Abstract
Context:
Current guidelines recommend parathyroidectomy in patients with primary hyperparathyroidism (PHPT) who have an estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2. It is unclear, however, whether values below this threshold of renal impairment affect bone and mineral metabolism in PHPT.
Objective:
The purpose of this study was to assess the effect of renal function on skeletal health in PHPT.
Design:
This is a retrospective analysis of PHPT patients with (eGFR < 60 ml/min per 1.73 m2) and without chronic kidney disease (CKD) from our previously described PHPT cohort recruited from 1984 to 1991.
Setting:
The study was conducted in a university hospital metabolic bone unit.
Participants:
One hundred thirty-eight women and men with PHPT were studied.
Outcome Measures:
We assessed bone mineral density (BMD) by dual-energy x-ray absorptiometry; quantitative histomorphometric indices from transiliac bone biopsies; and biochemical markers of mineral metabolism.
Results:
Although there was no difference in serum or urinary calcium or PTH level, calcitriol levels were lower and phosphate levels higher in patients with CKD. BMD adjusted for weight did not differ at any site between groups. Histomorphometric analysis (n = 30 of 138) revealed a 45% greater eroded surface in those with CKD (P = 0.02). Eroded surface negatively correlated with eGFR (r = −0.46, P = 0.02) and phosphate (r = −0.48, P = 0.02) and positively correlated with serum calcium level (r = 0.51, P = 0.009) but not with PTH, alkaline phosphatase, vitamin D metabolites, or urinary calcium excretion.
Conclusion:
Although cardinal biochemical indices (such as calcium and PTH) and BMD do not differ in PHPT patients with an eGFR below 60 ml/min per 1.73 m2, these patients have higher phosphate and histomorphometric evidence of altered bone remodeling compared with those without CKD.
Current guidelines for parathyroidectomy from the Third International Workshop on Asymptomatic Primary Hyperparathyroidism include an estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 [stage 3 chronic kidney disease (CKD)] (1). This recommendation is based, in part, on data from the general population without primary hyperparathyroidism (PHPT), in which the serum PTH level typically increases at an eGFR below this threshold (2). It was thought, furthermore, that the secondary elevation of PTH at this level of renal dysfunction would worsen the primary hyperparathyroid state. Although this recommendation seems to be a reasonable one, there is no evidence that in PHPT, PTH levels increase when this threshold is reached. In fact, recent work suggests that intact PTH increases in PHPT only when the eGFR falls below 30 ml/min per 1.73 m2 (3).
Data are also lacking on the effect of renal impairment on the skeleton, the other major organ affected by PHPT. Bone density data are very limited and there are essentially no histomorphometric data in this regard (4). Despite the paucity of data, there is certainly cause for concern. CKD is associated with a number of complex and detrimental changes in mineral and bone metabolism. Although many studies have focused on those with end-stage renal disease, a growing body of evidence also suggests that even those with less severe CKD have histomorphometric (5) and microstructural skeletal abnormalities (6) that may result in an increased risk of fracture (7, 8). Superimposed on alterations due to PHPT, these changes could lead to further abnormalities in bone remodeling, worse skeletal microstructure, and lower bone mineral density (BMD). The Third International Workshop on Asymptomatic Primary Hyperparathyroidism highlighted the need for further research on the effect of renal impairment on PHPT to adequately inform and manage such patients (1). This report represents a retrospective analysis of our previously described PHPT cohort (enrolled in our natural history study from 1984 to 1991) (9, 10) and was designed to assess the influence of renal function on biochemical, bone densitometric, and histomorphometric indices in patients with PHPT.
Participants and Methods
This investigation represents a retrospective analysis of baseline data according to renal function from our previously described PHPT cohort (9, 10). We enrolled 139 participants from 1984 to 1991 as part of a longitudinal study designed to evaluate the natural history of PHPT with or without parathyroidectomy (9, 10). PHPT was defined as the presence of hypercalcemia with a frankly elevated or an inappropriately normal PTH level. All but one subject had serum creatinine values available at the baseline visits and are included in this analysis (n = 138). None of the 138 participants was taking bisphosphonates at the time of the study enrollment. All participants, except those taking anticoagulants (n = 10), were asked to undergo transiliac bone biopsy. Histomorphometric data are presented for the subset of patients who had a bone biopsy (n = 30). All participants gave written, informed consent. This study was approved by the Institutional Review Board of Columbia University Medical Center.
