Abstract
The placenta is responsible for the transport of nutrients, gasses and growth factors to the fetus, as well as the elimination of wastes. Thus, defects in placental development have important consequences for the fetus and mother, and are a major cause of embryonic lethality. The major cell type of the fetal portion of the placenta is the trophoblast. Primary mouse placental trophoblast cells are a useful tool for studying normal and abnormal placental development, and unlike cell lines, may be isolated and used to study trophoblast at specific stages of pregnancy. In addition, primary cultures of trophoblast from transgenic mice may be used to study the role of particular genes in placental cells. The protocol presented here is based on the description by Thordarson et al.1, in which a percoll gradient is used to obtain a relatively pure trophoblast cell population from isolated mouse placentas. It is similar to the more widely used methods for human trophoblast cell isolation2-3. Purity may be assessed by immunocytochemical staining of the isolated cells for cytokeratin 74. Here, the isolated cells are then analyzed using a matrigel invasion assay to assess trophoblast invasiveness in vitro5-6. The invaded cells are analyzed by immunocytochemistry and stained for counting.
Keywords: Developmental Biology, Issue 59, placenta, primary trophoblast cells, mouse, invasion assay, matrigel
Protocol
1. Dissection of the mouse placenta
Set up mating pairs of mice.
On each of the following days, check for the presence of a copulatory plug. The day in which a plug is detected is considered 0.5 days post-coitus (dpc)7.
Placentas may be collected at the desired stage of pregnancy, with a clean dissection of the placenta possible beginning with the development of the mature placenta on d 10.5. Here, we demonstrate 14.5 dpc. At the given volumes, 40-60 placentas on 10.5 dpc, and 10-20 placentas on 14.5 dpc will yield a clearly visible trophoblast cell layer.
Euthanize the pregnant mouse, and remove the uterus, cutting at the cervix, and along the mesometrium.
Using sharp scissors, cut between each conceptus (Figure 1A).
Using two pairs of forceps, grasp the uterine wall at the cut edge, and pull apart, removing the myometrium (Figure 1B). One pair serrated, moderately fine (curved or straight) forceps plus one pair very fine forceps (#5, for example) is recommended.
Opposite the placenta, open the decidua with forceps or scissors, exposing placenta. Grasping uterine tissue with one forceps pair, slide another forceps beneath the placenta to separate (Figure 1C). If necessary, separate fetus and fetal membranes from placenta. It is not possible to remove all decidual tissue by dissection without also losing placental tissue, but additional decidual tissue (pale) can be removed from the surface of the placenta by gently peeling with forceps. We will rely on the Percoll gradient for further purification.
Place each dissected placenta in 25 ml ice cold dissociation buffer in a 50 ml conical tube until all dissections are complete. At later stages, coarsely mince placental tissue with scissors or razor blade prior to transfer into dissociation buffer.
2. Isolation of trophoblast cells
Loosely cap conical tube and place in a 37°C bath for 45-60 minutes. Mix the dissociation solution vigorously by pipetting approximately every 10 min. The total digestion time should be monitored closely and will likely require some trial and error to achieve optimal cell recovery. Tissues should be able to be passed through a 10 ml pipet, but visible pieces of tissue will remain. Overdigestion will result in poor cell survival and plating efficiency. Optimal cell survival and plating efficiency will occur when clumps of a few cells are present, rather than when complete dissociation is achieved. Conversely, underdigestion will lead to reduced purity following percoll separation. When the proper digestion point has been reached, place tube on ice to stop collagenase activity.
Pass the solution through a cell strainer to remove undigested materials, and centrifuge @ 500 x g for 5 minutes.
To wash, remove supernatant and resuspend cells in 10 ml wash solution. Centrifuge @ 500 x g for 5 minutes.
During the centrifugation, prepare percoll solution by mixing 9.6 ml percoll, 13.4 ml wash solution and 1.1 ml 10x medium 199 in a 50 ml tube appropriate for high speed centrifugation.
Remove supernatant and resuspend cell pellet in 2 mL wash solution.
Add cells to percoll solution and centrifuge @ 30,000 x g for 30 minutes at 4°C.
Remove tube from centrifuge without disturbing the gradient. To visualize the gradient, place tube in front of a bright light source. Locate the trophoblast band (Figure 2), and aspirate with a pipet, taking care not to disturb the red blood cell band below. Volume of the trophoblast band will be 5-8 ml.
Transfer trophoblast cells to a 50 ml conical tube. Add wash solution to a total volume of 45ml.
Centrifuge @ 500 x g for 5 minutes. Remove supernatant, and resuspend in culture medium (NCTC-135). Cells can be cultured on thin-layer matrigel or tissue culture plastic at 37°C and 5% CO2. At day 10.5, each placenta yields roughly 50,000 live cells, of which 80-90% are cytokeratin positive.
