This study showed the developmentally regulated expressions and distributions of NCAM and polysialic acid in embryonic and postnatal chick cornea, which suggests their potential functions in corneal innervation.
Abstract
Purpose.
To assay for expression and localization of neural cell adhesion molecule (NCAM) and polysialic acid (polySia) in the chick cornea during embryonic and postnatal development.
Methods.
Real time quantitative PCR and Western blot analyses were used to determine NCAM expression and polysiaylation in embryonic, hatchling, and adult chick corneas. Immunofluorescence staining for NCAM and polySia was conducted on cryosections of embryonic and adult corneas, whole embryonic corneas, and trigeminal neurons.
Results.
NCAM and ST8SiaII mRNA transcripts peaked by embryonic day (E)9, remained steady between E10 and E14 and slowly decreased thereafter during embryonic development. Both gene transcripts showed > 190-fold decline in the adult chick cornea compared with E9. In contrast, ST8SiaIV expression gradually decreased 26.5-fold from E6 to E19, increased thereafter, and rose to the early embryonic level in the adult cornea. Western blot analysis revealed NCAM was polysialylated and its expression developmentally changed. Other polysiaylated proteins aside from NCAM were also detected by Western blot analysis. Five NCAM isoforms including NCAM-120, NCAM-180 and three soluble NCAM isoforms with low molecular weights (87–96 kDa) were present in chick corneas, with NCAM-120 being the predominate isoform. NCAM was localized to the epithelium, stroma, and stromal extracellular matrix (ECM) of the embryonic cornea. In stroma, NCAM expression shifted from anterior to posterior stroma during embryonic development and eventually became undetectable in 20-week-old adult cornea. Additionally, both NCAM and polySia were detected on embryonic corneal and pericorneal nerves.
Conclusions.
NCAM and polySia are expressed and developmentally regulated in chick corneas. Both membrane-associated and soluble NCAM isoforms are expressed in chick corneas. The distributions of NCAM and polySia in cornea and on corneal nerves suggest their potential functions in corneal innervation.
The cornea is the most densely innervated and sensitive tissue on the surface of the body. During early chick embryonic development, corneal nerves are derived from neural crest cells located in the ophthalmic lobe of the trigeminal ganglion.1–4 Trigeminal nerve axon fascicles reach the corneal periphery by embryonic day (E)5. The nerves are repelled from entering the cornea and form a perilimbal ring around the cornea until E9.3–5 Beginning at E9, nerves from the ring invade the anterior stroma, branch, and extend toward the center of the cornea. By E12, the nerves migrate from the stroma, penetrate the basement membrane, intermingle with the epithelium, and reach the center of the cornea by E15.1,3 By E18 the nerves complete the innervation of the cornea.6,7 Recently work has shown that secreted neuronal guidance proteins, such as Semaphorin 3A and Slit2, are involved in orchestrating this pattern of nerve development and distribution.7–9 However, in addition, carbohydrate moieties on proteins, such as polysialic acid (polySia) on neural cell adhesion molecule (NCAM), are functionally significant during axon outgrowth, guidance, plasticity, neural repair, and regeneration in the central nervous system (CNS) and peripheral nervous system (PNS).10–17 Expression of NCAM and associated posttranslational modifications have not been studied previously during embryonic development of corneal nerves.
NCAM is an immunoglobulin superfamily cell adhesion molecule. The three major isoforms of NCAM are NCAM-120, -140, and -180. NCAM-120 is a glycosylphosphatidylinositol (GPI)-anchored membrane protein, whereas NCAM-140 and -180 are transmembrane proteins.18 Particularly high levels of NCAM are expressed in the nervous system,19 but also are expressed in nonneuronal tissues, such as lungs, muscles, kidneys, stomach, and heart.20 In addition to membrane-associated isoforms, soluble forms of NCAM have been found in rat brain, cerebrospinal fluid, and plasma21–23; human serum and amniotic fluid24; and culture media of chick retinal cells.25 Soluble NCAM exists in different isoforms, with various molecular weights ranging from 180 kDa to 100 kDa,26–31 which are produced via alternative splicing of the transcripts, enzymatic cleavage of the extracellular domain of membrane-associated NCAM, and detached NCAM-containing membrane fragments.32 All these NCAM isoforms can be modified posttranslationally with polySia.
PolySia is a unique and highly negatively charged homopolymer of sialic acid residues mostly with α2-8 linkage. The degree of polymerization of the polySia moieties on NCAM can be as high as 400 residues.33 The large, negatively charged, and highly hydrated structure of polySia on NCAM can increase the intermembrane space and disrupt the adhesive properties of NCAM,34–36 thus influencing cell-cell interaction and communication.37–39 During embryonic nervous system development, polySia on NCAM is regarded as a prominent regulator of neural cell migration and differentiation, nerve outgrowth, axon guidance, and targeting.12,34,40 It also contributes significantly to neurogenesis, synaptic plasticity, and repair in the postnatal nervous system.17,40–42
The function of polySia on soluble NCAM is not well understood. However, it has been demonstrated that soluble NCAM can interfere with the homophilic interaction between membrane-associated NCAM molecules and reduce NCAM-mediated cell adhesion. Thus, soluble NCAM can modulate neurite outgrowth and branching,31,43 and also can promote Schwann cell migration.44
In the CNS, the polySia on NCAM is downregulated during embryonic development and is persistently expressed at sites limited to ongoing neurogenesis or plasticity.11,42 In chick, NCAM is first detected in the gastrula (primitive streak) stage, continues to be expressed at high levels throughout the developing central and peripheral nervous system, and persists at low levels in the adult.45–48 Studies have shown that NCAM is expressed in chick retina and regulates retinal ganglion cell nerve outgrowth and guides ganglion cell axons.13,49 NCAM has also been localized in epithelium and endothelium of mouse cornea20,50 and endothelium of human cornea.51,52 To date, NCAM expression, distribution, and the extent to which it is polysiaylated in developing corneas have not yet been reported previously. In this work, the expression and localization of NCAM and polySia in chick corneas during embryonic and postnatal development were investigated, including polysialylation of NCAM and other proteins, and the distribution of NCAM and polySia in the chick cornea and on corneal and pericorneal nerves.
