Abstract
Context:
Graves' ophthalmopathy (GO) is characterized by expanded volume of the orbital fat and extraocular muscle tissues and elevated levels of TSH receptor autoantibodies (TRAb). The expansion of orbital tissues involves accumulation of hyaluronic acid (HA) within the orbit.
Objective:
The objective of the study was to determine whether a monoclonal stimulatory TRAb (M22) impacts HA synthesis in GO orbital cells and, if so, whether this might be blocked by an IGF-I receptor (IGF-IR)-blocking antibody (1H7) or inhibitors of various downstream signaling cascades.
Design:
GO orbital fibroblast cultures (n = 6) were treated with M22, bovine TSH (bTSH), or IGF-I in serum-free medium. Some cultures also received 1H7, LY294002, rapamycin, or protein kinase A inhibitor.
Main Outcome Measures:
HA production and phosphorylated Akt levels in media or immunoblotting for phosphorylated Akt were measured.
Results:
M22 or bTSH stimulated HA synthesis (2.1-fold with 100 ng/ml M22 and 1.9-fold with 10 U/liter bTSH; P < 0.05 each). M22-induced HA synthesis was inhibited by LY294002 or rapamycin but not by protein kinase inhibitor. HA synthesis stimulated by M22 or IGF-I was inhibited by 1H7 (mean 36.6 ± 5.6% and mean 45.8 ± 7.6%, respectively; P < 0.05 each). Similarly, M22- or IGF-I-stimulated Akt phosphorylation was inhibited by 1H7 (mean 54 ± 9.6 and 36.1 ±8.8%, respectively; P = 0.01 each).
Conclusions:
The stimulatory TRAb M22 increases HA production in undifferentiated GO orbital fibroblasts via phosphoinositide 3-kinase/phosphorylated AKT/mammalian target of rapamycin activation. Blockade of IGF-IR inhibits both HA synthesis and Akt phosphorylation induced by M22 or IGF-I in these cells, suggesting that TSH receptor and IGF-IR signaling may be closely linked in the GO orbit.
Graves' ophthalmopathy (GO) is an inflammatory autoimmune disorder of the orbital adipose tissue and extraocular muscles (1, 2). Many of the signs and symptoms of GO, including proptosis and ocular congestion, result from expansion of these tissues. The adipose tissue volume increases owing in part to new fat cell development (adipogenesis) within the orbital fat (2). The accumulation of hydrophilic glycosaminoglycans, primarily hyaluronic acid (HA), within the orbital adipose tissue and the perimysial connective tissue between the extraocular muscle fibers, further expands the fat compartments and enlarges the extraocular muscle bodies (3). HA is produced by fibroblasts residing within the orbital fat and extraocular muscles, and its synthesis in vitro is stimulated by several cytokines and growth factors, including IL-1 (4), interferon-γ (5), platelet-derived growth factor, and IGF-I (6).
In addition to cytokines and growth factors, HA production in GO orbital fibroblasts has been shown by the group of Smith and Hoa (7) to be augmented by the IgG fraction of pooled serum samples from patients with Graves' hyperthyroidism. The authors found this effect to be inhibited by a monoclonal antibody that blocks the IGF-I receptor (IGF-IR) α-subunit, termed 1H7. They concluded that HA production was stimulated in these cells by putative IGF-IR autoantibodies present in the Graves' IgG fraction signaling through that receptor, rather than by TSH receptor autoantibodies (TRAb) signaling through the TSH receptor (TSHR). We recently reported that a high-affinity human monoclonal IgG1 λ-chain stimulatory TSHR antibody, known as M22 (8, 9), enhances adipogenesis in GO orbital fibroblasts via phosphoinositide 3-kinase (PI3K) activation (10).
We undertook the current study to determine whether M22 might also impact HA synthesis in these cells and, if so, whether this might be blocked by the IGF-IR antagonist antibody 1H7. We additionally studied downstream signaling cascades activated by M22 in orbital preadipocytes to elucidate mechanisms involved and define pathways that might be targeted to develop novel therapeutic strategies for patients with GO.
Materials and Methods
Cell culture
Orbital adipose tissue specimens were obtained from euthyroid patients during the course of orbital decompression surgery for severe GO. Use of these samples was approved by the Mayo Clinic Institutional Review Board and studies carried out according to institutional review board guidelines. The tissues were transported to the laboratory, minced, and placed directly in plastic culture dishes, allowing preadipocyte fibroblasts to proliferate as described previously (11). Briefly, cells were propagated in medium 199 containing 20% fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT), penicillin (100 U/ml), and gentamicin (20 μg/ml) in a humidified 5% CO2 incubator at 37 C and maintained in 75-mm2 flasks with medium 199 containing 10% FBS and antibiotics.
In experiments to determine the ability of M22, bovine TSH (bTSH), or IGF-I to stimulate HA production, orbital cells were cultured in medium 199 containing 20% FBS in 24-well plates until nearly confluent. The cells were deprived of serum for 24 h before the start of experiments and maintained for the duration in serum-free media (199 or DMEM/F12). Cultures were treated for 48 h with M22 (10, 50, or 100 ng/ml; 67, 335, or 670 pm; Kronus, Boise, ID; no. M22-1b), bTSH (1, 5, or 10 U/liter; Sigma Aldrich Co., St. Louis, MO; no. T-8931), IGF-I (10 ng/ml; R&D Systems., Minneapolis, MN; no. 291-G1; used as positive control), or isotype control IgG2 (10 μg/ml; BD Biosciences, Franklin Lakes, NJ; no. 555574) or were untreated.
