Abstract
Context:
Cushing's Disease (CD) alters fat distribution, muscle mass, adipokine profile, and cardiovascular risk factors. It is not known whether remission entirely reverses these changes.
Objectives:
Our objective was to determine whether the adverse body composition and cardiovascular risk profile in CD change after remission.
Design, Setting, and Patients:
Fourteen CD patients were studied prospectively: before surgery (active disease) and again postoperatively 6 months after discontinuing oral glucocorticoids (remission). Whole-body magnetic resonance imaging was used to examine lean and fat tissue distributions.
Outcome Measures:
Body composition (skeletal muscle and fat in the visceral, bone marrow, sc, and inter-muscular compartments) and cardiovascular risk factors (serum insulin, glucose, leptin, high-molecular-weight adiponectin, C-reactive protein, and lipid profile) were measured in active CD and remission (mean 20 months after surgery).
Results:
Remission decreased visceral, pelvic bone marrow, sc (including trunk and limb sc), and total fat; waist circumference; and weight (P < 0.05). Remission altered fat distribution, resulting in decreased visceral/total fat (P = 0.04) and visceral fat/skeletal muscle ratios (P = 0.006). Remission decreased the absolute muscle mass (P = 0.015). Cardiovascular risk factors changed: insulin resistance, leptin, and total cholesterol decreased (P < 0.05), but adiponectin, C-reactive protein, and other lipid measures did not change.
Conclusions:
CD remission reduced nearly all fat depots and reverted fat to a distribution more consistent with favorable cardiovascular risk but decreased skeletal muscle. Remission improved some but not all cardiovascular risk markers. Remission from CD dramatically improves body composition abnormalities but may still be associated with persistent cardiovascular risk.
Glucocorticoids (GC) are essential in the regulation of metabolism and body composition (1). Patients with Cushing's syndrome, a state of chronic GC excess due to either a pituitary ACTH-producing tumor or an adrenal cortisol-producing tumor, have profound body composition changes, including increased central adiposity and decreased lean mass (1, 2). In other populations, central adiposity (3) and elevated proportion of visceral to total fat and visceral fat to thigh muscle ratio (4, 5) are associated with insulin resistance, hepatic steatosis, and the metabolic syndrome. Visceral adipose tissue (VAT) may (6) or may not (7) positively associate with bone marrow adipose tissue (BMAT), a depot that also inversely associates with bone density (8) but has largely unknown metabolic associations. VAT also positively associates with elevated systemic proinflammatory markers, including C-reactive protein (CRP) (9), and CRP is a marker of cardiovascular risk (10). Body composition is therefore strongly linked to cardiovascular risk.
The altered body composition of Cushing's syndrome (including both pituitary and adrenal causes) has been described (11, 12) but rarely followed over time with treatment (13, 14). To our knowledge, only one study directly measured visceral fat [by computed tomography (CT)] after treatment, with a maximum follow-up of 10 months (13). BMAT has never been measured in Cushing's, and whether it changes with treatment or positively associates with other fat depots or adipokines in Cushing's is not known. Cross-sectional (12, 15) and retrospective (16) studies have suggested that cardiovascular risk markers, including insulin resistance, elevated proinflammatory markers, increased trunk and total fat, and the metabolic syndrome, may persist in Cushing's patients despite successful treatment. One prospective study showed continued cardiovascular risk in Cushing's patients 1 yr after surgery but did not investigate body composition (17). All Cushing's studies except a few (15–18) have included cohorts of both pituitary Cushing's [Cushing's disease (CD)] and adrenal Cushing's syndrome (CS). Prospective studies of CD are needed to understand possible associations between previous or persistent abnormalities in adipose tissue (AT) distribution and cardiovascular risk markers in patients successfully treated for CD.
Whole-body magnetic resonance imaging (MRI), which offers the most accurate currently available method of assessing body composition components, has been previously used only by us (18) to study CD and has never been used to investigate body composition in CD over time with treatment. Therefore, in this study, we conducted whole-body MRI in CD patients before transsphenoidal surgery (TS) and 6 months after discontinuation of GC. We hypothesized that remission of CD would reduce VAT, trunk sc AT (TrSAT), and BMAT, and increase skeletal muscle (SM). We also aimed to investigate the relationship between cardiovascular risk markers (adipokines, insulin resistance, proinflammatory markers, and lipid profile) and fat distribution in this population.
Subjects and Methods
Study subjects
We prospectively studied 14 subjects (12 female) with active CD, all of whom underwent TS and entered postoperative biochemical remission. One patient (patient 8) required two TS (within a one month span) before entering remission. Patients' pre- and postoperative clinical and anthropometric data are shown in Table 1. Preoperative data in patients 1–11 were previously reported (18). Four patients included in the previous manuscript were not included in the present study: one did not enter immediate postoperative remission; two did not return for the follow-up visit; and one returned for her follow-up visit 33 months after discontinuing GC (instead of 6–8 months per study design). All had elevated 24-h urinary free cortisol (UFC) and normal or elevated ACTH levels, as measured by their referring physician. Median preoperative UFC was 169.9 μg/24 h (range 59.8–6959), mean preoperative plasma ACTH was 66.4 pg/ml (range 28–166), and mean preoperative serum cortisol was 25.4 μg/dl (range 10.1–57.3). No patient received pharmacological therapy for CD before study entry or during the study. Thirteen of the 14 patients had a diagnosis of hypertension, confirmed by resting blood pressure over 140/90 mm Hg or the use of antihypertensive medications. Two patients had known osteopenia, and one had osteoporosis, documented by dual-energy x-ray absorptiometry (DXA). Eight had hyperlipidemia, defined as total cholesterol (TC) over 200 mg/dl, low-density lipoprotein (LDL) over 130 mg/dl, or treatment with lipid-lowering medication. All were ambulatory with normal renal function and no liver disease.
Table 1.
