Abstract
Context:
Anti-Mullerian hormone (AMH) has emerged as a marker of ovarian reserve and a possible surrogate measure of reproductive aging.
Objective:
The aim of the study was to evaluate the predictive value of AMH levels in determining the median time to menopause for late reproductive age women and the predictive ability of AMH compared to FSH and inhibin b.
Design and Setting:
A 14-yr follow-up in the Penn Ovarian Aging Study, 1996–2010, was conducted for a randomly identified population-based cohort.
Subjects:
A total of 401 late reproductive age women participated in the study.
Main Outcome Measure:
Observed time to menopause was measured.
Results:
All participants were premenopausal, with a mean (sd) age of 41.47 (3.52) yr and a median AMH level of 0.68 ng/ml at baseline. AMH strongly predicted time to menopause; age further improved predictions. Among women with a baseline AMH level below 0.20 ng/ml, the median time to menopause was 5.99 yr [95% confidence interval (CI), 4.20–6.33] in the 45- to 48-yr age group and 9.94 yr (95% CI, 3.31–12.73) in the 35- to 39-yr age group. With higher baseline AMH levels above 1.50 ng/ml, the median time to menopause was 6.23 yr in the oldest age group and more than 13.01 yr in the youngest age group. Smoking significantly reduced the time to menopause (hazard ratio, 1.61; 95% CI, 1.19–2.19; P = 0.002). AMH was a stronger predictor of time to menopause than FSH or inhibin b.
Conclusions:
AMH is a strong predictor of median time to menopause in late reproductive age women. Age and smoking are significant and independent contributors to the predictions of AMH.
Anti-Mullerian hormone (AMH) has recently emerged as an important marker of ovarian reserve. AMH is produced by granulosa cells of small follicles in the ovary and is detectable in the peripheral circulation (1). This hormone appears to modulate two regulatory steps of folliculogenesis: inhibiting recruitment of primordial follicles and decreasing the sensitivity of small antral follicles to FSH (2).
Several studies have validated AMH as a direct biomarker for ovarian aging (1, 3–5). For example, AMH is significantly correlated with the number of antral follicles, and further evidence shows that AMH is a stronger predictor of late menopausal transition than age, antral follicle count, or ovarian volume (6). Research has demonstrated that AMH levels and antral follicle counts are highly associated with the ovarian primordial follicular pool (7). AMH measures may provide information in women with ovarian dysfunction and may have a role in predicting outcomes of individual treatment regimens, although this is not fully clarified at this time (8). AMH steadily decreases with age and generally becomes undetectable in the menopause transition (9–12). Investigations have suggested that AMH may be a better surrogate measure of reproductive age than chronological age alone (13).
Evaluating the predictive value of AMH in the naturally aging population is important for counseling women about reproductive planning and for planning treatments for women who experience hormone-sensitive gynecological conditions such as endometriosis and fibroids. Moreover, anticipating age at menopause may help to determine risks for hormone-related adverse health outcomes, such as breast cancer, endometrial cancer, osteoporosis, and cardiovascular disease (14). Two recent studies evaluated AMH as a predictor of age at menopause in naturally fertile or normoovulatory women (15, 16), but validation and further investigation are needed for AMH to become a clinical tool.
The objective of this study was to determine the association between AMH levels and time to menopause in late reproductive age women and compare the predictive ability of AMH with other reproductive hormones that were measured concurrently (FSH and inhibin b). This cohort allowed for the prospective evaluation of temporal effects during 14 yr of follow-up. We hypothesized that in addition to AMH and age, smoking, body mass index (BMI), and race were possible risk factors (17–23) and may confound the association between AMH and time to menopause.
Subjects and Methods
Cohort participants
The study evaluated 401 women who were randomly identified and enrolled in the Penn Ovarian Aging Study (POAS) and who had a baseline measure of AMH. The cohort was identified in 1966 using random-digit dialing to households in Philadelphia County, Pennsylvania; sampling was stratified to obtain equal numbers of African-American and white women, as described in previous reports (24). The Institutional Review Board of the University of Pennsylvania approved the study, and all participants provided written informed consent.
