Abstract
Context:
Progesterone promotes uterine relaxation during pregnancy and its withdrawal induces labor. Progesterone withdrawal in human parturition is mediated in part by changes in the relative levels of the nuclear progesterone receptor isoforms, PR-A and PR-B, in myometrial cells. Parturition also involves myometrial inflammation; however, the functional link between nuclear PR-mediated progesterone actions and inflammation in human myometrial cells is unclear.
Objective:
Our objective was to determine how PR-A and PR-B regulate progesterone action in human myometrial cells and specifically the expression of genes encoding contraction-associated proteins and proinflammatory mediators.
Design:
Effects of PR-A and PR-B on the capacity for progesterone to modulate gene expression was determined using an immortalized human myometrial cell line stably transfected with inducible PR-A and PR-B expression transgenes and conditioned to express various PR-A and PR-B levels. Gene expression was assessed by genome wide transcriptome analysis, quantitative RT-PCR and immunoblotting.
Results:
PR-A and PR-B were each transcriptionally active in response to progesterone and affected the expression of distinct gene cohorts. The capacity for progesterone to affect gene expression was dependent on the PR-A to PR-B ratio. This was especially apparent for the expression of proinflammatory genes. Progesterone decreased proinflammatory gene expression when the PR-A to PR-B ratio favored PR-B and increased proinflammatory gene expression when the ratio favored PR-A. Progesterone via PR-B increased expression of inhibitor-κBα, a repressor of the nuclear factor-κB transcription factor, and inhibited basal and lipopolysaccharide-induced proinflammatory gene expression. Both of those PR-B-mediated effects were inhibited by PR-A.
Conclusions:
Our data suggest that during most of human pregnancy, when myometrial cells are PR-B dominant, progesterone promotes myometrial quiescence through PR-B-mediated antiinflammatory actions. At parturition, the rise in PR-A expression promotes labor by inhibiting the antiinflammatory actions of PR-B and stimulating proinflammatory gene expression in response to progesterone.
The progesterone block hypothesis, first proposed by George Corner in 1942 (1) and later expanded by Arpad Csapo in the 1950s (2), posits that progesterone maintains pregnancy by blocking labor and that withdrawal of the progesterone block is a key trigger for parturition. Although studies in multiple species have supported this hypothesis, the molecular mechanisms by which progesterone promotes myometrial cell relaxation to prevent labor, and how those actions are controlled in the context of human pregnancy and parturition are not known.
Uterine contractility is determined mainly by the responsiveness of myometrial cells to uterotonic hormones [e.g. oxytocin (OXT), prostaglandin (PG)-F2α, PGI] that activate signaling cascades and ion channels leading to changes in intracellular free calcium levels, which in turn, through the regulation of specific kinases, affects activity of the actin-myosin contractile apparatus (3–5). In addition, contractility is affected by the formation of gap-junctions between myometrial cells that synchronize contractions over the entire uterus (6). Progesterone is thought to affect these processes in myometrial cells via genomic and nongenomic mechanisms. Progesterone could directly modulate signaling pathways that affect intracellular free calcium levels and the sensitivity of the contractile machinery to stimulatory uterotonins (nongemonic mode), or it could affect the expression of genes (genomic mode) encoding factors, collectively referred to as contraction-associated proteins (CAPs), that establish either a relaxed or laboring phenotype. Some important CAPs include connexins [e.g. connexin-43 (gap junction protein, α1 [GJA1])], which form gap junctions between myometrial cells, allowing rapid electrical and chemical signaling between adjacent cells to permit coordinated contractions; ion channels (e.g. calcium channels) that determine the resting membrane potential and basal excitability; uterotonin receptors (e.g. OXT and PG receptors) that determine responsiveness to hormones that directly induce or repress contractions; and enzymes that determine the rate of local PG synthesis [e.g. PG endoperoxide synthase-2 (PTGS2) and PG dehydrogenase]. These actions of progesterone in myometrial cells are mediated by a variety of progesterone receptors (PRs) (7). The nuclear PRs (nPRs) are especially important because inhibition of nPR activity (e.g. by treating pregnant women with antagonists such as mifepristone and onapristone) increases uterine excitability and contractility and in most cases induces labor at any stage of pregnancy (8–12). Thus, the progesterone block to labor is in large part mediated by nPR signaling, and inhibition of nPR activity alone is sufficient to induce labor. The mechanisms, however, by which nPRs mediate the relaxatory/labor-blocking actions of progesterone in human pregnancy myometrial cells and how nPR activity is withdrawn at parturition are not known.
It is now clear that tissue-level inflammation within the myometrium, decidua, and fetal membranes plays a major role in the human parturition process (13). In 1977 Siiteri et al. (14) proposed that labor is an inflammatory process and that progesterone blocks labor for most of pregnancy by suppressing inflammation. Their model suggests that labor is secondary to myometrial inflammation and that progesterone blocks labor indirectly via antiinflammatory actions. Recent studies have indeed shown that myometrium in pregnant women at term exhibits biochemical and histological characteristics of inflammation (e.g. increased expression of proinflammatory cytokines, increased infiltration of neutrophils, and macrophages) that may precede the onset of active labor and is independent of infection (15–19). Importantly, in vitro studies in human myometrial cells have shown that progesterone exerts antiinflammatory actions by inhibiting the proinflammatory nuclear factor-κB (NF-κBα) transcription factor complex and that this effect is due, in part, to nPR-induced expression of inhibitor-κB-α (NFKB1A; aka IκBα), a major NF-κB repressor (20). Thus, current evidence suggests that human parturition is associated with myometrial inflammation and that progesterone inhibits myometrial inflammation and desensitizes myometrial cells to proinflammatory stimuli.
Progesterone withdrawal is a key trigger for parturition in viviparous species. In most animals parturition is preceded by a decrease in circulating progesterone levels (i.e. systemic progesterone withdrawal) mediated by hormonal interactions that inhibit progesterone production by either the placenta or the corpus luteum (21). Parturition in women, however, occurs without a decrease in systemic progesterone levels and labor ensues, even though myometrial cells are exposed to high levels of progesterone (22–24). This suggests that human parturition does not require progesterone withdrawal. However, treatment of pregnant women with nPR antagonists induces labor at any stage of pregnancy (8–12), suggesting that human parturition can be triggered by functional, rather than systemic, progesterone withdrawal, whereby myometrial cells become refractory to nPR-mediated relaxatory actions of progesterone. Possible mechanisms for functional progesterone withdrawal include changes in the relative levels of nPR isoforms (25–28), decreased interaction of the nPRs with gene promoter targets (29), changes in the levels of nPR-associated transactivation coregulators (30), and increased expression of a specific endogenous nPR antagonists (31).
