Abstract
Context:
Mifepristone is a glucocorticoid and progestin antagonist under investigation for the treatment of Cushing's syndrome. Mifepristone decreases high-density lipoprotein (HDL) cholesterol (HDL-C) levels in treated patients, but the clinical significance of this is unclear because recent studies suggest that functional properties of HDL predict cardiovascular disease status better than does HDL-C concentration.
Objective:
The aim of the study was to characterize the impact of mifepristone administration on HDL particle concentration and function.
Design and Setting:
We conducted a double-blind, randomized, placebo-controlled trial at a single-site, clinical research center.
Participants:
Thirty healthy postmenopausal female volunteers participated in the study.
Intervention:
Individuals were randomized to receive daily oral mifepristone (600 mg) or placebo for 6 wk.
Main Outcome Measures:
We measured HDL-C, serum HDL particle concentration, and HDL-mediated cholesterol efflux by treatment group.
Results:
As expected, ACTH, cortisol, estradiol, and testosterone levels increased in the mifepristone group. Mifepristone treatment decreased HDL-C and HDL particle concentration by 26 and 25%, respectively, but did not alter pre-β HDL concentration. In contrast, the serum HDL-mediated cholesterol efflux decreased with mifepristone treatment by only 12%, resulting in an effective increase of the efflux capacity per HDL particle. No changes were observed in cholesterol ester transfer protein or lecithin:cholesterol acyltransferase activity.
Conclusions:
Treatment with mifepristone reduced HDL-C, HDL particle concentration, and serum HDL cholesterol efflux in postmenopausal women. However, on a per particle basis, the efflux capacity of serum HDL increased. These observations support the concept that a decrease in HDL-C may not represent proportional impairment of HDL function.
Chronic elevations in corticosteroids lead to central obesity, insulin resistance, and type 2 diabetes, hypertension, and an increased risk of atherosclerotic vascular disease in patients with Cushing's syndrome (1). Current therapies for Cushing's syndrome often result in poor control of these complications. Mifepristone is a potent glucocorticoid receptor antagonist and is under investigation for the treatment of Cushing's disease in patients who fail to respond to conventional therapy (2).
Central obesity and insulin resistance are typically associated with dyslipidemia characterized by elevated levels of triglyceride and small, dense low-density lipoprotein (LDL), and low levels of high-density lipoprotein (HDL) cholesterol (HDL-C) (3). Low levels of HDL-C are strongly associated with an increased risk of cardiovascular disease (CVD). Patients with Cushing's syndrome have elevated triglyceride levels and very low-density lipoprotein production rates consistent with their insulin-resistant state, but paradoxically also have elevated HDL-C compared with controls (4). In non-Cushing's subjects, a 1-month burst and taper of the glucocorticoid prednisone raised total cholesterol and HDL-C but not triglyceride levels (5). Taken together, these studies suggest an independent effect of glucocorticoids on HDL metabolism that is dissociated from longer-term effects of central weight gain and insulin resistance.
HDL is a complex of cholesterol, phospholipids, triglycerides, and proteins with apolipoprotein (apo) A-I (apoA-I), a single major protein constituting about 70% of the total HDL protein (6). HDL is thought to exert its cardioprotective effects primarily by promoting cholesterol efflux from macrophages in the artery wall (7, 8). The concentration of HDL in blood is monitored clinically as HDL-C. However, HDL is composed of a heterogeneous mixture of particles that carry a wide range of proteins (6, 9–12), and the relationship between HDL-C levels, HDL function, and specific populations of HDL particles is poorly understood. Several lines of evidence support the proposal that the cardioprotective effects of HDL can be dissociated from blood levels of HDL-C (12–15). Consistent with this hypothesis, recent studies indicate that the ability of serum HDL (serum depleted of apoB-containing particles) to promote cholesterol efflux from macrophages is independent of HDL-C and apoA-I levels (15). Moreover, the efflux capacity of serum HDL is a better predictor of CVD status than either HDL-C or apoA-I (14). These observations suggest that the function of HDL in cholesterol efflux may better predict CVD risk than does HDL-C concentration.
Recent studies suggest that mifepristone improves glycemic control but lowers HDL-C in patients with Cushing's disease (16), consistent with its glucocorticoid antagonist mechanism, but effects of mifepristone on HDL function have not been previously reported. To investigate the effects of glucocorticoid antagonism on HDL metabolism, healthy postmenopausal women were randomized to treatment with mifepristone or placebo. Our observations indicate that mifepristone lowers HDL-C and HDL particle concentration, while at the same time improving the specific efflux capacity of serum HDL per particle.
Subjects and Methods
Study population
Healthy postmenopausal women (absence of menses for 12 months and FSH >35 IU/ml), ages 45–65, euthyroid, and with a body mass index (BMI) of 18–30 kg/m2 were recruited by advertisement to a single study site (Diablo Clinical Research, Walnut Creek, CA). Serum HDL-C above 40 mg/dl and triglycerides below 200 mg/dl were required for inclusion. Major exclusion criteria were: acute or chronic disease state, significantly abnormal clinical laboratory test, concomitant or recent use of lipid-reducing drugs, drugs known to interfere with lipid metabolism, estrogen and/or progesterone replacement, smoking, consumption of more than one alcoholic beverage daily, signs and/or symptoms of adrenal insufficiency (e.g. orthostatic hypotension, fatigue, anorexia, nausea, abdominal pain, joint and muscle pain), endometrial thickness of more than 5 mm on transvaginal ultrasound, history of unexplained vaginal bleeding or cancer, recent or planned diet or exercise modification or use of diet mediations, diabetes mellitus, fasting blood glucose above 100 mg/dl and/or treatment with an antidiabetic medication, or renal insufficiency. Informed consent was obtained in all cases before any study procedures. The protocol was approved by the Aspire Institutional Review Board (La Mesa, CA).