Methods
Chick Husbandry and Corneal Isolation
Fertile White Leghorn chicken eggs, newly hatched chicks (1-day-old; 1 D) and adult chickens (20-week-old; 20 weeks) used in this work were all from a local hatchery. The fertile chicken eggs were incubated at 38°C and 45% humidity from E0. Hatched chickens were all handled under an approved Institutional Animal Care and Use Committee (IACUC) protocol. Corneas from chick embryos of the desired ages and chickens were dissected free of sclera and limbus tissue in sterile phosphate buffered saline (PBS), snap frozen in liquid nitrogen, and stored at −80°C until used.
RNA Isolation and Real Time Q-PCR
For each data point of real time quantitative (Q)-PCR, three separate RNA isolations, cDNA synthesis, and real time Q-PCR reactions were performed as previously described.53 Corneas of desired embryonic ages between E6 and E20, newly hatched (1 D), and adult (20 weeks), previously snap frozen in liquid nitrogen and stored at −80°C, were pooled and pulverized in a prechilled stainless steel pulverizer (Biopulverizer; BioSpec Products, Inc., Bartlesville, OK). The pulverized corneas were immediately transferred to the lysis buffer from kits (RNeasy Protect Kits; Qiagen, Valencia, CA) containing mercaptoethanol at room temperature, then further homogenized using a rotor (Pro 200; Pro Scientifc, Oxford, CT) at maximum speed for 1 minute, and stored at −80°C. Total RNA was isolated according to the manufacturer's protocol for tissues containing abundant connective tissue, including proteinase K digestion and column DNase digestion, and stored at −80°C. cDNA was synthesized from 1 μg total RNA using a cDNA synthesis kit (iScript; Bio-Rad, Hercules, CA) according to the manufacturer's instructions and stored at −20°C. Sequences and gene numbers of chicken NCAM, ST8SiaII, ST8SiaIV, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were obtained from GenBank (http://www.ncbi.nlm.nih.gov/GenBank). PCR primers for NCAM, ST8SiaII, ST8SiaIV, and housekeeping gene GAPDH were designed using specialized software (Beacon Designer 7.9; Premier Biosoft, Palo Alto, CA) to amplify fragments between 75 and 150 base pairs in length as listed in Table 1. Each primer set generates only one amplified band with chick cornea cDNA, and is between 90% and 110% efficient when analyzed over 10-fold cDNA dilutions. Housekeeping gene GAPDH was chosen for normalization of all gene expressions. All comparative real time Q-PCR reaction series consisted of duplicates for 10X and 1X cDNA solutions for each PCR primer pair.
Table 1.
Real Time Q-PCR Primers for GAPDH, NCAM, ST8SiaII, and ST8SiaIV
| Symbol | Gene Name/GI Number | Forward Primer | Reverse Primer |
|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate-dehydrogenase/4105595 | 5′-GCTGAGAACGGGAAACTTGTGA | 5′-GCACCTGCATCTGCCCATTT |
| NCAM | NCAM1 neural cell adhesion molecule-1/770798 | 5′-GTGCTGTCCAACAACTAC | 5′-AATGACCTGAATATCTTTGAAGT |
| ST8SiaII | ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase-2/414336 | 5′-GCAACCGCATCCATCTCT | 5′-CTTCAGGCTGTCGTAGTAGTG |
| ST8SiaIV | ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase-4/374155 | 5′-AGTATTCTCCGATTCTTGG | 5′-GATTACATCTCCTGGCTTA |
GI, GenInfo Identifier.
Total Protein Extraction
The previously snap frozen corneas of chick embryos (E7, E9, E10, E12, E14, E16, E18, and E20), newly hatched chicks (1 D), adult chickens (20 weeks) and E12 chick brain were pulverized in liquid nitrogen and homogenized in an NP-40 extraction buffer containing protease inhibitors (Total Protein Extraction Kit; Millipore, Billerica, MA). The extraction buffer was composed of HEPES (pH 7.9), MgCl2, KCl, EDTA, sucrose, glycerol, sodium deoxycholate, NP-40, and sodium orthovanadate. Total protein was extracted according to the manufacturer's protocol. The concentration of total protein was determined using an assay (BCA Protein Assay Kit; Pierce, Rockford, IL).
Digestion with Peptide-N-Glycosidase F
Each solution of 20 μg total protein extracted from corneas of the desired ages was diluted to 45 μL with deionized (DI) water followed by incubation with 2 μL peptide-N-glycosidase F (N-Glycanase; ProZyme, Hayward, CA) overnight at 37°C to deglycosylate proteins. The digestion was terminated by adding SDS-PAGE sample buffer and incubating at 70°C for 10 minutes.
Immunoblotting Analysis
Twenty micrograms of total protein, N-Glycanase-treated total protein from corneas of desired developmental stages or E12 chick brain were subjected to SDS-PAGE gel electrophoresis. Proteins in the SDS-PAGE gel were transferred to a nitrocellulose membrane, blocked with the blocking solution from a Western blotting kit (Invitrogen WesternBreeze Chromogenic Immunodetection Kit; Invitrogen, Carlsbad, CA), and then incubated with the antibody against the extracellular domain of NCAM (5e, 1:1000; Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, IA), the cytoplasmic domain of NCAM (4d, 1:1000; Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, IA), polySia-NCAM (2-2B, 1:500; Miltenyi Biotec Inc., Auburn, CA), or GAPDH (6C5, 1:1000; Thermo Scientific, Waltham, MA) in the blocking solution. This was followed by three washes in washing solution and incubation with an alkaline phosphatase-conjugated secondary antibody in the blocking solution. NCAM, GAPDH, or polySia was visualized with chromogenic solution (BCIP/NBT; Invitrogen). GAPDH was chosen as sample loading control.