The human nPR exist as two predominant isoforms: PR-A and PR-B. PR-A is a truncated (by 164 N terminal amino acids) form of PR-B. As with other steroid hormone receptors, PR-A and PR-B function primarily as ligand-activated transcription factors. In vitro studies using artificial reporters controlled by the canonical progesterone response element (PRE) showed that PR-B is the predominant mediator of progesterone-induced transcriptional activity, whereas PR-A has minimal transcriptional activity at the exogenous PRE (25, 27, 32, 33). In this experimental paradigm, PR-A inhibits the transcriptional activity of PR-B, especially when its levels dominate (i.e. the PR-A to PR-B ratio >1). This led to the hypothesis that PR-A is an endogenous repressor of PR-B and that genomic progesterone responsiveness is inversely related to the PR-A to PR-B ratio. In this context, functional progesterone withdrawal in human parturition could be mediated by an increase in the myometrial cell PR-A to PR-B ratio. Consistent with that hypothesis we found that myometrial cell PR-A levels increase late in human pregnancy causing the PR-A to PR-B ratio to transition from PR-B-dominance (PR-A to PR-B ratio ∼0.5) to PR-A dominance (PR-A to PR-B ratio ∼3.0) in association with advancing gestation and especially with the onset of labor (25). Those findings support the PR-A/PR-B hypothesis for functional progesterone withdrawal, which posits that progesterone promotes myometrial relaxation for most of pregnancy via PR-B-mediated genomic actions in myometrial cells and that this is inhibited by increased myometrial cell expression of PR-A. In the present study, we tested the PR-A/PR-B hypothesis using a genetically modified human myometrial cell line in which the levels of PR-A and PR-B can be experimentally controlled to mimic those observed in late gestation human pregnancy uterus. Our goal was to determine how the PR-A to PR-B ratio in myometrial cells affects the capacity for progesterone to modulate the expression of genes, especially those encoding CAPs and inflammatory mediators, involved with the onset of labor.
Materials and Methods
Cell culture
Cell cultures were maintained at 37 C in 95% air-5% CO2 in DMEM/Ham's F12 (1:1) containing 5% charcoal-stripped and fetal calf serum (Life Technologies, Grand Island, NY), 0.1 mg/ml geneticin, 2 mm l-glutamine and antibiotics (penicillin/streptomycin), and selection antibiotics (neomycin, hygromycin, and blasticidin; all from Life Technologies). Medium was refreshed every 48 h.
Transfection
DNA was introduced into cells by nucleofection (Lonza, Walkersville, MD). Briefly, cells were harvested by trypsinization, centrifuged, and resuspended in smooth muscle nucleofection solution (Lonza; 2 × 106 cells per 100 μl) containing 1–2 μg of DNA. The mixture was then transferred to an electroporation cuvette, placed in the Nucleofector device (Lonza), and subjected to program A33. Cells were then replated and allowed to stabilize for at least 16 h.
Production of the hTERT-HMA/B cell line
The hTERT-HMA/B cell line was derived from the human telomerase reverse transcriptase immortalized human myometrial cell line (hTERT-HM) provided by Dr. William Rainey (Medical College of Georgia, Augusta, GA) (34). hTERT-HM cells were first stably transfected with linearized TetON-advanced plasmid (Clontech, Mountain View, CA) and a neomycin resistance expression plasmid to produce hTERT-HM sublines that constitutively expresses the reverse tetracycline receptor complex. One neomycin-resistant subline was selected based on phenotypic similarity to the parental cell line (i.e. CAP gene expression, smooth muscle cells morphology, and proliferation rate) and transfected with linearized pTetON-advanced expression plasmid (Clontech) in which the PR-A open reading frame was inserted in the multiple cloning site. The cells were also transfected with linearized DNA that constitutively expresses a cassette of genes that confer resistance to hygromycin (Clontech). Hygromycin-resistant clonal cell lines were then established and screened to identify sublines in which PR-A levels (determined by immunoblotting) increased in response to doxycycline (DOX). One clonal subline (designated hTERT-HMA) was then transfected with a modified RheoSwitch expression plasmid (pRG-1015-G6-A; provided by New England Biolabs, Beverly, MA) containing the PR-B open reading frame in the multiple cloning site. The cells were also transfected with linearized DNA that constitutively expresses genes that confer resistance to blasticidin (Life Technologies). Blasticidin-resistant clonal cell lines were established and screened for dose-responsive RheoSwitch ligand (RSL)-induced PR-B expression and DOX-induced PR-A expression. One clonal subline, designated hTERT-HMA/B, was selected based on similarity to the parental cell line and myometrial cell phenotype.
RNA and protein extraction
Total RNA was isolated using Trizol reagent (Life Technologies), treated with deoxyribonuclease (Applied Biosciences, Carpinteria, CA), and quantified by light absorption at 260 nm. Total cell lysates were prepared using the radioimmunoprecipitation assay extraction buffer (Sigma, St. Louis, MO), supplemented with protease and phosphatase inhibitors (Roche, Indianapolis, IN; final concentrations: 0.5 mmol/liter phenylmethylsulfonyl fluoride, 86 μmol/liter leupeptin, 77 μg/ml aprotinin, 1.4 μmol/liter pepstatin A, and 100 μg/ml bacitracin) on ice. The protein concentration was assessed by the bicinchoninic acid method (Thermo Scientific, Rockford, IL).
Immunoblotting
Cells lysates (50–100 μg) were diluted in gel loading buffer (40% glycerol, 1 m Tris-HCl, 2.5% β-mercaptoethanol, 8% sodium dodecyl sulfate, and 0.01% bromophenol blue) heated for 5 min at 100 C and subjected to denaturing (in 10% SDS-PAGE) using the Novex Tris-glycine system (Life Technologies). Separated proteins were then electrotransferred to polyvinyl difluoride membranes (Bio-Rad Laboratories, Hercules, CA). For immunoblotting, the polyvinyl difluoride membranes were blocked with 5% nonfat milk in 20 mm Tris, 500 mm NaCl (pH 7.5) containing 0.05% Tween 20 (TTBS) for 1 h at room temperature and then were incubated with primary antibodies (anti-nPR, catalog no. PgR1294; Dako North America, Carpinteria CA; anti-PTGS2, catalog no. 4842; Cell Signaling, Boston, MA; anti-IL-8, catalog no. MAB208; R&D Systems, Minneapolis, MN; and antiglyceraldehyde 3-phosphate dehydrogenase, catalog no. sc-32233; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 C. The following day blots were washed three times with TTBS and incubated at room temperature with a horseradish peroxidase-conjugated secondary antibody (antimouse or antirabbit; Cell Signaling). The blots were then treated with chemiluminescent reagents (Amersham Life Sciences, Piscataway, NJ) and exposed to x-ray film (Hyperfilm ECL; Amersham Life Sciences). Films were scanned and intensity of bands quantified using digital densitometry (ImageJ, National Institutes of Health, http://imagej.nih.gov/ij/).
