistochemical detection of α smooth muscle actin was performed on lungs fixed with 10% NBF at constant inflation-pressure (25 cmH2O). Dako's retrieval solution was used for antigen retrieval (Dako, Carpinteria, CA) at pH 6.00, for 6 min at 100°C. Normal horse serum (10%; Jackson Immunoresearch, West Grove, PA) was used to prevent nonspecific binding. Primary antibody was polyclonal rabbit anti-alpha smooth muscle actin (1:100; Abcam Ab5694-100, 0.2 mg/ml). Secondary antibody was biotin-anti rabbit IgG (Vector BA-1000; Vector Laboratories, Burlingame, CA) diluted 1:500. Tertiary reagent was avidin-conjugated HRP (1:500; A-2004, Vector Laboratories). 3–3′-Diaminobenzidine was used as the substrate for the peroxidase reactions. All slides were counterstained with hematoxylin.
Lung fixation and processing for stereological analysis.
All lungs were inflation-fixed with 10% NBF at a pressure of 25 cmH2O for at least 30 min. The trachea was ligated to avoid deflation, and heart and lungs were removed en block and stored immersed in NBF. Prior to tissue sampling, total lung volume, V(lung) was measured by fluid displacement (37). Lungs were then embedded in 2.5% agar (Merck, Dietikon, Switzerland) in 0.9% NaCl (Merck) and cut perpendicularly to the longitudinal axis into series of 2-mm-thick slices (with random start) in a tissue slicer (13). Every second slice (with random start) was postfixed with Na-cacodylate buffered 1% osmium tetroxide (Simec Service, Aarberg, Switzerland), dehydrated in a graded series of acetone and embedded in glycol methacrylate resin (Technovit 7100, Simec Service). Parallel sections of 1.5 μm nominal thickness were cut from the embedded slices; the first and third sections were mounted on microscopy slides and stained with toluidine blue (Merck). Stereological analysis was performed using a computer-assisted system (NewCAST3.2; Visiopharm, Horsholm, Denmark) connected to an Olympus BH-2 light microscope (Olympus, Muenster, Germany). The following parameters were estimated using established design-based stereological methods (18, 30). Total alveolar volume [V(alv)] was obtained by point counting. The total alveolar surface area [S(alv)] was obtained by intersection counting. The alveolar number [N(alv)] was estimated by the physical disector method. The mean size of an individual alveolus [νN(alv)] was estimated by dividing total alveolar volume by alveolar number, and the mean thickness of interalveolar septa [τ(sep)] was estimated as twice the alveolar septal volume divided by the alveolar surface area.
Southern blot.
Southern blots analysis of transgene copy number was conducted according to manufacturer's instructions using 10 μg of BamHI-digested genomic tail DNA and Hybond N+ membrane (GE Healthcare Life Sciences, Piscataway, NJ) using the mouse Scnn1b cDNA as probe. To control for DNA loading, signal intensity of the transgene-specific band was normalized to the signal from an endogenous restriction fragment.
Statistical analyses.
Statistical analyses were performed using SigmaStat 3.1 or GraphPad Prism 5.0. Survival curves were compared using Kaplan-Meier log rank analysis and Holm-Sidak multiple comparison. t-Tests were used to compare Scnn1b-Tg mice vs. WT littermates when comparisons were made within the same strain. One-way ANOVA followed by Dunnett's post hoc test for multiple comparisons (comparing all groups vs. reference group, i.e., C57BL/6N) were used to determine significant differences among strains. For statistical analysis of stereology data pertaining to BALB/cJ Line A and Line B, we used the alternative hypothesis that the population medians are ordered, i.e., BALB/cJ Line A Scnn1b-Tg ≤ BALB/cJ Line B Scnn1b-Tg ≤ BALB/cJ Line A WT ≤ BALB/cJ Line B WT. Data were analyzed by the nonparametric Jonkheere-Terpstra test using StatXact software (Cytel, Cambridge, MA). This test is sensitive for analysis of data with adopted ordered alternative hypothesis and requires only limited assumptions (4). P < 0.05 was considered statistically significant, and “n” represents the number of mice in each experimental group. All data are expressed as means ± SE.
RESULTS
Survival of Scnn1b-Tg mice is strongly dependent upon genetic background.
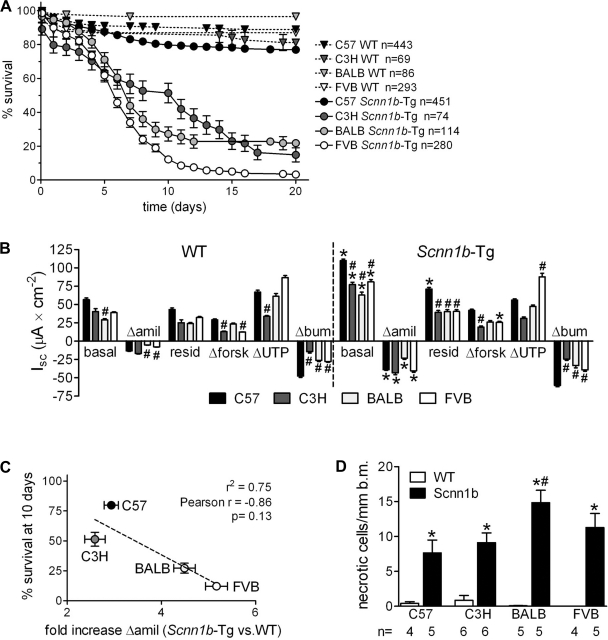
For all strains, pups were born at the expected Mendelian distribution of 50% WT and 50% Scnn1b-Tg (see n in Fig. 1A), but survival to PND 20 varied significantly (Fig. 1A). Compared with the mixed C3H:C57 strain, which had ∼50% survival at PND 20 (27), Scnn1b-Tg mice on the C57BL/6N background had increased survival (77 ± 2%), whereas all other backgrounds exhibited reduced survival (C3H/HeN 15 ± 4%, BALB/cJ 22 ± 4%, and FVB/NJ 3 ± 1% at PND 20) (Fig. 1A). For the C57BL/6N, C3H/HeN, and BALB/cJ strains, these differences became obvious early in the backcrossing process (N4-N5). Strikingly, the FVB/NJ strain showed very poor survival beginning at N1 (survival at PND 20 <10% n = 34), and it became impossible to conduct backcrosses beyond N4 (reached after ∼2 yr of active breeding). Interestingly, a single cross of high-surviving congenic C57BL/6N Scnn1b-Tg mice with FVB/NJ WT mice resulted in plummeting survival (0% at PND 10, n = 10), suggesting a dominant effect driven by the FVB/NJ genotype.
Fig. 1.
Variable survival in congenic sodium channel nonvoltage gated 1, beta subunit-transgenic (Scnn1b-Tg) mouse strains does not directly correlate with the levels of epithelial Na+ channel (ENaC) hyperactivity or airway epithelial cell necrotic degeneration. A: survival curves for congenic Scnn1b-Tg mice and wild-type (WT) littermates. n, Number of mice/group. B: ion transport properties of freshly excised tracheal tissues of 3-day-old Scnn1b-Tg and WT mice. “Basal” indicates the short circuit current (Isc) before drug application. The changes in Isc (Δ) after sequential addition of drugs are shown. “Residual” Isc is the Isc remaining after amiloride (amil) application. forsk, Forskolin; bum, bumetanide. *P < 0.05 vs. WT littermates, #P < 0.05 vs. C57BL/6N of the same genotype. n = 10 WT and 16 Scnn1b-Tg for C57BL/6N; 6 WT and 8 Scnn1b-Tg for C3H/HeN; 8 WT and 8 Scnn1b-Tg for BALB/cJ; 16 WT and 10 Scnn1b-Tg for FVB/NJ. C: correlation between the fold increase in amiloride-sensitive current in Scnn1b-Tg compared with WT littermates and survival at 10 days (from Fig. 1A). n = same as in B. D: morphometric quantification of necrotic cells in 5-day-old mice. b.m., Basal membrane; n, number of mice/group. *P < 0.05 vs. WT littermates, #P < 0.05 vs. C57BL/6N of the same genotype.