The Western blot analysis results obtained with deglycosylated total protein using the anti-NCAM extracellular domain antibody were scanned and profiled. The intensity and relative amount of each NCAM isoform was determined (ImageQuant TL; GE Health Care Biosciences Corp., Piscataway, NJ).
Immunostaining for NCAM on Corneal Sections
Localization of NCAM was performed using immunofluorescence staining on frozen sections of E7, E9, E14, E20, and 20 weeks corneas. Entire hemispheres, containing the cornea, from eyeballs of embryonic chicks, or one cornea of 20 weeks chickens, were embedded in OCT. Frozen sections (15 μm) were cut at −20°C using a cryostat (Bright Instrument Company Ltd., Huntingdon, England), mounted on slides (Fisher, Pittsburgh, PA) and stored at −80°C until used. The sections were then fixed in cold acetone at −20°C for 10 minutes, washed twice with PBS containing 0.025% Tween-20 for 5 minutes and then blocked with 10% bovine serum albumin (BSA) in PBS. NCAM was detected with anti-NCAM extracellular domain antibody (5e, 1:200), and a secondary antibody Alexa Fluor 488 goat anti-mouse IgG1 (Invitrogen). Negative controls were performed on each immunofluorescence staining with absence of primary antibody and showed negative staining. The sections were visualized and photographed using an epifluorescent microscope equipped with a digital camera (Leica MZ16F microscope and DFC 320 camera; Leica Microsystems, Wetzlar, Germany). This epifluorescent microscope was used in the following experiments unless stated otherwise. The fluorescence intensity across each corneal section was profiled using ImageJ (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html).
Whole-mount Double Immunostaining for NCAM or PolySia and Corneal Nerves
Whole-mount double immunofluorescence staining for neuronal-specific class III β-tubulin (β-tubulin) and NCAM or polySia was performed on E9 and E14 corneas. The corneas were dissected in PBS and fixed in 4% paraformaldehyde solution overnight at 4°C. After washing with PBS-T (PBS with 0.1% Triton X-100) three times for 5 minutes, the cornea was cut into two halves; one half was used for negative control with absence of primary antibody and the other half was for double immunofluorescence staining. The negative controls showed no staining. Each half cornea was slit through the pericornea to allow the antibodies to diffuse into the cornea stroma and epithelium.53,54 NCAM was detected using the anti-NCAM extracellular domain antibody (5e, 1:100) and a secondary antibody Alexa Fluor 488 goat anti-mouse IgG1 diluted to 1:200. Anti-polySia-NCAM antibody (2–2B, 1:50; Miltenyi Biotec Inc.) and a secondary antibody Alexa Fluor 488 goat anti-mouse IgM (1:200; Invitrogen) were used to detect polySia. Corneal and pericorneal nerves were stained using 5 μg/mL anti-neuronal-specific class III β-tubulin antibody (R&D Systems, Minneapolis, MN) and a secondary antibody Alexa Fluor 546 goat anti-mouse IgG2a (Invitrogen) diluted to 1:200.
Trigeminal Neuron Culture and Immunostaining
Trigeminal ganglia were dissected from E9 and E14 chick embryos and the proximal region containing the ophthalmic branch, which innervates the cornea,1 was cut into tissue explants using a tungsten needle (Fine Science Tools, Foster City, CA). Trigeminal explants were put in 2-well chamber slides coated with poly-d-lysine (100 μg/mL in borate buffer, pH 8.4; Sigma, St. Louis, MO) and laminin (20 μg/mL in water; Sigma) to support neuronal adhesion and outgrowth. Then the trigeminal explants were cultured at 37°C in a humidified CO2 incubator for 48 hours in media (Opti-MEM; Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum, antibiotics (100 units penicillin, 0.1 mg/mL streptomycin; Sigma) and 25 ng/mL nerve growth factor (Sigma) to support neurogenesis of the trigeminal explants.3,55 After culture, neuronal cell bodies and their neurite extensions were washed briefly in Howard Ringer's saline solution (7.2 g NaCl, 0.23 g CaCl2 · H2O, 0.37 g KCl in 1 liter DI water, pH 7.3), fixed at room temperature for 1 hour in 4% paraformaldehyde solution, followed by several washes in PBS-T. Neuronal tissue was incubated at room temperature for 1 hour in blocking solution (PBS, pH 7.2, containing 5% goat serum, 1% bovine serum albumin, 0.1% Triton X-100), followed by staining with anti-neuronal-specific class III β-tubulin (1:100), anti-NCAM extracellular domain (1:50), or anti-polySia-NCAM (1:50) in blocking solution overnight at 4°C with mild rocking. After several washes in PBS-T, tissue was incubated in appropriate secondary antibodies, Alexa Fluor 488 mouse IgG2a antibody (neuronal-specific class III β-tubulin), Alexa Fluor 488 goat anti-mouse IgG1 (NCAM) antibody or Alexa Fluor 488 goat anti-mouse IgM (polySia) antibody each used at a 1:100 dilution in blocking solution.