Immunohistochemistry
hTERT-HMA/B cells were cultured in multichamber Lab-Tek plates (Nalge Nunc International, Rochester, NY), treated with DOX and/or RSL for 48 h, washed in PBS, and fixed in 4% paraformaldehyde in PBS. Cells were then heated at 95 C in citrate buffer (10 mm citric acid; 0.05% Tween 20, pH 6.0) for 20 min, cooled to room temperature, and rinsed in TTBS, pretreated for 5 min in 3% H2O2, washed, and then incubated with either anti-nPR PgR1294 (Dako North America) or nonimmune mouse IgG (Santa Cruz Biotechnology) (each 1:250 diluted in 1% BSA) at room temperature for 1 h. Cells were then washed and incubated with biotinylated antimouse IgG (Dako North America) for 20 min at room temperature, washed, and incubated with streptavidin-horseradish peroxidase for 20 min at room temperature and then 3,5 diaminobenzidine tetrahydrochloride, rinsed in water, dehydrated in graded alcohols, cleared in xylene and mounted with coverslips, and examined by regular light microscopy.
Quantitative RT-PCR
Total RNA (300–600 ng) was reverse transcribed with random primers using Superscript II reverse transcriptase (Life Technologies). Primers (Table 1) were designed using the Primer Express software (Applied Biosystems) based on published sequences. Assays were optimized and validated for all primer sets by confirming that single amplicons of appropriate size and sequence were generated and that the priming and amplification efficiencies of all primer pairs were identical. PCR was performed in the presence of SYBR Green (Applied Biosystems) in an ABI PRISM 7500 sequence detector (Applied Biosystems). The cycling conditions were 50 C for 2 min, 95 C for 10 min, and 40 cycles of 95 C for 15 sec; and 60 C for 1 min. The cycle at which the fluorescence reached a preset threshold [cycle threshold (CT)] was used for quantitative analyses. The threshold in each assay was set at a level at which the rate of exponential increase in amplicon abundance was approximately parallel between all samples. mRNA abundance data were expressed relative to the abundance of the constitutively expressed α-actin mRNA using the ΔCT method [i.e. relative mRNA abundance = 2−(CT gene of interest − CT α-actin)].
Table 1.
Sequence of qRT-PCR primers
| NCBI Reference sequence no. | Name | Forward | Reverse |
|---|---|---|---|
| GU_143396.1 | ACTA2 | GCCTTGGTGTGTGACAATGG | AAAACAGCCCTGGGAGCAT |
| NM_001145777 | FKBP5 | ATGCCATTTACTGTGCAAACCAG | AAGAGAGTTGCATTCGAGGGAA |
| NM_000165 | GJA1 | TGGCCTTCTTGCTGATCCA | TTTGCAAGTGTAAACAGCACTCAA |
| NM_000584 | IL8 | TGGCAGCCTTCCTGATTTCT | TTAGCACTCCTTGGCAAAACTG |
| NM_000575 | IL1A | CGCCAATGACTCAGAGGAAGA | ACATTGCTCAGGAAGCTAAAAGGT |
| NM_000576 | IL1B | CACGATGCACCTGTACGATCA | CAGACATCACCAAGCTTTTTTGC |
| Np_001091 | NFKB1A | AAGTGATCCGCCAGGTGAAG | TGCTGCAGGTTGTTCTGGAA |
| NM_000963 | PTGS2 | ATGTTCCACCCGCAGTACAGA | CAGCATAAAGCGTTTGCGGTA |
Microarray-based transcriptome analyses
Microarray procedures were performed by the Expression and Genotyping Facility of the Case Comprehensive Cancer Center, Case Western Reserve University. Briefly, total RNA (∼100 ng) was used to generate labeled sense-strand cDNA using the Ambion WT expression kit (Life Technologies) according to the manufacturer's instructions. The labeled cDNA was added to the hybridization cocktail along with appropriate buffers, labeled control transcripts, and carrier molecules. Hybridization cocktails were then added to GeneChip 1.0 ST arrays (Affymetrix Inc., Santa Clara, CA) and incubated overnight at 45 C with agitation. The 1.0 ST arrays survey both gene expression and alternative splicing patterns across the whole genome with roughly four probes per exon and 40 probes per gene. Posthybridization, washing, and staining were performed in a Fluidics Station 450 (Affymetrix). Hybridization signal was measured using a GC3000 GeneChip scanner (Affymetrix) to detect the fluorescence at each probe loci. Signal values were generated using Expression Console 1.1 (Affymetrix) and normalized using the robust multichip analysis algorithm. For each RNA sample, a gene transcript abundance list was generate based on average and normalized fluorescence intensities. Comparisons of transcript levels for specific genes between sets was performed using a moderated t test with a false discovery rate adjustment of the P value. Transcripts with a false discovery rate P value less than 0.05 and a fold change (±) 1.5 or greater compared with appropriate control were considered to reflect genes whose expression was altered by the experimental treatment. Lists of altered gene were then subjected to biological/functional systems analysis using Pathway Studio 7.0 (Ariadne Inc., Rockville, MD) by assessing the cooccurrence of Gene Ontology (GO) functional annotation terms associated with affected genes within each treatment set. Default stringency parameters were applied and the Fisher exact test was used to measure the extent of enrichment of gene annotation terms (35, 36).
Statistical analyses
All cell culture conditions were performed in triplicate and each experiment was repeated at least three times. Data were compared by Student's t test (equal variance, two tailed). Correlation analysis was performed using Pearson's correlation coefficient analysis. Differences were considered statistically significant when P < 0.05.
Results
Conditional expression of PR-A and PR-B in hTERT-HMA/B cells
hTERT-HMA/B cells expressed PR-A from the TetON-PRA transgene in response to DOX and PR-B from the RheoSwitch-PRB transgene in response to RSL and each in a dose-dependent manner (Fig. 1A). In the basal state, the cells expressed low levels of PR-A and no PR-B (undetectable by immunoblotting). Using quantitative RT-PCR (qRT-PCR) with primers specific for the PR-A transgene, we confirmed that the basal expression of PR-A was derived from the TetON-PRA transgene (data not shown) and was likely due to trace amounts of tetracycline in the fetal bovine serum added to the cell culture media. Immunoreactive PR-A and PR-B increased in all cells in response to DOX and RSL, respectively, and each receptor localized mainly to the nucleus, especially in progesterone-treated cells, consistent with their function as ligand-activated transcription factors (Fig. 1B).
Fig. 1.