Scnn1b-tg mice of all strains exhibit ENaC hyperactivity and spotty airway epithelial necrotic degeneration during the neonatal period.
To test whether variations in Scnn1b function could account for differences in survival among strains, we measured the bioelectric properties of freshly excised tracheal epithelia from 3-day-old mice in Ussing chambers. Basal short circuit current (Isc) and amiloride-sensitive Isc were significantly greater in all Scnn1b-Tg mice compared with WT littermates (Fig. 1B, Scnn1b-Tg), indicating that the β-ENaC transgene was functional in all the strains tested and consistent with increased ENaC activity in Scnn1b-Tg mice as previously reported (27). We observed strain-specific differences in endogenous ENaC activity as indexed by amiloride-sensitive Isc (Fig. 1B, WT mice), but no differences among strains overexpressing Scnn1b (Fig. 1B, Scnn1b-Tg). We also compared the ratio of transgenic to WT amiloride-sensitive Isc. C57BL/6N Scnn1b-Tg mice had an approximately threefold increase in amiloride-sensitive current compared with their WT littermates, whereas the low-surviving BALB/cJ and FVB/NJ strains had about fivefold increase. The fold increase in amiloride-sensitive current (Δamil) was inversely correlated with survival (r2 = 0.75), but the correlation was not significant (two-tailed p value = 0.13, Fig. 1C). There was no correlation between survival and basal Isc or between survival and Δbasal Isc (Scnn1b-Tg basal minus WT basal, not shown).
Scnn1b overexpression in murine airways results in necrotic degeneration of Clara cells during the neonatal period (29). Because occurrence of necrotic cells coincides with early postnatal death and the presence of necrotic cells could be related to survival, the incidence of necrotic airway epithelial cells was measured in congenic strains. The incidence of this lesion for each strain was very similar to the original mixed strain. We observed a significantly increased number of necrotic cells in the airways of the low-surviving strain BALB/cJ compared with C57BL/6N, but not in other low-surviving strains (Fig. 1D).
Tracheal mucus obstruction contributes to the mortality of low surviving Scnn1b-tg strains.
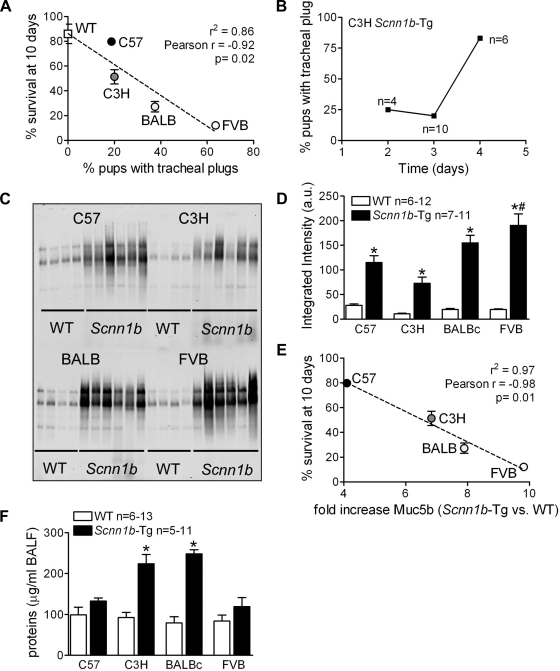
Our previous data indicated that the mortality of neonatal mixed background Scnn1b-Tg mice was strongly associated with the presence of tracheal mucus plugs, which were never observed in WT mice. A higher percentage of pups with tracheal mucus plugs at PND 3 correlated with worse survival outcome at PND 10 (Fig. 2A, r2 = 0.86), and the correlation was significant (P = 0.02). In the C3H/HeN strain, the formation of mucus plugs was time dependent and increased significantly at PND 4 (Fig. 2B), indicating the dynamics of mucus plug formation. Collectively, the results suggest that the extent of asphyxiating mucus plug formation varies among strains, contributing to overall mortality.
Fig. 2.
Tracheal mucus obstruction and increased bronchoalveolar lavage (BAL) mucus content are features of congenic Scnn1b-Tg mouse strains. A: correlation between the percentage of 3-day-old Scnn1b-Tg pups with visible tracheal mucus plugs at dissection and survival. n = same as in Fig. 1B. B: progressive incidence of tracheal mucus plugging in C3H/HeN mice. n = number of mice/time point. Control WT mice (n = 6 or 7 per time point) showed no evidence of tracheal mucus plugging (not shown). C: Agarose gel Western blots. D: corresponding densitometry of unfractionated BAL samples from 5-day-old Scnn1b-Tg mice and WT littermates from the various strains. Membranes were probed with a murine Muc5b antibody. n, Number of mice/group; a.u., arbitrary units. E: correlation between the fold increase in Muc5b BAL content in Scnn1b-Tg compared with WT littermates and survival at 10 days (from Fig. 1A). n, same as D. F: total protein content of BAL fluid from the samples studied in Fig. 2. C–E: *P < 0.05 vs. WT littermates. n, number of mice/group. For all panels, *P < 0.05 vs. WT littermates, #P < 0.05 vs. C57BL/6N of the same genotype.
Agarose western blots of unfractionated BAL isolated from 5-day-old mice demonstrated higher levels of Muc5b in all Scnn1b-Tg mice compared with WT mice (Fig. 2, C and D). Across strains, a higher fold increase of BAL Muc5b content for Scnn1b-Tg vs. WT mice strongly correlated with worse survival (Fig. 2E, r2 = 0.97, P = 0.02). Total BAL protein content was not different in WT vs. Scnn1b-Tg of the C57BL/6N or FVB strains. However, it was higher in Scnn1b-Tg mice of the C3H/HeN and BALB/cJ strains (Fig. 2F), suggestive of vascular leak due to more severe lung damage. As described later, both C3H/HeN and BALB/cJ strains exhibited parenchymal hemorrhage.
Bronchial mucus obstruction is a feature of all Scnn1b-tg strains.