At cohort enrollment, all participants were premenopausal, as defined by regular menstrual cycles in the reference range (22–35 d for the previous three menstrual cycles); were ages 35–48 yr; and had an intact uterus and at least one ovary. Exclusion criteria included current use of psychotropic or hormonal medications, including hormonal contraception and hormone therapies; pregnancy or breast feeding; serious health problems known to compromise ovarian function (e.g. diabetes mellitus, liver disease, breast or endometrial cancer); and alcohol or drug abuse in the past year.
Study design
The cohort was followed for 14 yr after enrollment. Follow-up assessments were at approximately 9-month intervals for the first 5 yr and then annually, with a 2-yr gap between assessments 10 and 11. At each assessment, there were two in-home visits to collect study data and blood samples for the hormone assays. All visits were timed to the early follicular phase (d 2–6) of the menstrual cycle and were conducted in two consecutive menstrual cycles or approximately 1 month apart in noncycling women.
The study was described to participants as a general women's health study. At each assessment, trained research interviewers obtained structured interview data on overall health, blood samples for the hormone assays, and anthropometric measures (height, weight, waist and hip circumference); participants completed a set of validated self-report measures to assess health and other behavioral variables of the study.
Study variables
The primary outcome variable was time to menopause. This was measured in years from the first study assessment (when all participants were premenopausal) to the first follow-up assessment where the participant reported no menstrual bleeding for at least 12 months. The point 1 yr before the 12 months of no menstrual bleeding was then defined as menopause.
The covariates that were selected as possible risk factors for time to menopause were obtained at the first assessment: age, race (African-American or white), BMI (kilograms per square meter), and current smoker (yes, no).
Hormone values were assayed from blood samples that were obtained at the scheduled study visits (d 2–6 of the menstrual cycle), centrifuged, and frozen in aliquots at −80 C. The AMH assays were conducted contemporaneously in 2011 in the Translational and Clinical Research Center of the University of Pennsylvania, using the first available frozen samples in assessments 1–3. AMH ELISA kits were used (Beckman Coulter Inc., Brea, CA). The intra- and interassay coefficients of variation were 4.6 and 6.8%, respectively. The lower limit of detection was 0.10 ng/ml. FSH was measured at assessment 1 by RIA using Coat-A-Count commercial kits (Siemens, Deerfield, IL). Interassay and intraassay coefficients of variation were less than 5%. Inhibin b assays at assessment 1 were conducted in the laboratory of Patrick Sluss, Ph.D., at the Massachusetts General Hospital (Boston, MA), using a solid-phase sandwich ELISA with plates coated with a monoclonal antibody specific for the α-subunit for detection. The assay was controlled in triplicate using samples with mean concentrations of 155.3, 316.3, and 919.3 pg/ml with interassay coefficients of variation of 11.6, 7.6, and 9.7%, respectively. The lower limit of detection was 15 pg/ml (coefficient of variation, 20%).
Statistical analysis
A priori power calculations assumed type I, α error of 5% and 80% power. Given 198 (49%) women who achieved menopause out of the cohort of 401, the study has sufficient power to detect hazard ratios of 1.6 or larger for AMH quartiles and other risk factors with a prevalence of 25% or greater.
Statistical analyses were performed using Kaplan-Meier estimations for time to menopause (25), log-rank tests (26), and univariate and multivariable Cox proportional hazards models (27) to evaluate the risk of menopause over the 14-yr follow-up period. AMH was evaluated as a continuous variable (natural log transformed) and as a group variable, using quartiles of the raw baseline values. In the proportional hazards analysis, hazard ratios with 95% confidence intervals (CI) indicate the estimated ratio of the risk of reaching menopause between the exposure [i.e. low AMH levels (Q1 or Q2 or Q3)] and the reference group [high AMH levels (Q4)] at a time point during the observation period. Hazard ratios above 1.0 indicate greater risk (shorter time to menopause), and those below 1.0 indicate less risk (longer time to menopause) in the exposure group compared with the reference group. Proportionality of hazards was evaluated by plots of transformed hazard estimates and smoothed residuals (28, 29). No violations of modeling assumptions were observed.
