Abstract
Clinical Context:
Sensitive tools are available to diagnose occult ischemic and atherosclerotic coronary disease, yet screening for coronary artery disease (CAD) has not been shown to reduce cardiac events in patients with type 2 diabetes mellitus (T2DM). Professional guidelines are inconsistent regarding CAD screening recommendations, but it is suggested that those at highest risk (10-yr risk ≥20%) for cardiac events may benefit.
Evidence Acquisition:
We reviewed bibliographies of professional CAD screening guidelines, review articles, and clinical trials published within the last 10 yr, although we have included relevant older studies. We excluded studies that did not focus on T2DM or explicitly analyze that subgroup.
Evidence Synthesis:
Although screening for coronary ischemia or atherosclerosis does provide incremental prognostic information in patients with T2DM and previously undiagnosed CAD, this has not been found to significantly impact outcomes. This appears to result from comparable efficacy of revascularization and optimal medical therapy in stable CAD. Limited evidence supports the hypothesis that those with more severe CAD (three-vessel, left main, proximal left anterior descending) amenable to bypass surgery may be potential beneficiaries of screening. However, the low prevalence of such candidates in the asymptomatic population, continuing advances with percutaneous intervention, and the lack of prospective trials makes such a recommendation currently unsupportable.
Conclusions:
Findings to date do not support widespread screening for CAD in patients with T2DM. A future strategy identifying those at highest risk as screening candidates may ultimately be effective, but the optimal method for selecting those subjects or subsequent treatment is unknown.
Why Screen?
It is estimated that 25 million people in the United States have diabetes (1). Approximately 90–95% have type 2 diabetes mellitus (T2DM), but type 1 and type 2 are both associated with increased risk of cardiovascular disease (2–5). At least 65% of these individuals will die from cardiovascular causes (6), with a significant fraction experiencing sudden death.
A substantial percentage of patients with T2DM have silent myocardial ischemia (SMI) as determined by stress testing (17–59%) (7–11), and those individuals with SMI are at greater risk for cardiovascular events (12). Therefore, knowledge of silent coronary disease may allow one to more accurately predict risk and possibly implement more aggressive risk reduction. Additionally, screening asymptomatic T2DM patients with myocardial perfusion imaging may produce a significant number of high-risk scans (11, 13). One retrospective study suggested that some of these scans will uncover patients with angiographic findings that merit surgical revascularization (left main, three-vessel, and/or proximal left anterior descending) (14), possibly leading to better survival with coronary artery bypass grafting (CABG) over medical therapy. In either scenario, screening could potentially prevent cardiac outcomes by intervening before a first event (15).
Current Guidelines
The 2011 guidelines from the American Diabetes Association (ADA) clearly discourage routine screening for coronary artery disease (CAD) in the asymptomatic diabetic population. The evidence behind this stance carries the highest grade of “A” (16). As recently as 2006, the ADA recommended screening those with a higher burden of risk factors, although that stance was admittedly controversial and was reversed in 2007 (17, 18). However, the hypothesis persists that those with very high CAD risk may ultimately benefit from screening (15, 18–20).
Reflecting this, a recent consensus statement from the American College of Cardiology/American Heart Association concluded that in asymptomatic patients with an interpretable baseline electrocardiogram (ECG), the use of radionuclide imaging in low- or moderate-risk patients [Adult Treatment Panel (ATP) III risk criteria] is inappropriate. However, they concluded that in asymptomatic patients with very high coronary heart disease (CHD) risk, screening with radionuclide imaging is appropriate (21, 22).
In general agreement with the above, a systematic compilation of recent professional guidelines found 14 quality guidelines on cardiac imaging in asymptomatic patients (23). Eight recommended against or found insufficient evidence for screening, whereas six actually recommended imaging in those at intermediate or high risk (based on 10-yr Framingham risk). Only a minority of the guidelines specifically factored cost into their recommendations.
Although opinions diverge, two conclusions are clear: 1) there is a pressing need for further studies to develop consistent recommendations; and 2) future studies should focus on those at highest risk because these individuals are most likely to benefit.
Risk Stratification
To be effective, screening of asymptomatic patients must provide prognostic information that allows risk stratification, which then triggers more effective risk reduction. Clinically, the simplest screening involves measurement of circulating markers (e.g. low-density lipoprotein, glycosylated hemoglobin, etc.) or readily assessable clinical measures (blood pressure, neuropathy) as is done with the Framingham, UK Prospective Diabetes Study (UKPDS), and other risk engines. However, current risk engines do not provide consistently reliable information (24–26). Emerging data on circulating markers such as high-sensitivity C-reactive protein (27) and high-sensitivity Troponin T (hsTroponin T) (28–30) report on aspects of atherosclerosis pathogenesis distinct from traditional risk factors and may ultimately improve the utility of risk engines in nominating candidates for further testing. To date, clinicians have relied on more sophisticated techniques such as exercise ECG, coronary calcium scoring (CCS), stress echocardiogram, and myocardial perfusion SPECT (single photon emission computed tomography) (MPS) imaging to detect silent ischemia. These techniques can provide prognostic information in the T2DM population and are briefly reviewed here.
The exercise tolerance test with ECG is relatively inexpensive and widely available, and it has been an important tool in the evaluation of cardiovascular disease for several decades (31, 32). In one of the largest and most recent studies in patients with diabetes, exercise stress testing was performed in 262 patients with no history of CAD; individuals were followed for cardiac events over 42 months (33). SMI was a predictor of future cardiac events (odds ratio, 4.1; P = 0.03); a negative test was reassuring with a negative predictive value of 97%. Stress ECG was a better predictor of future events than peripheral vascular disease, carotid disease, or clinical risk factors. However, the sensitivity and positive predictive value for future events was low. These results fit with two other smaller prospective studies focused on patients with diabetes without a diagnosis of CAD (10, 34). In those studies, a positive exercise stress test was a significant predictor of cardiac events, and a negative test carried a negative predictive value greater than 87%.