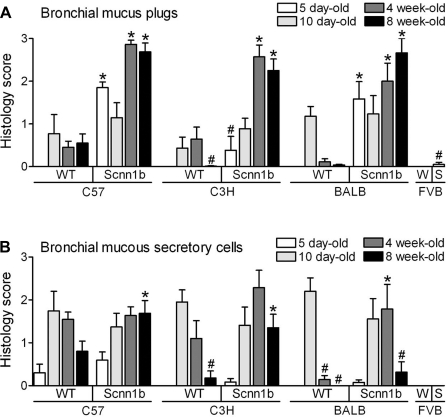
All Scnn1b-Tg strains developed progressive bronchial mucus obstruction, as assessed histologically, that was significantly different from WT littermates during (C57BL/6N and BALB/cJ) or immediately after (C3H/HeN) the neonatal period (Fig. 3A). FVB/NJ Scnn1b-Tg mice could not be evaluated for this trait after the early postnatal period due to very poor survival in this strain. The time dependence of mucous secretory cell (MuSC) abundance in neonatal WT mice (25) differed slightly among strains (Fig. 3B), but generally bronchial MuSCs peaked at PND 10 then waned. MuSCs persisted in the bronchi of Scnn1b-Tg mice of all strains well past PND 10, resulting in mucous cell metaplasia in adult Scnn1b-Tg mice. In contrast to tracheal mucus plug data illustrated in Fig. 2, the presence of bronchial mucus plugs (Fig. 3A) and bronchial MuSC (Fig. 3B) did not correlate with survival. In particular, C57BL/6N (high surviving) and BALB/cJ (low surviving) mice exhibited very similar bronchial mucus plugging. Mucus plugs were detected at later time points in C3H/HeN (low surviving) compared with C57BL/6N Scnn1b-Tg mice (Fig. 3A).
Fig. 3.
Congenic Scnn1b-Tg mouse strains develop bronchial mucus plugging and mucous secretory cell metaplasia. Semiquantitative histopathology scores for mucus plugs (A) and mucus secretory cells (B, AB-PAS positive) in the left lobe intrapulmonary main stem bronchus at different time points across congenic strains. 5 day-old mice: n = 5 WT and 10 Scnn1b-Tg for C57BL/6N; 7 WT and 6 Scnn1b-Tg for C3H/HeN; 8 WT and 8 Scnn1b-Tg for BALB/cJ; 14 WT (W) and 10 Scnn1b-Tg (S) for FVB/NJ. 10 day-old mice: n = 7 WT and 13 Scnn1b-Tg for C57BL/6N; 10 WT and 10 Scnn1b-Tg for C3H/HeN; 11 WT and 8 Scnn1b-Tg for BALB/cJ. 4 wk-old mice: n = 11 WT and 11 Scnn1b-Tg for C57BL/6N; 7 WT and 7 Scnn1b-Tg for C3H/HeN; 7 WT and 7 Scnn1b-Tg for BALB/cJ. 8 wk-old mice: n = 10 WT and 8 Scnn1b-Tg for C57BL/6N; 9 WT and 10 Scnn1b-Tg for C3H/HeN; 10 WT and 6 Scnn1b-Tg for BALB/cJ. *P < 0.05 vs. WT littermates, #P < 0.05 vs. C57BL/6N of the same genotype.
Airway inflammation and air-space enlargement are robust indexes of lung pathology in all Scnn1b-Tg strains.
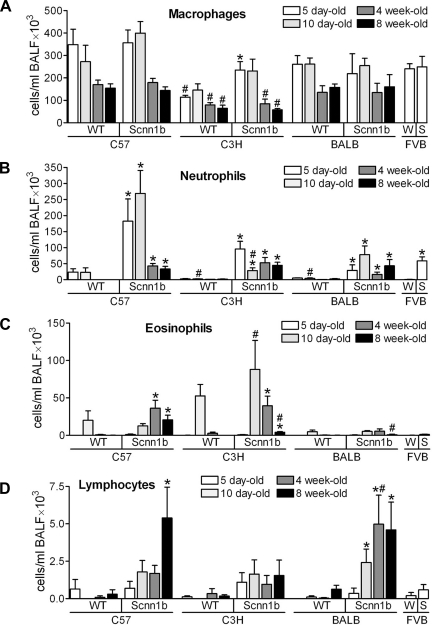
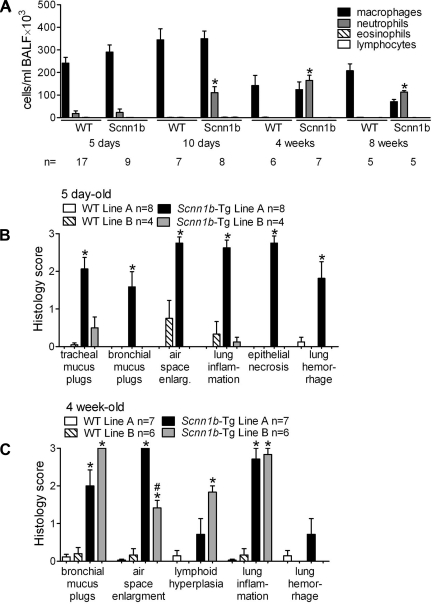
Longitudinal analyses of BAL inflammatory cells in Scnn1b-Tg and WT littermates indicated strain-specific quantitative differences, such as low BAL cellularity in the C3H/HeN strain and a constitutively lower number of eosinophils in BALB/cJ mice (Fig. 4). However, the overall composition and temporal pattern of inflammatory infiltrate in each congenic Scnn1b-Tg strain were qualitatively similar to that of the original mixed background strain, and survival did not correlate with any BAL parameter. In particular, Scnn1b-Tg macrophage number was significantly higher than WT littermates only in the C3H/HeN mice at 5 days and otherwise was similar across strains (Fig. 4A). Macrophages isolated from Scnn1b-Tg mice of all strains exhibited the morphologically activated phenotype originally described for the mixed strain (29) (not shown). All Scnn1b-Tg strains exhibited chronic, low-level neutrophilic infiltration that was quantitatively highest in neonatal C57BL/6N compared with other strains (Fig. 4B). Eosinophils were very rare in BAL from PND 5 mice but were found in WT mice of all strains at PND 10 (Fig. 4C), consistent with previously reported transient eosinophilia in neonatal inbred WT mice (25). In Scnn1b-Tg mice, eosinophilia persisted after PND 10 and, albeit quantitatively variable, was present in all strains tested. Finally, a lymphocytic infiltrate was detected in BAL starting at PND 10 and, at least for C57BL/6N and BALB/cJ strains, became more significant as the mice aged (Fig. 4D).
Fig. 4.
Congenic Scnn1b-Tg mouse strains develop varying degrees of airway inflammation. Differential BAL cell counts at different time points. A: macrophages; B: neutrophils; C: eosinophils; D: lymphocytes. 5 day-old mice: n = 6 WT and 8 Scnn1b-Tg for C57BL/6N; 16 WT and 13 Scnn1b-Tg for C3H/HeN; 14 WT and 10 Scnn1b-Tg for BALB/cJ; 12 WT (W) and 9 Scnn1b-Tg (S) for FVB/NJ. 10 day-old mice: n = 7 WT and 11 Scnn1b-Tg for C57BL/6N; 8 WT and 9 Scnn1b-Tg for C3H/HeN; 14 WT and 10 Scnn1b-Tg for BALB/cJ. 4 wk-old mice: n = 12 WT and 13 Scnn1b-Tg for C57BL/6N; 5 WT and 7 Scnn1b-Tg for C3H/HeN; 7 WT and 5 Scnn1b-Tg for BALB/cJ. 8 wk-old mice: n = 10 WT and 10 Scnn1b-Tg for C57BL/6N; 8 WT and 11 Scnn1b-Tg for C3H/HeN; 8 WT and 6 Scnn1b-Tg for BALB/cJ. *P < 0.05 vs. WT littermates, #P < 0.05 vs. C57BL/6N of the same genotype.