Patient clinical and anthropometric characteristics
| Patient | Age (yr) | Sex | Ethnicity | Sx (yr) | GC dose 6–8 months postoperative (mg)a | Postoperative GC (months)b | Menstrual status |
DM |
WC (cm) |
BMI (kg/m2) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | |||||||
| 1 | 39 | F | AA | 1 | HC 20/10 | 6 | Oligo | Oligo | Yes | No | 111.1 | 106.0 | 38.0 | 34.8 |
| 2 | 39 | F | A | 1 | HC 20/10 | 8 | Reg | Reg | IFG | No | 91.4 | 85.7 | 30.9 | 28.0 |
| 3 | 52 | F | C | 10 | Dex (0.25 mg) | 30 | Post | Post | No | No | 85.1 | 74.9 | 25.7 | 21.7 |
| 4 | 29 | F | A | 3 | HC 10/5 | 36 | Oligo, OCPS | Reg | No | No | 106.6 | 96.0 | 36.2 | 31.1 |
| 5 | 23 | F | AA | 3 | HC 15/10 | 16 | Oligo, OCPS | Reg | No | No | 96.5 | 73.7 | 25.1 | 18.3 |
| 6 | 27 | F | C | 0.5 | Off | 1 | Reg, OCPS | Reg, OCPS | No | No | 92.4 | 102.4 | 30.2 | 30.6 |
| 7 | 74 | F | AA | 4.5 | Off | 5 | Post | Post | Yes | Yes | 120.0 | 123.0 | 43.7 | 40.0 |
| 8 | 41 | F | C | 2 | HC 10/10 | Oligo | Oligo | No | No | 100.5 | 85.0 | 29.1 | 24.8 | |
| 9 | 24 | F | C | 2 | HC 10 | 14 | Oligo | Reg | No | No | 89.0 | 92.5 | 27.9 | 25.8 |
| 10 | 41 | F | AA | 2.5 | HC 10/10 | 11 | Oligo | Reg | IGT | No | 104.5 | 83.7 | 31.2 | 26.9 |
| 11 | 28 | F | C | 8 | HC 15/10 | 10 | Reg, OCPS | Reg, OCPS | IFG | No | 111.7 | 123.0 | 38.3 | 38.5 |
| 12 | 60 | M | C | 10 | HC 10/10 | 11 | NA | NA | No | No | 106.5 | 99.3 | 25.2 | 24.9 |
| 13 | 36 | M | C | 0.16 | HC 10/5 | 9 | NA | NA | No | No | 113.0 | 97.8 | 31.6 | 28.8 |
| 14 | 22 | F | AA | 4 | Off | 2 | Oligo | Oligo | No | No | 113.0 | 94.9 | 36.1 | 31.0 |
| Mean | 38.2 | 3.7 | 12.2 | Mean | 103.0 | 95.6c | 32.1 | 28.9d | ||||||
| sd | 15.1 | 3.3 | 10.2 | sd | 10.6 | 15.1 | 5.6 | 6.0 | ||||||
Patient 6 had a previous TS and presented as a recurrence 6 yr later. Patient 8 was hypopituitary at follow-up, taking hydrocortisone 10 mg in the morning and 5 mg at 1500 h, levothyroxine 50 μg daily, medroxyprogesterone 10 mg daily for 5 d every 3 months, and GH 0.3 mg by daily sc injection. A, Asian; AA, African-American; C, Caucasian; Dex, dexamethasone; DM, diabetes mellitus; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; OCPS, oral contraceptives; Oligo, oligomenorrhea; Post, postmenopausal; Reg, regular menses; Sx, duration of symptoms before diagnosis.
Milligrams hydrocortisone (HC) given as morning/afternoon dose.
Number of months on postoperative oral GC.
P < 0.05 vs. preoperative values (paired t test).
P < 0.01 vs. preoperative values (paired t test).
Preoperatively, seven patients had a microadenoma (less than 1 cm), and seven had a normal/inhomogeneous pituitary on pituitary MRI. The seven patients without a clear tumor seen on MRI had preoperative inferior petrosal sinus sampling, and all had a post-CRH central to peripheral ACTH ratio over 3:1 (patients 3, 5, 7, 8, and 11–13).
No patient had documented hypopituitarism before surgery, based on normal thyroid hormone values. Menstrual status for females is described in Table 1. The preoperative oligomenorrhea in some could be secondary to hyperandrogenism (19) rather than hypopituitarism. Of the five oligomenorrheic patients (patients 1, 8, 9, 10, and 14) at baseline who were not taking oral contraceptives (ensuring estrogen sufficiency), three had available preoperative gonadal steroid hormones (patients 9, 10, and 14), disproving hypopituitarism. The remaining two patients did not have available preoperative gonadal steroid values (patients 1 and 8).
No patient received perioperative GC, according to the standard protocol of our neurosurgeon (K.D.P.). Immunohistochemical stain was positive for ACTH adenoma in nine of the 14 patients. Eleven of the 14 patients had low (<5.0 μg/dl) 1- to 2-d postoperative cortisol (not on GC replacement). Postoperative GC replacement was initiated before discharge on postoperative d 2 and consisted of dexamethasone 1 mg twice daily for patients 1–11 and hydrocortisone 20 mg in the morning and 10 mg in the afternoon for patients 12–14, due to a change in standard protocol. Subsequent postoperative care, including GC dose and tapering schedule, was managed by each patient's primary endocrinologist. Each patient's GC dose at 6–8 months postoperatively is shown in Table 1. Remission was confirmed by normal 24-h UFC and resolution of clinical features, as previously defined (20). Patients returned for their postoperative visit 6–8 months after discontinuation of oral GC. The postoperative visit occurred at a range of 9–42 months since surgery (mean 20.1). At follow-up, hypertension had resolved in all but three patients (patients 3, 7, and 12).
Patient 8, who underwent two TS after study entry, developed hypopituitarism and was taking complete pituitary replacement, noted in Table 1. Two patients (patients 1 and 11) had low IGF-I values at follow-up, but GH deficiency was not confirmed by stimulation testing. No other patient had documented hypopituitarism at follow-up.
The study was approved by the Institutional Review Board at the Mount Sinai Medical Center. All subjects gave written informed consent before participation.