In addition to low sensitivity, the exercise tolerance test may have a limited role in diabetic patients who are typically deconditioned, overweight, and possibly ataxic from peripheral neuropathy. In some studies, over 50% of subjects are not able to complete the exercise stress test (35). To avoid this, pharmacological or exercise stimulation can be combined with MPS or echocardiogram to predict risk in asymptomatic patients. In a prospective study of 1737 diabetic patients without known CAD undergoing MPS, 39% of asymptomatic patients had SMI (36). The annual critical event rate in these patients was 1.6% after a normal MPS but 3.4% after an abnormal test (P = 0.009). Another study using MPS in 1427 diabetic patients demonstrated an abnormal stress test in 58%, with 18% of the total abnormal being high-risk scans (14). This was a retrospective database analysis, and assessment of symptoms was limited to available documentation and coding. Therefore, the true number of asymptomatic individuals was likely overstated. Regardless, annual mortality rates in the high-risk scan group were 5.9%, compared with 3.6% in the low-risk scan group. Other prospective studies have demonstrated the ability of MPS to predict hard cardiac outcomes (37, 38).
Echocardiography combined with pharmacological stress is capable of detecting silent CAD while avoiding the challenge of exercise and costs of MPS, but there is less published experience in diabetic patients (22, 39, 40). One study involving 204 asymptomatic subjects with T2DM found that dobutamine stress echo (DSE) provided similar predictive power for cardiovascular events as MPS during a 3-yr follow-up (41). Another study involving 56 patients did not examine outcomes but rather demonstrated the ability of DSE to predict stenosis on angiography similar to MPS (42). Although both demonstrated similar positive predictive values, the concordance of DSE and MPS was not high. Of 25 positive tests, only five were positive for both DSE and MPS (42).
CCS by electron beam tomography can provide prognostic information in patients with diabetes (43). In a large prospective observational trial of 10,377 asymptomatic patients (9% with diabetes), mortality increased with increasing baseline CCS in the diabetic cohort. Patients with CCS scores of 0–10 had a 0.3% annual all-cause mortality rate, and those with scores greater than 1000 had an annual rate of approximately 5%. Using a multivariable model, there was increased risk of death for each quartile of increasing calcium scores (11–100, 101–400, 401-1000, and >1000). Interestingly, T2DM with no calcium had a risk of death that was low and equivalent to nondiabetic patients.
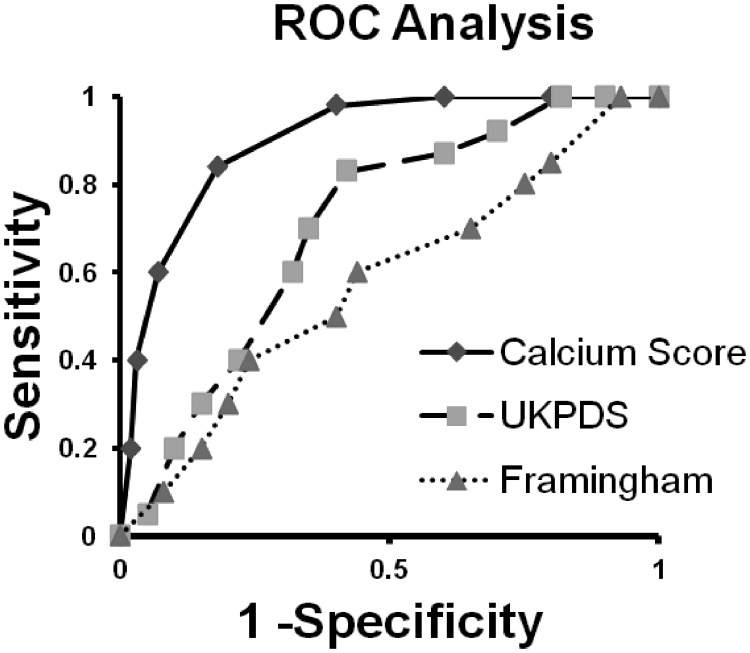
Another prospective CCS study focused solely on T2DM patients without CAD (44). In this study, 510 subjects were screened; 25% had Agatston scores that were above 100 (moderate or worse), and all patients with CCS above 100 underwent MPS. Patients were followed for a median of 2.2 yr. CCS was highly predictive of hard cardiac outcomes, with all-cause death or myocardial infarction (MI) occurring in 0, 3, 13, and 18% with increasing CCS quartiles (P < 0.0001). Using Framingham and UKPDS risk engines, all subjects' risk for cardiac outcome was compared with the observed event rate. The CCS was superior at predicting future events with an area under the receiver operator curve (AUC) of 0.92 vs. 0.74 and 0.60 for UKPDS and Framingham, respectively (P < 0.0001; see Fig. 1). The AUC for CCS is particularly impressive considering the poor predictive performance of the risk engines. The relative value and safety of CCS compared with MPS is illustrated by the fact that CCS yields similar prognostic information to MPS yet is associated with lower costs (with multidetector computed tomography) and less than one tenth the radiation exposure (45–47). Noninvasive contrast computed tomography coronary angiography and coronary magnetic resonance imaging have not been extensively studied in the asymptomatic T2DM population.
Fig. 1.
Demonstration of AUC values for three risk prediction tools. Calcium score AUC, 0.92; UKPDS AUC, 0.74; Framingham AUC, 0.60. ROC, Receiver operating characteristic. [Modified from D. V. Anand et al.: Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J 27:713–721, 2006 (44), with permission. © European Society of Cardiology.]