Study design
Forty-three subjects were screened, of whom 30 enrolled and received at least one dose of study medication. Six participants in the mifepristone group withdrew from the study before d 43; in four of these cases, withdrawal was due to the development of a rash. One participant withdrew consent, and a sixth withdrew due to a constellation of four mild adverse events (abdominal cramping, fatigue, muscle/body aches, and fluid retention). Of all adverse events observed, only the abdominal cramping required treatment. All complaints resolved without long-term sequelae. There were no serious adverse events. One participant was noted to have a small uterine fluid collection on d 43 that was stable at d 84 and did not require further evaluation.
After screening, participants were randomized in a 2:1 fashion to receive either mifepristone (two 300 mg once daily; Corcept Therapeutics) or placebo for 6 wk. The study drug was administered on d 1, 8, 15, and 29 in the clinic and was self-administered on other days. In addition to these dates, participants were evaluated at the clinical study site on d 43 and 84 (off treatment follow-up). Safety laboratories and a brief physical examination were performed at all study visits. Fasting (overnight, minimum 10 h) blood was obtained on d 1 (baseline), 8, 15, 43, and 84.
Safety laboratory measures
Standard lipid quantification and safety laboratory tests including serum electrolytes, creatinine, liver function tests, complete blood counts, and hormones were performed by a central clinical laboratory, and serum estradiol and total testosterone were measured by liquid chromatography tandem mass spectrometry (Quest Diagnostics, San Juan Capistrano, CA). Normal ranges for postmenopausal women are less than 10 pg/ml and 2–40 ng/dl for estradiol and testosterone, respectively.
Lipoprotein and apolipoprotein analyses
HDL subfraction measurements and apolipoprotein analyses were performed at Children's Hospital Oakland Research Institute on fasting serum samples obtained on d 1, 15, and 43. Concentrations of HDL particles were directly measured as a function of their size by ion mobility, a technique based on gas-phase differential electric mobility (9, 14). For the present analyses, two HDL subfractions were determined: large HDL2b (10.5–14.5 nm) and small HDL3 + 2a (7.6–10.4 nm). Total HDL particle concentration was calculated as the sum of HDL3 + 2a and HDL2b. Serum apoA-I, apoA-II, and apoB were measured by sandwich-style ELISA using primary antibodies (Biodesign International, Saco, ME). Assay controls were validated by Northwest Lipid Laboratory (Seattle, WA). Assays were performed in triplicate with an interassay variation of less than 10%.
Measurement of HDL pre-β particles was performed at the University of California, San Francisco. Sample plasma was electrophoresed in agarose, immunofixed by monospecific antihuman apoA-I antiserum and gels stained with Coomassie blue. The pre-β regions were quantified by densitometry. Pre-β-1 HDL concentration was estimated from a five-point calibration curve (log mg/dl vs. peak area, r = 0.98) run in the same gel. Within-run variation was controlled by normalizing test values to control plasma of known pre-β-1 HDL concentration (coefficient of variation = 10%). Samples were run in triplicate, paired by subject but blinded by treatment.
HDL-mediated cholesterol efflux
Cholesterol efflux capacity of serum HDL from cultured macrophages was measured using serum collected on d 1 and 43 at the University of Washington using the method described by Rader and colleagues (14, 15). J774 macrophages were labeled with [3H]cholesterol (1 μCi/ml; Perkin-Elmer, Waltham, MA) in DMEM containing 1 mg/ml fatty acid-free BSA and the acyl CoA; cholesterol acyltransferase inhibitor Sandoz 58-035 (5 μg/ml; Sigma, St. Louis, MO) overnight, and ATP-binding cassette transporter A1 was induced with cAMP (0.5 mm) overnight. The cells were then incubated with DMEM/fatty acid-free BSA with or without 2.8% apoB-depleted serum (serum HDL) for 4 h at 37 C. The apoB was depleted by precipitation with polyethylene glycol (14, 15). The [3H]cholesterol content of medium and cells was quantified, and serum HDL cholesterol efflux capacity was calculated as a fraction of total [3H]cholesterol released into the medium after subtraction of values obtained in the absence of serum.
LCAT and CETP enzyme activity
Lecithin:cholesterol acyltransferase (LCAT) and cholesterol ester transfer protein (CETP) activities were measured at the University of California, San Francisco, using a microplate method employing colorimetric cholesterol assays for total cholesterol and unesterified cholesterol (UC). LCAT activity was quantified in plasma from decrease of UC concentration after 4-h incubation at 37 C. CETP activity was measured as the change in HDL cholesterol ester content during a 6-h, 37 C incubation of plasma in the presence of LCAT inhibitor. The HDL plasma fraction was prepared by polyethylene glycol precipitation of LDL/very low-density lipoproteins, and cholesterol ester was calculated as the difference of total cholesterol and UC.