Inducible expression of functional PR-A and PR-B from stably incorporated TetON-Advanced (PR-A) and RheoSwitch (PR-B) transgenes in hTERT-HMA/B cells. A, Whole-cell lysates of hTERT-HMA/B cells treated with varying concentrations of DOX and RSL for 48 h assessed by immunoblot analysis for nPR (5 min exposure) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (30 sec exposure). B, nPR immunolocalization (PgR1294 antibody; bar, 100 μm) in hTERT-HMA/B cells exposed to vehicle or DOX (10 ng/ml) for 24 h. Consistent with the immunoblot outcomes (far right lane), the cells expressed low levels of nPR under basal conditions. DOX induced expression of PR-A (indicated by grey intensity) in all cells. Identical outcomes were obtained with cells exposed to RSL (data not shown).
Genes affected by progesterone via PR-A and PR-B in hTERT-HMA/B cells
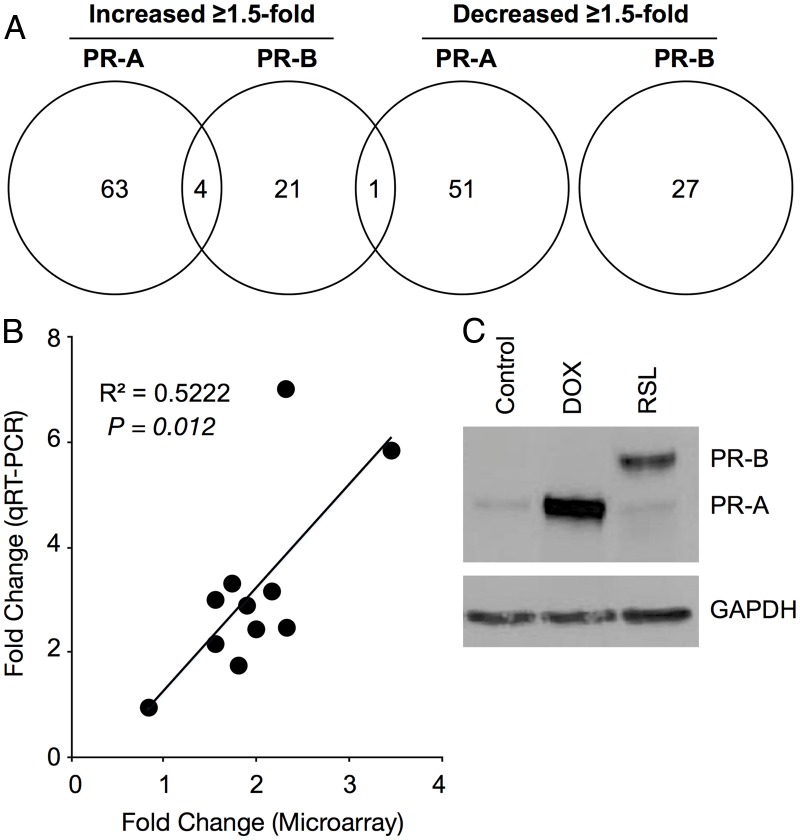
Genes affected by progesterone via PR-A and PR-B in hTERT-HMA/B cells were identified by microarray-based genome-wide transcriptome analyses. In response to 100 nm progesterone for 6 h, PR-A and PR-B affected (≥1.5-fold change compared with cells expressing each nPR but treated with vehicle instead of progesterone) the levels of transcripts representing distinct sets of genes. The full cohort of affected transcripts included multiple noncoding RNA and transcripts representing genomic areas with unknown function. Analyses were limited to transcripts representing annotated genes encoding proteins with known function (Fig. 2 and Supplement 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). In PR-A-dominant cells, progesterone affected the expression of 119 genes (67 increased and 52 decreased). In PR-B-dominant cells, progesterone affected the expression of 53 genes (26 increased and 27 decreased). Five genes were common to both sets; four (APOBEC3B, BRD7, PCDHB8, and PLAT) were increased by progesterone via PR-A and PR-B, and one (MST1) was increased by progesterone via PR-B and decreased by progesterone via PR-A. Expression of 11 genes of interest was assessed by qRT-PCR in separate cell cultures and we confirmed that changes induced by progesterone via PR-A and PR-B were in the same direction as detected by microarray analysis (Fig. 2B).
Fig. 2.
Effects of PR-A and PR-B on gene expression in hTERT-HMA/B cells. A, Venn diagram showing numbers of genes affected (≥1.5-fold change compared with nPR expressing cells in the absence of progesterone) by progesterone (100 nm for 6h) via PR-A and PR-B. Only annotated genes encoding known proteins were included in the analysis. B, Correlation analysis (Pearson's correlation analysis) between microarray and qRT-PCR outcomes for 11 different genes affected by PR-A and PR-B. C, nPR immunoblot analysis of total cell lysates from hTERT-HMA/B cells used for the microarray analysis. Cells were exposed to DOX (50 ng/ml) and RSL (300 nm) for 24 h. GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
Progesterone via PR-A- and PR-B-affected genes involved in multiple cellular processes as assessed by Pathway Studio (analysis) (Table 2). Twenty GO terms were significantly (P < 0.01) overrepresented in the list of genes whose expression was increased by progesterone via PR-A. Among those were mitosis, cell division, cell cycle, traversing start control point of mitotic cell cycle, regulation of cell cycle, inflammatory response, and neutrophil chemotaxis. Five GO terms (regulation of epithelial cell differentiation, glycoprotein metabolic process, organ morphogenesis, and Wnt receptor signaling) were significantly (P < 0.01) overrepresented in the list of genes whose expression was decreased by progesterone via PR-A. These outcomes suggest that progesterone via PR-A affects myometrial cell proliferation, inflammatory response, and differentiation. Four GO terms (translation, RNA metabolic process, translational initiation, and blood coagulation) were significantly overrepresented among the genes whose expression was increased by progesterone via PR-B and three GO terms (inflammatory response, cellular calcium ion homeostasis, and female pregnancy) were overrepresented among the genes decreased by progesterone via PR-B.
Table 2.