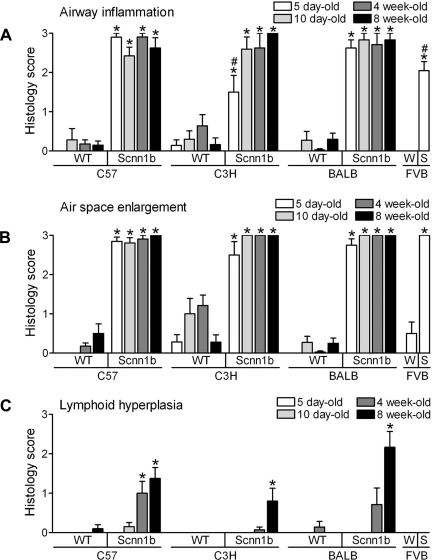
Histologically, Scnn1b-Tg mice of all strains exhibited airway inflammation (Fig. 5A) and air-space enlargement (Fig. 5B) when evaluated as previously described (25). All strains tested exhibited a near maximum phenotype when scored semiquantitatively. Lymphoid hyperplasia, or development of BALT, was also a common finding in 8 wk old Scnn1b-Tg mice of all strains (Fig. 5C).
Fig. 5.
Inflammatory lung disease and air-space enlargement is a feature of all congenic Scnn1b-Tg mouse strains. Semiquantitative histopathology score for airway inflammation (A), air-space enlargement (B), and lymphoid hyperplasia (C). n = same as Fig. 3. *P < 0.05 vs. WT littermates, #P < 0.05 vs. C57BL/6N of the same genotype.
New phenotypes, parenchymal hemorrhage and atelectasis, emerged in low-surviving congenic strains.
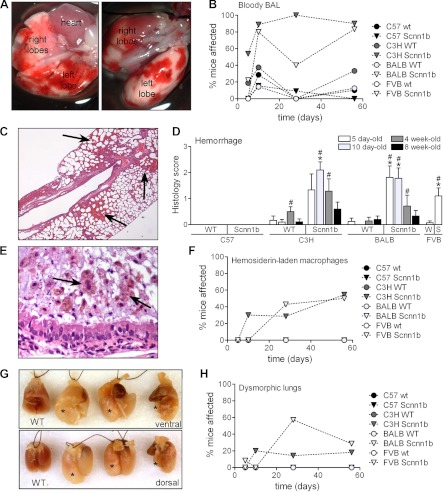
Two previously unobserved phenotypic consequences of Scnn1b-Tg expression were observed in the low-survival C3H/HeN and BALB/cJ strains. First, Scnn1b-Tg mice from these strains showed evidence of pulmonary hemorrhage as indicated by bloody lungs at dissection (Fig. 6A) and bloody BAL samples (Fig. 6B). Histopathological examination revealed regions of active hemorrhage (Fig. 6, C and D) and hemosiderin-laden macrophages (Fig. 6, E and F), suggesting chronicity of this lesion. C3H/HeN and BALB/cJ Scnn1b-Tg mice more frequently developed gross abnormalities, i.e., atelectasis or wrinkled lobes, which we collectively termed dysmorphic lungs (Fig. 6, G and H).
Fig. 6.
Pulmonary hemorrhage, pulmonary hemosiderosis, and dysmorphic lungs in congenic Scnn1b-Tg mouse strains. A: representative images of patchy hemorrhage observed at dissection in 10-day-old C3H/HeN Scnn1b-Tg mice, ventral and dorsal side, respectively. B: incidence of bloody BAL in congenic WT and Scnn1b-Tg strains (n = same as Fig. 4). C: representative photomicrograph of lung histology [hematoxylin and eosin (H&E) stain] in 10-day-old C3H/HeN Scnn1b-Tg mice. Arrows, areas of parenchymal hemorrhage. D: semiquantitative histopathology score for pulmonary hemorrhage. n, Same as Fig. 3. *P < 0.05 vs. WT littermates, #P < 0.05 vs. C57BL/6N of the same genotype. E: representative photomicrograph of infiltrating inflammatory cells encased in a mucus plug in the airways of a 10-day-old C3H/HeN Scnn1b-Tg mouse H&E stain. Hemosiderin-laden macrophages exhibiting typical brown granular inclusions are present (arrows). F: incidence of hemosiderosis in Scnn1b-Tg mice and WT littermates at different time points. n, Same as Fig. 3. G: representative image of dysmorphic lungs in 3 adult BALB/cJ Scnn1b-Tg mice compared with WT age-matched control (WT). *Overdistended (left and center) or atelectatic (right) lobes. H: incidence of dysmorphic lungs in Scnn1b-Tg mice and WT littermates at different time points. n, Same as Fig. 3.
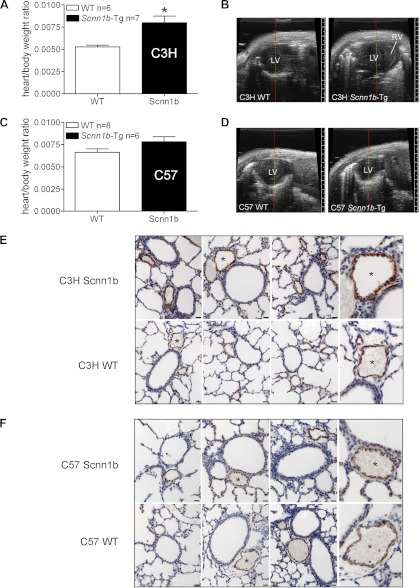
The presence of hemorrhage suggested pulmonary hypertension and/or disruption of the capillary/endothelial architecture in these strains. Ten-day-old C3H/HeN Scnn1b-Tg mice, which had the highest prevalence of pulmonary hemorrhage, exhibited an increased heart/body weight ratio compared with WT littermates (P = 0.0064) (Fig. 7A) and a high incidence of right ventricular enlargement (7/8), as assessed by two-dimensional echocardiographic scan (Fig. 7B). However, no differences in the ratio of right ventricle/(left ventricle + septum) weights were detected (WT 0.3655 ± 0.03269 n = 20, Scnn1b-Tg 0.3690 ± 0.02378 n = 21). These phenotypes were not observed in the C57BL/6N strain that did not exhibit pulmonary hemorrhage (Fig. 7, C and D).
Fig. 7.
C3H/HeN Scnn1b-Tg mice exhibit signs of pulmonary hypertension. A: comparison between heart/body weight ratio in 10-day-old C3H/HeN Scnn1b-Tg mice and WT littermates. n, Number of mice/group. *P = 0.0064 vs. WT littermates. B: representative echocardiographic images of heart in nonsedated C3H/HeN Scnn1b-Tg and WT littermates. LV, left ventricle; RV, right ventricle. The RV is clearly visible and enlarged in C3H/HeN Scnn1b-Tg mice compared with their WT littermates. C and D, as in A and B except that the mice are from the C57BL/6N strain. The RV in this strain is not prominent. E: representative images of immunohistochemical detection of α-smooth muscle actin in small pulmonary arteries from inflation-fixed lungs of 10-day-old C3H/HeN Scnn1b-Tg mice (top) and WT littermates (bottom). Scale bar, 20 μm. Panel at far right shows higher power magnification of selected arteries (*) to provide greater detail. F: as in E except mice are from the C57BL/6N strain.
α-Smooth muscle actin staining was visually more pronounced in small pulmonary arteries (∼100 μm diameter) of 10-day-old C3H/HeN Scnn1b-Tg mice compared with either WT littermates (Fig. 7E) or C57BL/6N Scnn1b-Tg mice (Fig. 7F). Vascular smooth muscle layer thickening suggests that these mice were experiencing early development of pulmonary hypertension.