One can take from the above studies that stress testing by one of several modalities allows clinicians to place patients into different risk groups. However, this by itself does not justify the costs and risks of the test. For screening to be effective, an intervention must exist that can be differentially applied according to the test results.
Medical Intervention—Risk Factor Reduction
Based upon several large studies, including the Heart Outcomes Prevention Evaluation Study (HOPE), Organization to Assess Strategies for Ischemic Syndromes (OASIS), and the Finnish population study, which showed that patients with T2DM and no prior history of CAD have a risk for cardiac events comparable to patients with known CAD but without diabetes (48–50), ATP III raised “persons with diabetes without CHD to the risk level of CHD risk equivalent” (51). Although these studies were not able to exclude all symptoms or prior history of CAD and thus possibly overestimated the cardiovascular risk, it is generally accepted that patients with diabetes have a risk that approaches that of patients with known CAD. This risk equivalence led to a recommendation for aggressive risk factor reduction in all T2DM patients. These medical interventions are powerful and set a high threshold for competing therapies. In the Heart Protection Study (HPS), 20,563 patients with known CHD or diabetes without known CHD (type 1 or type 2 diabetes) were randomized to simvastatin or placebo (52). There was a significant 18% decrease in coronary death rate and a 13% decrease in all-cause mortality. These reductions were similar for those with CAD and those with T2DM without known CAD.
In addition to lipid therapy, implementation of the entire array of risk-reducing agents (aspirin, glucose-lowering agents, blood pressure control including blockers of the renin-angiotensin system) to reach the specified targets of the ADA in the Steno-2 trial led to additional event reduction (53). The reductions in risk suggested that the additional strategies were additive to lipid therapy alone.
Despite these impressive benefits of optimal medical therapy (OMT), a residual risk for cardiovascular events persists even in those treated according to current guidelines. In the Steno-2 trial, the 10-yr incidence of major cardiac events remained at least 20% in the intensive therapy group (53). Although this was at least a 50% reduction compared with those in the conventional arm, the persistently high risk suggests that these patients have a residual risk despite our efforts. Therefore, an intervention greater than current OMT alone is warranted to further reduce these individuals' risk, and it is possible that additional risk stratification through screening may allow such an intervention.
Revascularization Interventions
Given the substantial reduction in cardiac events found in primary (Collaborative Atorvastatin Diabetes Study) and secondary (Scandinavian Simvastatin Survival Study) trials as well as in HPS and Treating to New Targets (TNT), any revascularization must provide incremental benefit over intensive medical therapy to justify screening (54–57). Two recent prospective trials have compared OMT vs. revascularization plus OMT in stable CAD patients. Although these patients had known CAD and are clearly different from those discovered in a screening population, one would expect a certain degree of similarity.
The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial randomized patients with stable CAD (33% had either type 1 or type 2 diabetes) to percutaneous coronary intervention (PCI) with OMT or OMT alone (58). Subjects with severe ischemic disease (persistent angina, markedly positive stress test, refractory heart failure, or ejection fraction <30%) were excluded. There was no benefit with PCI over OMT for the combined endpoint of death, MI, or stroke in the overall cohort or in a predefined T2DM subgroup analysis. The Bypass Angioplasty Revascularization Investigation (BARI) 2D trial also evaluated revascularization plus OMT vs. OMT alone (59). This was the first large-scale randomized trial designed to determine whether prompt revascularization (either CABG or PCI) is superior to OMT specifically in diabetic patients with stable CAD referred for angiography. It is noteworthy that, like COURAGE, patients were receiving maximal medical care for ischemic heart disease at baseline and throughout the study. Patients were stratified to either CABG or PCI by the responsible physician according to a baseline diagnostic heart catherization. After stratification, patients were randomized to either revascularization with that procedure or medical therapy alone. After 5 yr, the rates of all-cause mortality and major cardiovascular events did not differ between the revascularization group (combined CABG and PCI) and the medical-therapy group. However, in patients stratified to the CABG arm, there was a significant 8.1% absolute risk reduction in the secondary outcome (composite death, nonfatal MI, and stroke) favoring CABG over medical therapy.
The conclusion from these studies is that in patients with T2DM and stable CAD referred for PCI, prompt revascularization is not superior to OMT. Additionally, candidates for whom CABG is indicated appear to have better outcomes with surgery than with medical therapy. The finding that CABG improves mortality is in accordance with some (15, 60) but not all (61) prior studies. These latter studies suffer from significant limitations [retrospective, nonrandomized (15), subset analysis of symptomatic/unstable patients receiving balloon angioplasty (60), subset analysis of symptomatic patients underpowered for diabetic subjects (61)], thus making BARI 2D the most valid study extant.
Two aspects of BARI 2D merit caution. In the PCI arm, only 35% received a drug-eluting stent (DES). Therefore, one can question whether these results are still valid with today's PCI techniques. The recent prospective randomized Coronary Artery Revascularization in Diabetes (CARDia) trial suggested that PCI is noninferior to CABG in preventing major cardiovascular events when a DES is used in patients with symptomatic CAD and T2DM (62). Although this study was underpowered for the primary endpoint, longer-term follow-up from this trial, as well as the ongoing Future Revascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) Trial, may establish the optimal revascularization in patients with diabetes (63). If PCI with DES is found to be noninferior to CABG, then conclusions from BARI 2D will have to be reexamined. In addition, the majority of patients studied during BARI 2D were treated aggressively at baseline for ischemia (88% aspirin, 75% statin, 77% angiotensin-converting-enzyme inhibitor/angiotensin receptor blocker, 73% β-blockade) because they had known CAD. The high rate of use of these agents would not be expected in the general population of asymptomatic patients with T2DM. For example, in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study, which included diabetic patients without diagnosed CAD or CAD symptoms, the baseline use of these medications was much lower: angiotensin-converting-enzyme inhibitor, 39%; aspirin, 45%; statin, 39%; and β-blocker, 11% (37). BARI 2D found that revascularization was no better than coronary disease OMT, but it may be unreasonable to extrapolate the efficacy of antiischemia OMT to primary prevention therapy used in an asymptomatic population.