In experiments performed to assess the impact of inhibitors of cell-signaling pathways on M22-stimulated HA production, or the impact of 1H7 on M22- or IGF-I-stimulated HA production, orbital cells were propagated as above until confluent, serum starved for 24 h, and then pretreated for 24 h with each inhibitor individually, followed by 48 h treatment in serum-free medium with inhibitor alone or with the inhibitor and M22 (50 or 100 ng/ml) or were untreated for the same period of time. Inhibitors used in these studies were protein kinase inhibitor (PKI; 10 μm; Promega Co., Madison, WI; no. V5681; a protein kinase A inhibitor), LY294002 (10 μm; SA Biosciences, Fredrick, MD; an inhibitor of the enzyme PI3K required for Akt phosphorylation), or rapamycin [10 μm; Sigma Aldrich; no. R-8781; a mammalian target of rapamycin (mTOR) inhibitor]. Other experiments were performed similarly, except that cultures were treated for 48 h with IGF-I (10 nm) or M22 with or without 1H7 (5 μg/mL; BD Biosciences; no. 555998), instead of the inhibitors of cell signaling.
In experiments aimed at assessing the effect of 1H7 on M22- or IGF-I-stimulated Akt phosphorylation, orbital cells were propagated as above, seeded into six-well plates, grown to confluence, and serum starved for 24 h. Cultures were then pretreated with 1H7 (5 or 50 μg/ml) for 60 min to allow receptor binding, followed by treatment in serum-free media for between 5 and 30 min with 1H7 (5 or 50 μg/ml), M22 (10 or 100 ng/ml), IGF-I (10 nm), 1H7 plus M22, or 1H7 plus IGF-I or were not treated.
Measurement of HA production
Hyaluronic acid production was measured using a commercial hyaluronan ELISA kit (Echelon Biosciences Inc., Salt Lake City, UT), and the assay was performed as per the manufacturer's instructions. Results are expressed as fold change compared with parallel control cultures or as concentration of HA (nanograms per milliliter).
Measurement of Akt phosphorylation
Akt protein phosphorylation was measured using a commercial ELISA kit (CASE kit AKT S473 FE-001; SA Biosciences). This cell-based kit quantifies activated (phosphorylated serine 473) phospho-Akt protein (pAkt) relative to total Akt protein. Results are expressed as fold change in pAkt to Akt ratio relative to parallel control cultures.
Western blotting
Confluent cultures were serum starved for 24 h and then either untreated or pretreated for 24 h with 1H7 (5 μg/ml), followed by treatment for 1 h with either M22 (10 ng/ml), 1H7, or both. Cell protein was extracted using a complete lysis-M, EDTA-free protocol to extract total cytoplasmic and nuclear protein (Roche, Indianapolis, IN). Extracts were subjected to electrophoresis on 4–12% Bis-Tris gel, electrotransferred to polyvinylidene fluoride membrane, and blotted with primary antibody against Akt, ser473 pAkt, or glyceraldehyde 3-phosphate dehydrogenase (Cell Signaling Technology, Danvers, MA; no. 9272, no. 9271, and no. 2118, respectively) at 1:1000 dil. The appropriate secondary IgG-horseradish peroxidase-linked conjugate (Cell Signaling; no. 7074) at 1:2000 dil was applied, followed by enhanced chemiluminescence detection.
Statistical analyses
The paired t test was used to evaluate differences in means for continuous variables, with values presented as the mean ± sem. Differences between values was considered significant when P ≤ 0.05. The Mann-Whitney rank sum test was used to assess differences between groups.
Results
M22 and TSH stimulate HA synthesis in orbital fibroblasts from patients with GO
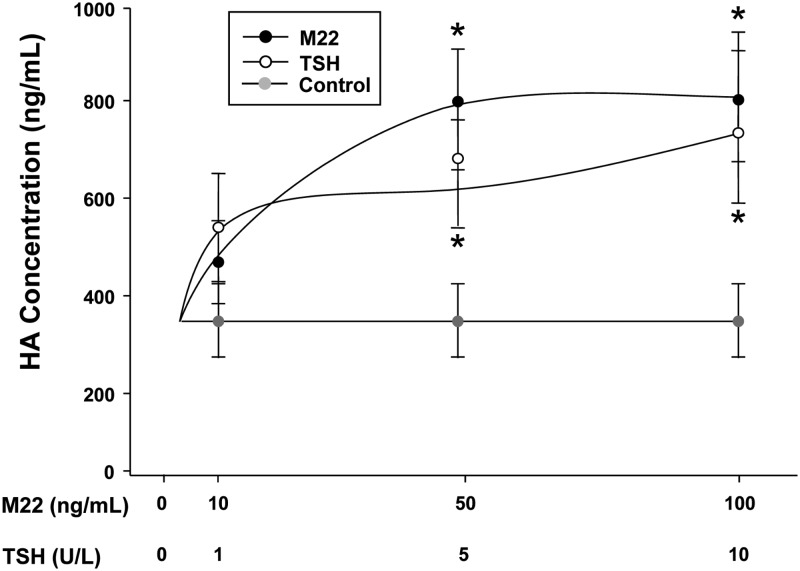
GO orbital fibroblast cultures (n = 6) in serum-free medium were treated for 48 h with M22 (10, 50, or 100 ng/ml), bTSH (1, 5, or 10 U/liter), or control isotype control IgG2 (10 μg/ml) or were not treated. Treatment with M22 at doses of 50 or 100 ng/ml resulted in stimulation of HA synthesis (mean 778 ± 112 ng/ml; 2.1-fold and mean 800 ± 141 ng/ml; 2.1-fold, respectively; P < 0.05 for each dose) relative to control media (mean 376 ± 61.3 ng/ml; Fig. 1). Treatment with bTSH (5 or 10 U/liter) also resulted in increased HA synthesis (mean 584 ± 152 ng/ml; 1.5-fold and mean 727 ± 181 ng/ml; 1.9-fold, respectively; P < 0.05 for each dose) relative to the same control media (Fig. 1). Treatment of cultures with the isotype control IgG2 had no impact on HA synthesis.