3. Trophoblast invasion Assay
Add 1-2 ml culture medium (NCTC-135) to each matrigel chamber and incubate at 37°, 5% CO2 for 30-90 min.
Remove medium, and add 1.5 ml x 105 freshly isolated cells in 2ml medium to upper chamber. Add 1 ml medium to lower chamber to cover lower membrane surface.
Culture cells in inserts for 18-24 hours.
Scrape cells and matrigel from the insert using a foam swab.
Rinse the top and bottom of the chamber in 2 ml PBS.
Fix for 5 min in 1 ml 4% paraformaldehyde in PBS.
Stain by filling lower chamber with 400 μl DAPI (300nM) and incubate for 30 min.
Wash three times briefly in PBS.
Remove membranes from inserts with a sharp scalpel blade.
Add a 10 μl drop of mounting medium (50% glycerol in PBS) to slide. Using forceps, lay membrane on drop. Add another 10 μl drop of medium, then coverslip carefully.
Photograph and count cells. Be sure to orient the membrane appropriately (cell-side up or cell-side down) for the microscope being used. Image J software (National Institutes of Health) includes a manual counting feature that can be used for this purpose, as can Adobe Photoshop or a manual counter.
4. Representative Results
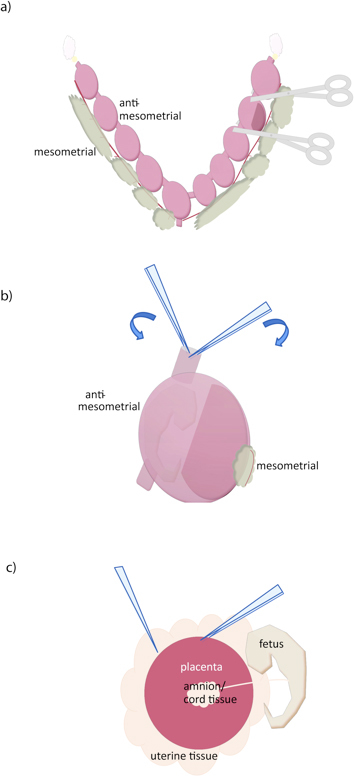 Figure 1. Dissection of the mouse placenta. (a) The uterus is removed by cutting along the oviducts (yellow), along the mesometrial surface, and at the cervix (bottom). Individual conceptuses can then be separated by cutting with scissors as shown. (b) The uterine wall is removed by placing two forceps in the cut uterine surface, and tearing along the anti-mesometrial side, near the fetus and away from the placenta. (c) The placenta is then isolated by grasping with a forceps and sliding another between the placenta and uterine tissue. Umbilical cord and other extraembryonic membranes can then be separated from the placenta.
Figure 1. Dissection of the mouse placenta. (a) The uterus is removed by cutting along the oviducts (yellow), along the mesometrial surface, and at the cervix (bottom). Individual conceptuses can then be separated by cutting with scissors as shown. (b) The uterine wall is removed by placing two forceps in the cut uterine surface, and tearing along the anti-mesometrial side, near the fetus and away from the placenta. (c) The placenta is then isolated by grasping with a forceps and sliding another between the placenta and uterine tissue. Umbilical cord and other extraembryonic membranes can then be separated from the placenta.
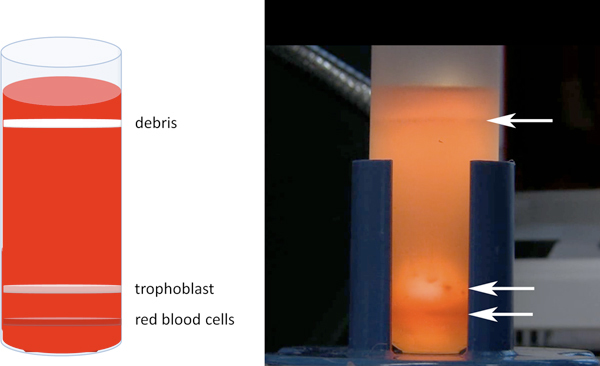 Figure 2. Percoll separation of trophoblast cells. After centrifugation, three major bands will be visible as shown in cartoon at left, and photograph at right. The upper band contains cellular debris and fibroblasts and will be most apparent. A tight (red) band near the bottom containing red blood cells will be visible just below the diffuse trophoblast band. Cell aggregates will likely seen in the trophoblast band, as in the two shown just to the left of the arrow in the photograph.