Study design
Laboratory testing and hormone assays
Fasting insulin, glucose, CRP, leptin, high-molecular-weight adiponectin (HMWApN), and lipid profile [TC, triglyceride (TG), high-density lipoprotein (HDL), and LDL] values were measured. Serum was frozen at −80 C. Each subject's samples were run in the same assay and in duplicate. Insulin was measured by Immulite, with an intraassay coefficient of variation (CV) of 5.3%, interassay CV of 6.0%, and sensitivity of 2 μIU/ml. Glucose was measured by the hexokinase method. CRP was measured by Immulite, with a functional sensitivity of 0.3 mg/liter and an imprecision at the upper limit of normal (3 mg/liter) of approximately 5% CV. Leptin was measured by RIA (Linco Research, Inc., St. Charles, MO) with an intraassay CV of 8.3% and interassay CV of 6.2%. HMWApN was measured by ELISA (Millipore, Billerica, MA) with an intra-assay CV of 3.0–8.8% and an inter-assay CV of 1.8–6.1%. Lipid profile was measured by the enzymatic in vitro method (Roche, Indianapolis, IN). Insulin sensitivity was measured by homeostasis model assessment for insulin resistance (HOMA-IR) scores (21).
Body composition testing
Each subject underwent measurements of anthropometrics, BMI, and whole-body MRI acquisition.
For anthropometric measurements, body weight was measured with a digital scale to the nearest 0.1 kg and height with an eye-level scale (Detecto, Webb City, MO) at the Mount Sinai General Clinical Research Center. BMI was calculated as the patient's weight in kilograms divided by the height in meters squared. Waist circumference (WC), per the World Health Organization, was measured as the lowest value between the xyphoid process and the umbilicus.
For MRI, total and regional body AT volumes were measured by whole-body multislice MRI on a 1.5-T scanner at the Mount Sinai Hospital Radiology Associates (General Electric, Milwaukee, WI) as previously reported by our group (18). Subjects were placed on the MRI platform with their arms extended above their heads. Approximately 40 axial images of 10 mm thickness at 40-mm intervals from head to toe were acquired. AT compartment volume [including sc adipose tissue (SAT), VAT, inter-muscular adipose tissue (IMAT), TrSAT, and BMAT] was calculated as:
where V is volume, Ai is each scan's cross-sectional area, h is the between-slice interval, t is the thickness of each slice, and N is the number of total slices (22). Total body SAT was defined as SAT from fingers to toes. Trunk AT was defined as all AT from the shoulder (upper limit defined as the separation between the arms and neck) to the pelvis (lower limit defined as the level of separation of the legs). The IMAT compartment was defined as the AT located between muscle groups and beneath the muscle fascia (23). BMAT was calculated according to previously published methods (8). Total BMAT included all BMAT excluding the skull and ribs. Spine BMAT was not reported separately due to inadequate quantities in some subjects. Pelvic BMAT was defined as the AT located from L4–L5 to the separation of legs from trunk including the femoral heads. Images were analyzed with SliceOmatic image analysis software (TomoVision, Montreal, Canada) in the Image Reading Center at St. Luke's-Roosevelt Hospital Center. MRI volume estimates were converted to mass using the assumed density of 0.92 kg/liter for AT and 1.04 kg/liter for SM (24). The CV for repeated measurements of the same scan by the same observer of MRI-derived AT volumes is 1.7% for SAT, 2.3% for VAT, and 5.9% for IMAT (23). The intraclass correlation coefficient for volume rendering of BMAT was 0.99 (8).
Statistical analysis
Normality of the data was assessed by visual inspection and the Shapiro-Wilk's W test. For normally distributed variables, parametric tests were used for analysis. Nonnormal data were either log transformed or analyzed with the appropriate nonparametric test. Each subject's preoperative VAT, SAT, TrSAT, total adipose tissue (TAT), IMAT, BMAT, SM mass, measures of AT distribution (e.g. VAT/TAT), and biochemical markers (e.g. HOMA-IR, CRP, leptin, HMWApN, and lipid profile) were compared with the postoperative value by paired t test. Pearson's or Spearman's correlation, when appropriate, was used to assess the relationships between the mass of each AT compartment and each biochemical marker. P values <0.05 were considered significant. Data are given as mean ± sd unless stated otherwise. Data were analyzed using SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Anthropometrics
Remission decreased weight, BMI, and WC (P ≤ 0.05, Table 1). At both visits, WC correlated with BMI (active disease correlation coefficient, R = 0.752, P = 0.002; remission R = 0.810, P = 0.0001). Active disease (R = −0.576, P = 0.031) and remission (R = −0.746, P = 0.002) weight correlated negatively with duration of postoperative oral GC exposure.
