Abstract
Context:
In nondiabetic pregnancy, cross-sectional studies have shown associations between maternal dyslipidemia and preeclampsia (PE). In type 1 diabetes mellitus (T1DM), the prevalence of PE is increased 4-fold, but prospective associations with plasma lipoproteins are unknown.
Objectives:
The aim of this study was to define lipoprotein-related markers and potential mechanisms for PE in T1DM.
Design and Settings:
We conducted a multicenter prospective study in T1DM pregnancy.
Patients:
We studied 118 T1DM women (26 developed PE, 92 remained normotensive). Subjects were studied at three visits before PE onset [12.2 ± 1.9, 21.6 ± 1.5, and 31.5 ± 1.7 wk gestation (means ± sd)] and at term (37.6 ± 2.0 wk). Nondiabetic normotensive pregnant women (n = 21) were included for reference.
Main Outcome Measures:
Conventional lipid profiles, lipoprotein subclasses [defined by size (nuclear magnetic resonance) and by apolipoprotein content], serum apolipoproteins (ApoAI, ApoB, and ApoCIII), and lipolysis (ApoCIII ratio) were measured in T1DM women with and without subsequent PE.
Results:
In women with vs. without subsequent PE, at the first and/or second study visits: low-density lipoprotein (LDL)-cholesterol, particle concentrations of total LDL and large (but not small) LDL, serum ApoB, and ApoB:ApoAI ratio were all increased (P < 0.05); peripheral lipoprotein lipolysis was decreased (P < 0.01). These early differences remained significant in covariate analysis (glycated hemoglobin, actual prandial status, gravidity, body mass index, and diabetes duration) but were not present at the third study visit. High-density lipoprotein and very low-density lipoprotein subclasses did not differ between groups before PE onset.
Conclusions:
Early in pregnancy, increased cholesterol-rich lipoproteins and an index suggesting decreased peripheral lipolysis were associated with subsequent PE in T1DM women. Background maternal lipoprotein characteristics, perhaps masked by effects of late pregnancy, may influence PE risk.
Preeclampsia (PE), a pregnancy-specific syndrome characterized by new-onset hypertension and proteinuria after 20 wk gestation, causes significant maternal and fetal morbidity and mortality (1). It affects 4–6% of pregnancies worldwide, but in the presence of maternal type 1 diabetes mellitus (T1DM) its prevalence is increased 4-fold (2, 3). PE has been associated with increased future risks of developing cardiovascular disease and diabetes in both mothers and offspring (4–6). Currently, there are no reliable predictors for PE and no effective preventive or treatment strategies (other than delivery). Better understanding of the disease mechanisms is urgently needed. For the prospective study of PE, the context of diabetic pregnancy brings two important advantages: a high case yield, and the potential to elucidate mechanisms not only for PE (in diabetes and in general), but also for the vascular complications in diabetes. PE may be viewed as a vascular complication of human diabetes that occurs within months—a “mouse-study time frame.”
Factors implicated as mechanisms for PE, mostly supported by cross-sectional, late-pregnancy studies in nondiabetic women, include endothelial dysfunction (7), imbalance between pro- and antiangiogenic factors (8), lipid peroxidation (9), oxidative stress (10), systemic inflammation (11), and dyslipidemia (9). Interestingly, these factors have also been implicated in the pathogenesis of the vascular complications of diabetes (12, 13), suggesting that common mechanisms might underlie PE and diabetic vascular complications. Our own studies show a general elevation of endoglin among T1DM women in later pregnancy (∼30 wk, but before PE onset) (14). Because endoglin is one of only two antiangiogenic factors that, together, are thought to constitute a prerequisite for PE (8), this could explain the high prevalence of PE in diabetes. However, abnormalities arising earlier in pregnancy are also of interest and could provide additional predictive and mechanistic insights (including reasons for elevation of endoglin). In this regard, we recently showed that lower levels of β-carotene early in pregnancy are associated with subsequent PE in T1DM women (15).
Dyslipidemia is implicated in endothelial dysfunction (16), and hence, potentially in the pathogenesis of PE (17). Normal pregnancy is characterized by progressive, physiological increases in serum total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and triglycerides (TG) (18, 19); these increases also occur in the presence of maternal T1DM (19). Existing studies addressing the relationship between maternal dyslipidemia and PE are mostly cross-sectional (cases vs. controls late in pregnancy in nondiabetic women), they either employ nonfasting samples or do not include positive confirmation of actual (rather than intended) fasting status, and they show the following associations with PE: high TG (9, 17, 20, 21); low (21) or normal (9) high-density lipoprotein cholesterol (HDL-C); and increased TC, LDL-C, and “small dense” low-density lipoprotein (LDL) (21, 22). Four prospective studies have addressed the relationship between lipid profiles early in pregnancy and subsequent PE (23–26). Of these, two employed nonfasting samples, and none involved women with diabetes. All found an association between hypertriglyceridemia and later PE. Our study is the first prospective investigation of the association between dyslipidemia and the increased prevalence of PE in pregnant T1DM women.
Our primary objective was to compare, starting in early pregnancy, lipid profiles of T1DM women who subsequently developed PE with those who remained normotensive. We aimed to determine whether detailed lipoprotein measures, including size-based and apolipoprotein-defined subclasses, might predict the onset of PE in T1DM and thus might provide potential new disease markers and mechanisms. We included a small cohort of nondiabetic, normotensive pregnant women to provide reference values and to enable a secondary comparison between “healthy” diabetic and nondiabetic women. Both by design and necessity (resource issues for a prospective study), our study does not address PE in the absence of T1DM.
Subjects and Methods
Study design and participants
The study was conducted according to the principles of the Declaration of Helsinki, and all study participants provided written informed consent at the study centers (Australia, Norway, and United States) as previously reported (14). In the first trimester of pregnancy, 151 women with established T1DM and 24 healthy nondiabetic women were recruited. Clinical data and specimens were collected at three study visits corresponding to late first, mid-second, and early third trimesters [12.2 ± 1.9, 21.6 ± 1.5, and 31.5 ± 1.7 wk gestation (means ± sd)] and at term (37.6 ± 2.0 wk). Participants were requested to fast overnight, and actual prandial status was recorded. Samples (serum, plasma, and urine) were obtained before insulin administration and stored at −80 C. Inclusion/exclusion criteria and other details have been described in our previous report (14). Of 151 T1DM women, 133 provided samples at all study visits and completed pregnancy. Of these, 26 developed PE, 12 developed pregnancy-induced hypertension (PIH), and 95 remained normotensive. Three of the 95 were excluded because of later detection of proteinuria in urine samples collected at visit 1. Of 24 healthy nondiabetic controls, 21 completed the entire pregnancy with no hypertensive complications; three were excluded because of PE (one), miscarriage (one), or loss to follow-up (one).