Fig. 1.
Effect of 48 h treatment with M22 (10, 50, or 100 ng/ml; dark filled circles), bTSH (1, 5, or 10 U/liter; open circles), or untreated control (light filled circles) on HA secretion in GO orbital fibroblast cultures (n = 6). ELISA results are expressed as mean ± sem nanograms per milliliter HA. *, P < 0.05.
M22-stimulated HA synthesis in GO orbital fibroblasts is inhibited by the PI3K inhibitor LY294002 or by the mTOR inhibitor rapamycin, but not by the protein kinase A (PKA) inhibitor PKI
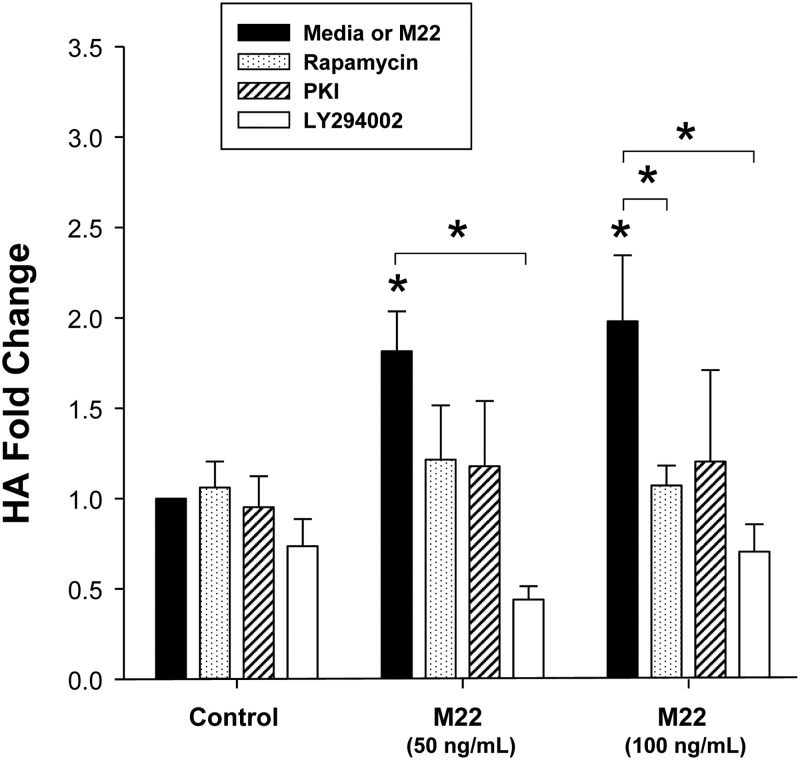
GO orbital fibroblast cultures (n = 6) in serum-free medium were treated for 48 h with M22 (50 or 100 ng/ml), LY294002 (10 μm), rapamycin (10 μm), PKI (10 μm), or M22 plus each of the inhibitors individually or were not treated. We again found stimulation of HA by either dose of M22 (P < 0.05 for each dose; Fig. 2). In addition, LY294002 inhibited HA synthesis stimulated by M22 at 50 or 100 ng/ml (mean 72.6 ± 5.4% decrease; P < 0.001 and mean 65.0 ± 3.3% decrease, respectively; P < 0.05; Fig. 2). Although there was a trend toward inhibition of M22-induced HA synthesis by rapamycin at both doses of M22, the effect was significant only for M22 at 100 ng/ml (mean 44.3 ± 7.8% decrease; P < 0.05; Fig. 2). PKI did not significantly impact M22-induced HA synthesis. In addition, there were no significant differences between any of the control conditions (i.e. rapamycin, PKI, or LY294002 alone) and basal levels of pAkt (i.e. media alone).
Fig. 2.
Effect of 48 h treatment with M22 (50 or 100 ng/ml), PI3K inhibitor LY294002 (10 μm), mTOR inhibitor rapamycin (100 nm), or PKA inhibitor PKI (10 μm) alone or each of the inhibitors in combination with M22 (either dose) on HA levels (fold change) in GO orbital fibroblast cultures (n = 6). ELISA results are expressed as mean ± sem fold change in HA relative to parallel untreated cultures (medium alone) or to parallel control cultures treated with M22 alone. *, P < 0.05.