Body composition and AT distribution
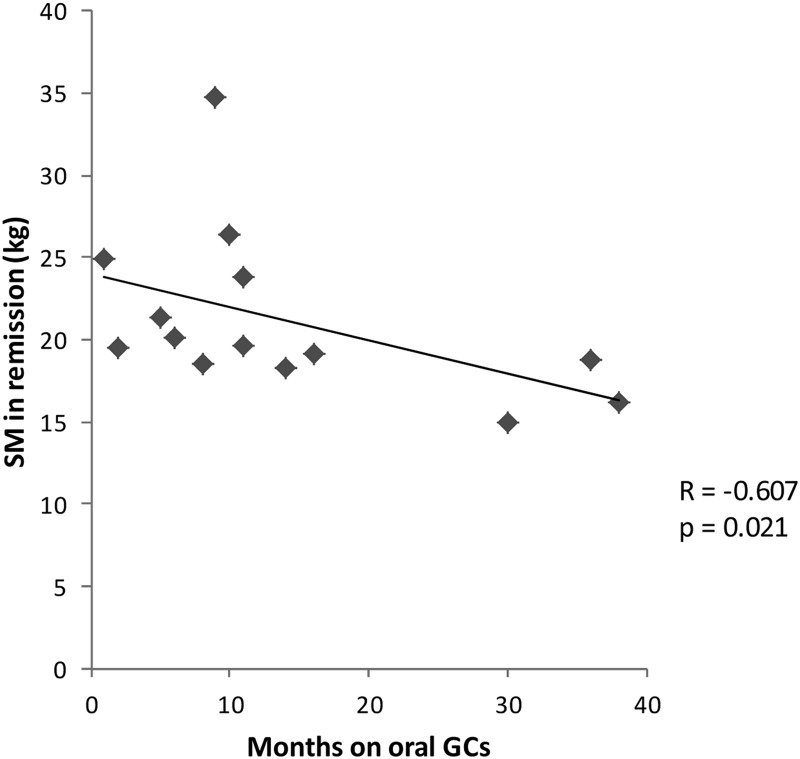
Mean AT mass in each compartment in active disease and remission are shown in Table 2. Remission decreased VAT (P = 0.004), pelvic BMAT (P = 0.012), TrSAT (P = 0.0005), limb SAT (P = 0.001), total SAT (P = 0.0001), and TAT (P = 0.0002). IMAT (P = 0.51) and total BMAT (P = 0.54) did not change. Total BMAT correlated with VAT in active disease (R = 0.571, P = 0.041) but not in remission. Remission decreased total SM (P = 0.02) but not limb SM (P = 0.12). SM in remission correlated negatively with duration of postoperative oral GC exposure (R = −0.607, P = 0.021) (Fig. 1). Remission decreased the mean ratio of VAT/SM (P = 0.006) and VAT/TAT (P = 0.04) but not SAT/TAT (P = 0.06) or IMAT/SM (P = 0.95).
Table 2.
Body composition measurements
| Measure (kg) | Active CD | Remission | Difference | Change (%) | Value decreased (no. of patients) | P valuea |
|---|---|---|---|---|---|---|
| VAT | 4.59 ± 2.68 | 3.21 ± 2.05 | −1.38 | −29.3 | 12 | 0.004 |
| Pelvic BMATb | 0.26 ± 0.11 | 0.19 ± 0.09 | −0.07 | −20.5 | 11 | 0.012 |
| TrSAT | 19.54 ± 7.35 | 15.72 ± 7.92 | −3.82 | −21.9 | 12 | 0.0005 |
| Limb SAT | 13.82 ± 7.33 | 12.01 ± 7.29 | −1.81 | −14.8 | 13 | 0.001 |
| Total SAT | 33.36 ± 14.10 | 27.69 ± 14.33 | −5.67 | −19.1 | 13 | 0.0001 |
| TAT | 39.21 ± 14.15 | 32.00 ± 15.43 | −7.21 | −20.5 | 12 | 0.0002 |
| IMAT | 1.18 ± 0.46 | 1.10 ± 0.57 | −0.08 | −4.8 | 9 | 0.512 |
| SM | 21.18 (19.4–22.9) | 19.58 (18.6–23.2) | −1.60 | −4.5 | 10 | 0.02 |
| Limb SM | 11.04 (9.92–12.66) | 10.86 (9.84–11.67) | −0.18 | −2.9 | 10 | 0.12 |
| VAT/SM | 0.20 ± 0.09 | 0.14 ± 0.07 | −0.06 | −26.1 | 12 | 0.006 |
| VAT/TAT | 0.13 ± 0.09 | 0.11 ± 0.08 | −0.02 | −13.9 | 13 | 0.04 |
Data are presented as mean ± sd or median (interquartile range). P values are from Ln (natural logarithm) values.
From paired t test.
For pelvic BMAT, n = 13 (patient 12 had metal artifact in right femur).
Fig. 1.
Inverse correlation between SM mass in remission and duration of postoperative oral glucocorticoid exposure (Spearman's correlation coefficient = −0.607, P = 0.021).
Results were similar after separately excluding the hypopituitary patient at follow-up (patient 8) and the patients taking oral contraceptives at both visits (patients 6 and 11). After excluding the postmenopausal patients (patients 3 and 7), the change in SM was not significant (P = 0.057), nor was the association between duration of GC exposure and SM in remission (P = 0.062). After excluding the male patients (patients 12 and 13), the changes in VAT/TAT (P = 0.102) and WC (P = 0.070) were not significant.
Cardiovascular risk markers
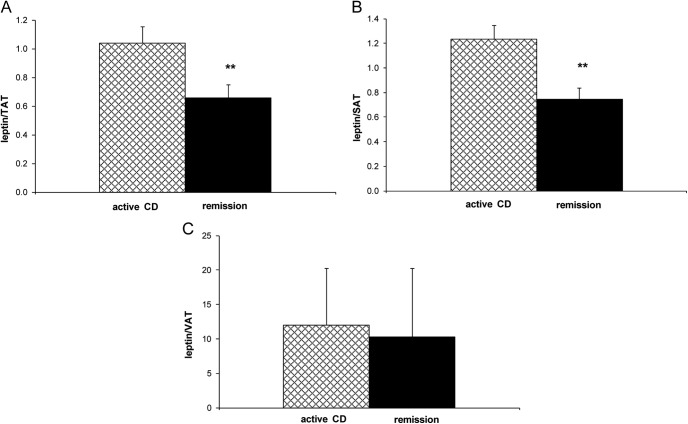
Table 3 shows HOMA-IR, leptin, CRP, HMWApN, and lipid concentrations in active disease and remission. Remission decreased HOMA-IR score (P = 0.0004) and leptin concentration (P = 0.00002). Remission decreased leptin/TAT (P = 0.0004) and leptin/SAT (P = 0.0003) but not leptin/VAT (P = 0.18) (Fig. 2). Remission did not change CRP (median active disease CRP was 5.2 vs. 1.8 mg/liter in remission, P = 0.69) or HMWApN (median active disease HMWApN was 6.0 vs. 6.1 μg/ml in remission, P = 0.43). Remission decreased TC (P = 0.03) but did not change any other lipid parameter or ratio. These results were unchanged after removal of the two patients taking lipid-lowering medications (patients 7 and 12).
Table 3.