Diagnosis of PE and PIH
PE and PIH were defined as previously (14). PE was defined as new-onset hypertension (systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg) and proteinuria (urinary excretion ≥0.3 g in 24-h collection in the absence of urinary tract infection) after 20 wk gestation in a previously normotensive woman. PIH was defined as new-onset hypertension without proteinuria.
Glycated hemoglobin (HbA1c) and urine analyses
HbA1c and urine analyses were conducted as previously described (14).
Conventional plasma lipid analyses
Plasma lipids including TC, LDL-C, TG, very low-density lipoprotein-cholesterol (VLDL-C), and HDL-C were analyzed at the University of Oklahoma Health Sciences Center clinical laboratory.
Nuclear magnetic resonance (NMR) (particle size-based) subclass analyses
NMR subclass analyses were conducted by a 400-MHz proton NMR analyzer at LipoScience Inc. (Raleigh, NC) as previously described (27).
Apolipoprotein-defined lipoprotein subclass (ADLS) analyses
According to the concept of ADLS, there are two major apolipoprotein AI (ApoAI)-containing lipoprotein subclasses—Lp-AI and Lp-AI:AII (28)—and five major apolipoprotein B (ApoB)-containing lipoprotein subclasses—Lp-B, Lp-B:E, Lp-B:C, Lp-B:C:E, and Lp-AII:B:C:D:E, all defined by immunoprecipitation (29). The structure, function, and clinical significance of these ADLS have been previously described in detail (28–30). We also measured apolipoprotein CIII (ApoCIII), which plays a significant role in the transport and catabolism of triacylglycerols. We have measured ApoCIII bound to ApoAI-containing lipoproteins (heparin-soluble, ApoCIII-HS) and ApoCIII bound to ApoB-containing lipoproteins (heparin precipitate, ApoCIII-HP). ApoCIII is measured by electroimmunoassay in each fraction (31). We also report ApoCIII-HS/HP or “ApoCIII ratio” (ApoCIII-R) as a useful index of catabolism of TG-rich lipoproteins (32).
Measures of serum apolipoproteins
Serum concentrations of ApoAI, ApoB, and lipoprotein(a) [Lp(a)] were analyzed by nephelometry (Beckman Coulter Instruments, Brea, CA) as previously described (33). ApoB:ApoAI ratios were calculated.
Statistical analyses
We compared women with T1DM who subsequently developed PE (DM PE+) with women with T1DM who remained normotensive (DM PE−). We did this first cross-sectionally (at visits 1, 2, and 3), and then longitudinally (from visit 1 to visit 3). Our main analyses used data before PE onset, i.e. from visits 1–3, in accordance with the primary goal of our prospective study. Data from the term visit are also presented. Secondary analyses evaluated differences between DM PE− and nondiabetic non-PE control pregnancies (DM−).
Results were expressed as means ± sd, as defined in Tables 1–6. For non-normally distributed measures (per Shapiro-Wilks test), results were log-transformed and reported as geometric means ± sd [LDL-C, TG, VLDL-C, total LDL particles, ApoB:ApoAI ratio, and Lp(a)]. Differences between groups at each visit (cross-sectional comparisons) were analyzed by Student t test for continuous measures and χ2 test for categorical measures. Time trend analyses (longitudinal changes) were also conducted in each group using general estimating equations for average change in parameters at visits 1–3, i.e. before PE onset. Analyses were conducted with and without inclusion of two sets of covariates: 1) HbA1c and actual prandial status; and 2) HbA1c, actual prandial status, gravidity, body mass index (BMI), and diabetes duration. Significance levels were not adjusted for multiple hypothesis testing; rather, the results were reviewed for consistencies. All tests were two-tailed, with P < 0.05 considered significant. Microsoft Excel 2007 (Microsoft Corp., Redmond, WA) and SPSS for Windows 15.0 (SPSS Inc., Chicago, IL) were used in the analyses.
Table 1.
Clinical characteristics of 118 T1DM and 21 nondiabetic women at visit 1 and gestational age at each visit
| Characteristic n | DM PE+ 26 | Pa | DM PE− 92 | Pb | DM− 21 |
|---|---|---|---|---|---|
| Age (yr) | 29 ± 6 | 0.41 | 30 ± 5 | 0.11 | 32 ± 5 |
| BMI (kg/m2) | 28 ± 6 | 0.06 | 26 ± 5 | 0.04 | 24 ± 4 |
| Age at diabetes onset (yr) | 12 ± 7 | 0.01 | 17 ± 8 | ||
| Duration of diabetes (yr) | 16 ± 7 | 0.04 | 13 ± 8 | 0.24 | 1.7 ± 1.0 |
| Insulin (IU/d) | 61 ± 20 | 0.15 | 54 ± 22 | ||
| Gravida (n) | 1.5 ± 1.0 | 0.03 | 2.1 ± 1.5 | ||
| HbA1c (%) | 7.3 ± 1.1 | 0.09 | 6.8 ± 1.1 | <0.001 | 5.0 ± 0.3 |
| Blood pressure (mm Hg) | |||||
| Systolic | 115 ± 13 | 0.35 | 112 ± 12 | 0.88 | 112 ± 9 |
| Diastolic | 67 ± 9 | 0.82 | 67 ± 8 | 0.89 | 66 ± 8 |
| Gestation age (wk) | |||||
| Visit 1 | 12 ± 2 | 0.74 | 12 ± 2 | 0.19 | 13 ± 2 |
| Visit 2 | 22 ± 2 | 0.29 | 22 ± 1 | 0.81 | 22 ± 1 |
| Visit 3 | 32 ± 2 | 0.57 | 31 ± 2 | 0.38 | 31 ± 1 |
| Term | 37 ± 1 | 0.28 | 38 ± 2 | <0.001 | 39 ± 2 |
Values are shown as mean ± sd.
P, DM PE+ vs. DM PE−;
P, DM PE− vs. DM−; P values <0.05 are shown in boldface.
Table 2.