Randomized Trials of Screening
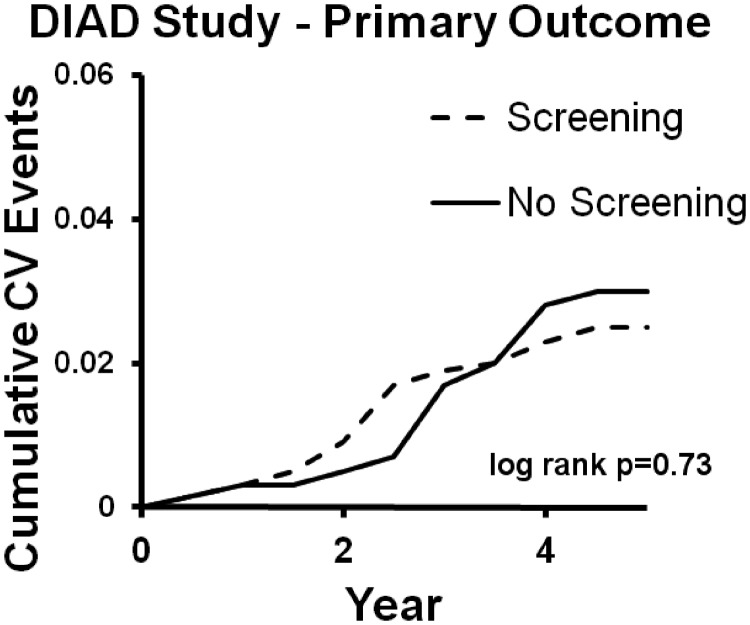
The DIAD study published in 2009 attempted to satisfy the need for a large, randomized trial testing the efficacy of widespread cardiac screening in T2DM, and it is still the only such trial (37). A total of 1123 subjects without CAD symptoms or baseline ECG consistent with ischemia were randomized to screening with stress MPS or no screening. Test results were sent directly to the patients and their personal physicians. Importantly, there was no mandated treatment for those with abnormal stress tests; thus, testing was intended to reflect what occurs in the practice setting. Patients in either arm could be sent for nonprotocol cardiac testing at any time. As shown in Fig. 2, there was no difference in the primary endpoints (composite MI and cardiac death) between the screened and unscreened arms.
Fig. 2.
No difference in primary outcome between screened and unscreened arms. CV, Cardiovascular. [Modified from L. H. Young et al.: Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 301:1547–1555, 2009 (37), with permission. © The American Medical Association.]
Consistent with the studies discussed above, the stress testing was prognostic of future events. In patients with small or no defects on stress myocardial perfusion imaging, the 5-yr incidence of a cardiac outcome was 2% for each, whereas those with moderate or large defects had an event rate of 12%. However, to be effective, screening must accomplish more than risk stratification; instead, it must result in an effective treatment delivered earlier than it would be without the screen. The intervention in this study is revascularization within 120 d after stress test; screening did result in nine procedures, whereas the nonscreened group underwent two. Of these nine procedures, six were CABG and three were PCI. Using the BARI 2D trial as a crude estimate of revascularization efficacy (although these are two different patient populations and the ability to extrapolate is limited), it is not surprising that the small number of interventions did not provide a significant difference.
Although it is a valuable study, several questions about DIAD should be considered. The 10-yr rate of cardiac events was 5.8% in all subjects, which ironically qualifies these patients as low risk. This rate is surprising compared with other large prospective T2DM trials (48, 64) conducted a decade earlier. Part of this lower rate may have been due to advances in risk factor reduction, but a modern prospective study showed persistently high cardiac events in a large sample of T2DM patients without CAD (65). To assess whether DIAD inadvertently recruited a low-risk group, a post hoc analysis was recently published (26). This demonstrated that the majority of subjects had either intermediate or high cardiovascular risk according to four different common risk metrics including the UKPDS risk engine. However, baseline characteristics such as average duration of diabetes less than 9 yr, average hemoglobin A1c of 7.1%, and a 22% rate of insulin therapy in the screened cohort suggest lower risk (66).
The large number of diagnostic procedures performed in the nonscreened group may have biased the results away from screening. The no-screening group underwent 170 nonprotocol stress tests vs. 118 in the screened group (P < 0.001), and the no-screening group underwent more CABG and percutaneous transluminal coronary angioplasty over the course of follow-up. In addition, of those screened, only 22% with an abnormal stress test proceeded to angiography within 120 d. This would have reduced any impact of the screen.
We are aware of two other randomized prospective studies that evaluated screening in T2DM. In the first, DSE combined with exercise ECG found screening to be successful at reducing cardiac events (67). The study's findings are limited by the small sample size, with 71 subjects screened; additionally, individuals with a positive test underwent a cardiology consultation and follow-up, whereas the controls lacked this advantage. It is noteworthy that after cardiology consultation, 14 of the 15 positive DSE led to a left heart catherization. This 93% catherization rate is significant compared with the equivalent 22% rate in DIAD. This suggests that screening test follow-up will be of major importance. A second study was the Do You Need to Assess Myocardial Ischemia in Type-2 diabetes trial (DYNAMIT) in which 631 patients with T2DM but without CAD were randomized to screening with either exercise ECG/MPS or to no screening (68). The trial was halted due to poor recruitment and low cardiac event rates, but there was no significant difference in the primary end point with screening.