GO term biological processes associated with genes affected by progesterone via PR-A and PR-B in hTERT-HMA/B cells (P < 0.01)
| P value | |
|---|---|
| Increased by P4 via PR-A | |
| Mitosis | 0.000002 |
| Cell division | 0.000010 |
| Cell cycle | 0.000157 |
| Traversing start control point of mitotic cell cycle | 0.000245 |
| Protein import into mitochondrial inner membrane | 0.000245 |
| Regulation of cell cycle | 0.000317 |
| Positive regulation of Ras protein signal transduction | 0.000392 |
| Inflammatory response | 0.000464 |
| Double-strand break repair via homologous recombination | 0.001032 |
| Chemotaxis | 0.001163 |
| Response to estrogen stimulus | 0.001172 |
| Protein targeting to mitochondrion | 0.001622 |
| Negative regulation of cell proliferation | 0.001851 |
| Regulation of transcription, DNA dependent | 0.001889 |
| Neutrophil chemotaxis | 0.002536 |
| Antiapoptosis | 0.003029 |
| Response to DNA damage stimulus | 0.003140 |
| Transcription | 0.003600 |
| Cell proliferation | 0.006396 |
| Negative regulation of transcription | 0.008941 |
| Decreased by P4 via PR-A | |
| Regulation of epithelial cell differentiation | 0.000276 |
| Glycoprotein metabolic process | 0.000337 |
| Organ morphogenesis | 0.002616 |
| Wnt receptor signaling pathway | 0.004044 |
| Transcription | 0.005704 |
| Increased by P4 via PR-B | |
| Translation | 0.000925 |
| RNA metabolic process | 0.001108 |
| Translational initiation | 0.001594 |
| Blood coagulation | 0.006655 |
| Decreased by P4 via PR-B | |
| Inflammatory response | 0.000024 |
| Cellular calcium ion homeostasis | 0.004004 |
| Female pregnancy | 0.004745 |
P4, Progesterone.
Progesterone is thought to affect uterine contractility by modulating CAP gene expression in myometrial cells. Therefore, we expected that progesterone via PR-B would inhibit the expression genes encoding stimulatory CAP [e.g. connexin-43 (GJA1)] and/or increase expression of genes encoding relaxatory CAP (e.g. PGI synthase). However, in our microarray data sets, the only recognized CAP gene affected by progesterone was PTGS2 (also known as cyclooxygenase-2), whose expression was increased 2.2-fold by progesterone in PR-A-dominant cells. Interestingly, other NF-κB-regulated proinflammatory genes, including IL-1α (IL1A), IL-8 (IL8), and pentraxin-related gene rapidly induced by IL-1β (PTX3) were also increased by progesterone in PR-A-expressing cells.
Combined effects of PR-A and PR-B on expression of proinflammatory/prolabor genes
More detailed experiments were performed to assess the combined effects of PR-A and PR-B on the capacity for progesterone to modulate proinflammatory and CAP gene expression in hTERT-HMA/B cells. For these studies cells were induced to coexpress PR-A and PR-B at relative levels similar to those observed in late gestation myometrium (25). Consistent with the microarray data, we found that in PR-A-dominant cells (PR-A to PR-B ratio ∼3; similar to term in labor myometrium) progesterone increased expression of PTGS2, IL8, and IL-1β (IL1B). In contrast, in PR-B-dominant cells (PR-A to PR-B ratio ∼0.5; similar to preterm quiescent myometrium) expression of the same genes was decreased by progesterone (Fig. 3A). Effects on PTGS2 and IL8 were confirmed at the protein level (Fig. 3B).
Fig. 3.
Effects of the PR-A to PR-B ratio on progesterone-induced gene expression in hTERT-HMA/B cells. A, Effect of PR-A to PR-B ratio levels on basal and progesterone-induced expression of PTGS2, IL8, IL1B, and GJA1 in hTERT-HMA/B cells conditioned with DOX and RSL to be in PR-B- or PR-A-dominant states (indicated by the immunoblot). Cells were exposed to progesterone or vehicle for 16 h and then various concentrations of DOX and RSL for 12 h to modify the PR-A and PR-B levels. (Data are relative to abundance of mRNA encoding α-actin in each experimental condition; mean ± se; n = 3; *, P < 0.05.) B, Immunoblot analysis of PTGS2 and IL-8 levels in hTERT-HMA/B cells exposed to RSL and various levels of DOX ± progesterone (100 nm). Data are representative of triplicate experiments.
Repressive effects of PR-A on PR-B-induced transactivation
A core tenet of the PR-A/PR-B hypothesis is that PR-A inhibits the transcriptional activity of PR-B. This was tested by determining whether PR-A affects the capacity for progesterone to modulate expression of two PR-B-responsive genes: FK506 binding protein 5 (FKBP5) and NFKB1A. As expected, progesterone increased expression of FKBP5 in PR-B-expressing cells. PR-A alone had no effect on FKBP5 expression and did not affect PR-B-induction of FKBP5 expression (Fig. 4). Similarly, expression of NFKB1A was increased by progesterone in PR-B-expressing cells; however, in this case PR-A inhibited the capacity for progesterone to increase NFKB1A expression via PR-B (Fig. 4). These data suggest that PR-A repression of PR-B-mediated transcriptional activity in human myometrial cells is gene promoter specific.
Fig. 4.
Effects of PR-A and PR-B on progesterone (100 nm, 24 h)-induced expression of NFKB1A and FKBP5 in hTERT-HMA/B cells. Progesterone via PR-B increased NFKB1A and FKBP5 expression. PR-A inhibited the effects of PR-B on NFKB1A expression in a dose-dependent manner but did not affect PR-B-mediated expression of FKBP5 (data are relative to abundance of mRNA encoding α-actin in each experimental condition; mean ± se; n = 3; *, P < 0.05).
Effect of the PR-A to PR-B ratio on responsiveness to proinflammatory stimuli
The induction of NFKB1A by progesterone via PR-B and the inhibition of that activity by PR-A suggests that in the PR-B-dominant state, progesterone inhibits NF-κB-induced gene expression (due to increased levels of IκBα) and that this is suppressed by PR-A. This hypothesis was tested by determining the effect of PR-A on the response of hTERT-HMA/B cells to lipopolysaccharide (LPS)-induced expression of PTGS2. We found that progesterone inhibited LPS-induced PTGS2 expression in PR-B-dominant cells and that this effect was blocked by PR-A (Fig. 5).
Fig. 5.