Conventional lipid profiles
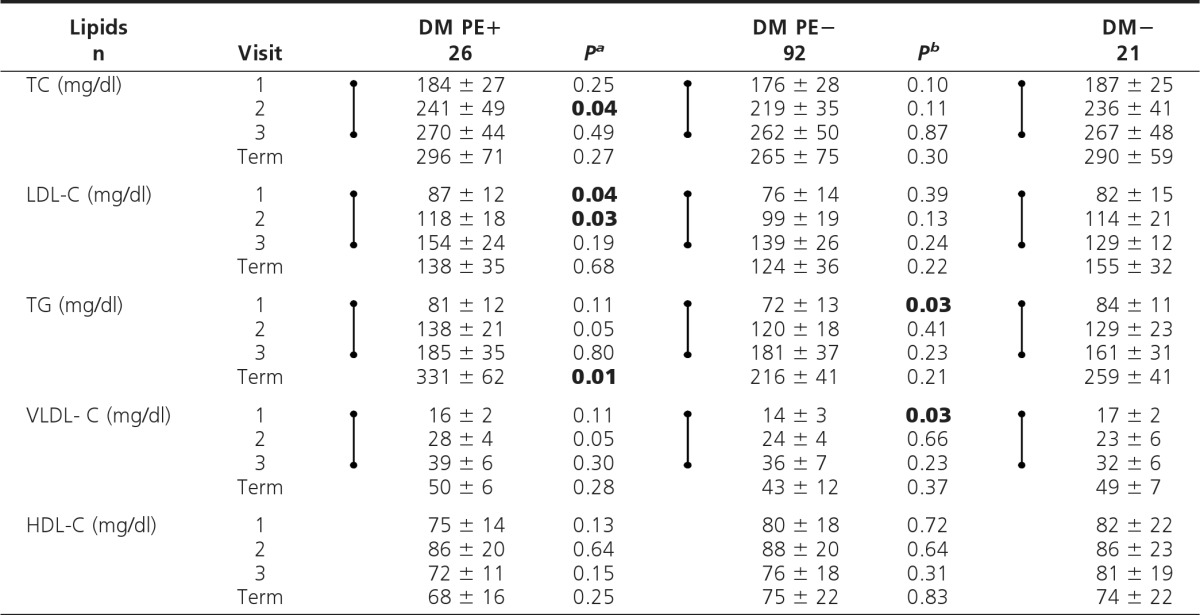
Values are shown as mean ± sd. For conversion to SI units (mmol/liter), multiply by 0.02586 for cholesterol and 0.01129 for TG. Dumbbell symbols indicate significant increase (time trend analyses, visits 1–3; P < 0.01).
aP, DM PE+ vs. DM PE−;
bP, DM PE− vs. DM−; P values <0.05 (visits 1–3) are shown in boldface.
Table 3.
NMR-derived lipid profiles
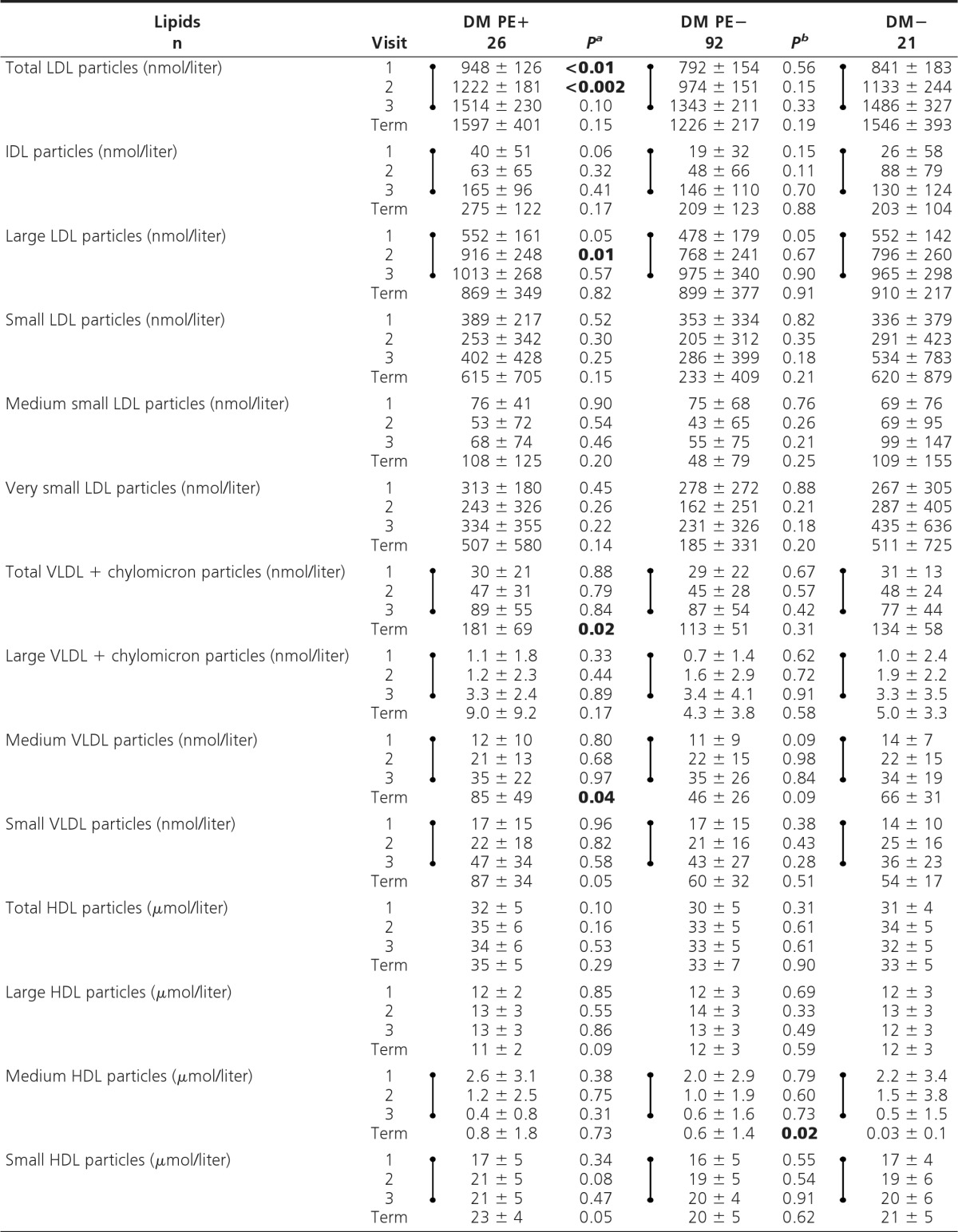
Values are shown as mean ± sd. Dumbbell symbols indicate significant increase (except decrease in medium HDL) (time trend analyses, visits 1–3; P < 0.01).
aP, DM PE+ vs. DM PE−;
bP, DM PE− vs. DM−; P values <0.05 (visits 1–3) are shown in boldface.
Table 4.