Spontaneous occurrence of new Scnn1b-Tg mice phenotypic variants in low-surviving strains.
In mouse strains exhibiting extremely high mortality, there is selective pressure for identification of mutations and/or epigenetic changes that improve survival. During backcrossing, a distinctly different substrain of BALB/cJ Scnn1b-Tg mice (named “Line B” to differentiate it from the original congenic BALB/cJ Scnn1b-Tg strain, “Line A”) was spontaneously generated. BALB/cJ Line B Scnn1b-Tg mice originated from an N9 founder male, whose progeny all had high survival at PND 20 (89 ± 4% and 79 ± 5% for Scnn1b-Tg mice, n = 55 and WT littermates, n = 58, respectively, not shown; compare with BALB/cJ Line A, Fig. 1A).
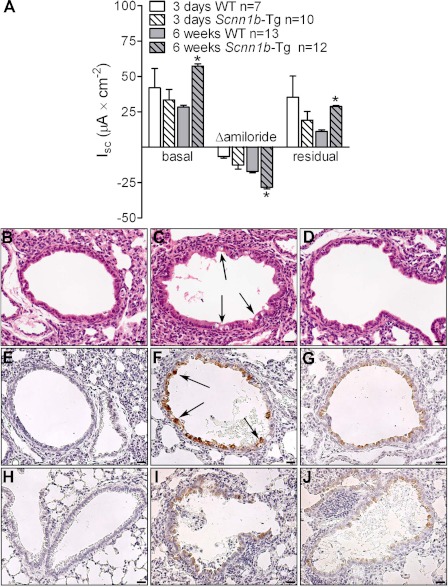
Southern blot of genomic DNA did not detect significant differences in transgene copy number among BALB/cJ Line A, Line B, or C57BL/6N Scnn1b-Tg mice, suggesting that a large rearrangement in the transgene insertion site was not the cause of the phenotypic variation (all strains showed an approximately twofold increase in transgene-specific Southern blot signal compared with the WT fragment when the Scnn1b cDNA was used as probe). Nonetheless, there was a noticeable difference in Scnn1b transgene function between BALB/cJ Line A and Line B Scnn1b-Tg mice, mainly delayed expression of ENaC hyperactivity (Fig. 8A). In contrast to Line A (Fig. 1B), basal, amiloride-sensitive, and residual Isc in Scnn1b-Tg Line B mice were not different from WT littermates in 3-day-old pups. However, evidence for ENaC hyperactivity was present in Line B by 6 wk of age (Fig. 8A). Notably, absence of ENaC hyperactivity in neonatal BALB/cJ Line B Scnn1b-Tg mice correlated with absence of airway epithelial necrosis at PND 3–5 (Fig. 8, B–D).
Fig. 8.
BALB/cJ Scnn1b-Tg Line B mice exhibited delayed onset of ENaC hyperactivity. A: ion transport properties of freshly excised tracheal tissues of 3-day-old and 6-wk-old BALB/cJ Line B Scnn1b-Tg and WT mice. Differences in airway bioelectric properties between WT and Scnn1b-Tg mice were not observed at early ages. Representative photomicrographs of lung histology in 5-day-old BALB/cJ Line B WT (B), BALB/cJ Line A Scnn1b-Tg (C), and BALB/cJ Line B Scnn1b-Tg mice (D), illustrating the presence of airway epithelial cell necrosis in BALB/cJ Line A Scnn1b-Tg mice (C, arrows) and its absence in BALB/cJ Line B Scnn1b-Tg mice. H&E stain. Scale bar = 20 μm. E–J: representative images of longitudinal analysis of Scnn1b overexpression in lung sections from BALB/cJ Scnn1b-Tg and WT mice, Line A and Line B. E: 5-day-old BALB/cJ Line A WT; F: 5-day-old BALB/cJ Line A Scnn1b-Tg; G: 5-day-old BALB/cJ Line B Scnn1b-Tg; H: 4-wk-old BALB/cJ Line A WT; I: 4-wk-old BALB/cJ Line A Scnn1b-Tg; J: 4-wk-old BALB/cJ Line B Scnn1b-Tg. Immunoreactivity for native Scnn1b was undetectable in WT mice (E, H). Arrows in F show cells with intense Scnn1b staining relative to all other panels. Scale bar = 20 μm.
Immunohistochemical detection of Scnn1b in lungs from neonatal (PND 5, Fig. 8, E–G) and adult (PND 28, Fig. 8, H–J) mice revealed that Scnn1b was overexpressed in both Line A and Line B compared with WT littermates at all ages tested. However, the expression pattern between Line A and Line B differed in neonatal mice, as Line A exhibited spotty, high-expressing epithelial cells (Fig. 8F), reminiscent of the pattern observed for necrotic cells (Fig. 8C), whereas in Line B Scnn1b expression was lower but more uniform (Fig. 8G). In adult Scnn1b-Tg mice, Scnn1b immunoreactivity was not qualitatively different between Line A and Line B (Fig. 8, I and J).
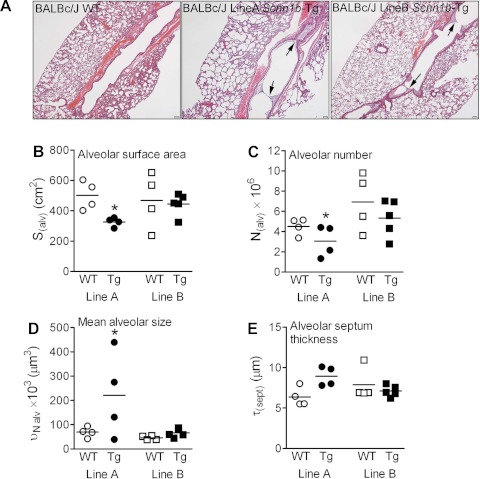
Importantly, delayed expression of ENaC hyperactivity in BALB/cJ Line B Scnn1b-Tg mice correlated with delayed onset of airway inflammation (Fig. 9A) and mucus obstruction (Fig. 9, B and C) compared with BALB/cJ Line A. By adulthood, most markers of lung pathology in BALB/cJ Line B Scnn1b-Tg mice were remarkably similar to those from Line A, except that air-space enlargement was significantly less (Fig. 9C and 10A). As assessed by unbiased stereology, BALB/cJ Line A Scnn1b-Tg mice exhibited decreased alveolar surface area (Fig. 10B), reduced alveolar number (Fig. 10C; p = 0.033 and p = 0.027, respectively), and increased mean alveolar size (Fig. 10D, p = 0.008) compared with WT littermates. Line A Scnn1b-Tg mice also showed a trend toward increased septal thickness (Fig. 10E, p = 0.080). Of note, none of these parameters differed between BALB/cJ Line B Scnn1b-Tg mice and WT littermates, indicating absence of emphysema-like lesions.
Fig. 9.
BALB/cJ Scnn1b-Tg Line B mice exhibited delayed development of airway inflammation and mucus obstruction, absence of neonatal epithelial necrosis, and reduced air-space enlargement. A: differential BAL cell counts for BALB/cJ Line B Scnn1b-Tg and WT mice at different time points. n, Number of mice/group. * < 0.05 vs. WT littermates. Semiquantitative histopathology scores for the pathologic changes characterizing neonatal [5 day old (B)] and adult [4 wk old (C)] Scnn1b-Tg mice in BALB/cJ Line A and Line B.