All hormone measures were transformed to natural log values to reduce the influence of their skewed distributions. Each covariate was evaluated individually for its association with time to menopause, using the first assessment values to emulate the clinical setting. All covariates were evaluated in multivariable models to identify the independent contributions after adjusting for the presence of the other variables. Each hormone was included singly in the multivariable model due to the high correlations among the hormone measures. In the Cox proportional hazards models, observations from participants who continued the study but did not reach menopause by study endpoint (n = 77) were considered censored at the last assessment. Participants who discontinued the study before reaching menopause were considered censored at their last observation (74 women in yr 1–5; 44 women in yr 6–10; and eight women in yr 11–15). Seventeen women who had a hysterectomy in the follow-up interval before menopause were censored at the time of hysterectomy. Exogenous estrogen/progestin use was low and not associated with time to menopause in the model and was not a confounder of the associations of other covariates. Two sensitivity analyses were conducted for the final multivariable Cox model: one excluded women who reported a hysterectomy before menopause; the second included only the participants with all hormone measures at visit 1. These results were nearly identical to those of the primary model. All analyses were conducted using the SAS 9.2 statistical package (SAS Institute Inc., Cary, NC). Statistical tests were two-sided with P < 0.05 considered significant.
Results
The mean (sd) age of the participants was 41.47 (3.52) yr (range, 35 to 48 yr) at assessment 1. All were premenopausal with regular menstrual cycles in the normal range as defined above. Table 1 shows the baseline values of the study variables. There were no significant differences in these variables compared between the study sample (n = 401) and the remainder of the cohort that did not have baseline AMH values due to lack of frozen serum samples (n = 35).
Table 1.
Baseline characteristics of the sample (n = 401)
| Variablea | Baseline (n = 401)b |
|---|---|
| Age (yr) | 41.47 (41.13–41.82) |
| Race, n (%) | |
| African-American | 198 (49.4) |
| White | 203 (50.6) |
| Smoker, n (%) | 157 (39.2) |
| BMI (kg/m2) | 29.33 (28.56–30.10) |
| AMH (ng/ml) | 1.08 (0.97–1.20) |
| FSH (mIU/ml) | 8.34 (7.82–8.87) |
| Inhibin b (pg/ml) | 77.62 (73.51–81.74) |
Continuous variables are presented as mean (95% CI). Hormones are raw baseline levels.
Thirty-five women in the randomly identified cohort were omitted due to no AMH baseline assay. There were no significant differences in baseline characteristics (P > 0.15) compared to the study sample (n = 401).
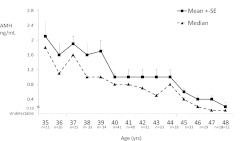
The AMH mean (sd) at baseline was 1.08 (1.19) ng/ml (median, 0.68 ng/ml; range, 0.10 to 7.80 ng/ml; interquartile range, 0.20 to 1.53 ng/ml). Figure 1 depicts the mean (se) and median levels of AMH for each year of age (35–48 yr). After cohort enrollment, 49% of the sample (198 of 401) were observed to reach natural menopause during the 14-yr follow-up. The median time to menopause from baseline AMH levels was 9.81 yr (95% CI, 9.24–10.36).
Fig. 1.
Raw mean (+se) and median AMH levels (ng/ml) by age at study baseline (n = 401).
To better understand the association with time to menopause, baseline AMH levels were divided into quartile groups. The median time to menopause in the lowest quartile (baseline AMH <0.20 ng/ml) was 6.09 yr (95% CI, 5.17–7.79). In the next quartiles, the median time was 9.02 yr (95% CI, 8.48–9.65) in Q2, 11.35 yr (95% CI, 10.03–12.52) in Q3, and 12.88 yr (95% CI, 12.51 not available) in the highest quartile (>1.50 ng/ml). The number of women who were observed to reach menopause in the study interval ranged from 61% in the lowest AMH quartile to 25% in the highest quartile.
Among women who reached menopause, the mean age (sd) at menopause was 50.93 (2.26) yr. Baseline age was significantly associated with time to menopause and remained a significant independent predictor of time to menopause in the adjusted analysis, as shown in Table 2.
Table 2.