Cardiovascular risk markers
| Measure | Active CD | Remission | Pa |
|---|---|---|---|
| HOMA-IR | 3.1 ± 1.9 | 1.3 ± 0.7 | 0.0004 |
| Leptin (ng/ml) | 41.3 ± 20.3 | 23.0 ± 17.8 | 0.00002 |
| CRP (mg/liter)b | 5.2 (1.1–12.5) | 1.8 (1.3–15.2) | 0.69 |
| HMWApN (μg/ml)b | 6.0 (2.6–8.4) | 6.1 (2.9–6.5) | 0.43 |
| TC (mg/dl) | 202.5 ± 36.1 | 186.4 ± 32.5 | 0.03 |
| TG (mg/dl) | 121.2 ± 55.2 | 109.4 ± 65.3 | 0.55 |
| LDL (mg.dl) | 117.2 ± 31.6 | 107.0 ± 25.1 | 0.08 |
| HDL (mg/dl) | 61.2 ± 16.4 | 57.4 ± 13.3 | 0.20 |
| TC/HDL | 3.5 ± 1.2 | 3.4 ± 0.8 | 0.53 |
| LDL/HDL | 2.1 ± 0.9 | 2.0 ± 0.6 | 0.55 |
Data are presented as mean ± sd or median (interquartile range). P values for CRP and HMWApN are from Ln (natural logarithm) values. HOMA-IR = [fasting serum insulin (microunits per milliliter) × fasting plasma glucose (millimoles per liter)/22.5]. CRP <1.0 mg/liter indicates low cardiovascular risk, 1.0–3.0 average risk, and >3.0 high risk (10). HOMA-IR ≥2.77 indicates insulin resistance (25). Two patients (7 and 12) were taking lipid-lowering medications at both visits.
From paired t test.
For these measures, n = 13. CRP was excluded in patient 6 due to cough and congestion at postoperative visit, and patient 13 had postoperative HMWApN values below the range of detection.
Fig. 2.
Mean leptin/TAT (A) (**, P = 0.0004) and leptin/SAT (B) (**, P = 0.0003) but not leptin/VAT (C) (P = 0.176) decreased with remission (paired t test).
Correlation between cardiovascular risk markers and AT compartments
Table 4 shows correlations between cardiovascular risk markers and AT compartments. Leptin values correlated with SAT (active disease and remission P = 0.0001) but not VAT (active disease P = 0.39, remission P = 0.90). Pelvic BMAT correlated positively with HMWApN values (active disease P = 0.032, remission P = 0.048) and negatively with HOMA-IR score (active disease P = 0.0001 and remission P = 0.001). CRP correlated with WC, VAT, and HOMA score in remission only (P < 0.05). CRP percent change correlated positively with TrSAT percent change (0.742, P = 0.004). TG correlated with HOMA-IR score (R = 0.548, P = 0.043), and HDL correlated with pelvic BMAT (R = 0.771, P = 0.002), both in remission only.
Table 4.
Correlation between measures of body composition and cardiovascular risk markers
| HOMA-IR |
CRP |
Leptin |
HMWApN |
|||||
|---|---|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | |
| WC | NS | NS | NS | 0.804 (0.002) | 0.594 (0.025) | 0.640 (0.014) | NS | NS |
| BMI | NS | 0.621 (0.018) | 0.720 (0.008) | 0.806 (0.002) | 0.696 (0.006) | 0.801 (0.001) | NS | NS |
| Pelvic BMAT | −0.841 (0.0001) | −0.809 (0.001) | NS | NS | NS | NS | 0.645 (0.032) | 0.636 (0.048) |
| SAT | NS | 0.585 (0.028) | 0.811 (0.001) | 0.658 (0.020) | 0.850 (0.0001) | 0.911 (0.0001) | NS | NS |
| VAT | NS | NS | NS | 0.688 (0.013) | NS | NS | NS | NS |
| HOMA-IR | NS | 0.621 (0.031) | NS | NS | −0.664 (0.026) | −0.842 (0.002) | ||
Data are presented as correlation coefficient (P value), from Pearson's or Spearman's when appropriate. HOMA-IR = [fasting serum insulin (microunits per milliliter) × fasting plasma glucose (millimoles per liter)/22.5]. NS, not significant.
Discussion
The present study demonstrates that remission of CD decreased nearly all fat depots, including VAT, pelvic BMAT, and trunk, total, and limb SAT, as assessed by total-body MRI. Remission improved fat distribution by reducing the proportion of TAT that was VAT (i.e. VAT/TAT) and the VAT/SM ratio, but the absolute SM mass also decreased, and most patients remained in the overweight (BMI >25 kg/m2) or obese (BMI >30 kg/m2) category. Cardiovascular risk factors changed; insulin resistance, leptin, and TC decreased, but HMWApN, CRP, and other lipid measures did not.
We previously showed that the increased TAT in active CD is due to increased VAT, given that the SAT was not larger compared with weight-matched controls (18). The elevated VAT/TAT ratio in CD decreases with remission in the current study, as does the VAT/SM ratio. Elevated VAT, VAT/TAT, and VAT/thigh SM ratios positively associate with hepatic steatosis and the metabolic syndrome in other cohorts (4, 5). The favorable changes in these ratios shown here could therefore play a role in reducing the HOMA-IR score to under the threshold for insulin resistance (25) in CD remission. IMAT, another depot associated with insulin resistance in other populations (26), was not elevated in active CD compared with controls (18), nor did it change over time with remission here. Although GC have been shown to increase intramyocellular lipid in rats (27), the role of GC in IMAT mass is not known.
Lack of an expected increase in CS patients' lean tissue postoperatively, measured by total body potassium counting (which measured body cell mass) (14) and CT (which measured SM plus skin) (13) has been shown in two small studies, with a maximum follow-up of 10 months. Here for the first time we measure SM longitudinally with MRI, with a maximum follow-up of 42 months, and show an unexpected decrease in SM. We found an inverse correlation between duration of oral GC replacement with SM mass in remission, suggesting continued SM loss resulting from physiologic oral GC replacement. After excluding the two postmenopausal women, the SM decrease was no longer significant (P = 0.057), nor was the correlation between duration of GC exposure and SM mass in remission (P = 0.062). This could reflect greater SM loss in the post- vs. premenopausal women due to greater vulnerability to GC-induced SM loss in the setting of estrogen deficiency, or insufficient sample size. The role of estrogens in the body composition and bone changes seen in CS is not fully understood. Pre- and postmenopausal women with CS have similar fat distributions (19), and estrogen-sufficient but not -deficient CS patients have lower bone mineral density (BMD) compared with controls (28), suggesting that the protective effect of estrogens on fat distribution and BMD is lost in CS. The effects of oral GC dose and type will need to be assessed in additional studies, as will the relative effect of weight loss (29) vs. normalization of cortisol on SM mass in CD remission.