Serum creatinine, calcium, phosphate, albumin and alkaline phosphatase activity, PTH, vitamin D metabolites, and urinary calcium were measured as previously described (9). All biochemistries, including creatinine, were measured on three separate occasions at baseline and values were averaged. The eGFR was calculated from the average creatinine value using the Modification of Diet in Renal Disease equation (11). Calcium values were corrected for low albumin (<4 mg/dl) using the following formula: (4-albumin)0.8 + serum calcium. Urinary hydroxyproline was measured by the method of Kivirikko et al. (12). BMD at the lumbar spine, femoral neck, and distal one third of the nondominant radius was measured as previously described (9, 10). The short-term in vivo precision error was 0.026 g/cm2 for L1-L4, 0.041 g/cm2 for the femoral neck, and 0.033 g/cm2 for the forearm. Dual-energy x-ray absorptiometry (DXA) instruments were calibrated with reference spine and hip phantoms to read BMD to within 1%. Additionally, a phantom was scanned daily and values reviewed every 3 months for drift.
Percutaneous transiliac bone biopsies were performed after double-tetracycline labeling. Biopsy specimens were obtained using a Bordier-type trephine with an inner diameter of 7.5 mm. Specimens were processed and subjected to histomorphometric analysis in our laboratory (13–15). All variables were expressed according to the recommendations of the American Society for Bone and Mineral Research Nomenclature Committee (16). Histomorphometric analyses represent previously measured data and biopsy samples were not freshly cut or remeasured for this analysis. The accuracy and reproducibility of histomorphometry measures were checked on a regular basis by repeated analysis of quality control slides.
Statistical methods
Data are expressed as mean ± sd or counts and percentages. Comparisons of group characteristics between those with eGFR less than 60 ml/min per 1.73 m2 and those with eGFR of 60 ml/min per 1.73 m2 or greater were evaluated by the χ2 or Fisher's exact test as appropriate (categorical variables) or independent two-sided Student's t test (continuous variables). Criterion values were adjusted for unequal variances where appropriate. Biochemistries and bone density variables for each site were first compared between the two groups without adjustment using two-sided Student's t tests. Bone density was compared again after adjustment for weight. The data were not adjusted for differences in postmenopausal status because only one premenopausal woman had CKD, but analyses were repeated, limiting the groups to postmenopausal women and men. The Pearson correlation was used to assess the association between continuous variables. For all analyses, a two-tailed P < 0.05 was considered to indicate statistical significance. A Sidak step-down approach and a repeated-measures analysis were used to adjust for the multiplicity of comparisons within measurement domains (biochemistries, bone density, and histomorphometry). Statistical analysis was performed using SAS, version 9.2 (SAS Institute, Cary, NC).
Results
This cohort had mild primary hyperparathyroidism (mean ± sd: calcium 10.7 ± 0.5 mg/dl and PTH 123 ± 64 pg/ml). Seventeen percent of patients (n = 23) had nephrolithiasis and thus had symptomatic disease, whereas none had osteitis fibrosa cystica. The percentage of participants who met other 1990 surgical guidelines for parathyroidectomy at the time of enrollment included the following: 30% were less than 50 yr of age, 19% had a one third radius Z-score less than −2.0; 10% had urinary calcium levels greater than 400 mg per 24 h; and 17% had a serum calcium 1 mg/dl or greater above the upper limit of normal.