Associations of study variables with time to menopause
| Variable | Unadjusted |
Adjusteda |
||||
|---|---|---|---|---|---|---|
| Hazard ratiob | 95% CI | P | Hazard ratiob | 95% CI | P | |
| AMH quartiles (ng/ml) | <0.0001 | <0.0001 | ||||
| <0.20 | 8.39 | 5.24–13.45 | <0.0001 | 4.85 | 2.91–8.11 | <0.0001 |
| 0.20–0.70 | 3.54 | 2.25–5.58 | <0.0001 | 3.37 | 2.11–5.37 | <0.0001 |
| >0.70–1.50 | 2.21 | 1.35–3.61 | 0.002 | 1.97 | 1.19–3.21 | 0.008 |
| >1.50 | 1.00 | Reference | 1.00 | Reference | ||
| Age (yr) | <0.0001 | <0.0001 | ||||
| 35–39 | 1.00 | Reference | 1.00 | Reference | ||
| 40–44 | 3.44 | 2.38–4.98 | <0.0001 | 3.26 | 2.23–4.78 | <0.0001 |
| 45–49 | 9.13 | 6.02–13.85 | <0.0001 | 8.12 | 5.01–13.15 | <0.0001 |
| Race | ||||||
| African-American | 0.96 | 0.73–1.27 | 0.781 | 0.62 | 0.46–0.84 | 0.002 |
| White | 1.00 | Reference | 1.00 | Reference | ||
| Smoking, current | 1.14 | 0.86–1.52 | 0.363 | 1.61 | 1.19–2.19 | 0.002 |
| BMI group ≥30 | 1.02 | 0.77–1.36 | 0.876 | NS | ||
NS, Not significant.
AMH quartile groups adjusted for age, smoking, race, hysterectomy censored.
Hazard ratio indicates risk of menopause for 1 U change in the covariate during the 14 yr of follow-up. Hazard ratio above 1.0 indicates greater risk (shorter time to menopause); hazard ratio below 1.0 indicates less risk (longer time to menopause).
When AMH quartile groups were adjusted for age, there were significant differences in the median time to menopause within each AMH group (Table 3). As examples, with a low AMH level at baseline (<0.20 ng/ml), women ages 45–48 yr had a median time to menopause of 5.99 yr (95% CI, 4.20–6.33), whereas women ages 35–39 yr had a median time to menopause of 9.94 yr (95% CI, 3.31–12.73). With a higher AMH level at baseline (>0.70–1.50 ng/ml), the median time to menopause increased to 8.72 yr for women ages 45–48 yr and to 12.63 yr for women ages 35–39 yr.
Table 3.
Median time to menopause by AMH quartiles adjusted for age at baseline
| Age (yr) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 35–39 |
40–44 |
45–48 |
|||||||
| n | Mediana | 95% CI | n | Mediana | 95% CI | n | Mediana | 95% CI | |
| AMH quartiles (ng/ml) | |||||||||
| >1.50 | 60 | >13.01 | 8.99–NAb | 41 | 12.51 | 10.85–12.88 | 2 | 6.23 | NAc |
| >0.70–1.50 | 40 | 12.63 | 12.26–12.79 | 44 | 10.02 | 9.08–11.02 | 9 | 8.72 | 6.18–12.05 |
| 0.20–0.70 | 35 | 12.03 | 9.28–12.69 | 47 | 8.76 | 8.10–9.52 | 24 | 7.99 | 6.12–8.67 |
| <0.20 | 14 | 9.94 | 3.31–12.73 | 39 | 7.99 | 5.01–9.45 | 46 | 5.99 | 4.20–6.33 |
NA, Not available.
Median time in years from Kaplan-Meier estimates (n = 401).
No estimate due to ongoing follow-up.
No 95% CI because there were only two subjects in category.