Effects of progesterone (P4) and the PR-A to PR-B ratio on basal and LPS-induced expression of PTGS2 in hTERT-HMA/B cells. As expected, LPS increased PTGS2 expression. In PR-B-dominant cells, progesterone inhibited basal and LPS-induced PTGS2 expression. In PR-A-dominant cells, progesterone increased basal PTGS2 expression and did not affect LPS-induced PTGS2 expression. Cells were exposed to vehicle (Veh) or RSL and DOX ± P4 (100 nm) for 24 h ± LPS (1 μg/ml; final 6 h). Immunoblot: nPR expression achieved by DOX and RSL treatment (data are relative to abundance of mRNA encoding α-actin in each experimental condition; mean ± se; n = 3; *, P < 0.05). GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
Discussion
Our goal was to test the PR-A/PR-B hypothesis for functional progesterone withdrawal in human parturition. The hypothesis posits that for most of human pregnancy, when myometrial cells express mainly PR-B (i.e. PR-A to PR-B ratio <1), progesterone promotes uterine relaxation via PR-B-mediated effects on gene expression and that functional progesterone withdrawal at parturition is mediated by increased expression of PR-A (i.e. PR-A to PR-B ratio >1), which inhibits the relaxatory actions of PR-B. Published data supporting the hypothesis were derived from studies in cultured human myometrial cells in which the PR-A and PR-B levels were experimentally manipulated by transient cotransfection of constitutively active PR-A and PR-B expression plasmids. In addition, progesterone-induced transcriptional activity was assessed using an artificial progesterone-responsive reporter plasmid controlled by the canonical PRE (25, 27, 32). Although those studies showed that PR-A repressed the transcriptional activity of PR-B, the interpretation of the data is problematic because the cotransfection approach likely produced heterogenous cultures consisting of cells transfected with the PR-A expression plasmid only, cells transfected with the PR-B expression plasmid only, cells transfected with both plasmids, and untransfected cells, with the proportion of each cell type determined by the relative amounts of PR-A and PR-B expression plasmids in the transfection cocktail. Thus, although immunoblot analyses of PR-A and PR-B levels in cell lysate from the entire culture showed expected changes in the PR-A to PR-B ratio, those outcome likely did not reflect PR-A to PR-B levels in individual cells but rather the proportion of cells in each transfection group. In addition, because the PR-A and PR-B expression plasmids were controlled by constitutive and highly active promoters (e.g. the cytomegalovirus promoter), each nPR would have been overexpressed, raising the possibility of squelching artifact due to excessive sequestration of coregulators (37). To address these shortcomings, we developed the hTERT-HMA/B cell line in which the cellular PR-A to PR-B ratio can be experimentally controlled over a broad range across the entire cell population (Fig. 1).
As a starting point to test the PR-A/PR-B hypothesis, we used the hTERT-HMA/B cells to identify genes affected by each receptor in response to progesterone. Using genome-wide transcriptome analyses, we found that PR-A and PR-B were both transcriptionally active in response to progesterone and that they affected the expression of distinct cohorts of genes. This finding is consistent with similar studies in breast cancer cells lines engineered to express PR-A and/or PR-B (38, 39) and studies in transgenic mice with selective ablation of either PR-A or PR-B (40–42). Our data support the growing consensus that PR-A is transcriptionally active upon ligand binding and mediates distinct action of progesterone. Importantly, our data show that PR-A is more than just a repressor of PR-B-mediated transactivation in human myometrial cells. Indeed, Pathway Studio analysis suggests that PR-A and PR-B mediate distinct and specific actions of progesterone some of which are relevant to myometrial function. For example, the myometrium undergoes remarkable growth and remodeling during pregnancy and exhibits inflammation at mensuration and parturition and during postpartum involution. Some functions, such as blood coagulation, are important for uterine remodeling after birth and during the menstruation. Thus, our data suggest that the diverse actions of progesterone in the myometrium are mediated by the combined and distinct genomic actions of PR-A and PR-B. In this context, we propose that the rise in PR-A expression at parturition mediates specific actions of progesterone on gene expression in myometrial cells.
The PR-A/PR-B hypothesis predicts that progesterone promotes myometrial relaxation via PR-B-mediated inhibition of stimulatory CAP genes. We were therefore interested in the effects of PR-A and PR-B on the expression of recognized CAP genes. Our microarray studies, however, showed that the only recognized CAP gene affected was PTGS2, whose expression was increased by progesterone only in cells expressing PR-A. PTGS2 encodes the key rate-limiting enzyme involved in PG synthesis and therefore is considered a prolabor and proinflammatory gene. Its expression is up-regulated by NF-κB and increases in myometrial cells in association with the onset of labor (15, 26), which is consistent with the hypothesis that labor involves myometrial inflammation (17). Recently, Mittal et al. (15) found that labor at term was associated with increased expression of multiple proinflammatory genes including PTGS2, IL8, IL1A, and PTX3 in lower uterine segment myometrium in the absence of detectable infection. We found that progesterone via PR-A increased expression of the same proinflammatory genes in hTERT-HMA/B cells. Our data suggest that the parturition-associated increase in proinflammatory gene expression in the human pregnancy myometrium is, at least in part, driven by progesterone via PR-A. Based on these findings, we propose that the PR-A/PR-B hypothesis be broadened to include the possibility that the parturition-associated rise in myometrial cell PR-A expression alters progesterone responsiveness such that it becomes a prolabor hormone by increasing expression of proinflammatory genes. Our study provides no indication of the mechanism by which PR-A increases proinflammatory gene expression in response to progesterone in myometrial cells. However, we speculate that the effect could be a direct transcriptional effect of PR-A at the promoters of the proinflammatory genes or as an indirect effect by the modulation of NF-κB activity.
In contrast to the proinflammatory actions of progesterone mediated by PR-A, PR-B mediated antiinflammatory actions by inhibiting the expression of proinflammatory genes (Fig. 3) and decreasing responsiveness to proinflammatory stimuli (Fig. 5). These findings are consistent with the proposal by Siiteri et al. (14) that a key mechanism by which progesterone maintains pregnancy is via antiinflammatory actions in myometrial cells. More recently Hardy et al. (20) showed that progesterone inhibits the interaction of NF-κB with targets in the PTGS2 promoter and augments NFKB1A expression in hTERT-HM cells. Our data suggest that antiinflammatory actions of progesterone in human myometrial cells (especially the increase in NFKB1A expression) are mediated exclusively by PR-B. As proposed by Hardy et al. (20), this may be a key mechanism by which progesterone promotes myometrial relaxation.
We found that PR-A inhibited the capacity for progesterone via PR-B to stimulate NFKB1A expression (Fig. 4). This supports the concept that PR-A represses that transcriptional activity of PR-B, which is a key tenet of the PR-A/PR-B hypothesis. Interestingly, the repressive effect of PR-A appeared to be gene specific because it did not repress the capacity for PR-B to augment expression of another PR-B-responsive gene, FKBP5, in response to progesterone. Thus, our data suggest that the repressive actions of PR-A on PR-B transcriptional activity are gene promoter specific. This could occur at PR-A-specific response elements in target gene promoters and/or involve PR-A-specific coregulators. It is also possible that PR-A acts as a competitive and transcriptionally inactive antagonist by displacing PR-B at common promoter targets. Further studies are needed to elucidate the features of the NFKB1A promoter, compared with the FKBP5 promoter, that confer PR-A-mediated repression of PR-B. The repressive effect of PR-A at the NFKB1A promoter may be a key mechanism for PR-A-mediated functional progesterone withdrawal because it would abrogate the antiinflammatory activity of progesterone mediated by PR-B and allow NF-κB-mediated proinflammatory stimuli to induce an inflammatory response. This hypothesis is supported by our finding that PR-A inhibited the capacity for progesterone via PR-B to repress LPS-induced PTGS2 expression (Fig. 5).