Mean eGFR was 74 ± 17 ml/min per 1.73 m2 in the group as a whole. eGFR was not associated with the severity of primary hyperparathyroidism as reflected in serum levels of PTH (r = 0.03, P = 0.74), serum calcium (r = 0.13, P = 0.14), or bone density Z-score at any site (r = −0.09 to −0.05, P = 0.32–0.64). There also was no association with 25-hydroxyvitamin D (25OHD) (r = −0.08, P = 0.37) levels. On the other hand, eGFR positively correlated with 1,25-dihydroxyvitamin D (r = 0.24, P = 0.004), serum alkaline phosphatase activity (r = 0.19, P = 0.03), urinary calcium excretion (r = 0.22, P = 0.02), and urinary hydroxyproline (r = 0.22, P = 0.01). The association with serum phosphate (r = −0.16, P = 0.06) was of borderline significance.
Twenty-two patients in our original cohort (16%) had an eGFR less than 60 ml/min per 1.73 m2 (CKD group). The mean eGFR in these individuals was 51 ml/min per 1.73 m2 (range 29–59 ml/min per 1.73 m2). All patients in the CKD group had stage 3 CKD as defined by the National Kidney Foundation Disease Outcomes Quality Initiative guidelines (eGFR 30–59 ml/min per 1.73 m2) except one, who had stage 4 CKD (eGFR 15–29 ml/min per 1.73 m2). Furthermore, the majority of patients in the CKD group (n = 18) had stage 3a disease (glomerular filtration rate 45–59 ml/min per 1.73 m2). Comparison of those with and without CKD revealed that patients with CKD were older and fewer were premenopausal, but there were no between-group differences in gender, weight, height, body mass index, or race/ethnicity (Table 1) or rates of nephrolithiasis (14% in those with CKD vs. 17% in those without CKD, P = 1.0).
Table 1.
Demographic data by renal function
| GFR <60 (n = 22) | GFR ≥60 (n = 116) | P value | |
|---|---|---|---|
| Age (yr) | 60.4 ± 9.9 | 53.8 ± 13.2 | 0.03 |
| Female (%) | 81.8 | 76.7 | 0.78 |
| Premenopausal (% of women) | 5.6 | 33.7 | 0.0005 |
| Weight (lb) | 167.3 ± 44.1 | 155.3 ± 35.8 | 0.23 |
| Height (in) | 64.6 ± 3.9 | 65.0 ± 3.5 | 0.68 |
| BMI (kg/m2) | 28.0 ± 5.7 | 26 ± 5.4 | 0.14 |
| Race | |||
| White (%) | 77.3 | 86.2 | 0.33 |
| Black (%) | 22.7 | 13.8 | |
| Ethnicity | |||
| Non-Hispanic (%) | 78 | 83 | 0.54 |
| Hispanic (%) | 22 | 17 | |
Values represent mean ± sd or percentages.
As shown in Table 2, there were no differences in serum calcium, PTH, alkaline phosphatase, albumin, albumin-corrected calcium, 25OHD, or urinary hydroxyproline levels between the groups. Calcitriol levels were significantly lower by 16% in the CKD group and phosphate was significantly higher by 7%. There was a trend toward lower urine calcium excretion in those with CKD (P = 0.09). There were no between-group differences in absolute BMD at any site. BMD was compared using Z-scores, given the between-group difference in age (Table 3). Lumbar spine Z-score was higher in the CKD group compared with those with normal renal function (P = 0.03), but this difference did not persist after adjusting for weight (P = 0.34). There were no differences in femoral neck or one third radius Z-scores before or after adjustment for weight. Analyses were repeated excluding premenopausal women, and results were unchanged except for the following: urinary calcium (176 ± 102 vs. 242 ± 118 mg/d, P = 0.03) was significantly lower in those with CKD, whereas the difference in 1,25-dihydroxyvitmain D was no longer significant (50 ± 19 vs. 58 ± 19 pg/ml, P = 0.08).
Table 2.