M22-stimulated HA synthesis is inhibited by the IGF-IRα blocking antibody 1H7
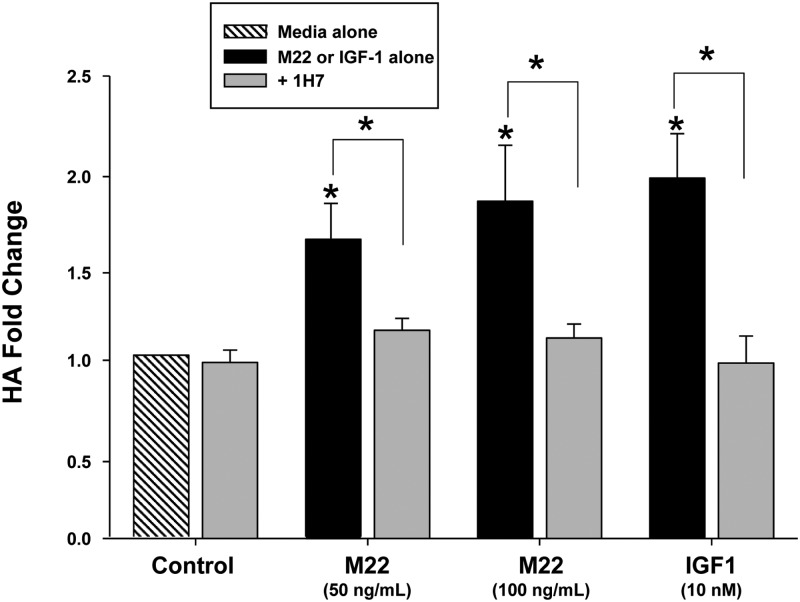
GO orbital fibroblast cultures (n = 6) in serum-free medium were treated for 48 h with M22 (10 or 100 ng/ml) or IGF-I (10 nm) with or without 1H7 (5 μg/ml) or were not treated. We found that 1H7 significantly attenuated HA synthesis stimulated by M22 at 50 or 100 ng/ml (mean 23.8 ± 2.2% decrease; P < 0.05 and mean 36.6 ± 5.6% decrease; P < 0.05, respectively; Fig. 3). Similarly, 1H7 blocked IGF-I-stimulated HA synthesis (mean 45.8 ± 7.6% decrease; P < 0.05; Fig. 3). Similar studies performed using orbital fibroblast strains obtained from normal orbits showed approximately 30–40% inhibition of M22-, TSH-, or IGF-I-induced HA secretion by 1H7 in three of three strains tested (data not shown). However, the mean percentage decrease in each case did not reach significance in the relatively small number of strains studied (data not shown).
Fig. 3.
Effect of 48 h treatment with M22 (50 or 100 ng/ml), IGF-I (10 ng/ml), the specific IGF-IRα blocking antibody 1H7 (5 μg/ml), or either M22 or IGF-I in combination with 1H7 on HA levels in GO orbital fibroblast cultures (n = 6). ELISA results are expressed as mean ± sem fold change in HA level relative to parallel untreated cultures (medium alone) or to parallel control cultures treated with M22 or IGF-I alone. *, P < 0.05.
M22- or IGF-I-stimulated Akt phosphorylation in GO orbital fibroblasts is inhibited by the IGF-IRα blocking antibody 1H7
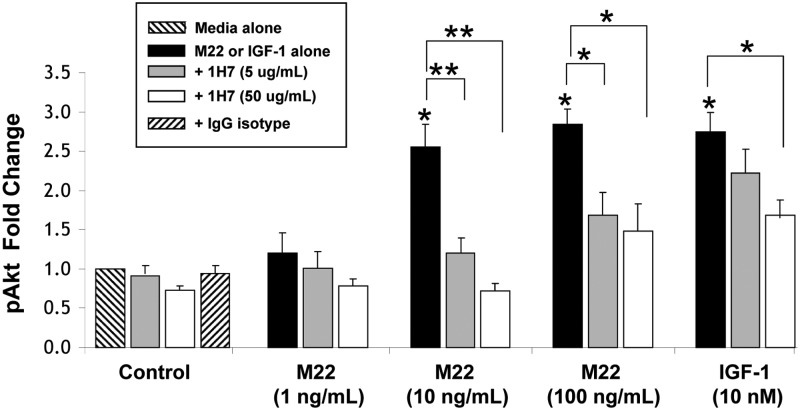
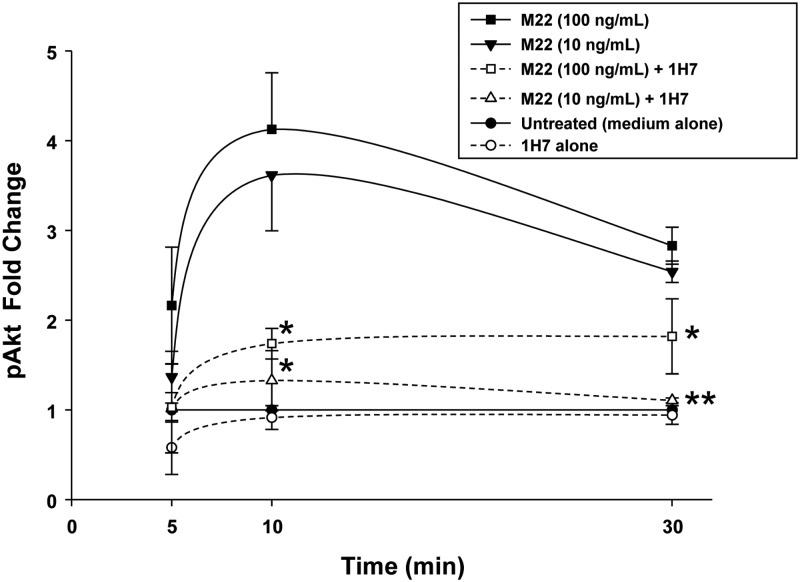
GO orbital fibroblast cultures (n = 6) in serum-free medium were treated for between 5 and 30 min with M22 (10 or 100 ng/ml) or IGF-I (10 nm) in the presence or absence of 1H7 (5 or 50 μg/ml) or were not treated. We found that M22 (at either dose) or IGF-I stimulate pAkt production at 30 min (P < 0.05 for each; Fig. 4). In addition, levels of M22-stimulated pAkt were significantly inhibited in cultures treated for 30 min with 1H7 (50 μg/ml) for M22 at 10 or 100 ng/ml (mean 75.2 ± 8.3% decrease; P < 0.001 and mean 54 ± 9.6% decrease, respectively; P = 0.01). Similarly, pAkt production stimulated by either dose of M22 was inhibited by the lower dose (5 μg/ml) of 1H7 (P < 0.05 for M22 100 ng/ml and P < 0.001 for M22 10 ng/ml; Fig. 4). Similarly, studies of IGF-I-treated cultures showed inhibition of Akt by 1H7 at 50 μg/ml (36.1 + 8.8% decrease; P = 0.01; Fig 4). No significant inhibition was seen using the lower dose of 1H7. Time-course experiments (n = 6) showed peak stimulation of pAkt at 10 min for M22 at 10 or 100 ng/ml (mean 4.0 ± 1.0-fold and mean 3.6 ± 1.0-fold, respectively, with inhibition of pAkt production by either dose of 1H7 (P < 0.05; Fig. 5).