ADLS-based lipid profiles
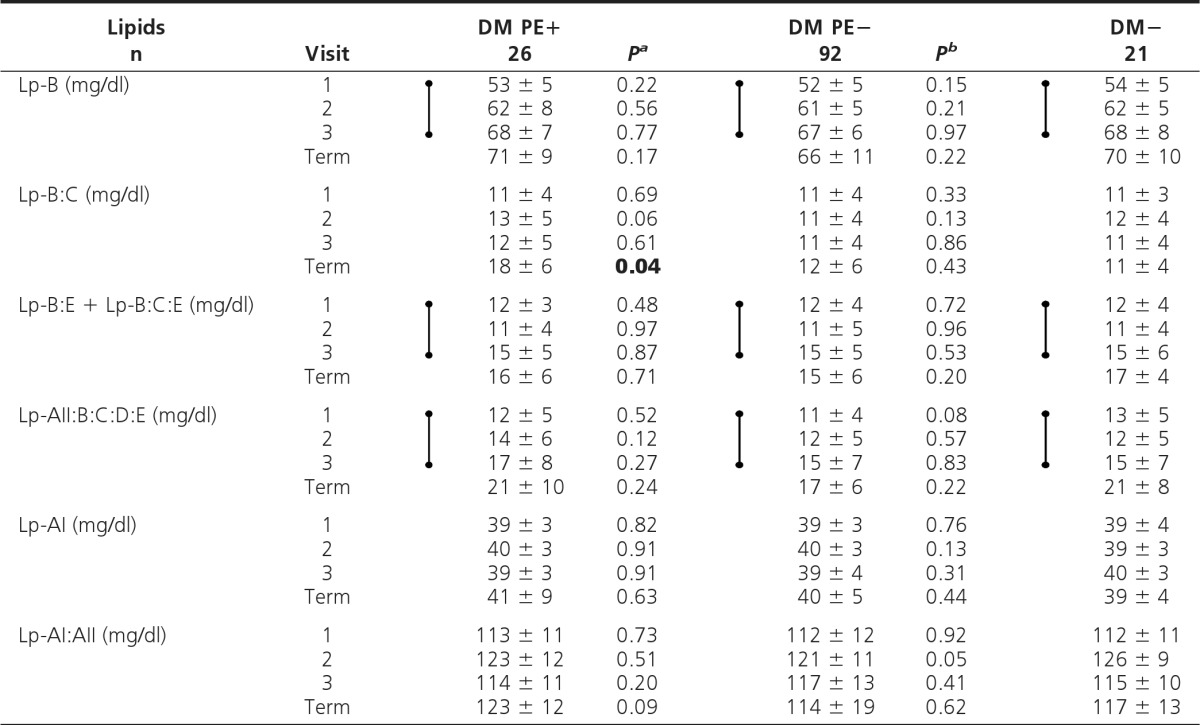
Values are shown as mean ± sd. Dumbbell symbols indicate significant increase (time trend analyses, visits 1–3; P < 0.01).
aP, DM PE+ vs. DM PE−;
bP, DM PE− vs. DM−; P values <0.05 (visits 1–3) are shown in boldface.
Table 5.
ApoCIII subfractions
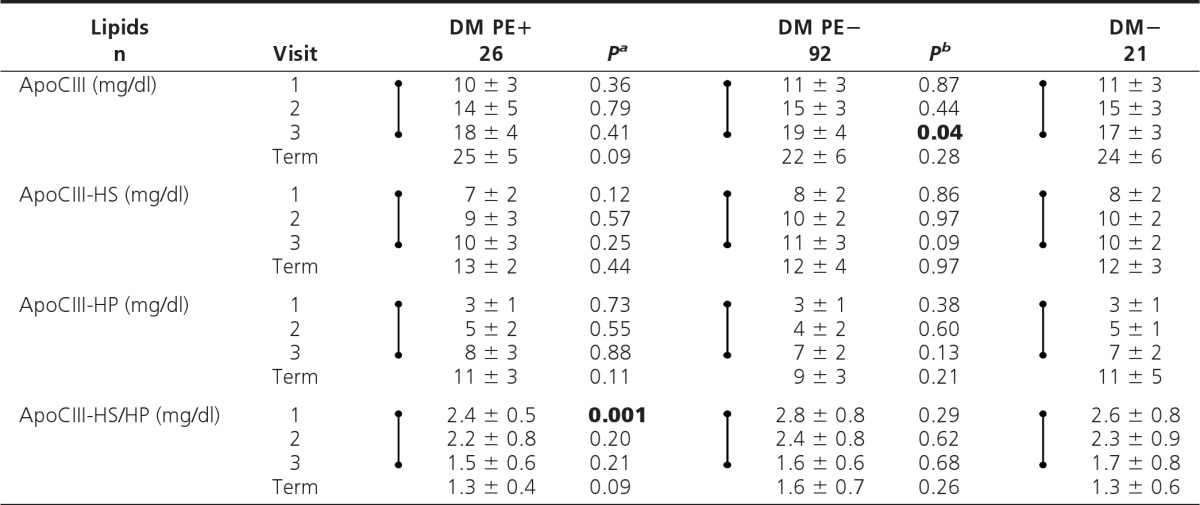
Values are shown as mean ± sd. Dumbbell symbols indicate significant increase (except decrease in ApoCIII-HS/HP) (time trend analyses, visits 1–3; P < 0.01).
aP, DM PE+ vs. DM PE−;
bP, DM PE− vs. DM−; P values <0.05 (visits 1–3) are shown in boldface.
Table 6.
Serum apolipoproteins

Values are shown as mean ± sd. Dumbbell symbols indicate significant increase (time trend analyses, visits 1–3; P < 0.01).
aP, DM PE+ vs. DM PE−;
bP, DM PE− vs. DM−; P values <0.05 (visits 1–3) are shown in boldface.
Results
Clinical characteristics at visit 1 (Table 1)
We excluded PIH patients (n = 12) in order to study clearly defined PE cases (n = 26) vs. normotensive and otherwise healthy diabetic pregnancies (n = 92). At visit 1, women with T1DM who subsequently developed PE had a younger age of diabetes onset (P < 0.01) and longer duration of diabetes (P < 0.05), and they tended to have higher BMI (P = 0.06) and HbA1c (P = 0.09) than those who remained normotensive. HbA1c levels did not differ significantly between these two groups throughout the study (14).
Fasting is challenging for a pregnant woman with T1DM. Actual prandial status revealed the following: DM PE+, DM PE−, and DM− participants fasted for 85, 67, and 84% of visits 1–3, respectively. Unfortunately, none of the DM PE+ women was fasting at term, limiting interpretation of data at this time point. Our primary comparisons are at visits 1–3 (i.e. before PE onset). Data were analyzed with and without adjustments for prandial status; we took the latter into account 1) as a covariate, and 2) by excluding all “nonfasting” data (Supplemental Tables 1–5, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). All these analytic methods yielded largely similar conclusions.