Indices of mineral metabolism by renal function
| GFR <60 (n = 22) Mean ± sd (range) | GFR ≥60 (n = 116) Mean ± sd (range) | Normal range | P value | |
|---|---|---|---|---|
| Creatinine (mg/dl) | 1.3 ± 0.3 | 0.9 ± 0.2 | NA | |
| eGFR | 51.0 ± 8.5 | 78.7 ± 13.8 | ||
| Serum PTH (pg/ml) | 126 ± 52 (69–249) | 123 ± 66 (22–368) | 10–65 | 0.84 |
| Albumin-corrected serum calcium (mg/dl) | 10.5 ± 0.6 (10.3–11.9) | 10.6 ± 0.6 (10.3–12.5) | 0.54 | |
| Serum phosphate (mg/dl) | 3.0 ± 0.5 (2.1–4.0) | 2.8 ± 0.4 (1.9–3.9) | 2.5–4.5 | 0.04 |
| Serum total alkaline phosphatase (U/liter) | 102 ± 38 (51–188) | 98 ± 39 (29–258) | <100 | 0.69 |
| 25OHD (ng/ml) | 25 ± 15 (9–76) | 21 ± 10 (3–65) | 9–52 | 0.25 |
| 1,25-dihydroxyvitamin D (pg/ml) | 51 ± 19 (19–91) | 61 ± 21 (26–147) | 15–60 | 0.03 |
| Urine calcium (mg/d) | 201 ± 152 (26–706) | 251 ± 117 (33–606) | <300 | 0.09 |
| Urinary hydroxyproline (mg/d) | 35 ± 24 (8–111) | 40 ± 19 (7–115) | <40 | 0.33 |
NA, Not applicable.
Table 3.
Bone mineral density by renal function
| GFR <60 (n = 22) Mean ± sd | GFR ≥60 (n = 116) Mean ± sd | P value | |
|---|---|---|---|
| Lumbar spine BMD (g/cm2) | 0.912 ± 0.13 | 0.896 ± 0.20 | 0.65 |
| Femoral neck BMD (g/cm2) | 0.654 ± 0.10 | 0.686 ± 0.12 | 0.28 |
| One-third radius BMD (g/cm2) | 0.565 ± 0.07 | 0.583 ± 0.092 | 0.41 |
| Lumbar spine BMD Z-score | 0.05 ± 1.1 | −0.57 ± 1.7 | 0.03a |
| Femoral neck BMD Z-score | −0.81 ± 0.79 | −0.97 ± 1.0 | 0.49 |
| Distal one-third radius BMD Z-score | −1.1 ± 0.89 | −1.2 ± 1.3 | 0.73 |
Remains significant when controlling for multiple comparisons.
PHPT patients who underwent bone biopsy (n = 30; Table 4) tended to have higher serum calcium (10.8 ± 0.7 vs. 10.5 ± 0.6 mg/dl, P = 0.052) and had greater urinary calcium excretion (290 ± 126 vs. 230 ± 121 mg per 24 h, P = 0.02) than those who did not undergo biopsy but did not differ in mean eGFR, age, gender, race/ethnicity, PTH level, vitamin D metabolites, or rates of nephrolithiasis. Biopsy patients with and without CKD did not differ by gender, race/ethnicity, years since menopause, or rates of nephrolithiasis. Those with renal dysfunction who underwent biopsy all had stage 3 CKD and none were taking bisphosphonates. There were no differences in any structural histomorphometric indices between those with and without CKD. However, among the remodeling indices, eroded surface (percent of surface occupied by Howship's lacunae) was 45% greater in those with CKD (P = 0.02), a difference that persisted after controlling for age (P = 0.03). Limiting the analysis to postmenopausal women and men did not change the significance of the comparison of eroded surface (12 0.0 ± 4.2 vs. 8.2 ± 2.8%, P = 0.03) or other histomorphometric results.
Table 4.