Statistical analyses
The primary endpoint of the study was the change in serum HDL-C concentration between groups at the end of treatment. Enrollment of 30 subjects (randomized 2:1, mifepristone:placebo) allowed for 90% power to detect a 10% change in HDL-C (variance = 25%; α = 0.05), but 80% power was maintained if 24 subjects completed the study. The primary analyses were performed on those subjects who completed the entire study (n = 14 for mifepristone, n = 10 placebo). Serum from one individual in the placebo group was not available for the efflux analyses. All variables were tested for normality using the method of Shapiro-Wilk. Because the majority of variables were not normally distributed at baseline, nonparametric methods were used as follows: Friedman's two-way ANOVA, for ranks for within-group comparison between baseline (d 1) and end-of-treatment (d 43); and a Wilcoxon rank sum test, applied to analyze between-group differences at baseline and changes over time. Correlation analysis was performed using Spearman's method to examine baseline variables (nonnormal distribution), and Pearson's method to examine relationships between longitudinal changes (because relative changes from baseline were normally distributed). All analyses were performed using either STATA version 10 (StataCorp, College Park, TX) or SPSS version 19 (IBM, Armonk, NY). In all cases, an α of less than 0.05 was considered significant.
Results
Study participants
There were no significant differences in baseline characteristics between the mifepristone and placebo-treated groups, including baseline hormone and lipid profiles (Table 1). The average (±sd) ages in the treatment and placebo groups were 59 ± 4 and 59 ± 5 yr, respectively. No significant differences in weight or BMI were observed in the two groups. Complete blood counts and serum chemistries, other than a small decrease in potassium (d 43 mean change = −0.54 mEq), were not affected by treatment and remained in the normal range in both groups throughout the study.
Table 1.
Effects of mifepristone treatment on body weight and serum glucose, hormones, and lipids levels
| Mifepristone (n = 14) |
Placebo (n = 10) |
|||
|---|---|---|---|---|
| Day 0 | Day 43 | Day 0 | Day 43 | |
| Weight (kg) | 70.0 (65, 81) | 69.7 (65, 82) | 62.5 (55, 71) | 63.6 (56, 71) |
| BMI (kg/m2) | 25.6 (23, 28) | 25.8 (24, 29) | 24.3 (21, 26) | 24.8 (21, 26) |
| ACTH (pg/ml) | 14.5 (11, 24) | 99 (70, 171)a,b | 13 (10, 18) | 13 (11, 17) |
| Cortisol (μg/dl) | 14.8 (13, 16) | 41 (39, 53)a,b | 14.6 (12, 20) | 12.1 (10, 14) |
| Estradiol (pg/ml) | 8.0 (0, 21) | 26.5 (10, 44)a,b | 8.0 (0, 13) | 8.5 (0, 24) |
| Total testosterone (ng/dl) | 15.0 (11, 21) | 51.5 (45, 71)a,b | 12.5 (7, 14) | 13.0 (7, 16) |
| Glucose (mg/dl) | 92.0 (85, 99) | 88.5 (85, 92) | 92.0 (85, 99) | 91.0 (86, 97) |
| Insulin (μU/ml) | 12.0 (8, 21) | 10.0 (7, 11)a | 11.0 (10, 14) | 10.5 (8, 18) |
| HOMA-IR | 2.5 (1.9, 5.2) | 2.2 (1.4, 2.4)a | 2.4 (2.2, 2.9) | 2.2 (1.7, 4.4) |
| Total cholesterol (mg/dl) | 226 (204, 238) | 180 (149, 222)a,b | 231 (206, 238) | 234 (213, 251) |
| Triglyceride (mg/dl) | 96 (78, 121) | 111 (82, 146) | 104 (72, 188) | 84 (69, 170) |
| LDL-C (mg/dl) | 119 (83, 129) | 100 (83, 129)b | 123 (112, 147) | 127 (111, 165) |
| HDL-C (mg/dl) | 70 (49, 59) | 53 (49, 59)a,b | 76 (46, 87) | 75 (52, 91) |
All data are presented as median (25th, 75th percentiles).
P < 0.05 vs. baseline (Friedman two-way ANOVA for ranks).
P < 0.05 for differences vs. placebo in change from baseline (Wilcoxon rank sum test).
Serum hormone concentrations
As expected, treatment with the glucocorticoid receptor antagonist mifepristone significantly increased morning serum ACTH and cortisol concentrations (Table 1), due to the loss of negative feedback at the pituitary. Similarly, serum sex steroid concentrations were significantly increased in the treatment group, likely driven by increased steroidogenesis in the setting of elevated ACTH (2, 17). TSH was slightly increased with mifepristone treatment (baseline = 2.0 ± 1.3 mIU/liter; d 43 = 4.9 ± 3.0 mIU/liter; P < 0.05), but free T4 was unchanged and both measures of thyroid function remained in the normal range throughout the study in both groups (data not shown).
Glucose metabolism
Fasting glucose concentrations were not different between treatment groups and were not affected by mifepristone administration. Fasting insulin concentrations decreased significantly in the mifepristone group at d 43 compared with baseline (P = 0.03; Table 1). This resulted in a significant decline in homeostatic model assessment of insulin resistance (HOMA-IR) (Table 1), consistent with a small increase in insulin sensitivity in the treatment group.