Cortisol is known to increase leptin production (30). In keeping with this, we show high leptin values in active CD, which decrease with remission, consistent with previous data (31). Remission reduces leptin/TAT and leptin/SAT, but not leptin/VAT, suggesting that the hypercortisolemia in active CD enhances leptin production preferentially in SAT vs. VAT. Accordingly, leptin does not correlate with VAT, consistent with our previous report (18) and data on healthy subjects showing higher leptin expression in SAT vs. VAT (32).
In contrast to leptin, HMWApN did not change with remission, in keeping with total ApN data from other CS studies (12). Interestingly, values were similar to those of nondiabetic healthy women from the Nurses Health Study (33). Higher ApN levels, particularly HMWApN levels, are associated with improved insulin sensitivity (34), and GC reduce ApN gene expression and mRNA regulation (35), so it is unclear why ApN does not increase with CD remission. Rat data show that ACTH increases whereas dexamethasone decreases ApN gene expression in SAT (36). This could suggest different effects of endogenous vs. exogenous GC and, due to reduced ACTH values with CD remission, would explain the lack of HMWApN increase shown here.
Although remission decreased TC, none of the other lipids measures (HDL, LDL, and TG) changed. In fact, HDL decreased in 11 of the 14 patients (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), but the change was not significant. Although remission did not change the TC/HDL and LDL/HDL ratios, mean values were in the desirable range (<4.5 for TC/HDL and <3.0 for LDL/HDL) in active disease, in contrast to other reports showing dyslipidemia in CD compared with controls (17, 37). Other longitudinal CD studies also have not shown a change in lipid profile with remission (17, 37). The fact that many patients in the present study remained either overweight (four of the 14 patients) or obese (six patients) despite remission could play a role in the lack of change in lipid profile, CRP, and HMWApN.
We did not find correlations between regional AT mass and markers of insulin resistance (by HOMA-IR) in active CD, consistent with baseline MRI data from our group (18) and other groups using CT (11), DXA (12), and WC measurements (37). This contrasts with data from other populations that show positive associations between elevated visceral and overall fat with insulin resistance and markers of proinflammation (3, 9, 10). Accordingly, CD patients in remission showed expected correlations between HOMA-IR score with BMI and SAT as well as with CRP (positive) and with HMWApN (negative). Similarly, CRP correlated with WC and VAT only in remission but not in active disease. IL-6, which may be elevated in CD (12), regulates CRP, along with other cytokines (38). GC-induced alterations in IL-6, therefore, may prevent CRP from being an accurate marker of insulin resistance and cardiovascular risk in active CD as it is in other populations.
It is unclear why the known association between WC and VAT with insulin resistance was not seen in active or treated CD, whereas the SAT correlated with HOMA score (in remission only). Others have suggested (12, 37) that hypercortisolemia-induced 11 β-hydroxysteroid dehydrogenase type 1 activation, resulting in differentiation of preadipocytes to adipocytes in the VAT (39), could result in persistent visceral adiposity after remission. Theoretically, this could result in dissociation of VAT mass and markers of insulin resistance in remission. However, although cross-sectional studies have found persistently elevated abdominal fat (by WC and DXA measurements) in remission (17, 37), we found a decrease in VAT after remission, using a prospective design and with each patient as their own control.
Existing data suggest that BMAT is independent from other depots and does not play a role in overall fat metabolism (7, 8), although evidence is conflicting (40). Data on associations with VAT are also conflicting (6, 7), and the relationship between BMAT and metabolic risk factors are largely unknown. We found that total BMAT correlated with VAT in active CD but not remission. However, larger studies in other populations show that the association between BMAT and VAT disappears after adjustment for age and menopausal status (8). Here, pelvic BMAT inversely correlated with total fasting insulin and HOMA score, both in active disease and remission. This finding needs further evaluation in a larger sample. Pelvic BMAT also positively correlated with HMWApN in active disease and remission, suggesting a role for ApN in the bone microenvironment in CD, consistent with recent data showing expression of ApN and its receptors on osteoblasts (41). BMAT, and in particular pelvic BMAT (a reflection of cancellous bone), inversely associates with BMD (8). Remission reduced pelvic BMAT, which may reflect improved BMD. Although BMD was not an endpoint in the present study, osteopenia and osteoporosis were observed at baseline, as expected. The possible inverse relationship between ApN and BMD (42) suggests that BMD may be the link between ApN and BMAT. The role of GC in bone marrow adipogenesis needs further clarification, and whether BMAT mass in CD is pathogenic in the reduced BMD is not yet understood.
One limitation of the study is the small sample, resulting from the rarity of the disease and our strict inclusion criteria (including only pituitary Cushing's patients who entered postoperative remission). Another limitation is the variation in timing of the follow-up visit, because length of GC requirement varied among patients. However, to study GC effects on body composition, it was imperative to control for GC status and study each patient after GC discontinuation. We cannot entirely rule out the role of elapsed time in the body composition changes seen after surgery, although visual inspection of the data does not suggest this. The diversity of the population in terms of gender, ethnicity, and estrogen status could be considered a limitation, but because each patient was their own control, this should not limit the strength of the data, and subgroup analyses did not change the major findings. Another limitation is inability of the T1-weighted MRI protocol used here to detect hepatic fat, which has been assessed in only one CS study, by CT (43), and needs further investigation. Strengths of the study include the novel use of MRI to quantify VAT, IMAT, BMAT, and SM in CD, the correlation of AT depots to cardiovascular risk markers, and the longitudinal follow-up of patients in remission.