Fig. 10.
Absence of emphysematous lesions in BALB/cJ Scnn1b-Tg Line B mice. A: representative photomicrographs of lung histology in 8-wk-old BALB/cJ Line B WT (left), BALB/cJ Line A Scnn1b-Tg (center), and BALB/cJ Line B Scnn1b-Tg mice (right), illustrating the absence of air-space enlargement in BALB/cJ Line B Scnn1b-Tg mice. H&E stain. Scale bar = 200 μm. B–E: structural parameters for lung emphysema as determined by stereology. B: alveolar surface area; C: alveolar number; D: alveolar size; E: alveolar septum thickness. *P < 0.05 vs. WT littermates.
DISCUSSION
The presence of key pathologic features in all strains of congenic Scnn1b-Tg mice, e.g., airway mucus obstruction and inflammation, highlights the importance of proper airway surface hydration for efficient mucus clearance and lung health (33). In Scnn1b-Tg mice, overexpression of the Scnn1b transgene led to both an elevation in the basal rate of Na+ transport (Fig. 1B) and a failure to regulate Na+ transport rates in response to airway surface hydration status (28). The observation that Scnn1b-Tg mice have intact endogenous Cl− channels (normal response to forskolin and UTP, Fig. 2B) supports the notion that ENaC regulation is critical for maintaining the balance of ion and fluid absorption and secretion required for airway surface homeostasis. Indeed, our studies with the different Scnn1b-Tg congenic strains and their spontaneous variants, e.g., BALB/cJ Line A and Line B, indicate that whenever ENaC is functionally hyperactive in the airways, mucus obstruction and lung disease result. However, this common phenotype was subject to quantitative and qualitative variations within the same environment, suggesting a role for genetic factors.
Survival of the Scnn1b-Tg lines was clearly affected by genetic background and correlated most strongly with the presence of asphyxiating early postnatal tracheal mucus plugs (Fig. 2A) and increased levels of Muc5b in BAL (Fig. 2E). Lethal mucus plugs are likely produced when mucus concentrations exceed critical values (Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. Personal communication). Epithelial Na+ hyperabsorption in Scnn1b-Tg mice concentrates mucus by depleting the airway surface liquid, and concentrated mucus adheres to airway surfaces and accumulates within the lung. Mucus concentration, however, is also determined by rates of mucin production and secretion. In this context, it is interesting to note that FVB/NJ mice, which are profoundly mucus obstructed in the presence of the β-ENaC transgene, express more Muc5ac in response to the toxicant acrolein compared with C57BL/6 mice (5) and exhibit increased mucus cell hyper-/metaplasia after ovalbumin challenge (W. K. O'Neal, unpublished observation). Thus, variation in genes that control mucin synthesis and secretion likely interact with a relatively constant Scnn1b-dependent increase in Na+ transport rates (Fig. 1B) to produce strain-dependent variations in mucus obstruction and survival. In addition, genes that control airway size might also contribute to differences in survival in the presence of airways mucus obstruction. Indeed, micro-CT imaging described larger-caliber airways in C57BL/6J mice than BALB/c mice, especially the main stem bronchi (44). With the use of recombinant inbred strategies, the survival differences between C57BL/6N and FVB/NJ Scnn1b-Tg mice will permit future mapping of key genes involved in the relative contributions of mucus secretion rates vs. airway caliber to the Scnn1b-Tg mouse phenotype.
The high mortality in some strains (such as BALB/cJ and C3H/HeN) might also relate to additional phenotypes that develop concurrently with the tracheal mucus plugging, such as pulmonary hemorrhage and atelectasis (Fig. 6) not previously associated with Scnn1b overexpression. Higher BAL protein in PND 5 C3H/HeN and BALB/cJ Scnn1b-Tg mice (Fig. 2E) suggested unique susceptibilities to early-onset lung damage, further supported by the presence of neonatal pulmonary hemorrhage (Fig. 6G) and hemosiderin-laden macrophages in surviving mice (Fig. 6H). Of note, regardless of the presence of these additional lesions, we did not observe significant mortality in any Scnn1b-Tg mouse strain in the postweaning period, suggesting that the early neonatal period represents a window of susceptibility to genetic and environmental factors that relate defects in airway mucus clearance to survival.
The high incidence of pulmonary hemorrhage in neonatal mice of susceptible strains was unexpected, since it was not observed in the original C3H:C57 Scnn1b-Tg mouse, and pulmonary hemorrhage is not a common phenotype in mice or in early-stage lung disease in humans. A number of studies were performed to elucidate the pathogenesis of pulmonary hemorrhage in the C3H/HeN Scnn1b-Tg strain. Because pulmonary hemorrhage is associated with severe pulmonary hypertension in humans (8), we tested for its surrogate markers. The higher heart/body mass ratio, enlarged right ventricle, and increased small pulmonary artery α smooth muscle actin in C3H/HeN vs. C57BL/6N Scnn1b-Tg mice (Fig. 7, A–C), suggest pulmonary hypertension as a cause for pulmonary hemorrhage. However, the right ventricle-to-left ventricle plus septal weight ratio, a known marker of pulmonary hypertension, was not different from WT littermates when measured at PND 10. The short survival period may not have allowed for the development of significantly altered cardiac structures characteristic of chronic pulmonary hypertension, and poor survival also made it difficult to assess any progression of cardiac changes in the C3H/HeN strain.
Pulmonary hemorrhage is also associated with abnormalities in pulmonary alveolar and vessel morphogenesis, and it has been observed in Foxf1-deficient mice (19), in moth-eaten mice (35), and upon airway-specific overexpression of the epithelial growth factor receptor ligands betacellulin and transforming growth factor-α, which disrupt capillary bed formation (22, 38), or the angiogenic growth factor VEGF (23). Mouse postnatal alveolar septation is genetically regulated, and C3H/HeJ mice have larger alveoli than C57BL/6 mice (40), potentially causing parenchymal fragility in the C3H/HeJ strain. Our observation that variant BALB/cJ Line B Scnn1b-Tg mice had delayed onset lung inflammation, no air-space enlargement and no hemorrhage suggests that interactions between inflammation and development of alveolar architecture may contribute to early onset pulmonary hemorrhage.
Transmural pressure changes may also contribute to the pulmonary hemorrhage phenotype. In humans, pulmonary hemorrhage and edema triggered by large, transient, negative intrathoracic pressures are a rare complication of acute upper airway obstruction (39). We speculate that local breaches in Scnn1b-Tg mouse vessels could have occurred due to large negative intrathoracic pressure swings resulting from the more severe tracheal mucus obstruction found in the lines with pulmonary hemorrhage, i.e., low-surviving BALB/cJ and FVB/NJ strains (Fig. 2A). Understanding the genes that are involved in the susceptibility to pulmonary hemorrhage in these mouse models is expected to provide insights into the development of pulmonary hemorrhage in humans.