Lipoprotein profiles
There were no differences between the two treatment groups in fasting lipid profiles at baseline (Table 1), and no significant changes were detected in any of the lipoproteins in the placebo group on d 43 (Table 2). Oral mifepristone treatment resulted in a significant, 20% decline in total cholesterol on d 43 (Table 1) and a decline in LDL-C with the treatment (P = 0.046) with no change in apoB concentration (Table 2). HDL-C declined by 26% with mifepristone treatment over the 43 d (P < 0.001 vs. baseline) (Table 1). Consistent with this decline in HDL-C, serum apoA-I, the most abundant protein within HDL particles, decreased in the mifepristone-treated group (19%; P < 0.001 vs. baseline) (Table 2), as did apoA-II concentration (11%; P = 0.033; Table 2). Fasting triglyceride levels did not significantly change in either group (Table 1).
Table 2.
Effects of mifepristone treatment on lipoproteins, HDL particles, and associated enzymes
| Mifepristone (n = 14) |
Placebo (n = 10) |
|||||
|---|---|---|---|---|---|---|
| Day 0 | Day 43 | P (day 0 vs. 43)a | Day 0 | Day 43 | P (day 0 vs. 43)a | |
| ApoB (mg/dl) | 89 (76, 105) | 89 (71, 104) | 0.285 | 87 (80, 123) | 94 (80, 114) | 1.0 |
| ApoA-I (mg/dl) | 154 (146, 170) | 125 (119, 135)b | <0.001 | 151 (139, 165) | 160 (143, 168) | 0.096 |
| ApoA-II (mg/dl) | 32.9 (30, 41) | 30.0 (26, 35)b | 0.033 | 30.7 (28, 35) | 31.2 (28, 36) | 0.527 |
| HDL particle no. (nmol/liter) | 7867 (7003, 9273) | 5955 (5122, 6854)b | 0.008 | 7974 (7014, 10169) | 9365 (7610, 10031) | 0.527 |
| HDL2b (nmol/liter) | 2882 (1653, 3496) | 977 (730, 1297)b | <0.001 | 2778 (1379, 3993) | 3172 (1746, 4295) | 0.206 |
| HDL3 + 2a (nmol/liter) | 5330 (5046, 6224) | 4990 (3889, 6123) | 0.593 | 5984 (4431, 6426) | 6057 (5553, 8072) | 1.0 |
| Pre-β-1 HDL (mg/dl) | 11.4 (10.4, 16.2) | 11.1 (5.4, 13.5) | 0.285 | 12.6 (10.9, 18.6) | 14.6 (10.4, 14.8) | 0.527 |
| LCAT activity (nmol/ml/h) | 79 (59, 95) | 61 (54, 67) | 0.285 | 68 (45, 85) | 65 (41, 91) | 0.206 |
| CETP activity (nmol/ml/h) | 12.5 (0, 15) | 10 (6, 20) | 0.248 | 12.5 (3, 16) | 11.5 (0, 19) | 0.527 |
All data are presented as median (25th, 75th percentiles).
P < 0.05 vs. baseline (Friedman two-way ANOVA for ranks).
P < 0.05 for differences vs. placebo in change from baseline (Wilcoxon rank sum test).
Lipoprotein subfraction analyses
To further investigate the observed decrease in HDL-C, we studied the impact of mifepristone treatment on the concentration of HDL particles measured by ion mobility (18) (Table 2). This approach directly measures lipoprotein particle concentration and is independent of apoA-I or HDL-C concentration measurements. Using this method, the total HDL particle concentration decreased by 25% (Table 2), similar to the decrease in apoA-I and HDL-C. Interestingly, mifepristone treatment exhibited potent differential effects on HDL particle subclasses. The decrease in total HDL particle number was completely accounted for by reduction in the larger HDL2b particles (58%), whereas no changes were observed for the smaller HDL3 + 2a particles (Table 2). These alterations in HDL particle concentrations occurred rapidly, within the first 2 wk of the treatment, and no further changes were observed between the d 15 and 43 (data not shown).
In parallel with the ion mobility measurements, we also evaluated the concentration of pre-β HDL particles, discoidal, and lipid-poor particles containing apoA-I. In vitro studies indicate that pre-β-1 HDL is an important acceptor of cellular cholesterol (11), whereas other studies found higher concentrations of pre-β-1 HDL in individuals with CVD (19, 20). There was no change in the pre-β-1 HDL in the mifepristone-treated group at d 43 compared with baseline or to the placebo group (Table 2). Similarly, the distribution of HDL particles by α-particle subtype was no different in the mifepristone group compared with placebo at either time point (data not shown).
Lastly, we investigated whether mifepristone altered LCAT and CETP activities, reasoning that these might be mechanisms whereby mifepristone lowered HDL-C and HDL particle concentration (11, 21). We found that mifepristone treatment had no impact on either LCAT or CETP enzymatic activity (Table 2).