Taken together, our data support two major predictions of the PR-A/PR-B hypothesis: 1) that the PR-A to PR-B ratio profoundly affects the capacity for progesterone to control gene expression in myometrial cells and 2) that PR-A represses the relaxatory actions of PR-B. In human pregnancy this is important because progesterone actions, and specifically progesterone withdrawal at parturition, are not controlled by changes in circulating progesterone levels, but instead it is hypothesized that progesterone withdrawal occurs by changes in myometrial cell progesterone responsiveness. The PR-A/PR-B hypothesis posits that an increase in the PR-A to PR-B ratio induces functional progesterone withdrawal. We propose (Fig. 6) that during most of human pregnancy, progesterone via PR-B maintains relaxation by inhibiting NF-κB activity (in part by increasing NFKB1A expression) and as such, decreases myometrial cells, proinflammatory gene expression, and responsiveness to NF-κB-mediated proinflammatory/prolabor stimuli.
Fig. 6.

Working model. Progesterone via PR-B inhibits proinflammatory gene expression and suppresses responsiveness of myometrial cells to proinflammatory/prolabor stimuli by stimulating expression of NFKB1A, which inhibits NF-κB activity. PR-A blocks the antiinflammatory actions of progesterone mediated by PR-B (e.g. by blocking PR-B induction of NFKB1A expression) and directly increases proinflammatory gene expression (indicated by PTGS2), leading to a proinflammatory state within the myometrial compartment. PR-A expression is increased by PGF2α whose levels increase in response to prolabor stimuli. As pregnancy progresses, prolabor stimuli (e.g. uterine distention, intrauterine infection, and fetal membrane PG production) increase local PGF2α levels within the gestational tissues, leading to increased myometrial cell PR-A expression and a gradual increase in the PR-A to PR-B ratio in myometrial cells. A PR-A to PR-B threshold exists above which PR-A represses the antiinflammatory activity of PR-B and begins to mediate proinflammatory effects of progesterone. At this point a positive-feedback proinflammatory loop develops within the myometrium whereby increased production of PGF2α by myometrial cells (due to progesterone/PR-A mediated increased PTGS2 expression) exerts an autocrine/paracrine effect that further augments PR-A expression that further increases the inflammatory state leading to a further increase in PGF2α. Eventually the PGF2α levels increase to a point whereby the hormone exerts potent uterotonic actions to induce labor. FP, PGF2α receptor; PGR, progesterone receptor.
Our data suggest that PR-A not only blocks the antiinflammatory actions of progesterone mediated by PR-B but also directly increases proinflammatory gene expression in response to progesterone. Based on this paradigm, the prepartum rise in myometrial cell PR-A expression becomes a critical early event in the parturition process. Our previous studies suggest that PGF2α increases PR-A but not PR-B expression in a human myometrial cell line (43). We propose that as pregnancy progresses the net cumulative effects of multiple prolabor/proinflammatory stimuli (e.g. uterine distention, intrauterine infection, fetal maturation signals, fetal membrane PG production, and maternal stress) increases local production of PGF2α by various cell types within the gestational tissues, leading to increased exposure of myometrial cells to PGF2α, which increases myometrial cell PR-A expression. This gradually increases in the myometrial cell PR-A to PR-B ratio until it reaches a threshold above which PR-A represses the antiinflammatory activity of PR-B. At this level myometrial cell responsiveness to progesterone changes to the PR-A-dominant pattern, whereby progesterone increases expression of proinflammatory genes. This leads to development of a positive-feedback proinflammatory state within the myometrium such that increased production of PGF2α by myometrial cells, due in part to progesterone/PR-A-mediated stimulation of PTGS2 expression, exerts an autocrine/paracrine effect that augments PR-A expression, which augments the inflammatory state leading to an increase in PGF2α. Eventually local PG synthesis increases to a point at which PGF2α levels exerts potent uterotonic actions to induce labor. The positive feedback proinflammatory interaction between PR-A and PGF2α may explain the functional link between progesterone and inflammation in the physiology of human parturition. Importantly, it explains why labor is difficult to stop once it starts and why labor can be initiated by proinflammatory stimuli such as intrauterine infection or administration of PGF2α.
Our model provides a foundation for understanding the functional interaction between inflammation and progesterone action in human pregnancy and parturition. Clinical studies in the last decade show that prophylactic progestin therapy decreases the incidence of preterm birth, albeit in a subset of women with elevated risk for preterm birth (44–47). The mechanism for the effect and why it is limited to relatively small subset of pregnancies is not known. A clear understanding of the molecular interactions and genomic processes through which PR-A and PR-B act to mediate and modulate progesterone actions in myometrial cells will lead to the development and more effective progestin-based strategies to control labor and prevent preterm birth.
Supplementary Material
Acknowledgments
This work was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Development Grants HD060127 and HD051563 and the March of Dimes Birth Defects Foundation Grant 21-FY10-173 (to S.M.). Support for the Gene Expression and Genotyping Facility of the Case Comprehensive Cancer Center was from the National Institutes of Health (grant CA43703).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CT
- Cycle threshold
- DOX
- doxycycline
- FKBP5
- FK506 binding protein 5
- GJA1
- gap junction protein, α1
- GO
- Gene Ontology
- hTERT-HM
- human telomerase reverse transcriptase immortalized human myometrial cell line
- LPS
- lipopolysaccharide
- NF-κB
- nuclear factor-κB
- NFKB1A
- inhibitor-κBα
- nPR
- nuclear PR
- OXT
- oxytocin
- PG
- prostaglandin
- PR
- progesterone receptor
- PRE
- progesterone response element
- PTGS2
- PG endoperoxide synthase-2
- qRT-PCR
- quantitative RT-PCR
- RSL
- RheoSwitch ligand
- TTBS
- Tris, NaCl, and Tween 20.