Figure 2. Percoll separation of trophoblast cells. After centrifugation, three major bands will be visible as shown in cartoon at left, and photograph at right. The upper band contains cellular debris and fibroblasts and will be most apparent. A tight (red) band near the bottom containing red blood cells will be visible just below the diffuse trophoblast band. Cell aggregates will likely seen in the trophoblast band, as in the two shown just to the left of the arrow in the photograph.
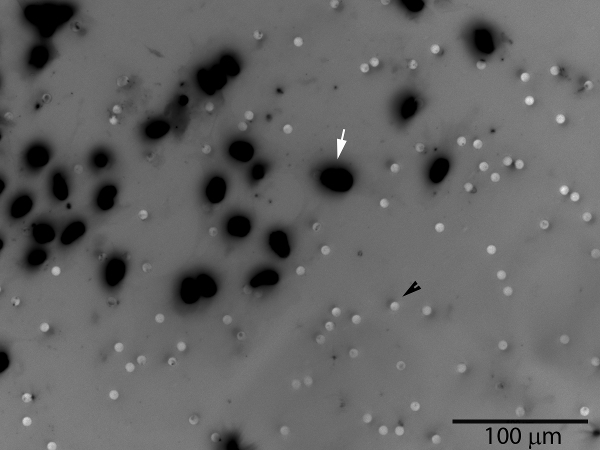 Figure 3. Invasive trophoblast cells (white arrow) will pass through the matrigel layer and the membrane pores, appearing on the bottom surface of the membrane. The pores are also visible (black arrowhead). The number of cells on the entire membrane surface or a representative sample area may be counted. A grayscale image of fluorescent DAPI nuclear staining has been inverted to improve visualization of cells.
Figure 3. Invasive trophoblast cells (white arrow) will pass through the matrigel layer and the membrane pores, appearing on the bottom surface of the membrane. The pores are also visible (black arrowhead). The number of cells on the entire membrane surface or a representative sample area may be counted. A grayscale image of fluorescent DAPI nuclear staining has been inverted to improve visualization of cells.
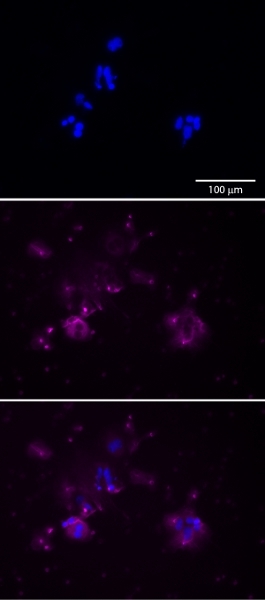 Figure 4.Trophoblast cells can be identified, and the purity of the isolated population assessed, by immunostaining for cytokeratin 7 (center,magenta). Cells are counterstained with DAPI (top,blue). Merged image, bottom.
Figure 4.Trophoblast cells can be identified, and the purity of the isolated population assessed, by immunostaining for cytokeratin 7 (center,magenta). Cells are counterstained with DAPI (top,blue). Merged image, bottom.
Discussion
In this video, we demonstrate the isolation of trophoblast cells from the mouse placenta. We then show an in vitro assay for trophoblast invasion. Using this procedure, a largely, but not entirely, pure population of trophoblast cells can be obtained. If further purity is required, magnetic bead separation or flow cytometry could be performed, as has been described for primary human trophoblast cells. The proper length of the collagenase dissociation step must be determined with each experiment, and will be learned with experience. Over-digestion will severely reduce cell recovery, whereas under-digestion may lead to reduced purity, as well as reduced numbers. Although we show the use of commercially prepared matrigel invasion chambers, they may also be prepared by adding matrigel to uncoated transwell chambers at the desired thickness.
Disclosures
We have nothing to disclose.
Acknowledgments
The authors wish to thank the Creative Services team at the University of Missouri Academic Support Center who produced this video. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health, grant number HD055231
References
- Thordarson G, Folger P, Talamantes F. Development of a placental cell culture system for studying the control of mouse placental lactogen II secretion. Placenta. 1987;8:573–585. doi: 10.1016/0143-4004(87)90028-2. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS. Isolation and culture of term human trophoblast cells. Methods. Mol. Med. 2006;121:203–217. doi: 10.1385/1-59259-983-4:201. [DOI] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Blaschitz A, Weiss U, Dohr G, Desoye G. Antibody reaction patterns in first trimester placenta: implications for trophoblast isolation and purity screening. Placenta. 2000;21:733–741. doi: 10.1053/plac.2000.0559. [DOI] [PubMed] [Google Scholar]
- Librach CL. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell. Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz LC, Widmaier EP. The effect of leptin on mouse trophoblast cell invasion. Biol. Reprod. 2004;71:1963–1967. doi: 10.1095/biolreprod.104.032722. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]