Current smoking was an independent contributor to the risk of menopause in adjusted analysis (Table 2). Smokers were significantly more likely to reach menopause compared with nonsmokers during the 14-yr follow-up (hazard ratio, 1.61; 95% CI, 1.19–2.10; P = 0.002) and reached menopause in a shorter time interval (9.52 yr compared with 10.02 yr for nonsmokers). The unadjusted association of smoking with time to menopause was not significant (Table 2), and there was no statistically significant interaction between AMH and smoking in relation to time to menopause until baseline age was considered (three-way interaction P value = 0.008). When stratified by age, the oldest age group had fewer smokers (21% compared with 45% in the 35–39 yr group and 43% in the 40–44 yr group). The oldest age group also had the greatest prevalence of undetectable AMH levels (41% compared with 21% in the younger age groups), and the interaction between AMH and smoking significantly differed within age groups: for ages 35–39 yr, P = 0.058; for ages 40–44 yr, P = 0.012; and for ages 40–44 yr, P = 0.435. These observations indicated that differences in AMH levels could not be observed between smokers and nonsmokers in relation to time to menopause when the age-related decrease of AMH reached low levels. Race was associated with time to menopause only in the multivariable model that adjusted for AMH, age, and smoking and indicated that the African-American women who reached menopause in the follow-up interval were slightly older compared with the white women (Table 2). There was no significant association of BMI with time to menopause in either adjusted or unadjusted analysis, and inclusion of BMI did not alter estimates of the other variables in the multivariable model.
During the 14-yr study, 73% of the cohort (292 of 401) were observed to reach undetectable AMH levels. Of these participants, 183 were observed to reach menopause in the study interval (hazard ratio, 2.53; 95% CI, 1.49–4.29; P < 0.0001). The median time to menopause from the point of undetectable AMH levels was 5.97 yr (95% CI, 5.55–6.29). This result is notably consistent with our findings for baseline AMH levels, where the median time to menopause was 6.09 yr for the lowest AMH quartile (<0.20 ng/ml) in unadjusted analysis.
We evaluated FSH and inhibin b levels, which were measured concomitantly with AMH, as predictors of the risk of menopause. Each hormone was a statistically significant predictor of time to menopause in both adjusted and unadjusted analysis. Table 4 shows that the strongest predictor was AMH, as indicated by the hazard ratio for each hormone adjusted for age, smoking, and race. The risk of menopause was decreased by 44% for each 1 sd increase in AMH (hazard ratio, 0.56; CI, 0.47–0.67; P < 0.0001); the risk of menopause was decreased by 21% for each sd increase in inhibin b and was increased by 19% for each sd increase in FSH. We also evaluated the predictive value of FSH and inhibin b when included with AMH and age. Both FSH and inhibin b became nonsignificant when included with AMH, and neither FSH nor inhibin b added to the predicted risk of menopause.
Table 4.
Associations of hormones with time to menopause
| Hormone | Unadjusted |
Adjustedc |
||||
|---|---|---|---|---|---|---|
| Hazard ratiob | 95% CI | P | Hazard ratioc | 95% CI | P | |
| log AMH (ng/ml)a | 0.44 | 0.38–0.52 | <0.0001 | 0.56 | 0.47–0.67 | <0.0001 |
| log FSH (mIU/ml)a | 1.44 | 1.27–1.64 | <0.0001 | 1.19 | 1.05–1.36 | 0.007 |
| log Inhibin b (pg/ml)a | 0.66 | 0.58–0.76 | <0.0001 | 0.79 | 0.68–0.92 | 0.003 |
Natural log of hormone as a continuous variable.
Hazard ratio indicates risk of menopause for each 1 sd increase in the log hormone. Hazard ratios greater than 1 indicate increased risk of menopause with each sd increase in the log hormone values (i.e. FSH). Hazard ratios less than 1 indicate a decreasing risk of menopause for each sd increase in log hormone values (AMH, inhibin b).
Adjusted for age, smoking, race. Each hormone entered singly due to high correlation of hormones in the adjusted models.
A sensitivity analysis omitted 17 participants who had a hysterectomy before reaching natural menopause (these women were censored in the primary analysis). The estimates were nearly identical to the results shown in Table 2 for the adjusted Cox model. The results of a second sensitivity analysis that included only participants who had all hormone measures at assessment 1 (n = 354) were also nearly identical to those of the adjusted model shown in Table 2.
Discussion
This prospective study indicated that AMH levels in the late reproductive years predicted median times to menopause, ranging on average from approximately 6 yr among women with AMH levels less than 0.20 ng/ml to approximately 13 yr among women with AMH levels more than 1.50 ng/ml.