Conventional lipid profile
In DM PE+ vs. DM PE−, TC at visit 2 and LDL-C at visits 1 and 2 were significantly higher (P < 0.05) (Table 2). However, no such differences were noted at visit 3. When adjusted for covariates, LDL-C remained significantly higher in early pregnancy (visits 1 and 2) in the DM PE+ group. TG-rich lipoproteins and HDL-C were similar between groups at all visits.
Time trend analyses revealed significant but similar increases in all conventional lipid measures (P < 0.01), except for HDL-C, which did not change, with advancing pregnancy in the two diabetic groups.
Secondary analysis (DM PE− vs. DM−) revealed no significant cross-sectional differences in conventional lipids (Table 2), except that TG and VLDL-C were significantly lower in DM PE− at visit 1 (P < 0.05). Time trend analyses in DM− showed the expected significant increases similar to the diabetic groups described above.
NMR-determined lipid profile
In DM PE+ vs. DM PE−, molar concentrations of total LDL (visits 1 and 2) and large LDL particles (visit 2) were significantly higher in early pregnancy (P < 0.05) and generally higher at all visits (Table 3). These findings were not affected by covariate analyses. Also of interest, no differences between groups were noted for small (or its constituent “medium small” and “very small”) LDL particles at any visit. TG-rich particles (VLDL-C and chylomicrons) and HDL-C particles also did not exhibit significant differences between these two groups at any visit prior to PE onset.
Time trend analyses showed significant but similar increases (P < 0.01) in total LDL, intermediate-density lipoprotein (IDL), and large LDL particles with advancing pregnancy, but in contrast, no changes occurred in concentrations of small, medium small, and very small LDL particles as pregnancy advanced. TG-rich particles [very low-density lipoprotein (VLDL) and chylomicrons] increased significantly with advancing pregnancy in both diabetic groups (P < 0.01), but again to a similar extent. In contrast, total and large high-density lipoprotein (HDL) particles did not change (similar to HDL-C), whereas medium HDL particles decreased (P < 0.01), and small HDL particles increased (P < 0.01) in both diabetic groups with advancing pregnancy.
Secondary analysis (DM PE− vs. DM−) showed no significant cross-sectional differences in any particle concentrations at any visit (Table 3). Time trend analyses in DM− showed changes in NMR profiles similar to the two diabetic groups.
ADLS-based lipoprotein profile
In DM PE+ vs. DM PE−, no significant differences in ApoB-containing cholesterol- or TG-rich subclasses or ApoA-containing subclasses (HDL) were noted at any visit (Table 4). ApoCIII ratio (ApoCIII-HS/HP or ApoCIII-R) was significantly lower in DM PE+ vs. DM PE− in early pregnancy (visit 1, P < 0.01), but no differences were seen in later pregnancy (Table 5). After adjustments for covariates, the low ApoCIII-R in women who later developed PE persisted as a trend (P = 0.07).
Time trend analyses revealed significant but similar increases in all ApoB-containing subclasses (except Lp-B:C), ApoCIII, and a significant decrease in ApoCIII-R with advancing pregnancy in the diabetic groups (P < 0.01). No differences were noted in ApoA-containing subclasses throughout pregnancy.
Secondary analysis (DM PE− vs. DM−) showed no significant differences in ApoB- or ApoA-containing subclasses at any visit (Table 4). Total ApoCIII was significantly higher in DM PE− vs. DM− at visit 3 only (P < 0.05) (Table 5). Time trend analyses in DM− showed changes in ADLS-based subclasses with advancing pregnancy similar to the two diabetic groups described above.
Serum ApoB and ApoB:ApoAI ratio
In DM PE+ vs. DM PE−, serum ApoB and ApoB: ApoAI ratio were significantly higher at visit 2 (P < 0.05) and generally higher at all visits (Table 6). These results were unaffected by covariate analyses. ApoAI and Lp(a) showed no significant differences at any visit. Time trend analyses revealed significant but similar increases in serum ApoAI, ApoB, and ApoB:ApoAI ratio with advancing pregnancy in the diabetic groups (P < 0.01).
Secondary analysis (DM PE− vs. DM−) showed no significant cross-sectional differences in any measure of serum apolipoproteins at any visit. Time trend analyses in DM− showed significant increases in serum ApoB and ApoB:ApoAI ratio with advancing pregnancy (P < 0.01), whereas no significant differences were found in serum ApoAI or Lp(a) (Table 6).
Discussion
This is the first detailed study of plasma lipoprotein subclasses over the course of T1DM pregnancy and the first to address their relationship with subsequent PE. We found that higher levels of certain cholesterol-rich particles and impaired peripheral lipolysis early in pregnancy were associated with later development of PE. In contrast, fasting TG, TG-rich subclasses, and most HDL measures bore no relation to the development of PE. This absence of an association with hypertriglyceridemia was surprising in view of published data from nondiabetic pregnancies. Strengths of our study include its prospective design (three visits before PE onset) and its assessment and statistical consideration of actual prandial status.
Conventional lipid profiles revealed higher TC and LDL-C early in pregnancy in T1DM women who later developed PE compared with those who did not. Existing late-pregnancy cross-sectional studies of nondiabetic women show higher TC and LDL-C in PE cases than in non-PE controls (21, 22), but these studies could not distinguish whether dyslipidemia preceded or followed PE onset. In other cross-sectional studies of nondiabetic women (9, 20, 21), hypertriglyceridemia was found in PE cases vs. controls. Ray et al. (34), in a meta-analysis of 22 mostly cross-sectional studies in nondiabetic women, conclude that maternal TG levels are positively associated with risk of PE. However, these results should be interpreted with caution; in 10 of the studies, subjects were nonfasting, and there was no adjustment for actual prandial status. In our prospective study of normolipidemic T1DM women, no association of TG with subsequent PE was observed. Only at the term visit did we observe higher serum TG in the PE cases, but at that visit, all PE subjects were nonfasting. Analyses of fasting samples only (see Supplemental Data) likewise failed to reveal any significant differences in TG between the two diabetic groups at visits 1–3, before PE onset. In contrast to our findings, four previously reported prospective studies in nondiabetic women show elevated TG to be associated with PE (23–26). Of these four studies, two used nonfasting samples (23, 24). Furthermore, the metabolic differences in our well-controlled T1DM cohort, who had lower TG levels than nondiabetic control subjects at visit 1, might also contribute to the lack of association between maternal TG and PE in our study. Thus, although none of our subjects had hypertriglyceridemia in early pregnancy, we still observed the high rate of PE characteristic of diabetes. HDL-C did not differ between the two diabetic groups at any visit.