Results
Developmental Expression of NCAM, ST8SiaII, and ST8SiaIV mRNA in the Chick Cornea
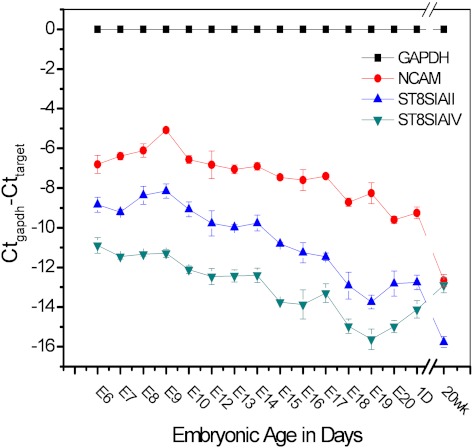
Among the six known α 2, 8-sialyltransferases, only ST8SiaII and ST8SiaIV can add polySia to NCAM.56 Both ST8SiaII and ST8SiaIV can modify all the isoforms of NCAM57 and cooperatively control the expression of polySia.58,59 Here we investigated the mRNA expression of both NCAM and the polysialyltransferases to understand the expression and polysialylation of NCAM in the chick cornea. To determine the mRNA expression, real time Q-PCR was performed with the total RNA from embryonic (E6 to E20), hatchling (1 D), and 20 weeks adult corneas. Figure 1 presents the Ct numbers of the target genes (NCAM, ST8SiaII and ST8SiaIV) normalized to the housekeeping gene, GAPDH. The mRNA expression levels of NCAM and ST8SiaII show similar patterns of changes during embryonic and postnatal development. NCAM and ST8SiaII transcript levels first increased approximately 3.5-fold and 1.6-fold respectively from E6 to E9. NCAM and ST8SiaII expression peaked on E9, and then their expression levels remained steady during E10 to E14 and decreased gradually during later embryonic stages. By E20, just before hatching, NCAM expression was 23-fold lower than that at E9. Respectively, ST8SiaII expression was approximately 25-fold lower than that at E9. The same expression levels were found in 1 D cornea. When compared with E9, dramatic drops occurred in 20 weeks adult chick corneas of 191-fold approximately for NCAM and 195-fold for ST8SiaII. Expression of ST8SiaIV mRNA showed a different, more moderate pattern of change during embryonic development and into adulthood. During the embryonic stages between E6 and E14, ST8SiaIV expression steadily declined by approximately 2.8-fold. There was an approximately10-fold decline from E14 to E19, while the expression gradually increased thereafter. By 20 weeks after hatchling, ST8SiaIV expression rose back to early embryonic levels.
Figure 1.
Real time Q-PCR analysis of NCAM, and two polysialyltransferases, ST8SiaII and ST8SiaIV, which are involved in the polysiaylation of NCAM, in corneal tissue during embryonic development, hatchling and adult stages. The y-axis is the Ct number of each target gene normalized to the Ct number of the housekeeping gene, GAPDH. The expressions of NCAM and ST8Sia II were downregulated from embryo to postnatal 20 weeks and decreased approximately 191-fold and 195-fold, respectively, in adult corneas compared with E9. In contrast, the expression of ST8Sia IV gradually decreased, first from E6 to E19, and then increased thereafter.
Characterization of NCAM in the Chick Cornea
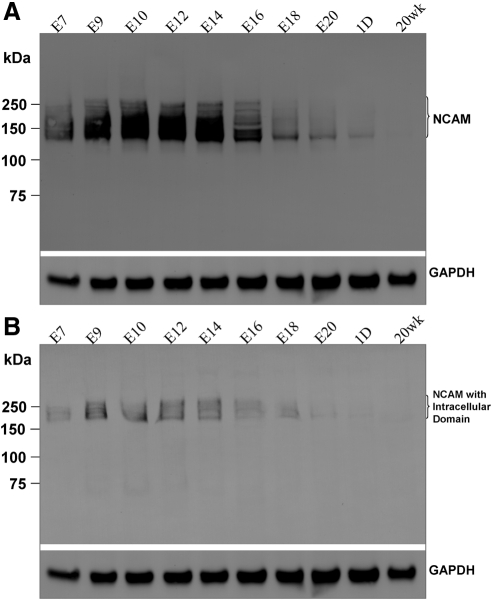
The presence of NCAM protein in the chick cornea was confirmed by Western blot analysis using antibodies against NCAM (Fig. 2). A smeared band with a molecular weight (MW) range of approximately 130 to 250 kDa was obtained using anti-NCAM extracellular domain antibody for each desired stage (Fig. 2A), whereas a narrower smeared band with a MW range of approximately 180 to 250 kDa was detected using the anti-NCAM cytoplasmic domain antibody for the selected stages (Fig. 2B). The presence of a smeared band instead of a single band in each lane in Figures 2A and 2B demonstrates that NCAM in the chick cornea is polysialylated. Consistent with the real time Q-PCR results, NCAM protein expression levels varied during development and after hatchling. The amount of NCAM protein increased from E7 to E10, remained constant to E14 and then decreased dramatically thereafter. Eventually, NCAM became undetectable in 20 weeks adult cornea.
Figure 2.
Western blot analysis for NCAM in embryonic and postnatal corneas. NCAM was detected either by the anti-NCAM extracellular domain antibody (A) or anti-NCAM cytoplasmic domain antibody (B) at specified developmental stages. GAPDH was used as a sample loading control. NCAM in chick corneas was most abundant during embryonic stages, E9–E14.