Hope for Effective Screening
Findings from both a nonrandomized retrospective study (15) of asymptomatic patients and from the prospective BARI 2D (59) of patients with known CAD suggest that patients with T2DM and the most unfavorable CAD fare better when revascularization with CABG is added to OMT. Although these data are not adequate to draw firm conclusions, they suggest that the bar by which a screening test is measured is not just the ability to detect those with silent ischemia, but rather the ability to differentiate those with the most severe disease. This poses a considerable challenge because the number of potential surgical candidates (due to either coronary disease burden or comorbidities) may be very small in the asymptomatic population. For example, in a large French prospective cohort of 688 T2DM subjects (without known CAD) screened with MPS, 28% were diagnosed with silent ischemia and subsequently evaluated with coronary angiography (12). Ultimately, only six subjects of the 688 originally screened underwent CABG. This 1% rate of bypass surgery is informative, considering that all subjects had at least one risk factor in addition to diabetes and that the low number of surgical candidates is similar to other prospective studies in asymptomatic cohorts (37, 67–69). This suggests that CAD optimally treated by surgical intervention is infrequent in the asymptomatic T2DM population.
To increase the yield of screening tests, a testing strategy will need to focus on those at higher risk. Such a strategy was attempted in a post hoc analysis of the DIAD cohort whereby cardiovascular risk was estimated in all 1123 participants by using four well-known criteria (Framingham risk score, UKPDS risk engine, French Speaking Association for the Study of Diabetes and Metabolic Diseases/French Society of Cardiology high-risk criteria, and metabolic syndrome criteria), and the effect of screening was assessed only in those predicted to be at intermediate/high risk (26). As in the larger trial, major cardiac events were not reduced by limiting screening to intermediate-/high-risk individuals. This analysis was underpowered to detect a statistically significant difference, and this cohort still suffered the limitations of the larger trial. However, it is suggested that these four risk systems are not adequate by themselves for selecting individuals likely to benefit from screening with MPS. It is notable that the UKPDS risk engine was superior to the Framingham engine at predicting primary cardiac events (26).
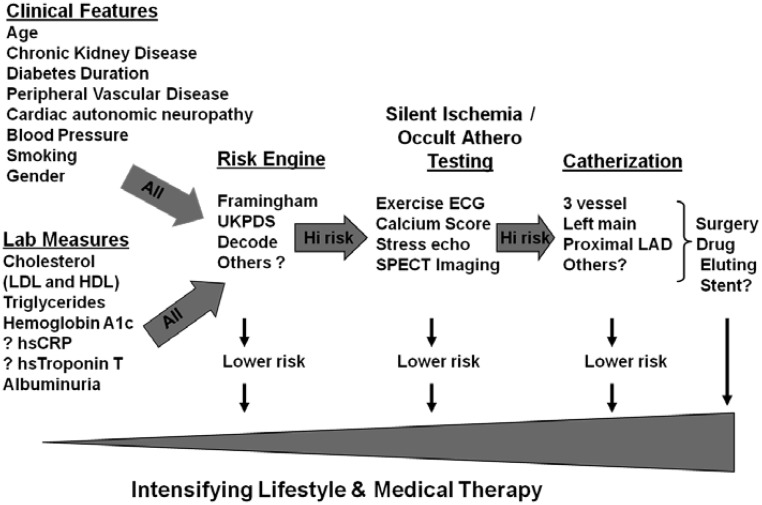
What is needed is a more insightful method for selecting patients who should proceed to testing for atherosclerosis or high-risk ischemia. Figure 3 illustrates how such a schema might work. There is ample evidence that a number of readily accessible clinical variables (chronic renal disease, cardiac autonomic neuropathy, duration of diabetes, etc.) impact the likelihood of identifying individuals with more severe CAD that might warrant surgical intervention. Screening may also provide additional risk information that leads to more aggressive medical therapy. It was recently shown in a prospective study that knowledge of SMI and silent CAD leads to superior risk predication (12). This underscores the need for additional effort whereby multiple inexpensive clinical and biochemical risk factors are correlated with findings on coronary angiography, ischemia evaluations, and clinical outcomes to improve the predictive power of clinical tools. This might be accomplished with either new studies or reanalysis of older prospective studies already completed.
Fig. 3.
Proposed flow chart for hypothetical screening process. hsCRP, High-sensitivity C-reactive protein; UKPDS, UK Prospective Diabetes Study; LAD, left anterior descending; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
For example, one study using stress echocardiogram in 1899 asymptomatic patients with T2DM correlated traditional risk factors with findings on angiography (40). Those with a positive stress test and at least two clinical risk factors were more likely to have three-vessel disease (33.3 vs. 7.6%) and diffuse disease (54.9 vs. 18.8%). Similarly, a retrospective analysis of asymptomatic patients demonstrated that several variables (glycosylated hemoglobin, age, and male gender) were associated with high-risk MPS (14). Using a different approach, a recent study demonstrated that hsTroponin T levels are increased in patients (24% with diabetes) with stable CAD (70). Importantly, hsTroponin T significantly correlated with the number of narrowed coronary arteries on angiography, thus raising the possibility that we may someday predict atherosclerotic burden with a lab test.
Given that risk reduction for patients with diabetes may take more than a decade to manifest benefit on cardiovascular outcomes (53, 71), it is likely that improvements in medical therapy over the last 20 yr will have an increasingly significant impact on the incidence of cardiovascular events. Similarly, improvements made in risk factor reduction over the last decade demonstrate that we as clinicians are making progress in implementing evidence-based prevention and can expect to see further decreases in cardiac events (72). If that is the case, demonstrating a benefit with screening will become even more formidable.