Fig. 4.
Effect of 30 min treatment with M22 (1, 10, or 100 ng/ml), IGF-I (10 nm), 1H7 (5 or 50 μg/ml), or either M22 or IGF-I in combination with 1H7 on pAkt levels in GO orbital fibroblast cultures (n = 6). Levels of pAkt are expressed as mean ± sem fold change relative to parallel untreated cultures (medium alone) or to parallel control cultures treated with M22 or IGF-I alone. *, P < 0.05; **, P < 0.001.
Fig. 5.
Effect of 5, 10, or 30 min treatment with M22 (10 or 100 ng/ml; filled symbols) alone or in combination with 1H7 (5 μg/ml) on pAkt protein levels in GO orbital fibroblast cultures (n = 6). Levels of pAkt are expressed as mean ± sem fold change relative to parallel control cultures treated with M22 alone. *, P < 0.05; **, P < 0.001.
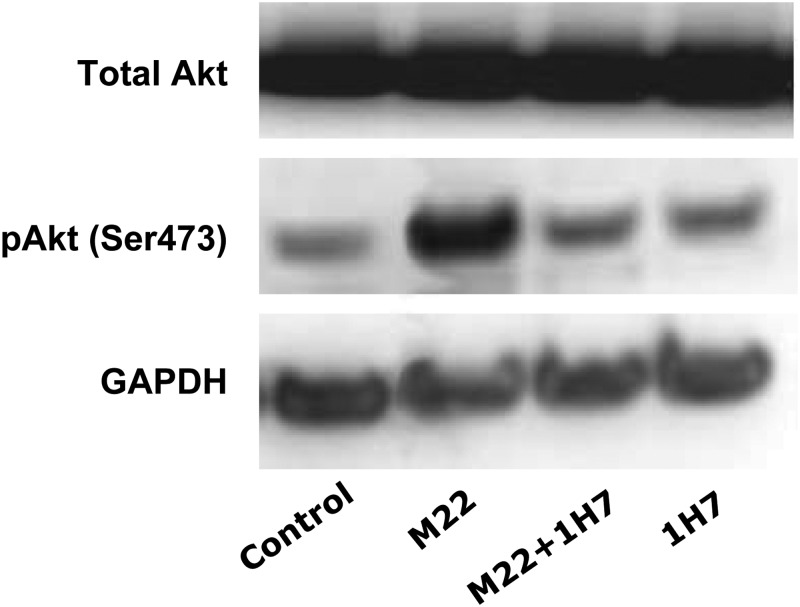
Western blotting of protein extracts from GO orbital fibroblast cultures (n = 3) treated for 60 min with M22 (10 ng/ml), M22 plus 1H7 (5 μg/ml), or 1H7 alone showed increased phosphorylation of Akt in cultures treated with M22 alone and inhibition of this effect in M22-treated cells exposed to 1H7 (Fig. 6). Cultures treated with 1H7 alone showed levels of pAkt similar to those found in untreated control cultures. The presence of a light pAkt band in control cultures reflects some small degree of activation of Akt under basal (nondifferentiating) culture conditions.
Fig. 6.
Representative Western blot showing total Akt, pAkt (pAkt Ser473), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) protein bands in confluent GO orbital fibroblast cultures exposed for 1 h to the indicated treatments. Left, 1) Control (medium alone); 2) M22 (10 ng/ml); 3) M22 plus 1H7 (5 μg/ml); or 4) 1H7 alone.