Cholesterol efflux
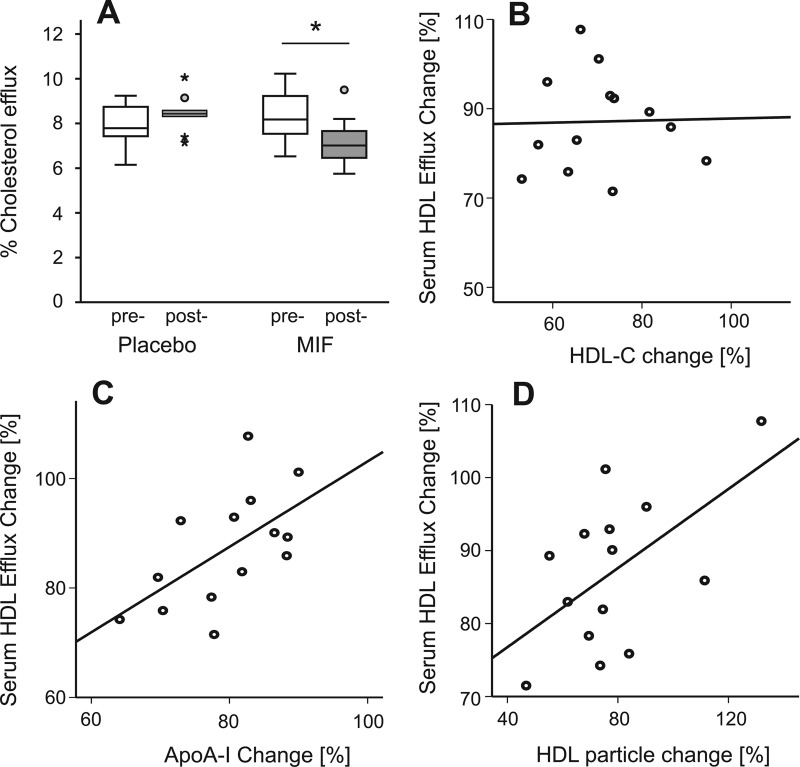
Because the mifepristone treatment significantly reduced HDL-C and HDL particle concentration, we evaluated the effect of mifepristone on HDL function by measuring the ability of serum HDL to mediate cholesterol efflux from macrophages. At baseline, there were no differences in the sterol efflux capacity between the two groups, and efflux capacity correlated with serum apoA-I concentration (n = 23; r = 0.51; P = 0.013), total HDL particle concentration (n = 23; r = 0.53; P = 0.009); the efflux capacity of serum HDL also increased with increasing HDL-C (n = 23; r = 0.40; P = 0.057). The 6 wk of mifepristone treatment resulted in significant attenuation of HDL efflux capacity by 12% compared with no change in the placebo-treated group (P = 0.002) (Fig. 1A). This decrease was considerably less than the decreases in HDL-C (26%), apoA-I (19%), or total HDL particle concentration (25%). Moreover, the decrease in HDL-C with mifepristone treatment was not correlated with the decrease in the serum HDL sterol efflux capacity (Fig. 1B; r = 0.029; P = 0.902). In contrast, changes in both apoA-I concentration and total HDL particle concentration were significantly correlated with the change in the serum HDL sterol efflux capacity (Fig. 1, C and D; r = 0.588, P = 0.027; and r = 0.562, P = 0.036, respectively). These results indicate that HDL-C may not be the optimal surrogate measure of HDL functionality.
Fig. 1.
Effect of mifepristone (MIF) on serum HDL sterol efflux capacity. Serum HDL sterol efflux capacity was significantly decreased by mifepristone treatment (A) (*, P = 0.002, treatment d 1 vs. d 43). Relative change of sterol efflux induced by mifepristone treatment was not correlated with relative decrease of HDL-C (r = 0.029; P = 0.902 (B), but significantly correlated with relative decrease of ApoA-I (r = 0.588; P = 0.027) (C) and HDL particles (r = 0.562; P = 0.036) (D). Relative changes were calculated for each subject as a change of the variable from d 1 to 43 divided by value at d 1 [(d43 − d1)/d1]. Bars represent median with interquartile range.
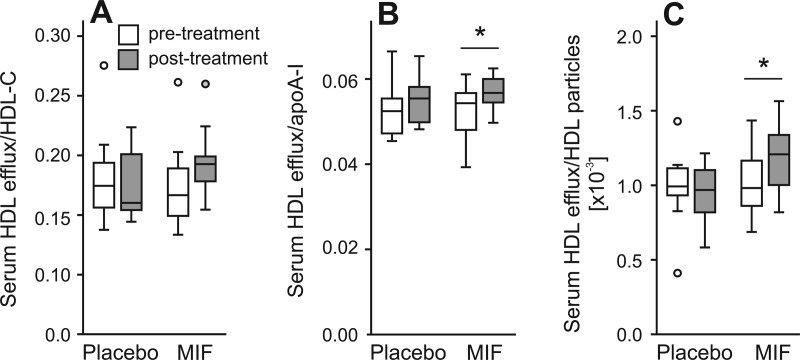
To further investigate the relationship between the serum HDL efflux capacity and other HDL measures, we normalized the efflux capacity to plasma HDL-C, apoA-I, and HDL particle concentration, respectively (Fig. 2). The HDL efflux capacity per unit of HDL-C, apoA-I, or HDL particle was increased by mifepristone treatment by 20, 7, and 18%, respectively (Fig. 2), suggesting that mifepristone induced changes in HDL particles that improved their ability to promote cholesterol efflux from lipid-loaded macrophages.
Fig. 2.