Conclusion
Screening cardiac tests can provide incremental prognostic information regarding cardiovascular risk in asymptomatic patients with T2DM. However, all diabetic patients are considered risk equivalent to patients with known CAD and therefore automatically receive aggressive risk reduction. This appears to mitigate the value of screening in the general T2DM population. The potential gain from a positive test may be improved survival after surgical revascularization in patients with advanced but remediable CAD, or it may be more aggressive medical therapy. Achieving cost-effective gains with screening to identify those who will benefit has not been determined. In the future, risk engines better able to predict those most likely to have severe disease, cheaper screening tools, or more effective interventions may make widespread cardiac screening feasible.
Acknowledgments
This work was supported by National Institutes of Health Grant T32DK007320.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the receiver operator curve
- CABG
- coronary artery bypass grafting
- CAD
- coronary artery disease
- CCS
- coronary calcium scoring
- CHD
- coronary heart disease
- DES
- drug-eluting stent
- DSE
- dobutamine stress echo
- ECG
- electrocardiogram
- hsTroponin T
- high-sensitivity Troponin T
- MI
- myocardial infarction
- MPS
- myocardial perfusion SPECT
- OMT
- optimal medical therapy
- PCI
- percutaneous coronary intervention
- SMI
- silent myocardial ischemia
- SPECT
- single photon emission computed tomography
- T2DM
- type 2 diabetes mellitus.
References
- 1. Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289:76–79 [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. 1998. Prediction of coronary heart disease using risk factor categories. Circulation 97:1837–1847 [DOI] [PubMed] [Google Scholar]
- 3. National Diabetes Information Clearinghouse 2008. Diabetes overview. NIH Publication 09-3873. Bethesda, MD: National Institutes of Health [Google Scholar]
- 4. Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, Rand LI, Christlieb AR, Bradley RF, Kahn CR. 1987. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol 59:750–755 [DOI] [PubMed] [Google Scholar]
- 5. Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. 2005. Report of the National Heart, Lung, and Blood Institute—National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 111:3489–3493 [DOI] [PubMed] [Google Scholar]
- 6. Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. 1999. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100:1134–1146 [DOI] [PubMed] [Google Scholar]
- 7. Rutter MK, McComb JM, Brady S, Marshall SM. 1999. Silent myocardial ischemia and microalbuminuria in asymptomatic subjects with non-insulin-dependent diabetes mellitus. Am J Cardiol 83:27–31 [DOI] [PubMed] [Google Scholar]
- 8. Langer A, Freeman MR, Josse RG, Steiner G, Armstrong PW. 1991. Detection of silent myocardial ischemia in diabetes mellitus. Am J Cardiol 67:1073–1078 [DOI] [PubMed] [Google Scholar]
- 9. Naka M, Hiramatsu K, Aizawa T, Momose A, Yoshizawa K, Shigematsu S, Ishihara F, Niwa A, Yamada T. 1992. Silent myocardial ischemia in patients with non-insulin-dependent diabetes mellitus as judged by treadmill exercise testing and coronary angiography. Am Heart J 123:46–53 [DOI] [PubMed] [Google Scholar]
- 10. Rutter MK, Wahid ST, McComb JM, Marshall SM. 2002. Significance of silent ischemia and microalbuminuria in predicting coronary events in asymptomatic patients with type 2 diabetes. J Am Coll Cardiol 40:56–61 [DOI] [PubMed] [Google Scholar]
- 11. Miller TD, Rajagopalan N, Hodge DO, Frye RL, Gibbons RJ. 2004. Yield of stress single-photon emission computed tomography in asymptomatic patients with diabetes. Am Heart J 147:890–896 [DOI] [PubMed] [Google Scholar]
- 12. Cosson E, Nguyen MT, Chanu B, Banu I, Chiheb S, Balta C, Takbou K, Valensi P. 2011. Cardiovascular risk prediction is improved by adding asymptomatic coronary status to routine risk assessment in type 2 diabetic patients. Diabetes Care 34:2101–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsujimoto T, Kajio H, Takahashi Y, Kishimoto M, Noto H, Yamamoto-Honda R, Kamimura M, Morooka M, Kubota K, Shimbo T, Hiroe M, Noda M. 2011. Asymptomatic coronary heart disease in patients with type 2 diabetes with vascular complications: a cross-sectional study. BMJ Open 1:e000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ. 2005. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol 45:43–49 [DOI] [PubMed] [Google Scholar]
- 15. Sorajja P, Chareonthaitawee P, Rajagopalan N, Miller TD, Frye RL, Hodge DO, Gibbons RJ. 2005. Improved survival in asymptomatic diabetic patients with high-risk SPECT imaging treated with coronary artery bypass grafting. Circulation 112:I311–I316 [DOI] [PubMed] [Google Scholar]
- 16. 2011. Standards of medical care in diabetes–2011. Diabetes Care 34(Suppl 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. 2006. Standards of medical care in diabetes–2006. Diabetes Care 29(Suppl 1):S4–S42 [PubMed] [Google Scholar]
- 18. Bax JJ, Young LH, Frye RL, Bonow RO, Steinberg HO, Barrett EJ. 2007. Screening for coronary artery disease in patients with diabetes. Diabetes Care 30:2729–2736 [DOI] [PubMed] [Google Scholar]
- 19. Valensi P, Cosson E. 2010. It is not yet the time to stop screening diabetic patients for silent myocardial ischaemia. Diabetes Metab 36:91–96 [DOI] [PubMed] [Google Scholar]
- 20. Shah PK. 2010. Screening asymptomatic subjects for subclinical atherosclerosis: can we, does it matter, and should we? J Am Coll Cardiol 56:98–105 [DOI] [PubMed] [Google Scholar]
- 21. Hendel RC, Budoff MJ, Cardella JF, Chambers CE, Dent JM, Fitzgerald DM, Hodgson JM, Klodas E, Kramer CM, Stillman AE, Tilkemeier PL, Ward RP, Weigold WG, White RD, Woodard PK. 2009. ACC/AHA/ACR/ASE/ASNC/HRS/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SIR 2008 Key data elements and definitions for cardiac imaging: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Cardiac Imaging). J Am Coll Cardiol 53:91–124 [DOI] [PubMed] [Google Scholar]