Histomorphometry by renal function
| GFR <60 (n = 5) | GFR ≥60 (n = 25) | P value | |
|---|---|---|---|
| Structural indices | |||
| Cortical width (μm) | 695 ± 184 | 626 ± 209 | 0.51 |
| Cancellous bone volume (%; BV/TV) | 22.1 ± 6.2 | 23.2 ± 7.0 | 0.75 |
| Trabecular number (1/mm) | 1.82 ± 0.23 | 1.93 ± 0.42 | 0.61 |
| Trabecular separation (μm) | 435 ± 92 | 424 ± 134 | 0.88 |
| Trabecular width (μm) | 121 ± 35 | 119 ± 25 | 0.89 |
| Remodeling indices | |||
| Osteoid surface (%) | 25.2 ± 12.7 | 29.3 ± 12.6 | 0.52 |
| Osteoid width (no. lamellae) | 13.3 ± 1.3 | 13.5 ± 3.1 | 0.88 |
| Mineralization lag time (d) | 34 ± 14 | 50 ± 34 | 0.59 |
| Mineralizing surface (%) | 19.0 ± 11.3 | 19.3 ± 10.3 | 0.95 |
| Mineral apposition rate (μm/d) | 0.65 ± 0.09 | 0.63 ± 0.12 | 0.69 |
| Bone formation rate (μm3/μm2 · d) | 0.13 ± 0.09 | 0.11 ± 0.06 | 0.63 |
| Eroded surface (%) | 12.0 ± 4.2 | 8.3 ± 2.7 | 0.02a |
| Activation frequency (cycles/yr) | 0.62 ± 0.15 | 1.07 ± 0.62 | 0.25 |
Values represent mean ± sd. BV/TV, Bone volume/tissue volume.
Statistically significant when controlling for multiple comparisons.
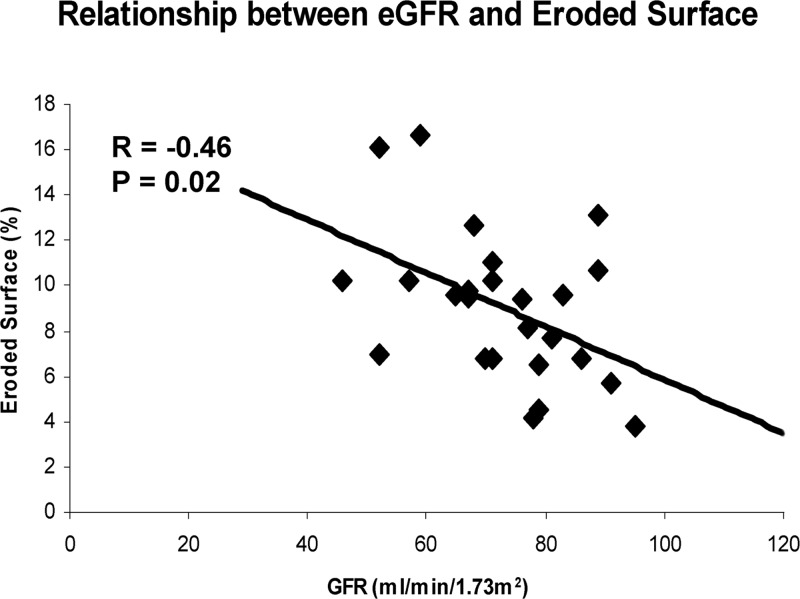
In the biopsy group, eroded surface was negatively correlated with eGFR (r = −0.46, P = 0.02; Fig. 1) and phosphate (r = −0.48, P = 0.02) but positively correlated with serum calcium level (r = 0.51, P = 0.009). eGFR was not correlated with PTH, alkaline phosphatase, vitamin D metabolites, or urinary calcium in this subgroup. In those who had CKD (n = 5), there was also a positive association between eGFR and osteoid surface (r = 0.94, P = 0.02).
Fig. 1.
Scatter plot indicating the association between eroded surface and eGFR.
Discussion
CKD, a common disorder of aging, is not infrequent among patients with PHPT, given its demographics (17). This is the first study to demonstrate that patients with PHPT and CKD have histomorphometric evidence of altered bone remodeling compared with those with normal renal function. Furthermore, our data suggest that this may occur in the setting of mild CKD (stage 3) in the absence of apparent secondary hyperparathyroidism or an effect on BMD as measured by DXA.