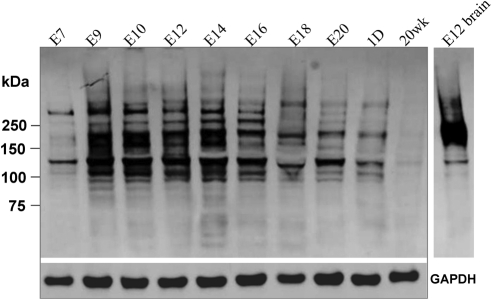
Western blot analysis using anti-polySia-NCAM antibody revealed several wide, smeared bands ranging from 120 kDa to 350 kDa for each embryonic developmental stage examined, with total protein from E12 chick brain60 as a positive control (Fig. 3). The expression of polySia showed similar developmental regulation as that of NCAM (Fig. 2). The intensity of the stained bands increased dramatically from E7 to E9 and then continued with high intensity to E16 and declined thereafter. Weak staining detected in 20 weeks corneas showed the persistent presence of polySia in adult corneas. Significantly, Western blot analysis, using antibodies against NCAM and polySia, showed very different band profiles. Firstly, the MW range of the staining using anti-polySia-NCAM antibody was much broader than that obtained using anti-NCAM extracellular domain antibody which can bind all NCAM isoforms (Fig. 2A). In addition, many bands that stained positive for polySia did not correspond in position to bands staining positive for NCAM. This suggests that there are other protein(s) in addition to NCAM that carry polySia residues.
Figure 3.
Western blot analysis of protein extracts from the chick corneas of specified developmental stages using anti-polySia-NCAM antibody. GAPDH was used as sample loading control. Protein extract from E12 chick brain was used as positive control.60 The broad range of multiple bands indicates the existence of other polysialylated proteins in chick corneas.
NCAM Isoforms in the Chick Cornea
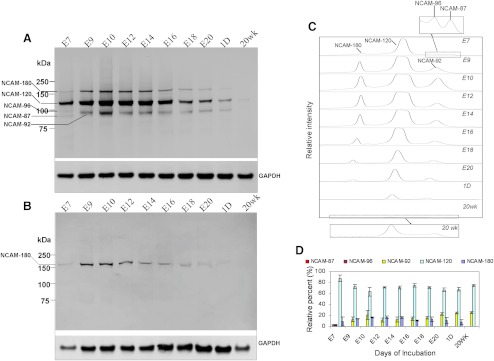
The anti-NCAM extracellular domain antibody binds all NCAM isoforms, whereas the anti-NCAM cytoplasmic domain antibody specifically recognizes the isoforms NCAM-140 and NCAM-180, which have intracellular domains. The Western blot analysis using anti-NCAM extracellular domain antibody detected bands of a wider MW range than using anti-NCAM cytoplasmic domain antibody (Figs. 2A, 2B). This demonstrates the existence of NCAM isoform(s) other than transmembrane isoform(s). To determine these NCAM isoforms in the chick cornea, we used peptide-N-glycosidase F (N-Glycanase) to remove the polySia chains which are attached to NCAM through N-glycans.61 Four NCAM isoforms with MWs of 87 kDa, 96 kDa, 110 kDa, and 160 kDa were detected in E7 cornea (Fig. 4A). When the embryo developed to E9, the isoforms with MWs of 87 kDa and 96 kDa disappeared and an isoform with MW of 92 kDa became present for the first time. This 92 kDa NCAM isoform, along with the two isoforms (110 kDa and 160 kDa), were consistently present from E9 to E20 and in hatchling chick corneas. In contrast to Western blot analysis of the native NCAM forms (polysialylated forms) (Fig. 2A), NCAM isoforms with MWs of 92 kDa and 110 kDa were detected in 20 weeks adult corneas. This occurs because the deglycosylation allowed each broadly spread native isoform with diverse polysialylic acid chains33 to migrate instead as one single band in SDS-PAGE gel (Fig. 4A).
Figure 4.
Western blot analysis of peptide-N-glycosidase F (N-Glycanase)-treated total protein extracted from chick embryonic, hatchling, and adult corneas. NCAM isoforms were detected using the anti-NCAM extracellular domain antibody (A) and anti-NCAM cytoplasmic domain antibody (B). Four NCAM isoforms, NCAM-180, -120, -96, and -87 with apparent MWs of 160 kDa, 110 kDa, 96 kDa, and 87 kDa, respectively, were found in E7 chick cornea. NCAM-180, -120, and another isoform, -92, with an apparent MW of 92 kDa were detected in later developmental stages. NCAM-120 and -92 were detected in 20 weeks adult corneas. Only one isoform which was identified as NCAM-180 showed immunoreactivity to the antibody against the intracellular domain (B). The intensity of the bands on each lane in (A) was profiled using commercial software (ImageQuant TL; GE Health Care Biosciences Corp.) (C). The relative amount of each isoform of NCAM at the selected stages was determined (ImageQuant TL; GE Health Care Biosciences Corp.) (D). The insets in (C) are zoom-in profiles of NCAM isoforms in E7 and 20 weeks adult corneas.
PolySias are attached to NCAM through the terminal α2–3(6) sialic acid of N-linked glycans.61 Previous studies have shown that after removing polySia with endoneuaminidase, the membrane-associated NCAM isoforms show the apparent MWs of 180 kDa, 140 kDa, and 120 kDa, commonly designated as NCAM-180, NCAM-140, and NCAM-120.60 Respectively, further removal of the N-glycans (using N-Glycanase) yields polypeptide chains with smaller apparent MWs of 160 kDa, 130 kDa, and 110 kDa.62–64 Therefore the bands with MWs of 160 kDa and 110 kDa in Figure 4A (produced by digestion of corneal proteins with N-Glycanase) were identified as NCAM-180 and NCAM-120. Other isoforms with lower MWs of 87 kDa, 92 kDa, and 96 kDa are defined respectively as NCAM-87, NCAM-92, and NCAM-96 in this work. A summary of NCAM isoforms in chick corneas and their MWs after desialylation and deglycosylation is listed in Table 2.
Table 2.