Longitudinal increases in conventional lipids (except HDL-C), as observed in our study, have previously been reported in nondiabetic pregnancies, as well as in those complicated by T1DM, type 2 diabetes mellitus (T2DM), and gestational diabetes mellitus (18, 19, 35). However, in the present study, the extent of these increases was not predictive of subsequent PE.
Consistent with the conventional lipid analyses, NMR revealed elevated molar concentrations of LDL and large LDL particles at visits 1 and 2 in DM PE+ vs. DM PE− groups. Interestingly, there were no significant differences between these groups in small LDL or in its constituent medium small and very small subclasses at any visit. These are novel findings showing that specific LDL measures in early T1DM pregnancy may be associated with subsequent PE. A few cross-sectional studies of PE in nondiabetic women in late pregnancy have shown higher concentrations of small, dense LDL (22, 36) or large LDL particles (37) in PE cases vs. controls, but in this well-controlled T1DM cohort, we do not confirm these findings. Again, consistent with our conventional lipid profile data, NMR-derived TG-rich particles (chylomicrons and VLDL) and HDL particles did not differ at any visit before PE between the two diabetic groups.
Our time trend observations show significant increases in NMR-derived molar concentrations of most cholesterol-rich particles (LDL, IDL, and large LDL particles), TG-rich particles (chylomicrons and VLDL), and small HDL subclasses as pregnancy advances. In contrast, there were no significant longitudinal changes in small (or medium small or very small) LDL, or in total and large HDL particles in any group. Medium HDL declined in all groups. Although these detailed longitudinal changes in NMR lipoprotein profiles in T1DM pregnancy have not previously been reported, prospective studies in nondiabetic pregnancy have reported increasing concentrations of small, dense LDL particles using other techniques (18, 38). The discrepancies between those findings and our own may be due to differences in the population studied (well-controlled T1DM patients have comparable lipid profiles to nondiabetic “healthy controls”) (39) and/or the methodologies used.
We did not observe any differences in ADLS-derived lipid profiles between the two diabetic groups at any visit. Thus, although both conventional lipid and NMR profiles provide significant evidence of LDL abnormalities in early pregnancy in T1DM before PE onset, no differences were noted in ApoB-containing ADLS. This may be explained by the overlap of these subclasses between cholesterol- and TG-rich subclasses and across size-based LDL subclasses. For example, Lp-B:C or Lp-B:E/B:C:E that is within the LDL density range could occur within small, medium, or large LDL subclasses as defined by NMR, and both of these subclasses may be found as VLDL (29, 30).
ApoCIII-R (ApoCIII-HS/HP ratio) was significantly decreased in early pregnancy in women with T1DM who later developed PE, suggesting decreased catabolism of TG-rich lipoproteins preceding PE (31, 32). This novel finding needs confirmation but suggests that reduced peripheral lipoprotein lipolysis in early pregnancy may be implicated in subsequent PE in the T1DM cohort. Impaired lipolysis would be supported by an increase in serum TG, which was not found in our fasting samples. This raises the intriguing possibility that an oral fat tolerance test, to reveal postprandial hyperlipidemia, might be of value, and/or that studies of postprandial lipoproteins might be more revealing than fasting lipoproteins, especially in the well-controlled T1DM cohort represented in our study.
Our time trend observations revealed significant increases in ApoB-containing particles (with the exception of atherogenic Lp-B:C) and ApoCIII subclasses in all groups with advancing pregnancy, whereas antiatherogenic ApoA-containing subclasses did not change in any group.
Consistent with our conventional lipid and NMR results, we found that higher levels of serum ApoB and ApoB:ApoAI ratio early in T1DM pregnancy were associated with subsequent PE, suggesting that an early-pregnancy “atherogenic” serum apolipoprotein profile might confer risk. ApoAI showed no such association, consistent with the findings for HDL. Late-pregnancy cross-sectional studies of PE in nondiabetic women have reported conflicting observations regarding serum apolipoproteins—no difference in ApoAI or ApoB (40), lower ApoAI (21), or higher ApoB (41) in PE vs. non-PE controls—but these are not directly comparable to the present study.
Time trend observations show similar increases in serum ApoB and ApoB:ApoAI in all groups with advancing pregnancy. ApoAI, like “small HDL” as measured by NMR, but unlike all other HDL measures, also increased with advancing pregnancy.
We found no significant differences in serum Lp(a) at any time point. These data are consistent with some previous findings (42). Thus, at present, Lp(a) is not clearly implicated in PE, but future studies to define its genotype-phenotype would be of interest.
Our study is limited by its small sample size, especially in the numbers of T1DM pregnancies complicated by PE. Larger confirmatory studies are needed. It would have been of great interest to include nondiabetic participants with PE, but this was not part of our study design (our goal was to seek markers and mechanisms for PE specifically in the context of T1DM, where the prevalence of PE is greatly increased) and was precluded by the resources it would demand (anticipated 4% case yield). Fasting is challenging for pregnant women, especially those with T1DM; we addressed this by: 1) taking actual prandial status into account as a covariate, and 2) analyzing only those data from visits positively confirmed as fasting (see Supplemental Tables). Although we did not correct for multiple hypothesis testing, our results concerning cholesterol-rich particles in early pregnancy are biologically plausible, and most variables remain significant at lower threshold (e.g. P ∼ 0.01) but need to be confirmed in larger studies. Unfortunately, it was not feasible to collect data on our study cohort either before or after the pregnancy, and the absence of dietary data is a weakness. Lipoprotein characteristics observed at visit 1 are, however, likely to reflect prepregnancy profiles and may reveal underlying maternal characteristics that are subsequently masked by pregnancy-associated changes.
The strengths of our study include a detailed prospective investigation of lipids and lipoprotein profiles in T1DM pregnancies preceding the onset of PE. Our combined analyses of serum cholesterol- and TG-rich lipoproteins using conventional lipid profiles, NMR, ADLS, serum apolipoproteins, and Lp(a) have not been previously reported in any study of human pregnancy, and the differential effects of pregnancy on large vs. small LDL, large vs. medium, vs. small HDL, and on certain TG-rich ADLS are all novel.
Our results show significant abnormalities in cholesterol-rich lipoproteins (LDL) and related apolipoproteins early in pregnancy in those who subsequently developed PE. Interestingly, these did not persist past visit 2, possibly due to the masking effects of increasing lipids caused by advancing pregnancy. Somewhat surprisingly, there were no associations between TG-rich lipoproteins and subsequent PE, and this may be explained by the excellent lipid profiles in young healthy T1DM women and our rigorous approach to the definition of fasting status. Future studies in T1DM should define these relationships in greater detail, and prospective studies of pregnancies complicated by T2DM and by gestational diabetes mellitus are also needed.