- 22. Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. 2010. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 122:e584–e636 [DOI] [PubMed] [Google Scholar]
- 23. Ferket BS, Genders TS, Colkesen EB, Visser JJ, Spronk S, Steyerberg EW, Hunink MG. 2011. Systematic review of guidelines on imaging of asymptomatic coronary artery disease. J Am Coll Cardiol 57:1591–1600 [DOI] [PubMed] [Google Scholar]
- 24. Coleman RL, Stevens RJ, Retnakaran R, Holman RR. 2007. Framingham, SCORE, and DECODE risk equations do not provide reliable cardiovascular risk estimates in type 2 diabetes. Diabetes Care 30:1292–1293 [DOI] [PubMed] [Google Scholar]
- 25. van der Heijden AA, Ortegon MM, Niessen LW, Nijpels G, Dekker JM. 2009. Prediction of coronary heart disease risk in a general, pre-diabetic, and diabetic population during 10 years of follow-up: accuracy of the Framingham, SCORE, and UKPDS risk functions: the Hoorn Study. Diabetes Care 32:2094–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bansal S, Wackers FJ, Inzucchi SE, Chyun DA, Davey JA, Staib LH, Young LH. 2011. Five-year outcomes in high-risk participants in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study: a post hoc analysis. Diabetes Care 34:204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. 2005. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 294:326–333 [DOI] [PubMed] [Google Scholar]
- 28. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. 2010. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA 304:2503–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. 2010. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 304:2494–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hallén J, Johansen OE, Birkeland KI, Gullestad L, Aakhus S, Endresen K, Tjora S, Jaffe AS, Atar D. 2010. Determinants and prognostic implications of cardiac troponin T measured by a sensitive assay in type 2 diabetes mellitus. Cardiovasc Diabetol 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ashley EA, Myers J, Froelicher V. 2000. Exercise testing in clinical medicine. Lancet 356:1592–1597 [DOI] [PubMed] [Google Scholar]
- 32. Lauer M, Froelicher ES, Williams M, Kligfield P. 2005. Exercise testing in asymptomatic adults: a statement for professionals from the American Heart Association Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention. Circulation 112:771–776 [DOI] [PubMed] [Google Scholar]
- 33. Cosson E, Paycha F, Paries J, Cattan S, Ramadan A, Meddah D, Attali JR, Valensi P. 2004. Detecting silent coronary stenoses and stratifying cardiac risk in patients with diabetes: ECG stress test or exercise myocardial scintigraphy? Diabet Med 21:342–348 [DOI] [PubMed] [Google Scholar]
- 34. Rubler S, Gerber D, Reitano J, Chokshi V, Fisher VJ. 1987. Predictive value of clinical and exercise variables for detection of coronary artery disease in men with diabetes mellitus. Am J Cardiol 59:1310–1313 [DOI] [PubMed] [Google Scholar]
- 35. Vanzetto G, Halimi S, Hammoud T, Fagret D, Benhamou PY, Cordonnier D, Denis B, Machecourt J. 1999. Prediction of cardiovascular events in clinically selected high-risk NIDDM patients. Prognostic value of exercise stress test and thallium-201 single-photon emission computed tomography. Diabetes Care 22:19–26 [DOI] [PubMed] [Google Scholar]
- 36. Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, Pfisterer ME, Berman DS. 2004. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J 25:543–550 [DOI] [PubMed] [Google Scholar]
- 37. Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE. 2009. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes. The DIAD study: a randomized controlled trial. JAMA 301:1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Scholte AJ, Bax JJ, Wackers FJ. 2006. Screening of asymptomatic patients with type 2 diabetes mellitus for silent coronary artery disease: combined use of stress myocardial perfusion imaging and coronary calcium scoring. J Nucl Cardiol 13:11–18 [DOI] [PubMed] [Google Scholar]
- 39. Heller GV. 2005. Evaluation of the patient with diabetes mellitus and suspected coronary artery disease. Am J Med 118(Suppl 2):9S–14S [DOI] [PubMed] [Google Scholar]
- 40. Scognamiglio R, Negut C, Ramondo A, Tiengo A, Avogaro A. 2006. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol 47:65–71 [DOI] [PubMed] [Google Scholar]
- 41. Jacqueminet S, Barthelemy O, Rouzet F, Isnard R, Halbron M, Bouzamondo A, Le Guludec D, Grimaldi A, Metzger JP, Le Feuvre C. 2010. A randomized study comparing isotope and echocardiography stress testing in the screening of silent myocardial ischaemia in type 2 diabetic patients. Diabetes Metab 36:463–469 [DOI] [PubMed] [Google Scholar]
- 42. Penfornis A, Zimmermann C, Boumal D, Sabbah A, Meneveau N, Gaultier-Bourgeois S, Bassand JP, Bernard Y. 2001. Use of dobutamine stress echocardiography in detecting silent myocardial ischaemia in asymptomatic diabetic patients: a comparison with thallium scintigraphy and exercise testing. Diabet Med 18:900–905 [DOI] [PubMed] [Google Scholar]
- 43. Raggi P, Shaw LJ, Berman DS, Callister TQ. 2004. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol 43:1663–1669 [DOI] [PubMed] [Google Scholar]
- 44. Anand DV, Lim E, Hopkins D, Corder R, Shaw LJ, Sharp P, Lipkin D, Lahiri A. 2006. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J 27:713–721 [DOI] [PubMed] [Google Scholar]
- 45. Raman V, McWilliams ET, Holmberg SR, Miles K. 2012. Economic analysis of the use of coronary calcium scoring as an alternative to stress ECG in the non-invasive diagnosis of coronary artery disease. Eur Radiol 22:579–587 [DOI] [PubMed] [Google Scholar]