Including age with AMH levels significantly improved the prediction of menopause. Among women with AMH levels less than 0.20 ng/ml, the predicted median time to menopause was approximately 6 yr on average for ages 45–48 yr, but considerably longer (approximately 10 yr) for women ages 35–39 yr. With higher AMH levels (e.g. 0.70–1.50 ng/ml), the median time to menopause was about 9 yr on average for women ages 45–48 yr and nearly 13 yr for women ages 35–39 yr.
The study clearly demonstrated the age-related decline of AMH levels in the late reproductive years but also indicated the considerable variability of age in this decline. Only two women in the oldest age group (45–48 yr) had high AMH levels, whereas the youngest women were distributed in all AMH quartile groups. Fourteen women in the youngest age group (35–39 yr) approached or had undetectable AMH levels (<0.20 ng/ml). The clinical meaning of very low AMH levels in younger regularly menstruating women is unclear. However, the observations in our study were similar to the prevalence of undetectable AMH levels in young healthy women in the general population: the 5th percentile of AMH levels was less than 0.30 ng/ml in a study of over 2700 reportedly healthy women ages 25 to 43 yr (12). Undetectable AMH levels have been investigated primarily in the infertile population, where they indicate decreased ovarian reserve and appear to predict ovarian response to controlled ovarian stimulation (8). The present findings clearly show that AMH and age are each strong and independent predictors of time to menopause and together provide a stronger prediction of time to menopause than either variable alone, but more information is needed to fully understand the basic physiology and clinical utility of AMH.
This evaluation of AMH levels was conducted among women in their late reproductive years, a time period when women want to know the number of years before they reach menopause. Other recent AMH studies predicted age at menopause (15, 16) and estimated time to menopause from the point of reaching undetectable AMH levels (10). Our investigation supports these studies and substantially strengthens the evidence by providing estimates from a large, population-based cohort. It extends information by evaluating the independent associations of age, smoking, BMI, race, and the relative strength of AMH compared with FSH and inhibin b in the predictions of time to menopause.
In the present study, smokers reached menopause in a shorter time interval compared with nonsmokers. This is consistent with our previous findings that current smoking was one of the strongest predictors of entry into early stages of the menopause transition and increased the likelihood of entering each transition stage by about 30% (23). Natural menopause appears to occur up to 3 yr earlier in smokers (30, 31). A report from the Michigan Bone Health and Metabolism Study showed that smokers had an earlier age at menopause and that smoking modified the association of AMH, with a more rapid decline of AMH relative to age at menopause (22). Another community sample of women ages 38–50 yr found smoking to be associated with decreased AMH levels compared with nonsmokers (18). The mechanism behind this association is likely related to the toxic effect of smoking on ovarian follicles, which results in accelerated ovarian follicular depletion and may lead to diminished ovarian reserve at earlier reproductive ages (30, 32).
Obesity was evaluated as a covariate but had no significant associations with time to menopause in this study. There are conflicting reports of the association between obesity and AMH (19–21, 33), possibly due to differences in age or menopausal stage of the study participants, and the association remains an open question. We previously found that AMH levels were significantly lower in obese compared with nonobese premenopausal women but that antral follicle counts did not differ by body size (20, 21). This suggested that lower AMH levels in obese women did not result from decreased ovarian reserve (which might lead to an earlier menopause) but from other physiological processes in hormone metabolism, sequestration, or clearance (34–36). It is also possible that the age-related decline of AMH to nondetectable levels obscures differences between obese and nonobese women in this and other studies.
In this study, we found that race was associated with time to menopause only in the multivariable model. The African-American women who reached menopause in the follow-up interval were slightly older compared with the white women when adjusted for AMH, age, and smoking. Previous studies are conflicting and report both earlier and later ages at menopause for African-American women (37, 38), or no difference compared with white women in the Study of Women's Health Across the Nation (39). Associations of race with menopause appear to be associated with other covariates as indicated in this study and remain an open question.
The findings from generally healthy women may not be applicable to women with ovulatory infertility, menstrual cycle irregularities, or other health problems. Women who had disease conditions such as endometriosis or polycystic ovary syndrome were not included in this cohort, although the possible influence of these or other disease conditions on AMH levels cannot be excluded. Other limitations to consider are differences in AMH assays that require caution in comparing AMH levels among studies and underscore the need for a standardized AMH assay that is sensitive at low levels. Our models predict median times to menopause but do not convert to precise estimates for individual women and should be used carefully when counseling patients about their remaining time to menopause.