In conclusion, we have demonstrated that remission of CD decreases weight and nearly all AT depots, including VAT, pelvic BMAT, and TrSAT, although most patients remained in the overweight or obese category. AT distribution became more favorable, with decreases in VAT/TAT, and VAT/SM ratios, in keeping with the reductions in HOMA-IR score and leptin concentration. Unexpectedly, remission also reduced SM mass, and HMWApN and CRP profiles did not improve. Whether the decrease in insulin resistance and abdominal fat shown here confers a longstanding reduction in risk for the metabolic syndrome will be determined by continued follow-up of this patient cohort. Similarly, longer follow-up postoperatively will show whether SM ultimately increases and whether VAT mass plateaus or continues to decline. Further investigation of the relationship between excess GC, AT mass and distribution, and metabolic parameters is required to understand the metabolic and cardiovascular risk of active CD and CD in remission.
Supplementary Material
Acknowledgments
We thank Mark Punyanitya and Rahul Peethala of the Image Analysis Lab of the New York Obesity Research Center for assistance with MRI analysis and John Waselus and Thomas Eitel at Mount Sinai Medical Center for performing some of the MRI scans.
This work was supported by National Institutes of Health Grants K23 DK 082617 (to E.B.G.), K24 DK 073040 (to P.U.F.), P30-DK26687 (to the New York Obesity Research Center), and Mount Sinai General Clinical Research Center Clinical Research Feasibility Fund Award MO1-RR-00071 and Clinical and Translational Sciences Award UL1RR029887.
Disclosure Summary: E.B.G, W.S., E.S., K.D.P., and P.U.F. have nothing to declare.
Footnotes
- AT
- Adipose tissue
- BMAT
- bone marrow adipose tissue
- BMD
- bone mineral density
- CD
- Cushing's disease
- CRP
- C-reactive protein
- CS
- Cushing's syndrome
- CT
- computed tomography
- CV
- coefficient of variation
- GC
- glucocorticoid
- DXA
- dual-energy x-ray absorptiometry
- HDL
- high-density lipoprotein
- HMWApN
- high-molecular-weight adiponectin
- HOMA-IR
- homeostasis model assessment for insulin resistance
- IMAT
- inter-muscular adipose tissue
- LDL
- low-density lipoprotein
- MRI
- magnetic resonance imaging
- SAT
- sc adipose tissue
- SM
- skeletal muscle
- TAT
- total adipose tissue
- TC
- total cholesterol
- TrSAT
- trunk sc AT
- TS
- transsphenoidal surgery
- UFC
- urinary free cortisol
- vAT
- visceral adipose tissue
- WC
- waist circumference.
References
- 1. Rebuffé-Scrive M, Krotkiewski M, Elfverson J, Björntorp P. 1988. Muscle and adipose tissue morphology and metabolism in Cushing's syndrome. J Clin Endocrinol Metab 67:1122–1128 [DOI] [PubMed] [Google Scholar]
- 2. Wajchenberg BL, Bosco A, Marone MM, Levin S, Rocha M, Lerário AC, Nery M, Goldman J, Liberman B. 1995. Estimation of body fat and lean tissue distribution by dual energy x-ray absorptiometry and abdominal body fat evaluation by computed tomography in Cushing's disease. J Clin Endocrinol Metab 80:2791–2794 [DOI] [PubMed] [Google Scholar]
- 3. Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. 1996. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes 45:633–638 [DOI] [PubMed] [Google Scholar]
- 4. Ducluzeau PH, Manchec-Poilblanc P, Roullier V, Cesbron E, Lebigot J, Bertrais S, Aubé C. 2010. Distribution of abdominal adipose tissue as a predictor of hepatic steatosis assessed by MRI. Clin Radiol 65:695–700 [DOI] [PubMed] [Google Scholar]
- 5. Lim KI, Yang SJ, Kim TN, Yoo HJ, Kang HJ, Song W, Baik SH, Choi DS, Choi KM. 2010. The association between the ratio of visceral fat to thigh muscle area and metabolic syndrome: the Korean Sarcopenic Obesity Study (KSOS). Clin Endocrinol (Oxf) 73:588–594 [DOI] [PubMed] [Google Scholar]
- 6. Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. 2011. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity 19:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Iorgi N, Mittelman SD, Gilsanz V. 2008. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond) 32:1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. 2007. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int 18:641–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diamant M, Lamb HJ, van de Ree MA, Endert EL, Groeneveld Y, Bots ML, Kostense PJ, Radder JK. 2005. The association between abdominal visceral fat and carotid stiffness is mediated by circulating inflammatory markers in uncomplicated type 2 diabetes. J Clin Endocrinol Metab 90:1495–1501 [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM, Buring JE, Cook NR, Rifai N. 2003. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 107:391–397 [DOI] [PubMed] [Google Scholar]
- 11. Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. 2003. Computed tomography assessment of fat distribution in male and female patients with Cushing's syndrome. Eur J Endocrinol 149:561–567 [DOI] [PubMed] [Google Scholar]
- 12. Barahona MJ, Sucunza N, Resmini E, Fernández-Real JM, Ricart W, Moreno-Navarrete JM, Puig T, Farrerons J, Webb SM. 2009. Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing's syndrome. J Clin Endocrinol Metab 94:3365–3371 [DOI] [PubMed] [Google Scholar]
- 13. Lönn L, Kvist H, Ernest I, Sjöström L. 1994. Changes in body composition and adipose tissue distribution after treatment of women with Cushing's syndrome. Metabolism 43:1517–1522 [DOI] [PubMed] [Google Scholar]
- 14. Pirlich M, Biering H, Gerl H, Ventz M, Schmidt B, Ertl S, Lochs H. 2002. Loss of body cell mass in Cushing's syndrome: effect of treatment. J Clin Endocrinol Metab 87:1078–1084 [DOI] [PubMed] [Google Scholar]
- 15. Colao A, Pivonello R, Spiezia S, Faggiano A, Ferone D, Filippella M, Marzullo P, Cerbone G, Siciliani M, Lombardi G. 1999. Persistence of increased cardiovascular risk in patients with Cushing's disease after five years of successful cure. J Clin Endocrinol Metab 84:2664–2672 [DOI] [PubMed] [Google Scholar]