Summary of the Molecular Weight Change of NCAM Isoforms in Chick Cornea Treated with Neuraminidase and Peptide-N-Glycosidase F (N-Glycanase)
| Native (kDa) | Desialylated (kDa) (Neuraminidase-treated60) | Deglycosylated (kDa) (N-Glycanase–treated, Fig. 4) | |
|---|---|---|---|
| NCAM-87 | — | — | 87 |
| NCAM-92 | — | — | 92 |
| NCAM-96 | — | — | 96 |
| NCAM-120 | — | 120 | 110 |
| NCAM-180 | 180–250 | 180 | 160 |
NCAM-120 is a GPI-anchored isoform and NCAM-180 is a transmembrane isoform. Besides the membrane-associated isoforms, NCAM can also exist in soluble form with various MWs ranging from 180 to100 kDa. Thus, it can be predicted that NCAM-87, NCAM-92, and NCAM-96 are in soluble form. To confirm this, Western blot analysis were performed on the deglycosylated NCAM using the anti-NCAM cytoplasmic domain antibody. Only one isoform with an apparent MW of 160 kDa, which is NCAM-180, was detected (Fig. 4B). As expected, other isoforms showed no immunoreactivity to the antibody and therefore do not include an intracellular domain.
It has been reported that the anti-NCAM extracellular domain antibody shows the same binding ability to all NCAM isoforms.60 The lanes in Figure 4A were scanned and the intensity of each band was profiled using software (ImageQuant TL; GE Health Care Biosciences Corp.) as shown in Figure 4C. The distribution of the NCAM isoforms on each lane was determined as shown in Figure 4D. All NCAM isoforms showed developmental changes resembling their polysialylated forms, as shown in Figure 2A. NCAM-120 is the most abundant isoform, constituting 90% of the total NCAM isoforms at E7, with amounts greater than 70% during later developmental and postnatal stages (Fig. 4D). The relative amount of NCAM-180 increased at early embryonic stages, decreased after E14, and lasted until undetectable in the 20 weeks adult cornea. The proportion of NCAM-92 increased slightly during embryonic stages and in postnatal corneas.
Localization of NCAM by Immunostaining in Chick Corneas
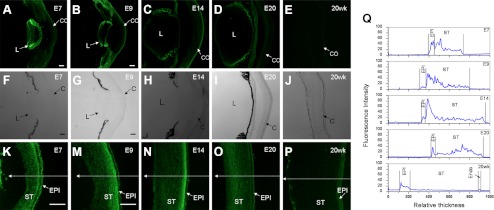
To localize NCAM in chick corneas, immunofluorescence staining was performed on frozen sections of E7, E9, E14, E20, and 20 weeks adult corneas. These developmental ages were selected due to their differential NCAM expression. NCAM was detected in the epithelium and stroma of embryonic corneas (Figs. 5K, 5M–O), and in the epithelium of embryonic lens (Figs. 5A–D). However, in 20 weeks adult cornea, NCAM was only found in the epithelium and endothelium (Fig. 5P). The fluorescence intensities across the anterior-posterior axis of corneas (along the arrows as shown in Figs. 5K, 5M–P) were profiled as in Figure 5Q. The continuous and high fluorescence intensity across the embryonic corneal stroma suggests that NCAM was expressed in the extracellular matrix (ECM) of the embryonic corneal stroma. This is consistent with the previous Western blot results indicating soluble NCAM isoforms in embryonic corneas. We also noticed a difference in the fluorescence intensities of the anterior versus the posterior stroma during embryonic development (Fig. 5Q). During early embryonic development stages from E7 to E14, NCAM is highly expressed in the anterior stroma with a decreased expression in epithelium from E7 to E9. By E20, higher amounts of NCAM were detected in posterior stroma cornea. No NCAM was found in 20 weeks adult stroma. Overall, NCAM is present in both embryonic and adult corneal epithelium. NCAM expression did not show obvious change in epithelial presence during embryonic and postnatal development. In the stroma, NCAM seems to shift from the anterior stroma to posterior stroma during embryonic development and disappear eventually in adult corneal stroma.
Figure 5.
Immunofluorescence staining on frozen sections of embryonic (E7, E9, E14, and E20) anterior eye fronts (A–D) or corneas (K, M–O) and adult cornea (20 weeks) (E, P) using the anti-NCAM extracellular domain antibody. The phase contrast images of the anterior eye fronts and corneas are shown in (F–J). All immunostaining images were taken under the same conditions. The fluorescence intensities along the arrows across the corneas in (K, M–P) were profiled using ImageJ as shown in (Q). NCAM was highly expressed in anterior stroma during early embryonic stages from E7 to E14. The expression shifted from anterior stroma to posterior stroma in E20 cornea and was undetectable in both locations in 20 weeks cornea. CO, cornea; Endo, endothelium; EPI, epithelium; L, lens; ST, stroma.
Localization of NCAM and PolySia to Corneal Nerves
To determine whether NCAM is expressed on corneal and pericorneal nerves, whole-mount immunofluorescence stainings for NCAM and polySia were performed on corneas and the surrounding periocular mesenchyme as well as isolated neurons from the ophthalmic lobe of the trigeminal ganglion (OTG) of E9 and E14 embryos.