Effect of mifepristone (MIF) on normalized HDL sterol efflux capacity. Mifepristone treatment improved specific serum HDL mediated efflux when normalized to HDL-C (A), apoA-I (B), or HDL particle concentrations (C). #*, P < 0.01. Normalized sterol efflux was calculated by dividing % serum HDL sterol efflux by HDL-C, HDL particle, or apoA-I concentration, respectively. Bars represent median and interquartile range.
Relationships between changes in HDL-C, cholesterol efflux, and serum hormones
Using univariate analyses, we investigated the relationship between changes in serum hormones and changes in HDL-C and HDL particle concentration in the subjects treated with mifepristone (n = 14). There was no significant correlation between changes in total testosterone, estradiol, ACTH, cortisol, or insulin and changes in HDL-C or HDL particle concentration (data not shown). Changes in free testosterone were weakly but significantly inversely correlated with changes in total HDL particle concentration (r = −0.54; P = 0.047). We also examined whether changes in cholesterol efflux correlated with changes in any of the serum hormones. Only in the case of changes in serum cortisol did we observe a significant, negative, association with changes in sterol efflux (n = 13; r = −0.62; P = 0.02) in the mifepristone-treated group.
Discussion
Our observations indicate that administration of the glucocorticoid antagonist mifepristone significantly lowered total cholesterol, HDL-C, apoA-I, and HDL particle concentration in healthy postmenopausal women. In contrast, mifepristone treatment did not alter the concentration of pre-β-HDL particles. Although the decrease in HDL-C was accompanied by a decrease in HDL-mediated cholesterol efflux from macrophages, efflux on a per particle basis was increased by mifepristone treatment. Moreover, the change in HDL-mediated cholesterol efflux correlated with a change in HDL particle concentration but not with a change in total HDL-C.
Low levels of large HDL particles (as assessed by two-dimensional, nondenaturing electrophoresis) have been associated with CVD in a number of studies (19, 22–24) and may even be predictive of CVD risk (25). However, quantification of large and small HDL has not proven to be a more effective predictive tool for CVD risk assessment than measurement of HDL-C alone (18). On the other hand, a recent study demonstrated that serum HDL from people with similar levels of HDL-C can have dramatically different capacity to mediate cholesterol efflux from lipid-loaded macrophages (15). Furthermore, serum HDL sterol efflux capacity was strongly and negatively associated with CVD status in two different populations of subjects (14). This inverse relationship between sterol efflux and CVD persisted in multivariate models even after correction for HDL-C and apoA-I concentrations, and sterol efflux capacity was a strong independent predictor of CVD status (hazard ratio, 0.7). Moreover, recent prospective, randomized interventional studies do not support the notion that increases in HDL-C reduce CVD events (26, 27). Collectively, these studies suggest that HDL function—rather than HDL-C level—may be an important factor in determining CVD risk (14, 28, 29). Our data suggest that measurement of HDL particle concentration may reflect HDL sterol efflux capacity better than HDL-C. Furthermore, the data raise the possibility that decreases in HDL-C and HDL particle concentration induced by mifepristone treatment may be considerably mitigated by improved function of HDL. Alternatively, because the concentration of small HDL particles was not decreased by mifepristone treatment, it is possible that this population of particles promotes sterol efflux from macrophages more effectively than larger HDL particles.
How mifepristone treatment mediates changes in HDL-C and efflux capacity is unknown. Within the treatment group, increases in serum cortisol inversely correlated with cholesterol efflux. Although it has been suggested that high levels of circulating cortisol might play a role in the pathophysiology of CVD through effects on risk factors (including HDL-C) (30), interpreting the physiological relevance of increases in serum cortisol that are the consequence of a glucocorticoid antagonist (which by definition blocks cortisol action) is difficult. Antagonism of cortisol actions in peripheral tissues would be expected to reverse high triglyceride levels (4), central obesity (31, 32), and insulin resistance (33), all of which should raise HDL-C. Insulin resistance as measured by HOMA-IR did indeed improve with mifepristone therapy. Despite this improved insulin resistance (and unchanged triglyceride levels), however, mifepristone treatment decreased HDL-C in these healthy postmenopausal women. On the other hand, it is possible that more direct effects of glucocorticoid receptor antagonism on HDL metabolism occur in the liver and gut, such as through regulation of apoA-I gene expression (34) and secretion (35, 36).
The observed increases in serum ACTH, testosterone, and estradiol in the treatment group, resulting from blockade of both the glucocorticoid and progestin receptors by mifepristone, did not correlate with alterations in serum lipids or sterol efflux in our study, although our study may have been underpowered to observe such a relationship. On the whole, however, these hormone changes would have been expected to raise, not lower, HDL-C (37, 38). Another possibility is that cortisol or mifepristone might alter HDL protein composition (9), in turn affecting HDL function, which we did not analyze here. Finally, with regard to effects on activities of serum enzymes that affect HDL-C levels, we did not observe changes in CETP or LCAT enzymatic activity levels with treatment; however, given the observed effect size, larger studies including nearly three times the sample size enrolled here would be required for sufficient power to rule out an effect of mifepristone on these secondary endpoints.