Lobar collapse (atelectasis) was more common in low-surviving BALB/cJ and C3H/HeN strains. In humans, and especially in CF, atelectasis may result from mucus blockage of bronchi or bronchioles. Airway blockage is likely the etiology in Scnn1b-Tg mice exhibiting atelectasis. However, C3H:C57 and C57BL/6N Scnn1b-Tg mice also exhibit significant mucus plugging, but not atelectasis. This apparent discrepancy suggests that the magnitude of mucus dehydration and adhesion must be viewed in the context of bronchial tree structure. As mentioned above, the larger caliber of C57BL/6 airways (44) would decrease the tendency for obstruction. C57BL/6 mice are also resistant to atelectasis due to acute lung injury induced by nickel sulfate aerosol, which activates macrophages, inhibits surfactant, and is toxic to epithelial cells (24). Finally, hemorrhage and atelectasis are rarely noted in the resistant C3H:C57 and C57BL/6N strains but simultaneously occur in sensitive strains, suggesting related underlying mechanisms.
Congenic Scnn1b-Tg strains uncovered the range of adverse phenotypic responses to airway surface dehydration likely regulated by genetic background. Backcrossing of the low surviving strains also enabled detection of genetic responses producing a survival advantage. In particular, a spontaneous BALB/cJ variant (Line B) provided insights regarding factors contributing to the development of lung pathology. BALB/cJ Line B Scnn1b-Tg mice exhibited delayed onset of ENaC hyperactivity, which correlated with high survival, absence of epithelial necrosis at PND 3–5, delayed development of airway inflammation and mucus obstruction, and reduced air-space enlargement (Figs. 8–10). The cause of delayed ENaC hyperactivity is unclear. It was not associated with lower Scnn1b transgene copy number but may be due to subtle rearrangements in the insertion site, which we have shown by in situ hybridization to map to mouse chromosome 5 near the B-C1 chromosome band junction, epigenetic modifications, or changes in genes close to the transgene insertion site that could modulate transgene expression and/or function. Regardless, the BALB/cJ Line B phenotype suggests that transient neonatal epithelial cell necrosis is not essential to drive lung disease development, pointing to mucus stasis as the primary trigger of chronic inflammation. Additionally, despite persistent neutrophilic inflammation and mucus plugging, adult Line B mice did not exhibit the air-space enlargement (Fig. 10) seen in all other strains. This finding dissociates chronic bronchitis from the alveolar enlargement phenotype in Scnn1b-Tg mice and indicates that neonatal obstruction and inflammation is essential to the development of emphysema-like lesions in this mouse model.
Variable lung disease in Scnn1b-Tg mice with different genetic backgrounds highlights both the robustness of the primary disease-causing mechanism, i.e., increased ENaC activity leading to impaired mucus clearance, and the role of genetic factors to alter the downstream consequences. This scenario is similar to the highly penetrant genetic disease CF in the human population that has a variable pulmonary clinical course despite presence of identical homozygous ΔF508 mutations, due to both genetic modifiers (11) and environmental factors (10). Collectively, our data indicate that Scnn1b-Tg mice will serve as a resource for identifying genetic and/or environmental factors influencing the development of lung pathology in the setting of defective mucus clearance. These mice will be useful for confirming and/or validating genetic modifiers identified in genome-wide association studies of multiple human lung diseases. Moreover, they could be used to sensitize mice from large genetic collections, such as the Collaborative Cross (45), to develop obstructive lung disease and subsequently to identify modifiers linked to specific phenotypes.
GRANTS
Funded by Cystic Fibrosis Foundation (CFF) Grant LIVRAG04I0 (to A. Livraghi-Butrico); National Institute of Health (NIH) P30 DK-065988, NIH subcontract on P40 RR-016049-09S1, CFF Grants R026-CR02, ONEAL07G0, and MALL03G0 (to W. K. O'Neal); NIH Grants P50 HL-060280, P01 HL-034322, P30 DK-065988, and P50 HL-084934, CFF Grant R026-CR11 (to R. C. Boucher), and CFF Grant RANDEL07P0 (to S. H. Randell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.L.-B., B.R.G., S.H.R., R.C.B., and W.K.O. conception and design of research; A.L.-B., B.R.G., E.J.K., K.J.W., and H.Y. performed experiments; A.L.-B., B.R.G., and H.Y. analyzed data; A.L.-B., B.R.G., M.G., S.H.R., R.C.B., and W.K.O. interpreted results of experiments; A.L.-B. prepared figures; A.L.-B. and W.K.O. drafted manuscript; A.L.-B., B.R.G., M.G., S.H.R., R.C.B., and W.K.O. edited and revised manuscript; A.L.-B., M.G., S.H.R., R.C.B., and W.K.O. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kimberly Burns, Donald Joyner, and Tracy Eldred for technical assistance with histology; Rodney Gilmore for assistance with mouse genotyping; Brian Brighton for assistance with immunohistochemistry and airway necrotic cell scoring; Troy Rogers for Ussing chamber studies; Christoph Wigge for technical assistance with lung sampling, embedding, and stereology; J. Hüsler for statistical analysis of stereological data; the UNC Michael Hooker Microscopy Facility; Dr. Mauricio Rojas and Jackie Kylander at the UNC Mouse Cardiovascular Models Core Laboratory for performing echocardiography on neonatal mice and interpreting the results; and Dr. Susan Smyth for advice on methods and data interpretation pertaining pulmonary hypertension phenotypes.
REFERENCES
- 1. Ackerman KG, Huang H, Grasemann H, Puma C, Singer JB, Hill AE, Lander E, Nadeau JH, Churchill GA, Drazen JM, Beier DR. Interacting genetic loci cause airway hyperresponsiveness. Physiol Genomics 21: 105–111, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Allen HD, Driscoll DJ, Shaddy RE, Feltes TF. Moss and Adams' Heart Disease in Infants, Children, and Adolescents. Wolters Kluver-Lippincott Williams & Wilkins, 2008 [Google Scholar]
- 3. Belvisi MG, Bolser DC. Summary: animal models for cough. Pulm Pharmacol Ther 15: 249–250, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bewick V, Cheek L, Ball J. Statistics review 10: further nonparametric methods. Crit Care 8: 196–199, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borchers MT, Wesselkamper S, Wert SE, Shapiro SD, Leikauf GD. Monocyte inflammation augments acrolein-induced Muc5ac expression in mouse lung. Am J Physiol Lung Cell Mol Physiol 277: L489–L497, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med 160: 1150–1156, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Cannon ML, Bauman LA. Pulmonary hemosiderosis in scimitar syndrome after prolonged management of pulmonary hypertension. Pediatr Crit Care Med 2: 274–279, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat 197: 361–372, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR. Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function. J Pediatr 157: 802–807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann NY Acad Sci 1214: 57–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ganguly K, Stoeger T, Wesselkamper SC, Reinhard C, Sartor MA, Medvedovic M, Tomlinson CR, Bolle I, Mason JM, Leikauf GD, Schulz H. Candidate genes controlling pulmonary function in mice: transcript profiling and predicted protein structure. Physiol Genomics 31: 410–421, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Geiser M, Cruz-Orive LM, Im Hof V, Gehr P. Assessment of particle retention and clearance in the intrapulmonary conducting airways of hamster lungs with the fractionator. J Microsc 160: 75–88, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Grubb BR. Bioelectric measurement of CFTR function in mice. Methods Mol Med 70: 525–535, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Guo X, Pace RG, Stonebraker JR, Commander CW, Dang AT, Drumm ML, Harris A, Zou F, Swallow DM, Wright FA, O'Neal WK, Knowles MR. Mucin variable number tandem repeat polymorphisms and severity of cystic fibrosis lung disease: significant association with MUC5AC. PLoS One 6: e25452, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Hollingsworth JW, Whitehead G, Berman KG, Tekippe EM, Gilmour MI, Larkin JE, Quackenbush J, Schwartz DA. Genetic basis of murine antibacterial defense to streptococcal lung infection. Immunogenetics 59: 713–724, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Howard CV, Reed MG. Unbiased stereology. Three-dimensional Measurement in Microscopy. Oxford: Bios, 2005 [Google Scholar]