Supplementary Material
Acknowledgments
We acknowledge the following for technical or clinical assistance: J. Mauldin and M. Myers (Medical University of South Carolina); Jill Cole and Nancy Sprouse (Spartanburg Regional Hospital); Myrra Windau (University of Colorado); C. Knight, J. Conn, P. England, S. Hiscock, J. Oats, and P. Wein (University of Melbourne); and Julie Stoner and Lori Doyle (University of Oklahoma). At the University of Oklahoma, Kenneth Wilson assisted with sample processing and the conduct of laboratory work.
This work was supported by research grants from the Juvenile Diabetes Research Foundation (JDRF 1-2001-844); National Institutes of Health (NIH) (National Center on Minority Health and Health Disparities) Grant P20 MD000528-05 (to T.J.L.); and by NIH (National Center for Research Resources) Grants M01-RR-1070 and M01-RR-14467 (to the General Clinical Research Centers at the Medical University of South Carolina and the University of Oklahoma Health Sciences Center, respectively). Support from Novo Nordisk enabled the participation of the Barbara Davis Diabetes Center for Childhood Diabetes. This publication was made possible by NIH Grant P20 RR 024215 from the Centers of Biomedical Research Excellence Program of the National Center for Research Resources.
Disclosure Summary: The authors report no conflict of interest.
Footnotes
- ADLS
- Apolipoprotein-defined lipoprotein subclasses
- ApoAI
- apolipoprotein AI
- BMI
- body mass index
- ApoB
- apolipoprotein B
- ApoCIII
- apolipoprotein CIII
- ApoCIII-HP
- ApoCIII in heparin-manganese precipitate
- ApoCIII-HS
- ApoCIII in supernatant fraction after heparin-manganese precipitation
- ApoCIII-R
- ApoCIII ratio
- DM−
- nondiabetic controls
- DM PE+
- women with T1DM who subsequently developed PE
- DM PE−
- women with T1DM who did not develop PE
- HbA1c
- glycated hemoglobin
- HDL
- high-density lipoprotein
- HDL-C
- HDL-cholesterol
- IDL
- intermediate-density lipoprotein
- LDL
- low-density lipoprotein
- LDL-C
- LDL-cholesterol
- Lp(a)
- lipoprotein(a)
- Lp-A
- ApoAI-containing lipoproteins
- Lp-B
- ApoB-containing lipoproteins
- NMR
- nuclear magnetic resonance
- PE
- preeclampsia
- PIH
- pregnancy-induced hypertension
- TC
- total cholesterol
- T1DM
- type 1 diabetes mellitus
- T2DM
- type 2 diabetes mellitus
- TG
- triglycerides
- VLDL
- very low-density lipoprotein
- VLDL-C
- VLDL cholesterol.
References
- 1. Roberts JM, Pearson G, Cutler J, Lindheimer M. 2003. Summary of the NHLBI Working Group on Research on Hypertension during Pregnancy. Hypertension 41:437–445 [DOI] [PubMed] [Google Scholar]
- 2. Hanson U, Persson B. 1993. Outcome of pregnancies complicated by type 1 insulin-dependent diabetes in Sweden: acute pregnancy complications, neonatal mortality and morbidity. Am J Perinatol 10:330–333 [DOI] [PubMed] [Google Scholar]
- 3. Evers IM, de Valk HW, Visser GH. 2004. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 328:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manten GT, Sikkema MJ, Voorbij HA, Visser GH, Bruinse HW, Franx A. 2007. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy 26:39–50 [DOI] [PubMed] [Google Scholar]
- 5. Tenhola S, Rahiala E, Martikainen A, Halonen P, Voutilainen R. 2003. Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. J Clin Endocrinol Metab 88:1217–1222 [DOI] [PubMed] [Google Scholar]
- 6. Libby G, Murphy DJ, McEwan NF, Greene SA, Forsyth JS, Chien PW, Morris AD. 2007. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: an intergenerational study from the Walker cohort. Diabetologia 50:523–530 [DOI] [PubMed] [Google Scholar]
- 7. Ferris TF. 1991. Pregnancy, preeclampsia, and the endothelial cell. N Engl J Med 325:1439–1440 [DOI] [PubMed] [Google Scholar]
- 8. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. 2004. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350:672–683 [DOI] [PubMed] [Google Scholar]
- 9. Hubel CA, McLaughlin MK, Evans RW, Hauth BA, Sims CJ, Roberts JM. 1996. Fasting serum triglycerides, free fatty acids, and malondialdehyde are increased in preeclampsia, are positively correlated, and decrease within 48 hours post partum. Am J Obstet Gynecol 174:975–982 [DOI] [PubMed] [Google Scholar]
- 10. Hubel CA. 1999. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 222:222–235 [DOI] [PubMed] [Google Scholar]
- 11. Szarka A, Rigó J, Jr, Lázár L, Beko G, Molvarec A. 2010. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamagishi S, Imaizumi T. 2005. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des 11:2279–2299 [DOI] [PubMed] [Google Scholar]
- 13. Xu J, Zou MH. 2009. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation 120:1266–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu Y, Jenkins AJ, Nankervis AJ, Hanssen KF, Scholz H, Henriksen T, Lorentzen B, Clausen T, Garg SK, Menard MK, Hammad SM, Scardo JC, Stanley JR, Dashti A, May K, Lu K, Aston CE, Wang JJ, Zhang SX, Ma JX, Lyons TJ. 2009. Anti-angiogenic factors and pre-eclampsia in type 1 diabetic women. Diabetologia 52:160–168 [DOI] [PubMed] [Google Scholar]
- 15. Azar M, Basu A, Jenkins AJ, Nankervis AJ, Hanssen KF, Scholz H, Henriksen T, Garg SK, Hammad SM, Scardo JA, Aston CE, Lyons TJ. 2011. Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: a longitudinal study. Diabetes Care 34:1258–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Boven AJ, Jukema JW, Paoletti R. 1994. Endothelial dysfunction and dyslipidemia: possible effects of lipid lowering and lipid modifying therapy. Pharmacol Res 29:261–272 [DOI] [PubMed] [Google Scholar]
- 17. Lorentzen B, Henriksen T. 1998. Plasma lipids and vascular dysfunction in preeclampsia. Semin Reprod Endocrinol 16:33–39 [DOI] [PubMed] [Google Scholar]