Strengths of the study include the prospective identification of menopause during the 14-yr follow-up, prospective measurement of AMH levels and concomitant measures of other reproductive hormones in the late reproductive years, hormone measurement on cycle d 2–6 when fluctuations are minimal, the ability to compare measures between African-American and white women, and adequate statistical power in a randomly identified, population-based cohort.
Health care providers require information to predict time to menopause to counsel patients about their concerns for reproductive planning, menopausal symptoms, and hormone therapy. This information may also enable clinicians to address adverse health outcomes known to be associated with reproductive hormone changes (e.g. increasing bone loss, increased risk of coronary heart disease, depression, and vasomotor symptoms) that occur in the menopause transition. This study indicates that a median time to menopause can be predicted for late reproductive age women based on AMH level, age, and current smoking. Further studies are needed to confirm the reliability and clinical utility of these predictions and develop greater precision for individual women.
Acknowledgments
This work was supported by National Institutes of Health Grants RO1-AG-12745 (to E.W.F., Principal Investigator) and RR024134 (to Clinical and Translational Research Center, School of Medicine).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- Anti-Mullerian hormone
- BMI
- body mass index
- CI
- confidence interval.
References
- 1. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. 2002. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril 77:357–362 [DOI] [PubMed] [Google Scholar]
- 2. Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. 2003. Anti-Müllerian hormone and its role in ovarian function. Mol Cell Endocrinol 211:85–90 [DOI] [PubMed] [Google Scholar]
- 3. Feyereisen E, Méndez Lozano DH, Taieb J, Hesters L, Frydman R, Fanchin R. 2006. Anti-Müllerian hormone: clinical insights into a promising biomarker of ovarian follicular status. Reprod Biomed Online 12:695–703 [DOI] [PubMed] [Google Scholar]
- 4. Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH, teVelde ER. 2003. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod 18:700–706 [DOI] [PubMed] [Google Scholar]
- 5. van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, Themmen AP. 2002. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod 17:3065–3071 [DOI] [PubMed] [Google Scholar]
- 6. Yang YS, Hur MH, Kim SY, Young K. 2011. Correlation between sonographic and endocrine markers of ovarian aging as predictors for late menopausal transition. Menopause 18:138–145 [PubMed] [Google Scholar]
- 7. Hansen KR, Hodnett GM, Knowlton N, Craig LB. 2011. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 95:170–175 [DOI] [PubMed] [Google Scholar]
- 8. La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS; ESHRE Special Interest Group for Reproductive Endocrinology–AMH Round Table 2009. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod 24:2264–2275 [DOI] [PubMed] [Google Scholar]
- 9. van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, de Jong FH, Fauser BJ, Themmen AP, te Velde ER. 2005. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83:979–987 [DOI] [PubMed] [Google Scholar]
- 10. Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, Harlow S, Randolph JF., Jr 2008. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab 93:3478–3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seifer DB, Baker VL, Leader B. 2011. Age-specific serum anti-Müllerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril 95:747–750 [DOI] [PubMed] [Google Scholar]
- 12. Shebl O, Ebner T, Sir A, Schreier-Lechner E, Mayer RB, Tews G, Sommergruber M. 2011. Age-related distribution of basal serum AMH level in women of reproductive age and a presumably healthy cohort. Fertil Steril 95:832–834 [DOI] [PubMed] [Google Scholar]
- 13. van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, Broekmans FJ. 2008. Relationship of serum antimüllerian hormone concentration to age at menopause. J Clin Endocrinol Metab 93:2129–2134 [DOI] [PubMed] [Google Scholar]
- 14. Schnatz PF, Jiang X. 2011. Predicting age of menopause: what is the future of the antimüllerian hormone biomarker? Menopause 18:727–729 [DOI] [PubMed] [Google Scholar]