In summary, our data indicate that whereas mifepristone treatment lowers HDL-C, HDL particle concentration, and serum HDL cholesterol efflux capacity, it fails to affect pre-β particles and small HDL (HDL3 + 2a) particles. By decreasing specifically large HDL particles, which carry the major portion of cholesterol, mifepristone improves macrophage sterol efflux by serum HDL on a per particle basis. Because the mifepristone-induced decrease in serum HDL sterol efflux capacity strongly correlated with the decrease in HDL particles, but not with HDL-C, our data also suggest that HDL-C may not accurately reflect functional properties of HDL. Further studies will be needed to conclusively establish the relationship between the various HDL particles and the sterol efflux capacity of serum HDL, whether specific HDL particle populations associate with CVD status, and whether other factors that have been proposed to mediate the cardioprotective effects of HDL (such as its protein cargo) are altered by mifepristone treatment. The impact of mifepristone treatment on HDL in patients with Cushing's syndrome and the mechanisms underlying our observations warrant further study.
Acknowledgments
We thank all of our study participants, without whom this work would not be possible, and Katie Wojnoonski and J. Casey Geaney for the ion mobility and apolipoprotein measurements.
This study was supported by Corcept Pharmaceuticals Therapeutics (Menlo Park, CA). In addition, the investigators' work was supported by the National Institutes of Health through the following grants: Eunice Kennedy Shriver National Institute of Child Health and Human Development cooperative agreement U54 HD42454, and Diabetes and Endocrinology Research Center Grant DK017047 from the National Institute of Diabetes and Digestive and Kidney Diseases (to S.T.P.); Grants K23 DK002689 and R01 DK068146 (to J.Q.P.); National Heart, Lung and Blood Institute Grants R01 HL086798, P01 HL092969, and P01 HL030086 (to J.W.H.); and the University of Washington Nutrition and Obesity Research Center (NIH P30DK035816), R01 HL089504, and the American Heart Association (0830231N) (to T.V.).
Current address for B.I.: Boston Heart Diagnostics, Framingham, Massachusetts 01701.
Disclosure Summary: S.T.P., B.I., J.K.A., P.M.S., C.J.C., C.T., and T.V. have nothing to disclose. R.M.K., J.W.H., J.K., and J.Q.P. served as consultants for Corcept Therapeutics in the design and conduct of the study. R.L.W. was paid by Corcept Therapeutics to serve as Principal Investigator at the clinical site. C.G. is a Corcept Therapeutics, Inc., employee.
Footnotes
- apo
- Apolipoprotein
- BMI
- body mass index
- CETP
- cholesterol ester transfer protein
- CVD
- cardiovascular disease
- HDL
- high-density lipoprotein
- HDL-C
- HDL cholesterol
- HOMA-IR
- homeostatic model assessment of insulin resistance
- LCAT
- lecithin:cholesterol acyltransferase
- LDL
- low-density lipoprotein
- UC
- unesterified cholesterol.
References
- 1. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. 2006. Cushing's syndrome. Lancet 367:1605–1617 [DOI] [PubMed] [Google Scholar]
- 2. Johanssen S, Allolio B. 2007. Mifepristone (RU 486) in Cushing's syndrome. Eur J Endocrinol 157:561–569 [DOI] [PubMed] [Google Scholar]
- 3. Brunzell JD, Hokanson JE. 1999. Dyslipidemia of central obesity and insulin resistance. Diabetes Care 22(Suppl 3):C10–C13 [PubMed] [Google Scholar]
- 4. Taskinen MR, Nikkilä EA, Pelkonen R, Sane T. 1983. Plasma lipoproteins, lipolytic enzymes, and very low density lipoprotein triglyceride turnover in Cushing's syndrome. J Clin Endocrinol Metab 57:619–626 [DOI] [PubMed] [Google Scholar]
- 5. Ettinger WH, Klinefelter HF, Kwiterovitch PO. 1987. Effect of short-term, low-dose corticosteroids on plasma lipoprotein lipids. Atherosclerosis 63:167–172 [DOI] [PubMed] [Google Scholar]
- 6. Davidson WS, Thompson TB. 2007. The structure of apolipoprotein A-I in high density lipoproteins. J Biol Chem 282:22249–22253 [DOI] [PubMed] [Google Scholar]
- 7. Tall AR, Yvan-Charvet L, Terasaka N, Pagler T, Wang N. 2008. HDL, ABC transporters, and cholesterol efflux: implications for the treatment of atherosclerosis. Cell Metab 7:365–375 [DOI] [PubMed] [Google Scholar]
- 8. Oram JF, Heinecke JW. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev 85:1343–1372 [DOI] [PubMed] [Google Scholar]
- 9. Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. 2007. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest 117:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sorci-Thomas MG, Thomas MJ. 2002. The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc Med 12:121–128 [DOI] [PubMed] [Google Scholar]
- 11. Rosenson RS, Brewer HB, Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. 2011. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem 57:392–410 [DOI] [PubMed] [Google Scholar]
- 12. Francis GA. 2010. The complexity of HDL. Biochim Biophys Acta 1801:1286–1293 [DOI] [PubMed] [Google Scholar]
- 13. Schultz JR, Verstuyft JG, Gong EL, Nichols AV, Rubin EM. 1993. Protein composition determines the anti-atherogenic properties of HDL in transgenic mice. Nature 365:762–764 [DOI] [PubMed] [Google Scholar]