- 18. Belo L, Caslake M, Santos-Silva A, Castro EM, Pereira-Leite L, Quintanilha A, Rebelo I. 2004. LDL size, total antioxidant status and oxidised LDL in normal human pregnancy: a longitudinal study. Atherosclerosis 177:391–399 [DOI] [PubMed] [Google Scholar]
- 19. Toescu V, Nuttall SL, Martin U, Nightingale P, Kendall MJ, Brydon P, Dunne F. 2004. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci (Lond) 106:93–98 [DOI] [PubMed] [Google Scholar]
- 20. Murai JT, Muzykanskiy E, Taylor RN. 1997. Maternal and fetal modulators of lipid metabolism correlate with the development of preeclampsia. Metabolism 46:963–967 [DOI] [PubMed] [Google Scholar]
- 21. Bayhan G, Kocyigit Y, Atamer A, Atamer Y, Akkus Z. 2005. Potential atherogenic roles of lipids, lipoprotein (a) and lipid peroxidation in preeclampsia. Gynecol Endocrinol 21:1–6 [DOI] [PubMed] [Google Scholar]
- 22. Llurba E, Casals E, Domínguez C, Delgado J, Mercadé I, Crispi F, Martín-Gallán P, Cabero L, Gratacós E. 2005. Atherogenic lipoprotein subfraction profile in preeclamptic women with and without high triglycerides: different pathophysiologic subsets in preeclampsia. Metabolism 54:1504–1509 [DOI] [PubMed] [Google Scholar]
- 23. Clausen T, Djurovic S, Henriksen T. 2001. Dyslipidemia in early second trimester is mainly a feature of women with early onset pre-eclampsia. BJOG 108:1081–1087 [DOI] [PubMed] [Google Scholar]
- 24. Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. 2004. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens 17:574–581 [DOI] [PubMed] [Google Scholar]
- 25. Lorentzen B, Drevon CA, Endresen MJ, Henriksen T. 1995. Fatty acid pattern of esterified and free fatty acids in sera of women with normal and pre-eclamptic pregnancy. Br J Obstet Gynaecol 102:530–537 [DOI] [PubMed] [Google Scholar]
- 26. Gratacós E, Casals E, Sanllehy C, Cararach V, Alonso PL, Fortuny A. 1996. Variation in lipid levels during pregnancy in women with different types of hypertension. Acta Obstet Gynecol Scand 75:896–901 [DOI] [PubMed] [Google Scholar]
- 27. Lyons TJ, Jenkins AJ, Zheng D, Klein RL, Otvos JD, Yu Y, Lackland DT, McGee D, McHenry MB, Lopes-Virella M, Garvey WT. 2006. Nuclear magnetic resonance-determined lipoprotein subclass profile in the DCCT/EDIC cohort: associations with carotid intima-media thickness. Diabet Med 23:955–966 [DOI] [PubMed] [Google Scholar]
- 28. Alaupovic P. 1991. Apolipoprotein composition as the basis for classifying plasma lipoproteins. Characterization of ApoA- and ApoB-containing lipoprotein families. Prog Lipid Res 30:105–138 [DOI] [PubMed] [Google Scholar]
- 29. Alaupovic P. 1996. Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzymol 263:32–60 [DOI] [PubMed] [Google Scholar]
- 30. Alaupovic P. 2003. The concept of apolipoprotein-defined lipoprotein families and its clinical significance. Curr Atheroscler Rep 5:459–467 [DOI] [PubMed] [Google Scholar]
- 31. Attman PO, Samuelsson O, Alaupovic P. 1993. Lipoprotein metabolism and renal failure. Am J Kidney Dis 21:573–592 [DOI] [PubMed] [Google Scholar]
- 32. Alaupovic P. 1981. David Rubinstein Memorial Lecture: the biochemical and clinical significance of the interrelationship between very low density and high density lipoproteins. Can J Biochem 59:565–579 [DOI] [PubMed] [Google Scholar]
- 33. Lyons TJ, Jenkins AJ, Zheng D, Lackland DT, McGee D, Garvey WT, Klein RL. 2004. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci 45:910–918 [DOI] [PubMed] [Google Scholar]
- 34. Ray JG, Diamond P, Singh G, Bell CM. 2006. Brief overview of maternal triglycerides as a risk factor for pre-eclampsia. BJOG 113:379–386 [DOI] [PubMed] [Google Scholar]
- 35. Göbl CS, Handisurya A, Klein K, Bozkurt L, Luger A, Bancher-Todesca D, Kautzky-Willer A. 2010. Changes in serum lipid levels during pregnancy in type 1 and type 2 diabetic subjects. Diabetes Care 33:2071–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sattar N, Bendomir A, Berry C, Shepherd J, Greer IA, Packard CJ. 1997. Lipoprotein subfraction concentrations in preeclampsia: pathogenic parallels to atherosclerosis. Obstet Gynecol 89:403–408 [DOI] [PubMed] [Google Scholar]
- 37. Winkler K, Wetzka B, Hoffmann MM, Friedrich I, Kinner M, Baumstark MW, Zahradnik HP, Wieland H, März W. 2003. Triglyceride-rich lipoproteins are associated with hypertension in preeclampsia. J Clin Endocrinol Metab 88:1162–1166 [DOI] [PubMed] [Google Scholar]
- 38. Sattar N, Greer IA, Louden J, Lindsay G, McConnell M, Shepherd J, Packard CJ. 1997. Lipoprotein subfraction changes in normal pregnancy: threshold effect of plasma triglyceride on appearance of small, dense low density lipoprotein. J Clin Endocrinol Metab 82:2483–2491 [DOI] [PubMed] [Google Scholar]
- 39. Jenkins AJ, Klein RL, Chassereau CN, Hermayer KL, Lopes-Virella MF. 1996. LDL from patients with well-controlled IDDM is not more susceptible to in vitro oxidation. Diabetes 45:762–767 [DOI] [PubMed] [Google Scholar]
- 40. Koçyigit Y, Atamer Y, Atamer A, Tuzcu A, Akkus Z. 2004. Changes in serum levels of leptin, cytokines and lipoprotein in pre-eclamptic and normotensive pregnant women. Gynecol Endocrinol 19:267–273 [DOI] [PubMed] [Google Scholar]
- 41. Hubel CA, Lyall F, Weissfeld L, Gandley RE, Roberts JM. 1998. Small low-density lipoproteins and vascular cell adhesion molecule-1 are increased in association with hyperlipidemia in preeclampsia. Metabolism 47:1281–1288 [DOI] [PubMed] [Google Scholar]
- 42. Rymer J, Constable S, Lumb P, Crook M. 2002. Serum lipoprotein (A) and apolipoproteins during pregnancy and postpartum in normal women. J Obstet Gynaecol 22:256–259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