Discussion
In this report, we demonstrate that the stimulatory TRAb M22, as well as bTSH, stimulate HA synthesis in GO orbital fibroblasts that have not undergone adipocyte differentiation. Previous studies examining HA synthesis in differentiated or undifferentiated GO orbital fibroblasts used various methods to activate TSHR, including treatment of cells with other monoclonal TRAb (12), or human recombinant TSH (rhTSH) (7, 14, 15), bTSH (12) or by transfecting the cells with an activating mutant TSHR (12). Smith and Hoa (7) studied undifferentiated GO orbital fibroblasts treated with the IgG fraction of pooled sera from patients with Graves' disease (termed Graves' IgG), which, by definition, contain TRAb as well as other IgG. They found that GD-IgG increases HA production in GO orbital fibroblasts but did not observe HA stimulation with rhTSH treatment. In contrast, van Zeijl et al. (14) found neither GD-IgG nor rhTSH to increase HA production in undifferentiated GO orbital fibroblasts. However, in a subsequent study using GO orbital fibroblasts that had undergone adipocyte differentiation, this group showed HA stimulation by GD-IgG but again not by rhTSH treatment (15). A study by Zhang et al. (12) demonstrated stimulation of HA synthesis by bTSH, as well as by two monoclonal TRAb (one stimulatory and one neutral), in normal undifferentiated orbital fibroblasts but not in GO fibroblasts. In studies using GO fibroblasts transfected with an activating mutant TSHR, this same group showed induction of hyaluronan synthases 1 and 2 and increased HA production (12). The differences between our results and those of the other three groups may be in part explained by the greater affinity and potency of the monoclonal TRAb used in the studies by Zhang et al. and by our group, compared with the heterogeneous TRAb (stimulating, blocking, and neutral) of various affinities and differing potency that would be present in GD-IgG, as was used in the studies by van Zeijl and Smith. In addition, we and Zhang et al. used bTSH in our studies, whereas Smith and van Zeijl (7, 14) used rhTSH that has lower affinity for human TSHR and lesser signaling activity than does bTSH (16, 17). Finally, it appears that relatively high levels of TSHR expression, or potent TSHR activation, are necessary. Preadipocyte fibroblasts that have undergone adipocyte differentiation, as were used in the study by van Zeijl et al. (15), exhibit fairly abundant TSHR expression (18, 19), and cells containing the transfected TSHR-activating mutation, as were used by Zhang et al., would have both high expression levels and potent receptor activation. It may be that the very high affinity and potency of the M22 antibody or bTSH used in our studies allowed for potent stimulation of HA production in our undifferentiated cells that would have relatively low TSHR expression (19).
In the next series of experiments, we explored the downstream signaling pathways used by M22 in stimulating HA production. We found that M22-stimulated HA production in undifferentiated orbital fibroblasts is markedly inhibited in cultures containing either the PI3K inhibitor LY294002 or the mTOR inhibitor rapamycin. In contrast, the specific PKA inhibitor PKI did not significantly impact HA production in M22-treated cultures. Our results suggest that M22 stimulates HA synthesis primarily via PI3K/pAkt/mTOR signaling. That TRAb activate this pathway as well as the cAMP cascade has been previously documented (20) and well characterized in thyrocytes (21). We previously showed engagement of the PI3K/pAkt pathway by M22 in GO orbital fibroblasts with enhancement of adipogenesis (10). The PI3K/pAkt/mTOR cascade was recently identified as a positive regulator of hyaluronan synthase (HAS)-2 transcription in a human breast cancer cell line (22). Three mammalian HAS isoforms have been identified, the most important to HA synthesis being HAS2. Although we measured only HA accumulation in our cultures, rather than HAS2 transcription or the impact of hyaluronidases on HA levels, our findings support a role for PI3K/pAkt/mTOR signaling in HA synthesis.
A recent study by Zhang et al. (23) demonstrated that adipogenesis itself (induced by culture in adipogenic medium) increases both HAS2 transcripts and HA accumulation by GO orbital fibroblasts. In contrast, they showed that sc preadipocyte fibroblasts respond to adipogenesis by decreasing HAS2 mRNA levels and HA secretion. To explain these findings, the investigators presented evidence of a possible phosphorylation-dependent repressor of HAS2 transcript accumulation in the orbital cells, thus linking adipogenesis with HA accumulation in the GO orbit. We do not believe that the increase in HA secretion found in our current study represents an indirect effect of differentiation induced by cell confluence. The experiments were performed under conditions (i.e. in serum free growth media lacking inducers of differentiation) in which we do not detect spontaneous adipogenesis, even after 14 d of culture (data not shown). It is possible, however, that M22 may have stimulated very early adipogenesis during these 48-h experiments and that this resulted in increased HA accumulation. If so, our findings would suggest that 1H7 primarily inhibited M22-enhanced adipogenesis and only secondarily decreased HA secretion, lending further support to the concept that adipogenesis and HA secretion are closely linked in GO orbital fibroblasts.
Several studies have shown that treatment of orbital or other fibroblasts with the cAMP mimetic dibutyryl cAMP stimulates HA production (12, 24, 25). Our results do not run counter to those studies but rather suggest that adenylate cyclase signaling in the face of activated PI3K may play a lesser role in HA production in GO fibroblasts. van Zeijl et al. (15) found that although GD-IgG induced only moderate cAMP response in differentiated orbital fibroblasts, this preparation stimulated HA production markedly in the majority of cultures. From these studies, they concluded that GD-IgG induce HA synthesis either by ligation of TSHR with activation of signaling pathways other than cAMP or through activation of IGF-IR. Our studies, using a specific TRAb rather than GD-IgG, suggest that the former explanation is the more likely and we demonstrate that the PI3K/pAkt/mTOR effector cascade is activated by TRAb in these cells.