No other studies have evaluated bone histomorphometric indices in PHPT based on renal function. It is generally accepted that the percutaneous bone biopsy is more sensitive for detecting renal osteodystrophy than biochemical or radiographic evaluations (5). Our data suggest that eroded surface is associated with serum calcium and phosphate levels and with extent of renal dysfunction but not with PTH level. However, a relationship between eroded surface and PTH may exist in patients with worse kidney function (stage 4–5 CKD), because this study mainly included those with stage 3 CKD. It is also possible that we might have detected a correlation between PTH level and indices of bone disease if we had evaluated PTH with an assay that measured only PTH (1–84) because the intact PTH assay used in our patients also measures inactive large fragments. We did not find histomorphometric evidence of altered bone formation in those with CKD, but given the relatively small sample size, we cannot rule out the possibility that a larger study might reveal such differences. The positive association between eGFR and osteoid surface suggests that bone formation is reduced in those with the worst renal function. Taken together, these results imply that there is uncoupling of bone formation and resorption in the setting of mild CKD in patients with PHPT, similar to that in a number of disease states, such as postmenopausal osteoporosis and glucocorticoid-induced osteoporosis (18).
Unfortunately, our study cannot directly address the mechanism of altered bone remodeling in these patients. An increase in eroded surface can result from one of the following: 1) an increase in bone resorption without an increase in formation, 2) a decrease in bone formation without a decrease in resorption, or 3) both a decrease in bone formation and increase in resorption. Because we did not measure osteoclast number, we cannot determine whether the increase in eroded surface is due to active resorption or failure to fill in resorptive cavities in prior bone-remodeling cycles. We speculate that abnormalities associated with renal failure aside from PTH, such as increased sclerostin levels among others, could alter the balance between bone formation and resorption leading to increased eroded surface (19). Further work is necessary to confirm our findings and elucidate the pathophysiology of our observations.
It is noteworthy that we did not observe lower BMD in those with CKD as might have been expected. This may be explained by the fact that most patients had stage 3a CKD, but it also suggests that lower BMD as measured by DXA may become evident only in PHPT patients with more impaired renal function. To our knowledge, only one other study has assessed BMD in PHPT patients with CKD. Using a threshold above that set by the Third International Workshop, Gianotti et al. (4) demonstrated, in a group of 161 patients, that those with minimally reduced glomerular filtration rate have lower BMD compared with those with normal renal function (eGFR <70 vs. >70 ml/min per 1.73 m2). These data are not, however, applicable to mild PHPT because approximately 50% of patients in the Gianotti study had symptomatic PHPT, including 19% with osteitis fibrosa cystica.
We did not detect the increase in PTH from secondary hyperparathyroidism that has been observed in CKD patients without PHPT, and that was anticipated by the Third International Workshop's guideline. Likewise, there was no difference in serum calcium between those with and without CKD. Tassone et al. (3) found that PTH levels increase further in PHPT only when renal dysfunction is reduced below a GFR of 30 ml/min per 1.73 m2, a level lower than that selected by the Third International Workshop. Other data are contradictory, with a glomerular filtration rate limit less than 70 ml/min per 1.73 m2 reported to be associated with a further elevation of PTH in PHPT in one study but not in another (4, 20). These discrepancies may be explained by differences in 25OHD level, which is likely to be an important effect modifier in determining the threshold of glomerular filtration rate at which an increase in PTH occurs. In the three studies that failed to demonstrate an increase in PTH with glomerular filtration rate less than 60 or 70 ml/min per 1.73 m2, 25OHD level was at least 24 ng/ml or greater, whereas the mean level was clearly in the deficient range (15 ng/ml) in the only study that reported an increase in PTH. Alternatively, these discrepancies could be due to differences in the number of participants with severely impaired renal function between studies (3, 4, 20).
We did find that 1,25-dihydroxyvitamin D levels were lower and phosphate levels higher in those with a glomerular filtration rate less than 60 ml/min per 1.73 m2. The former finding has been reported and is an expected consequence of reduced 1α-hydroxylase activity from diminished functioning renal mass and inhibition from fibroblast growth factor-23 (20–22). The higher phosphate and trend toward lower urine calcium is consistent with reduced filtration of phosphate and calcium (4, 20).