References
- 1. Corner GW. 1946. The hormones in human reproduction. London: Princeton University Press [Google Scholar]
- 2. Csapo A. 1956. Progesterone block. Am J Anat 98:273–291 [DOI] [PubMed] [Google Scholar]
- 3. Sanborn BM. 2000. Relationship of ion channel activity to control of myometrial calcium. J Soc Gynecol Investig 7:4–11 [DOI] [PubMed] [Google Scholar]
- 4. Sanborn BM, Yue C, Wang W, Dodge KL. 1998. G protein signalling pathways in myometrium: affecting the balance between contraction and relaxation. Rev Reprod 3:196–205 [DOI] [PubMed] [Google Scholar]
- 5. Sanborn BM. 1995. Ion channels and the control of myometrial electrical activity. Semin Perinatol 19:31–40 [DOI] [PubMed] [Google Scholar]
- 6. Garfield RE, Hayashi RH. 1981. Appearance of gap junctions in the myometrium of women during labor. Am J Obstet Gynecol 140:254–260 [DOI] [PubMed] [Google Scholar]
- 7. Mesiano S, Wang Y, Norwitz ER. 2011. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 18:6–19 [DOI] [PubMed] [Google Scholar]
- 8. Neilson JP. 2000. Mifepristone for induction of labour. Cochrane Database Syst Rev CD002865. [DOI] [PubMed] [Google Scholar]
- 9. Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. 1991. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril 56:385–393 [DOI] [PubMed] [Google Scholar]
- 10. Chwalisz K. 1994. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod 9(Suppl 1):131–161 [DOI] [PubMed] [Google Scholar]
- 11. Chwalisz K, Garfield RE. 1994. Antiprogestins in the induction of labor. Ann NY Acad Sci 734:387–413 [DOI] [PubMed] [Google Scholar]
- 12. Chwalisz K, Stockemann K, Fuhrmann U, Fritzemeier KH, Einspanier A, Garfield RE. 1995. Mechanism of action of antiprogestins in the pregnant uterus. Ann NY Acad Sci 761:202–223 [DOI] [PubMed] [Google Scholar]
- 13. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. 2007. The role of inflammation and infection in preterm birth. Seminars in reproductive medicine 25:21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siiteri PK, Febres F, Clemens LE, Chang RJ, Gondos B, Stites D. 1977. Progesterone and maintenance of pregnancy: is progesterone nature's immunosuppressant? Ann NY Acad Sci 286:384–397 [DOI] [PubMed] [Google Scholar]
- 15. Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L, Mazaki-Tovi S, Hassan SS, Mesiano S, Kim CJ. 2010. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 38:617–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Norman JE, Bollapragada S, Yuan M, Nelson SM. 2007. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth 7(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. 2003. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 9:41–45 [DOI] [PubMed] [Google Scholar]
- 18. Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. 1999. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 14:229–236 [PubMed] [Google Scholar]
- 19. Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. 2002. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 66:445–449 [DOI] [PubMed] [Google Scholar]
- 20. Hardy DB, Janowski BA, Corey DR, Mendelson CR. 2006. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-κB activation of cyclooxygenase 2 expression. Mol Endocrinol 20:2724–2733 [DOI] [PubMed] [Google Scholar]
- 21. Young IR, Renfree MB, Mesiano S, Shaw G, Jenkin G, Smith R. 2010. The comparative physiology of parturition in mammals: hormones and parturition in mammals. In: Norris D, Lopez K, eds. Hormones and reproduction in vertebrates. London: Academic Press [Google Scholar]
- 22. Walsh SW, Stanczyk FZ, Novy MJ. 1984. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab 58:629–639 [DOI] [PubMed] [Google Scholar]
- 23. Boroditsky RS, Reyes FI, Winter JS, Faiman C. 1978. Maternal serum estrogen and progesterone concentrations preceding normal labor. Obstet Gynecol 51:686–691 [PubMed] [Google Scholar]
- 24. Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. 1972. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol 112:1095–1100 [DOI] [PubMed] [Google Scholar]
- 25. Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. 2007. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab 92:1927–1933 [DOI] [PubMed] [Google Scholar]
- 26. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. 2002. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 87:2924–2930 [DOI] [PubMed] [Google Scholar]
- 27. Condon JC, Hardy DB, Kovaric K, Mendelson CR. 2006. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-κB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 20:764–775 [DOI] [PubMed] [Google Scholar]
- 28. Haluska GJ, Wells TR, Hirst JJ, Brenner RM, Sadowsky DW, Novy MJ. 2002. Progesterone receptor localization and isoforms in myometrium, decidua, and fetal membranes from rhesus macaques: evidence for functional progesterone withdrawal at parturition. J Soc Gynecol Investig 9:125–136 [PubMed] [Google Scholar]
- 29. Henderson D, Wilson T. 2001. Reduced binding of progesterone receptor to its nuclear response element after human labor onset. Am J Obstet Gynecol 185:579–585 [DOI] [PubMed] [Google Scholar]
- 30. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. 2003. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA 100:9518–9523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong X, Shylnova O, Challis JR, Lye SJ. 2005. Identification and characterization of the protein-associated splicing factor as a negative co-regulator of the progesterone receptor. J Biol Chem 280:13329–13340 [DOI] [PubMed] [Google Scholar]
- 32. Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. 2001. Interaction between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod 7:875–879 [DOI] [PubMed] [Google Scholar]
- 33. Giangrande PH, Kimbrel EA, Edwards DP, McDonnell DP. 2000. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol Cell Biol 20:3102–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Condon J, Yin S, Mayhew B, Word RA, Wright WE, Shay JW, Rainey WE. 2002. Telomerase immortalization of human myometrial cells. Biol Reprod 67:506–514 [DOI] [PubMed] [Google Scholar]
- 35. Huang da W, Sherman BT, Lempicki RA. 2009. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- 37. Cahill MA, Ernst WH, Janknecht R, Nordheim A. 1994. Regulatory squelching. FEBS Lett 344:105–108 [DOI] [PubMed] [Google Scholar]
- 38. Graham JD, Yager ML, Hill HD, Byth K, O'Neill GM, Clarke CL. 2005. Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endocrinol 19:2713–2735 [DOI] [PubMed] [Google Scholar]
- 39. Yudt MR, Berrodin TJ, Jelinsky SA, Hanna LA, Brown EL, Chippari S, Bhat RA, Winneker RC, Zhang Z. 2006. Selective and opposing actions of progesterone receptor isoforms in human endometrial stromal cells. Mol Cell Endocrinol 247:116–126 [DOI] [PubMed] [Google Scholar]
- 40. Conneely OM, Mulac-Jericevic B, Lydon JP. 2003. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68:771–778 [DOI] [PubMed] [Google Scholar]
- 41. Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. 2003. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. 2000. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- 43. Madsen G, Zakar T, Ku CY, Sanborn BM, Smith R, Mesiano S. 2004. Prostaglandins differentially modulate progesterone receptor-A and -B expression in human myometrial cells: evidence for prostaglandin-induced functional progesterone withdrawal. J Clin Endocrinol Metab 89:1010–1013 [DOI] [PubMed] [Google Scholar]
- 44. Meis PJ, Aleman A. 2004. Progesterone treatment to prevent preterm birth. Drugs 64:2463–2474 [DOI] [PubMed] [Google Scholar]
- 45. Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O'Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S. 2003. Prevention of recurrent preterm delivery by 17α-hydroxyprogesterone caproate. N Engl J Med 348:2379–2385 [DOI] [PubMed] [Google Scholar]
- 46. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. 2007. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med 357:462–469 [DOI] [PubMed] [Google Scholar]
- 47. da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. 2003. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 188:419–424 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.