Smith and Hoa (7) reported that the IGF-IR-blocking monoclonal antibody 1H7 completely attenuates HA synthesis in orbital fibroblasts induced by either GD-IgG or IGF-I. They concluded that HA synthesis in the GO orbit is mediated by circulating autoantibodies that target the IGF-IR rather than the TSHR. To further explore this concept, we undertook the current set of studies using the same IGF-IR blocking antibody, but rather than treating GO orbital fibroblast cultures with GD-IgG or rhTSH, we used M22 or bTSH. As expected, when IGF-IR was blocked by 1H7, we found inhibition of IGF-I-stimulated HA synthesis in these cells. However, we also showed that 1H7 blocks HA synthesis stimulated by M22. Other studies showed that 1H7 additionally blocks M22-stimulated phosphorylation of Akt. Our findings suggest that blocking IGF-IR with 1H7 may also impact M22-activated TSHR signaling. The cross-reactive targeting of TSHR by 1H7 or of IGF-IR by M22 appears less likely as both monoclonal antibodies are highly specific to their antigenic receptor (9, 26). Contamination of the M22 preparation by IGF-I- or an IGF-I-like compound is extremely unlikely because this antibody is a highly purified human monoclonal IgG1 λ-chain antibody (8, 9). We suggest that the stimulation of HA production by GD-IgG reported by Smith and Hoa (7) reflected TRAb in the sera, rather than putative IGF-IR autoantibodies. Indeed, elevated levels of IGF-IR autoantibodies are not detectable in the sera of GO patients in a luminescent immunoprecipitation assay using human embryonic kidney cells stably transfected with IGF-IR (27).
In another study, Smith and colleagues (28) immunoprecipitated both IGF-IR and TSHR from preparations of human thyrocytes or orbital fibroblasts using specific monoclonal antibodies directed against either receptor and also demonstrated colocalization of the two receptors in the perinuclear and cytoplasmic compartments of orbital fibroblasts. They concluded, as had earlier investigators (13), that TSHR and IGF-IR may interact as physical and/or functional complexes. Whether our current results reflect the presence of such interactions, or suggest that 1H7 may also directly target TSHR and thus not be specific for IGF-IR, awaits further study.
In summary, we demonstrate that the stimulatory TRAb M22 increases HA production in undifferentiated GO orbital fibroblasts, as does IGF-I, and that this effect is primarily mediated via PI3K/pAkt/mTOR activation. We also show that a blocking antibody directed against IGF-IRα inhibits both the phosphorylation of Akt and the synthesis of HA induced by M22 in these cells. These results suggest that TSHR and IGF-IR signaling may be closely linked in the GO orbit. This might take the form of a physical association between the receptors and/or be manifest through indirect modulation of common downstream signaling cascades. It is possible that IGF-I within the GO orbit acts in an autocrine or paracrine manner to modulate both HA synthesis and adipogenesis. Taken together with our previous report demonstrating M22-enhanced adipogenesis in GO orbital fibroblasts (10), our current findings suggest that circulating TRAb (perhaps in concert with IGF-I) may target TSHR and act via PI3K/Akt/mTOR signaling to foster development of the cardinal histologic features of GO, namely increased orbital adipose tissue volume and the accumulation of HA.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant DK77814.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bTSH
- Bovine TSH
- GO
- Graves' ophthalmopathy
- FBS
- fetal bovine serum
- HA
- hyaluronic acid
- HAS
- hyaluronan synthase
- IGF-IR
- IGF-I receptor
- mTOR
- mammalian target of rapamycin
- pAkt
- phospho-Akt protein
- PI3K
- phosphoinositide 3-kinase
- PKA
- protein kinase A
- PKI
- protein kinase inhibitor
- rhTSH
- recombinant human TSH
- TRAb
- TSH receptor autoantibody
- TSHR
- TSH receptor.
References
- 1. Bahn RS. 2010. Graves' ophthalmopathy. N Engl J Med 362:726–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar S, Leontovich A, Coenen MJ, Bahn RS. 2005. Gene expression profiling of orbital adipose tissue from patients with Graves' ophthalmopathy: a potential role for secreted frizzled-related protein-1 in orbital adipogenesis. J Clin Endocrinol Metab 90:4730–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prabhakar BS, Bahn RS, Smith TJ. 2003. Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev 24:802–835 [DOI] [PubMed] [Google Scholar]
- 4. Korducki JM, Loftus SJ, Bahn RS. 1992. Stimulation of glycosaminoglycan production in cultured human retroocular fibroblasts. Invest Ophthalmol Vis Sci 33:2037–2042 [PubMed] [Google Scholar]
- 5. Smith TJ, Bahn RS, Gorman CA, Cheavens M. 1991. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab 72:1169–1171 [DOI] [PubMed] [Google Scholar]
- 6. Imai Y, Odajima R, Inoue Y, Shishiba Y. 1992. Effect of growth factors on hyaluronan and proteoglycan synthesis by retroocular tissue fibroblasts of Graves' ophthalmopathy in culture. Acta Endocrinol (Copenh) 126:541–552 [DOI] [PubMed] [Google Scholar]
- 7. Smith TJ, Hoa N. 2004. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab 89:5076–5080 [DOI] [PubMed] [Google Scholar]