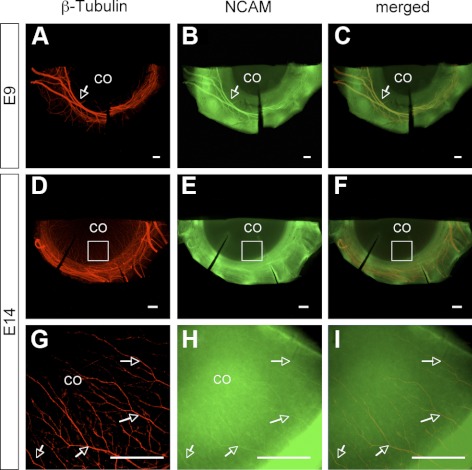
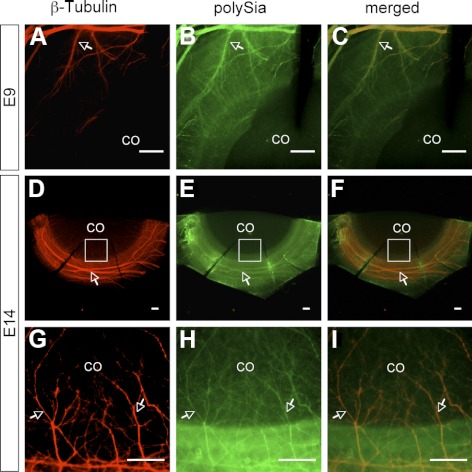
Corneas of E9 and E14 embryos were doubly-stained with antibodies against neuronal-specific class III β-tubulin and NCAM extracellular domain or polySia-NCAM. On E9, trigeminal sensory nerve bundles formed a ring around the cornea and started to invade the cornea (Fig. 6A). By E14, corneal nerves extended from the ring of nerve bundles around the cornea and migrated into the stroma along the whole circumference of the cornea (Fig. 6D). The colocalization of NCAM and β-tubulin in E9 (Figs. 6A–C) and E14 (Figs. 6G–I) corneas suggests NCAM is expressed on the surface of corneal and pericorneal nerves. The high fluorescent intensity in cornea obtained from immunofluorescence staining for NCAM (Fig. 6H) also reveals its existence in corneal epithelium and/or stroma (i.e., at sites other than corneal nerves), which is consistent with immunofluorescence staining results obtained on frozen sections (Fig. 5), indicating the presence of NCAM in epithelium and stroma. Similar results were obtained from the double immunostaining for β-tubulin and polySia. PolySia was detected on pericorneal nerve bundles and corneal nerves of both E9 (Figs. 7A–C) and E14 (Figs. 7D–I) embryos.
Figure 6.
Double immunofluorescence staining for β-tubulin and NCAM in E9 (A–C) and E14 (D–I) corneas. Immunofluorescence staining for β-tubulin (A), NCAM (B), and the merged image (C) in E9 cornea suggests the localization of NCAM to trigeminal sensory nerve bundles shortly before their invasion into cornea. Immunofluorescence staining on E14 for β-tubulin (D, G), NCAM (E, H), and the merged images (F, I) revealed the expression of NCAM on both corneal and pericorneal nerves. The high-magnification image of immunofluorescence staining for NCAM in E14 cornea (H) also showed high fluorescent intensity in cornea aside from corneal nerves indicating the expression of NCAM in epithelium and/or stroma, consistent with the results obtained using cryosections as shown in Figure 5. Arrows show NCAM staining on corneal nerves and pericorneal nerves.
Figure 7.
Double immunofluorescence staining for β-tubulin and polySia in E9 (A–C) and E14 (D–I) corneas. The colocalization of polySia and β-tubulin indicates the expression of polySia on embryonic corneal and pericorneal nerves. Arrows indicate the colocalization of β-tubulin and polySia on corneal and pericorneal nerves.
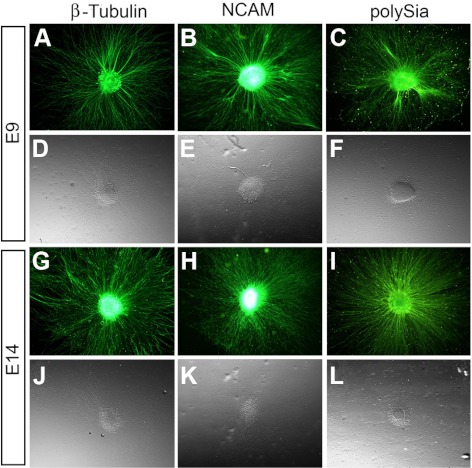
The majority of sensory nerves associated with the cornea are derived from the OTG.1,65,66 To confirm the expression of NCAM and polySia on nerves associated with the cornea and pericorneal nerve ring, we isolated explanted pieces of tissue from the OTG of E9 and E14 chick embryos and cultured them under conditions which promote neurite outgrowth. After 2 days in culture, E9 and E14 trigeminal neurons were immunostained with antineuronal-specific class III β-tubulin antibody, showing that neurites grew out radially in all directions from the explant (Figs. 8A, 8D, 8G, 8J). Next, we examined the expression of NCAM and polySia on trigeminal neurons after 2 days in culture. The results indicate the persistent expression of NCAM and polySia on the trigeminal neuronal cell bodies and all along their neurite extensions at both E9 (Figs. 8B, 8C, 8E, 8F) and E14 (Figs. 8H, 8I, 8K, 8L). These data support our findings from whole-mount corneas stained for NCAM and polySia to further suggest that nerves associated with the pericorneal ring and within the cornea express NCAM and polySia.
Figure 8.
NCAM and polySia expression on OTG neurons. (A–C) Cultures of explanted tissue from E9 OTG showing radial neurite outgrowth are positively labeled with antibodies for the neuronal marker β-tubulin (A) and also NCAM (B) and polySia (C). (D–F) Phase contrast images are shown directly below their accompanying fluorescent image for each antibody staining. (G–I) Staining for β-tubulin (G), NCAM (H), and polySia (I) on OTG explants harvested at a later stage, E14, remain positive for each antibody. (J–L) Phase contrast images are shown below their accompanying fluorescent image.
Discussion
In the present work, the expression and localization of NCAM and polySia in chick corneas and corneal nerves were investigated during embryonic and postnatal development.
Our work shows, for the first time, that NCAM is present in developing chick corneas and its expression and polysialylation are developmentally regulated in embryonic and postnatal chick corneas.