- 16. Webb SM, Mo D, Lamberts SW, Melmed S, Cavagnini F, Pecori Giraldi F, Strasburger CJ, Zimmermann AG, Woodmansee WW. 2010. Metabolic, cardiovascular, and cerebrovascular outcomes in growth hormone-deficient subjects with previous Cushing's disease or non-functioning pituitary adenoma. J Clin Endocrinol Metab 95:630–638 [DOI] [PubMed] [Google Scholar]
- 17. Faggiano A, Pivonello R, Spiezia S, De Martino MC, Filippella M, Di Somma C, Lombardi G, Colao A. 2003. Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing's disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab 88:2527–2533 [DOI] [PubMed] [Google Scholar]
- 18. Geer EB, Shen W, Gallagher D, Punyanitya M, Looker HC, Post KD, Freda PU. 2010. MRI assessment of lean and adipose tissue distribution in female patients with Cushing's disease. Clin Endocrinol (Oxf) 73:469–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garrapa GG, Pantanetti P, Arnaldi G, Mantero F, Faloia E. 2001. Body composition and metabolic features in women with adrenal incidentaloma or Cushing's syndrome. J Clin Endocrinol Metab 86:5301–5306 [DOI] [PubMed] [Google Scholar]
- 20. Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M. 2008. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 22. Shen W, Wang Z, Tang H, Heshka S, Punyanitya M, Zhu S, Lei J, Heymsfield SB. 2003. Volume estimates by imaging methods: model comparisons with visible woman as the reference. Obes Res 11:217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. 2005. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 81:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. 1975. Report of the Task Group on Reference Man. International Commission on Radiological Protection, no 23. Oxford, UK: Pergamon Press [Google Scholar]
- 25. Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, Targher G, Alberiche M, Bonadonna RC, Muggeo M. 1998. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes 47:1643–1649 [DOI] [PubMed] [Google Scholar]
- 26. Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, Janumala I, Burkey B, Heshka S, Gallagher D. 2005. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 82:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Korach-André M, Gao J, Gounarides JS, Deacon R, Islam A, Laurent D. 2005. Relationship between visceral adiposity and intramyocellular lipid content in two rat models of insulin resistance. Am J Physiol Endocrinol Metab 288:E106–E116 [DOI] [PubMed] [Google Scholar]
- 28. Barahona MJ, Sucunza N, Resmini E, Fernández-Real JM, Ricart W, Moreno-Navarrete JM, Puig T, Wägner AM, Rodriguez-Espinosa J, Farrerons J, Webb SM. 2009. Deleterious effects of glucocorticoid replacement on bone in women after long-term remission of Cushing's syndrome. J Bone Miner Res 24:1841–1846 [DOI] [PubMed] [Google Scholar]
- 29. Chaston TB, Dixon JB, O'Brien PE. 2007. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 31:743–750 [DOI] [PubMed] [Google Scholar]
- 30. Papaspyrou-Rao S, Schneider SH, Petersen RN, Fried SK. 1997. Dexamethasone increases leptin expression in humans in vivo. J Clin Endocrinol Metab 82:1635–1637 [DOI] [PubMed] [Google Scholar]
- 31. Masuzaki H, Ogawa Y, Hosoda K, Miyawaki T, Hanaoka I, Hiraoka J, Yasuno A, Nishimura H, Yoshimasa Y, Nishi S, Nakao K. 1997. Glucocorticoid regulation of leptin synthesis and secretion in humans: elevated plasma leptin levels in Cushing's syndrome. J Clin Endocrinol Metab 82:2542–2547 [DOI] [PubMed] [Google Scholar]
- 32. Hube F, Lietz U, Igel M, Jensen PB, Tornqvist H, Joost HG, Hauner H. 1996. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm Metab Res 28:690–693 [DOI] [PubMed] [Google Scholar]
- 33. Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. 2008. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med 149:307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, Okuno T, Inoue T, Node K, Tobe T, Inukai T, Nakano Y. 2006. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes 55:1954–1960 [DOI] [PubMed] [Google Scholar]
- 35. Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. 2002. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 290:1084–1089 [DOI] [PubMed] [Google Scholar]
- 36. Paschke L, Zemleduch T, Rucinski M, Ziolkowska A, Szyszka M, Malendowicz LK. 2010. Adiponectin and adiponectin receptor system in the rat adrenal gland: ontogenetic and physiologic regulation, and its involvement in regulating adrenocortical growth and steroidogenesis. Peptides 31:1715–1724 [DOI] [PubMed] [Google Scholar]
- 37. Giordano R, Picu A, Marinazzo E, D'Angelo V, Berardelli R, Karamouzis I, Forno D, Zinna D, Maccario M, Ghigo E, Arvat E. 2011. Metabolic and cardiovascular outcomes in patients with Cushing's syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf) 75:354–360 [DOI] [PubMed] [Google Scholar]
- 38. Heinrich PC, Castell JV, Andus T. 1990. Interleukin-6 and the acute phase response. Biochem J 265:621–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooper MS, Stewart PM. 2009. 11Beta-hydroxysteroid dehydrogenase type 1 and its role in the hypothalamus-pituitary-adrenal axis, metabolic syndrome, and inflammation. J Clin Endocrinol Metab 94:4645–4654 [DOI] [PubMed] [Google Scholar]
- 40. Lecka-Czernik B. 2011. Marrow fat metabolism is linked to the systemic energy metabolism. Bone 50:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. 2004. Adiponectin and its receptors are expressed in bone-forming cells. Bone 35:842–849 [DOI] [PubMed] [Google Scholar]
- 42. Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. 2003. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33:646–651 [DOI] [PubMed] [Google Scholar]
- 43. Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. 2003. Hepatic steatosis in Cushing's syndrome: a radiological assessment using computed tomography. Eur J Endocrinol 149:543–548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.