- 8. Sanders J, Jeffreys J, Depraetere H, Evans M, Richards T, Kiddie A, Brereton K, Premawardhana LD, Chirgadze DY, Núñez Miguel R, Blundell TL, Furmaniak J, Rees Smith B. 2004. Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid 14:560–570 [DOI] [PubMed] [Google Scholar]
- 9. Sanders J, Evans M, Premawardhana LD, Depraetere H, Jeffreys J, Richards T, Furmaniak J, Rees Smith B. 2003. Human monoclonal thyroid stimulating autoantibody. Lancet 362:126–128 [DOI] [PubMed] [Google Scholar]
- 10. Kumar S, Nadeem S, Stan MN, Coenen M, Bahn RS. 2011. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3-kinase activation in orbital preadipocytes from patients with Graves' ophthalmopathy. J Mol Endocrinol 46:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bahn RS, Gorman CA, Johnson CM, Smith TJ. 1989. Presence of antibodies in the sera of patients with Graves' disease recognizing a 23 kilodalton fibroblast protein. J Clin Endocrinol Metab 69:622–628 [DOI] [PubMed] [Google Scholar]
- 12. Zhang L, Bowen T, Grennan-Jones F, Paddon C, Giles P, Webber J, Steadman R, Ludgate M. 2009. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem 284:26447–26455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tramontano D, Cushing GW, Moses AC, Ingbar SH. 1986. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves'-IgG. Endocrinology 119:940–942 [DOI] [PubMed] [Google Scholar]
- 14. van Zeijl CJ, Fliers E, van Koppen CJ, Surovtseva OV, de Gooyer ME, Mourits MP, Wiersinga WM, Miltenburg AM, Boelen A. 2010. Effects of thyrotropin and thyrotropin-receptor-stimulating Graves' disease immunoglobulin G on cyclic adenosine monophosphate and hyaluronan production in nondifferentiated orbital fibroblasts of Graves' ophthalmopathy patients. Thyroid 20:535–544 [DOI] [PubMed] [Google Scholar]
- 15. van Zeijl CJ, Fliers E, van Koppen CJ, Surovtseva OV, de Gooyer ME, Mourits MP, Wiersinga WM, Miltenburg AM, Boelen A. 2011. Thyrotropin receptor-stimulating Graves' disease immunoglobulins induce hyaluronan synthesis by differentiated orbital fibroblasts from patients with Graves' ophthalmopathy not only via cyclic adenosine monophosphate signaling pathways. Thyroid 21:169–176 [DOI] [PubMed] [Google Scholar]
- 16. Rapoport B, Takai NA, Filetti S. 1982. Evidence for species specificity in the interaction between thyrotropin and thyroid-stimulating immunoglobulin and their receptor in thyroid tissue. J Clin Endocrinol Metab 54:1059–1062 [DOI] [PubMed] [Google Scholar]
- 17. Mueller S, Kleinau G, Szkudlinski MW, Jaeschke H, Krause G, Paschke R. 2009. The superagonistic activity of bovine thyroid-stimulating hormone (TSH) and the human TR1401 TSH analog is determined by specific amino acids in the hinge region of the human TSH receptor. J Biol Chem 284:16317–16324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. 2003. Adipose thyrotrophin receptor expression is elevated in Graves' and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol 30:369–380 [DOI] [PubMed] [Google Scholar]
- 19. Valyasevi RW, Erickson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, Bahn RS. 1999. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab 84:2557–2562 [DOI] [PubMed] [Google Scholar]
- 20. Van Sande J, Lejeune C, Ludgate M, Munro DS, Vassart G, Dumont JE, Mockel J. 1992. Thyroid stimulating immunoglobulins, like thyrotropin activate both the cyclic AMP and the PIP2 cascades in CHO cells expressing the TSH receptor. Mol Cell Endocrinol 88:R1–R5 [DOI] [PubMed] [Google Scholar]
- 21. Morshed SA, Latif R, Davies TF. 2009. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology 150:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kultti A, Kärnä R, Rilla K, Nurminen P, Koli E, Makkonen KM, Si J, Tammi MI, Tammi RH. Methyl-β-cyclodextrin suppresses hyaluronan synthesis by down-regulation of hyaluronan synthase 2 through inhibition of Akt. J Biol Chem 285:22901–22910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Grennan-Jones F, Lane C, Rees DA, Dayan CM, Ludgate M. 2012. Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves' orbitopathy. J Clin Endocrinol Metab 97:653–662 [DOI] [PubMed] [Google Scholar]
- 24. Shishiba Y, Imai Y, Odajima R, Ozawa Y, Shimizu T. 1992. Immunoglobulin G of patients with circumscribed pretibial myxedema of Graves' disease stimulates proteoglycan synthesis in human skin fibroblasts in culture. Acta Endocrinol (Copenh) 127:44–51 [DOI] [PubMed] [Google Scholar]
- 25. Tomida M, Koyama H, Ono T. 1977. Effects of adenosine 3′:5′-cyclic monophosphate and serum on synthesis of hyaluronic acid in confluent rat fibroblasts. Biochem J 162:539–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kusada Y, Morizono T, Matsumoto-Takasaki A, Sakai K, Sato S, Asanuma H, Takayanagi A, Fujita-Yamaguchi Y. 2008. Construction and characterization of single-chain antibodies against human insulin-like growth factor-I receptor from hybridomas producing 1H7 or 3B7 monoclonal antibody. J Biochem 143:9–19 [DOI] [PubMed] [Google Scholar]
- 27. Minish W, Eckstein A, Dehina N, Kohrle J, Schomburg L. Detection of autoantibodies against the insulin-like growth factor-1 receptor in patients with Graves' orbitopathy by luminescent immunoprecipitation analysis. Proc International Thyroid Congress, Paris, France, 2010, pp 39 (Abstract OC-074) [Google Scholar]
- 28. Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. 2008. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol 181:4397–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]