- 15. Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. 2011. Predicting age at menopause from serum antimüllerian hormone concentration. Menopause 18:766–770 [DOI] [PubMed] [Google Scholar]
- 16. Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, Laven JS, de Jong FH, Te Velde ER, Fauser BC, Broekmans FJ. 2011. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 96:2532–2539 [DOI] [PubMed] [Google Scholar]
- 17. Sun L, Tan L, Yang F, Luo Y, Li X, Deng HW, Dvornyk V. 2012. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause 19:126–132 [DOI] [PubMed] [Google Scholar]
- 18. Plante BJ, Cooper GS, Baird DD, Steiner AZ. 2010. The impact of smoking on antimüllerian hormone levels in women aged 38 to 50 years. Menopause 17:571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steiner AZ, Stanczyk FZ, Patel S, Edelman A. 2010. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception 81:245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su HI, Sammel MD, Freeman EW, Lin H, DeBlasis T, Gracia CR. 2008. Body size affects measures of ovarian reserve in late reproductive age women. Menopause 15:857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd 2007. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril 87:101–106 [DOI] [PubMed] [Google Scholar]
- 22. Sowers MR, McConnell D, Yosef M, Jannausch ML, Harlow SD, Randolph JF., Jr 2010. Relating smoking, obesity, insulin resistance, and ovarian biomarker changes to the final menstrual period. Ann NY Acad Sci 1204:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sammel MD, Freeman EW, Liu Z, Lin H, Guo W. 2009. Factors that influence entry into stages of the menopausal transition. Menopause 16:1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freeman EW, Sammel MD, Gracia CR, Kapoor S, Lin H, Liu L, Nelson DB. 2005. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril 83:383–392 [DOI] [PubMed] [Google Scholar]
- 25. Kaplan EL, Meier P. 1958. Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481 [Google Scholar]
- 26. Mantel N. 1966. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170 [PubMed] [Google Scholar]
- 27. Cox DR. 1972. Regression models and life-tables. J R Stat Soc Series B 34:187–220 [Google Scholar]
- 28. Kleinman DG, Klein M. 2005. Survival analysis: a self-learning text. 2nd ed New York: Springer-Verlag; 140 [Google Scholar]
- 29. Hess KR. 1995. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 14:1707–1723 [DOI] [PubMed] [Google Scholar]
- 30. van Asselt KM, Kok HS, van Der Schouw YT, Grobbee DE, te Velde ER, Pearson PL, Peeters PH. 2004. Current smoking at menopause rather than duration determines the onset of natural menopause. Epidemiology 15:634–639 [DOI] [PubMed] [Google Scholar]
- 31. Fleming LE, Levis S, LeBlanc WG, Dietz NA, Arheart KL, Wilkinson JD, Clark J, Serdar B, Davila EP, Lee DJ. 2008. Earlier age at menopause, work, and tobacco smoke exposure. Menopause 15:1103–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paszkowski T, Clarke RN, Hornstein MD. 2002. Smoking induces oxidative stress inside the Graafian follicle. Hum Reprod 17:921–925 [DOI] [PubMed] [Google Scholar]
- 33. Halawaty S, ElKattan E, Azab H, ElGhamry N, Al-Inany H. 2010. Effect of obesity on parameters of ovarian reserve in premenopausal women. J Obstet Gynaecol Can 32:687–690 [DOI] [PubMed] [Google Scholar]
- 34. de Jong PE, Verhave JC, Pinto-Sietsma SJ, Hillege HL; PREVEND study group 2002. Obesity and target organ damage: the kidney. Int J Obes Relat Metab Disord 26(Suppl 4):S21–S24 [DOI] [PubMed] [Google Scholar]
- 35. Hall JE, Jones DW, Kuo JJ, da Silva A, Tallam LS, Liu J. 2003. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep 5:386–392 [DOI] [PubMed] [Google Scholar]
- 36. Ledoux S, Campos DB, Lopes FL, Dobias-Goff M, Palin MF, Murphy BD. 2006. Adiponectin induces periovulatory changes in ovarian follicular cells. Endocrinology 147:5178–5186 [DOI] [PubMed] [Google Scholar]
- 37. Bromberger JT, Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. 1997. Prospective study of the determinants of age at menopause. Am J Epidemiol 145:124–133 [DOI] [PubMed] [Google Scholar]
- 38. Palmer JR, Rosenberg L, Wise LA, Horton NJ, Adams-Campbell LL. 2003. Onset of natural menopause in African American women. Am J Public Health 93:299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. 2001. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 153:865–874 [DOI] [PubMed] [Google Scholar]