- 14. Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. 2010. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 30:796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fleseriu M, Biller BMK, Findling JW, Molitch ME, Schteingart DE, Gross C, Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with refractory Cushing syndrome: results from the study of the efficacy and safety of mifepristone in the treatment of endogenous Cushing syndrome. Program of the 93rd Annual Meeting of the Endocrine Society, Boston, Massachusetts, 2011 (Abstract OR09-5) [Google Scholar]
- 17. Klijn JG, de Jong FH, Bakker GH, Lamberts SW, Rodenburg CJ, Alexieva-Figusch J. 1989. Antiprogestins, a new form of endocrine therapy for human breast cancer. Cancer Res 49:2851–2856 [PubMed] [Google Scholar]
- 18. Krauss RM. 2010. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol 21:305–311 [DOI] [PubMed] [Google Scholar]
- 19. Guey LT, Pullinger CR, Ishida BY, O'Connor PM, Zellner C, Francone OL, Laramie JM, Naya-Vigne JM, Siradze KA, Deedwania P, Redberg RF, Frost PH, Seymour AB, Kane JP, Malloy MJ. 2011. Relation of increased preβ-1 high-density lipoprotein levels to risk of coronary heart disease. Am J Cardiol 108:360–366 [DOI] [PubMed] [Google Scholar]
- 20. Asztalos BF, Cupples LA, Demissie S, Horvath KV, Cox CE, Batista MC, Schaefer EJ. 2004. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol 24:2181–2187 [DOI] [PubMed] [Google Scholar]
- 21. Khera AV, Rader DJ. 2009. Discovery and validation of new molecular targets in treating dyslipidemia: the role of human genetics. Trends Cardiovasc Med 19:195–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Watanabe H, Söderlund S, Soro-Paavonen A, Hiukka A, Leinonen E, Alagona C, Salonen R, Tuomainen TP, Ehnholm C, Jauhiainen M, Taskinen MR. 2006. Decreased high-density lipoprotein (HDL) particle size, preβ-, and large HDL subspecies concentration in Finnish low-HDL families: relationship with intima-media thickness. Arterioscler Thromb Vasc Biol 26:897–902 [DOI] [PubMed] [Google Scholar]
- 23. Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engström G, Williams PT, Kathiresan S, Melander O, Krauss RM. 2009. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol 29:1975–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. 2009. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 119:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan J, Carey C, Lincoff A, Capuzzi D. 2004. High-density lipoprotein subfractions and risk of coronary artery disease. Curr Atheroscler Rep 6:359–365 [DOI] [PubMed] [Google Scholar]
- 26. Sharma M. 2011. Combination therapy for dyslipidemia. Curr Opin Cardiol 26:420–423 [DOI] [PubMed] [Google Scholar]
- 27. Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. 2007. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 357:2109–2122 [DOI] [PubMed] [Google Scholar]
- 28. Shao B, Oda MN, Oram JF, Heinecke JW. 2010. Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem Res Toxicol 23:447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. 2004. Antiinflammatory properties of HDL. Circ Res 95:764–772 [DOI] [PubMed] [Google Scholar]
- 30. Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. 2009. Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701 [DOI] [PubMed] [Google Scholar]
- 31. Wajchenberg BL, Bosco A, Marone MM, Levin S, Rocha M, Lerário AC, Nery M, Goldman J, Liberman B. 1995. Estimation of body fat and lean tissue distribution by dual energy X-ray absorptiometry and abdominal body fat evaluation by computed tomography in Cushing's disease. J Clin Endocrinol Metab 80:2791–2794 [DOI] [PubMed] [Google Scholar]
- 32. Mayo-Smith W, Hayes CW, Biller BM, Klibanski A, Rosenthal H, Rosenthal DI. 1989. Body fat distribution measured with CT: correlations in healthy subjects, patients with anorexia nervosa, and patients with Cushing syndrome. Radiology 170:515–518 [DOI] [PubMed] [Google Scholar]
- 33. Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. 2001. A transgenic model of visceral obesity and the metabolic syndrome. Science 294:2166–2170 [DOI] [PubMed] [Google Scholar]
- 34. Saladin R, Vu-Dac N, Fruchart JC, Auwerx J, Staels B. 1996. Transcriptional induction of rat liver apolipoprotein A-I gene expression by glucocorticoids requires the glucocorticoid receptor and a labile cell-specific protein. Eur J Biochem 239:451–459 [DOI] [PubMed] [Google Scholar]
- 35. Staels B, van Tol A, Chan L, Verhoeven G, Auwerx J. 1991. Variable effects of different corticosteroids on plasma lipids, apolipoproteins, and hepatic apolipoprotein mRNA levels in rats. Arterioscler Thromb 11:760–769 [DOI] [PubMed] [Google Scholar]
- 36. Mahley RW, Gray ME, Hamilton RL, LeQuire VS. 1968. Lipid transport in liver. II. Electron microscopic and biochemical studies of alterations in lipoprotein transport induced by cortisone in the rabbit. Lab Invest 19:358–369 [PubMed] [Google Scholar]
- 37. Knopp RH, Zhu X. 1997. Multiple beneficial effects of estrogen on lipoprotein metabolism. J Clin Endocrinol Metab 82:3952–3954 [DOI] [PubMed] [Google Scholar]
- 38. Berg AL, Nilsson-Ehle P. 1994. Direct effects of corticotropin on plasma lipoprotein metabolism in man—studies in vivo and in vitro. Metabolism 43:90–97 [DOI] [PubMed] [Google Scholar]