- 46. Budoff MJ, Karwasky R, Ahmadi N, Nasserian C, Pratt F, Stephens J, Chang WW, Flores FR, Rizzo JA, Gunnarsson CL, McKay CR. 2009. Cost-effectiveness of multidetector computed tomography compared with myocardial perfusion imaging as gatekeeper to invasive coronary angiography in asymptomatic firefighters with positive treadmill tests. J Cardiovasc Comput Tomogr 3:323–330 [DOI] [PubMed] [Google Scholar]
- 47. Scott-Moncrieff A, Yang J, Levine D, Taylor C, Tso D, Johnson M, Heilbron B, Leipsic J. 2011. Real world estimated effective radiation doses from commonly used cardiac testing and procedural modalities. Can J Cardiol 27:613–618 [DOI] [PubMed] [Google Scholar]
- 48. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. 1998. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234 [DOI] [PubMed] [Google Scholar]
- 49. Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. 2000. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 102:1014–1019 [DOI] [PubMed] [Google Scholar]
- 50. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. 2000. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342:145–153 [DOI] [PubMed] [Google Scholar]
- 51. 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421 [PubMed] [Google Scholar]
- 52. 2002. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360:7–22 [DOI] [PubMed] [Google Scholar]
- 53. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. 2008. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358:580–591 [DOI] [PubMed] [Google Scholar]
- 54. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. 2004. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 364:685–696 [DOI] [PubMed] [Google Scholar]
- 55. 1994. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344:1383–1389 [PubMed] [Google Scholar]
- 56. Collins R, Armitage J, Parish S, Sleigh P, Peto R. 2003. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomized placebocontrolled trial. Lancet 361:2005–2016 [DOI] [PubMed] [Google Scholar]
- 57. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. 2005. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 352:1425–1435 [DOI] [PubMed] [Google Scholar]
- 58. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. 2007. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 356:1503–1516 [DOI] [PubMed] [Google Scholar]
- 59. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. 2009. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 360:2503–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. 1997. Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease: the Bypass Angioplasty Revascularization Investigation (BARI). Circulation 96:1761–1769 [DOI] [PubMed] [Google Scholar]
- 61. Abizaid A, Costa MA, Centemero M, Abizaid AS, Legrand VM, Limet RV, Schuler G, Mohr FW, Lindeboom W, Sousa AG, Sousa JE, van Hout B, Hugenholtz PG, Unger F, Serruys PW. 2001. Clinical and economic impact of diabetes mellitus on percutaneous and surgical treatment of multivessel coronary disease patients: insights from the Arterial Revascularization Therapy Study (ARTS) trial. Circulation 104:533–538 [DOI] [PubMed] [Google Scholar]
- 62. Kapur A, Hall RJ, Malik IS, Qureshi AC, Butts J, de Belder M, Baumbach A, Angelini G, de Belder A, Oldroyd KG, Flather M, Roughton M, Nihoyannopoulos P, Bagger JP, Morgan K, Beatt KJ. 2010. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients: 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 55:432–440 [DOI] [PubMed] [Google Scholar]
- 63. Farkouh ME, Dangas G, Leon MB, Smith C, Nesto R, Buse JB, Cohen DJ, Mahoney E, Sleeper L, King S, 3rd, Domanski M, McKinlay S, Fuster V. 2008. Design of the Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) Trial. Am Heart J 155:215–223 [DOI] [PubMed] [Google Scholar]
- 64. 1998. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853 [PubMed] [Google Scholar]
- 65. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, Wilson PW, Alberts MJ, D'Agostino R, Liau CS, Mas JL, Röther J, Smith SC, Jr, Salette G, Contant CF, Massaro JM, Steg PG. 2010. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 304:1350–1357 [DOI] [PubMed] [Google Scholar]
- 66. Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Wittlin SD, Heller GV, Filipchuk N, Engel S, Ratner RE, Iskandrian AE. 2004. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care 27:1954–1961 [DOI] [PubMed] [Google Scholar]
- 67. Faglia E, Manuela M, Antonella Q, Michela G, Vincenzo C, Maurizio C, Roberto M, Alberto M. 2005. Risk reduction of cardiac events by screening of unknown asymptomatic coronary artery disease in subjects with type 2 diabetes mellitus at high cardiovascular risk: an open-label randomized pilot study. Am Heart J 149:e1–e6 [DOI] [PubMed] [Google Scholar]
- 68. Lièvre MM, Moulin P, Thivolet C, Rodier M, Rigalleau V, Penfornis A, Pradignac A, Ovize M. 2011. Detection of silent myocardial ischemia in asymptomatic patients with diabetes: results of a randomized trial and meta-analysis assessing the effectiveness of systematic screening. Trials 12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Janand-Delenne B, Savin B, Habib G, Bory M, Vague P, Lassmann-Vague V. 1999. Silent myocardial ischemia in patients with diabetes: who to screen. Diabetes Care 22:1396–1400 [DOI] [PubMed] [Google Scholar]
- 70. Ndrepepa G, Braun S, Schulz S, Mehilli J, Schömig A, Kastrati A. 2011. High-sensitivity troponin T level and angiographic severity of coronary artery disease. Am J Cardiol 108:639–643 [DOI] [PubMed] [Google Scholar]
- 71. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. 2005. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ford ES. 2011. Trends in the risk for coronary heart disease among adults with diagnosed diabetes in the U.S.: findings from the National Health and Nutrition Examination Survey, 1999–2008. Diabetes Care 34:1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]