We recognize that our study has several limitations including its retrospective and cross-sectional design. The small number of participants with CKD who underwent bone biopsy may have limited our ability to detect differences in histomorphometry other than eroded surface. For example, we would have needed 13 participants per group to detect the observed between-group differences in activation frequency. The small biopsy sample may have also restricted our capacity to detect associations between markers of bone metabolism and eGFR (for example between alkaline phosphatase and eGFR, which was associated in the entire cohort but not in those who underwent biopsy). Given the absence of data in this area, we believe that these results provide useful information to guide future research in this area.
Several other limitations must be recognized. Those with and without CKD were imperfectly matched with regard to osteoporosis risk factors. Although we attempted to adjust for these, we cannot be certain that residual confounding does not exist. Additionally, only about 20% of the cohort underwent biopsy and this group had higher serum and urinary calcium levels than those who did not. Therefore, the results may not be completely representative of the entire cohort. Some helpful information is not available on this cohort. First, we can not say how many participants would meet current surgical guidelines because T-scores (widely adopted in 1994) were not available when baseline measures included in this analysis were obtained. Data were also not collected on etiology of renal insufficiency, and more sensitive assays for PTH (1–84) and bone turnover were unavailable. Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease calculation, which may be less accurate in extremes of glomerular filtration rate. However, calculating estimated glomerular filtration rate from the average of multiple serum creatinine values (compared with a single measure) increases the likelihood that renal function is correctly categorized. Furthermore, these data may not be applicable to those with moderate to severe kidney dysfunction (stage 4–5) because all but one of our patients had stage 3 CKD. Lastly, we could not determine whether parathyroidectomy is beneficial to skeletal health in PHPT patients with CKD, given their incomplete follow-up in the longitudinal portion of this study. Among the strengths of the study are the well-characterized PHPT cohort and the availability of histomorphometric information, which revealed a key element of altered bone remodeling that would not have been evident by other analyses. In particular, eroded surface as determined by bone biopsy analysis reveals the accumulated effects of resorption over time (23).
In summary, patients with PHPT and stage 3 CKD have altered bone remodeling that is related to the degree of renal dysfunction. The data suggest that even mild renal dysfunction may be associated with worse bone quality in PHPT, which provides some evidence to support the decision of the Third International Workshop on the Management of Asymptomatic PHPT to set a glomerular filtration rate of 60 ml/min per 1.73 m2 as a threshold for concern. Future investigations using sensitive tools are needed to confirm and extend our understanding of the effect of CKD on the skeleton in PHPT and to assess the effect of parathyroidectomy on bone health and renal function in subjects with CKD.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grants DK32333 and DK074457.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- Bone mineral density
- CKD
- chronic kidney disease
- DXA
- dual-energy x-ray absorptiometry
- eGFR
- estimated glomerular filtration rate
- PHPT
- primary hyperparathyroidism
- 25OHD
- 25-hydroxyvitamin D.