- 19. Kalinichenko VV, Lim L, Stolz DB, Shin B, Rausa FM, Clark J, Whitsett JA, Watkins SC, Costa RH. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the Forkhead Box f1 transcription factor. Dev Biol 235: 489–506, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Kelada SN, Wilson MS, Tavarez U, Kubalanza K, Borate B, Whitehead GS, Maruoka S, Roy MG, Olive M, Carpenter DE, Brass DM, Wynn TA, Cook DN, Evans CM, Schwartz DA, Collins FS. Strain-dependent genomic factors affect allergen-induced airway hyperresponsiveness in mice. Am J Respir Cell Mol Biol 45: 817–824, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleeberger SR, Levitt RC, Zhang LY, Longphre M, Harkema J, Jedlicka A, Eleff SM, DiSilvestre D, Holroyd KJ. Linkage analysis of susceptibility to ozone-induced lung inflammation in inbred mice. Nat Genet 17: 475–478, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Le Cras TD, Hardie WD, Deutsch GH, Albertine KH, Ikegami M, Whitsett JA, Korfhagen TR. Transient induction of TGF-alpha disrupts lung morphogenesis, causing pulmonary disease in adulthood. Am J Physiol Lung Cell Mol Physiol 287: L718–L729, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Le Cras TD, Spitzmiller RE, Albertine KH, Greenberg JM, Whitsett JA, Akeson AL. VEGF causes pulmonary hemorrhage, hemosiderosis, and air space enlargement in neonatal mice. Am J Physiol Lung Cell Mol Physiol 287: L134–L142, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Leikauf GD, McDowell SA, Wesselkamper SC, Hardie WD, Leikauf JE, Korfhagen TR, Prows DR. Acute lung injury: functional genomics and genetic susceptibility. Chest 121: 70S–75S, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Livraghi A, Grubb BR, Hudson EJ, Wilkinson KJ, Sheehan JK, Mall MA, O'Neal WK, Boucher RC, Randell SH. Airway and lung pathology due to mucosal surface dehydration in β-epithelial Na+ channel-overexpressing mice: role of TNF-alpha and IL-4R-alpha signaling, influence of neonatal development, and limited efficacy of glucocorticoid treatment. J Immunol 182: 4357–4367, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O'Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucos Immunol [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Mall MA, Button B, Johannesson B, Zhou Z, Livraghi A, Caldwell RA, Schubert SC, Schultz C, O'Neal WK, Pradervand S, Hummler E, Rossier BC, Grubb BR, Boucher RC. Airway surface liquid volume regulation determines different airway phenotypes in liddle compared with betaENaC-overexpressing mice. J Biol Chem 285: 26945–26955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mall MA, Harkema JR, Trojanek JB, Treis D, Livraghi A, Schubert S, Zhou Z, Kreda SM, Tilley SL, Hudson EJ, O'Neal WK, Boucher RC. Development of chronic bronchitis and emphysema in beta-epithelial Na+ channel-overexpressing mice. Am J Respir Crit Care Med 177: 730–742, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ochs M. A brief update on lung stereology. J Microsc 222: 188–200, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Prows DR, Leikauf GD. Quantitative trait analysis of nickel-induced acute lung injury in mice. Am J Respir Cell Mol Biol 24: 740–746, 2001 [DOI] [PubMed] [Google Scholar]
- 32. Prows DR, Shertzer HG, Daly MJ, Sidman CL, Leikauf GD. Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat Genet 17: 471–474, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Randell SH, Boucher RC, University of North Carolina Virtual Lung Group Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 35: 20–28, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reinhard C, Meyer B, Fuchs H, Stoeger T, Eder G, Ruschendorf F, Heyder J, Nurnberg P, de Angelis MH, Schulz H. Genomewide linkage analysis identifies novel genetic Loci for lung function in mice. Am J Respir Crit Care Med 171: 880–888, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Rossi GA, Hunninghake GW, Kawanami O, Ferrans VJ, Hansen CT, Crystal RG. Motheaten mice–an animal model with an inherited form of interstitial lung disease. Am Rev Respir Dis 131: 150–158, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol 31: 69–77, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26: 57–60, 1970 [PubMed] [Google Scholar]
- 38. Schneider MR, Dahlhoff M, Herbach N, Renner-Mueller I, Dalke C, Puk O, Graw J, Wanke R, Wolf E. Betacellulin overexpression in transgenic mice causes disproportionate growth, pulmonary hemorrhage syndrome, and complex eye pathology. Endocrinology 146: 5237–5246, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Schwartz DR, Maroo A, Malhotra A, Kesselman H. Negative pressure pulmonary hemorrhage. Chest 115: 1194–1197, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Soutiere SE, Mitzner W. Comparison of postnatal lung growth and development between C3H/HeJ and C57BL/6J mice. J Appl Physiol 100: 1577–1583, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 281: L394–L402, 2001 [DOI] [PubMed] [Google Scholar]
- 42. Tankersley CG, Fitzgerald RS, Kleeberger SR. Differential control of ventilation among inbred strains of mice. Am J Physiol Regul Integr Comp Physiol 267: R1371–R1377, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Tankersley CG, Fitzgerald RS, Levitt RC, Mitzner WA, Ewart SL, Kleeberger SR. Genetic control of differential baseline breathing pattern. J Appl Physiol 82: 874–881, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Thiesse J, Namati E, Sieren JC, Smith AR, Reinhardt JM, Hoffman EA, McLennan G. Lung structure phenotype variation in inbred mouse strains revealed through in vivo micro-CT imaging. J Appl Physiol 109: 1960–1968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Threadgill DW, Miller DR, Churchill GA, de Villena FP. The collaborative cross: a recombinant inbred mouse population for the systems genetic era. ILAR J 52: 24–31, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Wesselkamper SC, Prows DR, Biswas P, Willeke K, Bingham E, Leikauf GD. Genetic susceptibility to irritant-induced acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol 279: L575–L582, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Whitehead GS, Burch LH, Berman KG, Piantadosi CA, Schwartz DA. Genetic basis of murine responses to hyperoxia-induced lung injury. Immunogenetics 58: 793–804, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. Am J Physiol Lung Cell Mol Physiol 285: L32–L42, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat 198: 207–221, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wills-Karp M, Ewart SL. The genetics of allergen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 156: S89–S96, 1997 [DOI] [PubMed] [Google Scholar]
- 51. Wright FA, Strug LJ, Doshi VK, Commander CW, Blackman SM, Sun L, Berthiaume Y, Cutler D, Cojocaru A, Collaco JM, Corey M, Dorfman R, Goddard K, Green D, Kent JW, Jr, Lange EM, Lee S, Li W, Luo J, Mayhew GM, Naughton KM, Pace RG, Pare P, Rommens J, Sandford A, Stonebraker JR, Sun W, Taylor C, Vanscoy LL, Zou F, Blangero J, Zielenski J, O'Neal WK, Drumm ML, Durie PR, Knowles MR, Cutting GR. Genome-wide association and linkage identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q132. Nat Genet 43: 539–546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]