References
- 1. Bilezikian JP, Khan AA, Potts JT., Jr 2009. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab 94:335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fajtova VT, Sayegh MH, Hickey N, Aliabadi P, Lazarus JM, LeBoff MS. 1995. Intact parathyroid hormone levels in renal insufficiency. Calcif Tissue Int 57:329–335 [DOI] [PubMed] [Google Scholar]
- 3. Tassone F, Gianotti L, Emmolo I, Ghio M, Borretta G. 2009. Glomerular filtration rate and parathyroid hormone secretion in primary hyperparathyroidism. J Clin Endocrinol Metab 94:4458–4461 [DOI] [PubMed] [Google Scholar]
- 4. Gianotti L, Tassone F, Cesario F, Pia A, Razzore P, Magro G, Piovesan A, Borretta G. 2006. A slight decrease in renal function further impairs bone mineral density in primary hyperparathyroidism. J Clin Endocrinol Metab 91:3011–3016 [DOI] [PubMed] [Google Scholar]
- 5. Hamdy NA, Kanis JA, Beneton MN, Brown CB, Juttmann JR, Jordans JG, Josse S, Meyrier A, Lins RL, Fairey IT. 1995. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ 310:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bacchetta J, Boutroy S, Vilayphiou N, Juillard L, Guebre-Egziabher F, Rognant N, Sornay-Rendu E, Szulc P, Laville M, Delmas PD, Fouque D, Chapurlat R. 2010. Early impairment of trabecular microarchitecture assessed with HR-pQCT in patients with stage II-IV chronic kidney disease. J Bone Miner Res 25:849–857 [DOI] [PubMed] [Google Scholar]
- 7. Nickolas TL, McMahon DJ, Shane E. 2006. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17:3223–3232 [DOI] [PubMed] [Google Scholar]
- 8. Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR. 2007. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med 167:133–139 [DOI] [PubMed] [Google Scholar]
- 9. Rubin MR, Bilezikian JP, McMahon DJ, Jacobs T, Shane E, Siris E, Udesky J, Silverberg SJ. 2008. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab 93:3462–3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. 1999. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255 [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470 [DOI] [PubMed] [Google Scholar]
- 12. Kivirikko KI, Laitinen O, Prockop DJ. 1967. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem 19:249–255 [DOI] [PubMed] [Google Scholar]
- 13. Dempster DW, Parisien M, Silverberg SJ, Liang XG, Schnitzer M, Shen V, Shane E, Kimmel DB, Recker R, Lindsay R, Bilezikian JP. 1999. On the mechanism of cancellous bone preservation in postmenopausal women with mild primary hyperparathyroidism. J Clin Endocrinol Metab 84:1562–1566 [DOI] [PubMed] [Google Scholar]
- 14. Parisien M, Cosman F, Mellish RW, Schnitzer M, Nieves J, Silverberg SJ, Shane E, Kimmel D, Recker RR, Bilezikian JP, et al. 1995. Bone structure in postmenopausal hyperparathyroid, osteoporotic, and normal women. J Bone Miner Res 10:1393–1399 [DOI] [PubMed] [Google Scholar]
- 15. Parisien M, Mellish RW, Silverberg SJ, Shane E, Lindsay R, Bilezikian JP, Dempster DW. 1992. Maintenance of cancellous bone connectivity in primary hyperparathyroidism: trabecular strut analysis. J Bone Miner Res 7:913–919 [DOI] [PubMed] [Google Scholar]
- 16. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. 1987. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- 17. Peacock M. 2002. Primary hyperparathyroidism and the kidney: biochemical and clinical spectrum. J Bone Miner Res 17(Suppl 2):N87–N94 [PubMed] [Google Scholar]
- 18. Dalle Carbonare L, Bertoldo F, Valenti MT, Zenari S, Zanatta M, Sella S, Giannini S, Cascio VL. 2005. Histomorphometric analysis of glucocorticoid-induced osteoporosis. Micron 36:645–652 [DOI] [PubMed] [Google Scholar]
- 19. Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, Haas M, Malluche HH. 2011. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamashita H, Noguchi S, Uchino S, Watanabe S, Murakami T, Ogawa T, Masatsugu T, Takamatsu Y, Miyatake E, Yamashita H. 2003. Influence of renal function on clinico-pathological features of primary hyperparathyroidism. Eur J Endocrinol 148:597–602 [DOI] [PubMed] [Google Scholar]
- 21. Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. 2005. Circulating FGF-23 is regulated by 1α,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem 280:2543–2549 [DOI] [PubMed] [Google Scholar]
- 22. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. 2004. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19:429–435 [DOI] [PubMed] [Google Scholar]
- 23. Meunier PJ, Bressot C. 1982. Endocrine influences on bone cells and bone remodeling evaluated by clinical histomorphometry. In: Parsons JA, ed. Endocrinology of calcium metabolism. New York: Raven Press [Google Scholar]