Both NCAM mRNA (Fig. 1) and protein (Fig. 2) showed increased expression levels from early developmental stage (E6/7) to E9 and maintained the high expression level from E9 to E14, coincident with the timing when trigeminal nerves start to invade the cornea at points around its entire circumference and migrate into the entire area of the cornea.3 The high amounts of NCAM in the cornea at these developmental stages might be related to corneal innervation.
ST8SiaII and ST8SiaIV mRNA showed very different expression patterns in embryonic and adult chick corneas. ST8SiaII becomes downregulated from embryonic stages to adulthood. However, in the adult corneas, ST8SiaIV showed a distinct regulation pattern and is the predominate polysialyltransferase. Hildebrandt et al.67 reported similar regulation for ST8SiaII and ST8SiaIV during rat brain development. ST8SiaII dominates during embryonic development and is undetectable in 6-month-old postnatal brains, whereas ST8SiaIV persists at relatively high levels in postnatal rat brains. They did not propose the mechanism of the upregulation of ST8SiaIV in mature rat brains. However, they localized the persistent ST8SiaIV expression in the subependymal layer, the glomerular layer of the olfactory bulb, the granule cell layer of the dentate gyrus, and in some widely dispersed cells of the isocortex where the polysialylated NCAM is expressed in postnatal brains. Here, in the chick cornea, the downregulation of NCAM and ST8SiaII mRNA expression and upregulation of ST8SiaIV mRNA expression in adult cornea imply that polySia is persistently expressed in adult corneas. The Western blot analysis using anti-polySia-NCAM antibody confirmed the presence of polySia in 20 weeks adult corneas (Fig. 3). The analysis also reveals that there are other polysialylated proteins besides NCAM in chick corneas. The persistent expression of polySia in adult corneas may be related to postnatal corneal plasticity.
Another contribution of this work is that we identified the NCAM isoforms in chick corneas. Membrane-associated isoforms, NCAM-120 and NCAM-180, and three soluble NCAMs were identified in the chick cornea, with NCAM-120 being the predominate isoform (Fig. 4). The membrane-associated NCAM isoforms arise from the alternative splicing of a single gene.68 However, the mechanism by which NCAM isoforms are generated is not clear. It has been reported that the expression of NCAM isoforms is developmentally regulated in the chick brain. NCAM-140 was expressed first followed by NCAM-180 which appeared after E3, and NCAM-120 was not detected until E14.60 Sunshine et al.60 also reported the differential expression of NCAM isoforms in the brains of frog and chick and concluded that the expression of NCAM isoforms was related to species. Rutishauser and Goridis69 suggest that the expression of the NCAM isoforms depends on cell type and age. NCAM-180 is produced by neurons, but not by muscle cells, whereas NCAM-140 is expressed by both cell types. NCAM-120 is prominent on astrocytes and muscle cells and appears later during embryonic development than other isoforms. The lack of NCAM-140 in the chick cornea and consistent expression of NCAM-120 and NCAM-180 indicates that NCAM isoform expression is tissue related. The existence of NCAM-180 in E7, before the nerve ingrowth, reveals that NCAM-180 expression is not restricted to neurons.
The NCAM isoforms have been reported to be associated with different biological functions. NCAM-120 is the most adhesive form, whereas NCAM-180 is associated with cell motility due to the interaction of its long intracellular domain with the cytoskeleton.70 The high amounts of NCAM-120 may indicate the important functions of cell adhesion in cornea. Soluble NCAMs are also involved in the peripheral nervous system development.44 In the present work, we did not determine the source of the soluble NCAMs in the chick cornea. However, we noticed that there were differential soluble NCAM isoforms during development between stages E7 and E9 (Fig. 4A) when the sensory nerves start to invade the cornea. Thus, the soluble NCAMs may be involved in nerve growth cone invasion into cornea.
Also significant in this work, is the demonstration of localization of NCAM to the embryonic corneal stroma and corneal nerves. Prior reports revealed that NCAM was expressed in both the postnatal corneal epithelium and endothelium.20,50–52 In our work, NCAM was also detected in embryonic corneal stroma and its distribution in stroma is developmentally regulated from anterior stroma to posterior stroma (Fig. 5). Particular attention should be paid to the developmental stages between E9 and E14 during which NCAM is highly expressed in anterior stroma, coincident with the location of initiation of significant corneal innervation3 and stromal development.71
NCAM can be expressed by Schwann cells, satellite cells of sensory and sympathetic ganglia of PNS.72 Inhibition of polySia in chicken sensory neuron cultures increases the thickness of neurite bundles and reduces neurite outgrowth.14 PolySia on NCAM can reduce the axon-axon interaction and increase nerve branching10,73,74 and promote neuronal regeneration.14,16,75 In the present work, both NCAM and polySia are highly expressed on corneal and pericorneal nerves of embryos (Figs. 6, 7, 8) suggesting their potential functions in corneal nerve development and regeneration.
In summary, NCAM and polySia are expressed and developmentally regulated in the chick cornea. Our results also showed there are other proteins that carry polySia in the chick cornea. NCAM-120, NCAM-180, and three soluble NCAMs were found in chick corneas while NCAM-120 is the predominate isoform. NCAM was localized in the whole embryonic cornea including the corneal epithelium and stroma, and only in the epithelium and endothelium of the adult chick cornea. Both NCAM and polySia were detected on embryonic pericorneal and corneal nerves. In full, the expression, regulation, and localization of NCAM and polySia suggest their possible biological functions in sensory nerve innervation and development of the chick cornea.
Acknowledgments
The authors thank Stella Lee for the access to an MZ16F Leica epifluorescent microscope.
Footnotes
Supported by Grants NIH 5RO1EYE952–38 (GWC) and F32EY021708 (TS) awarded by the National Eye Institute, National Institutes of Health.
Disclosure: X. Mao, None; T. Schwend, None; G.W. Conrad, None
References
- 1. Lwigale PY. Embryonic origin of avian corneal sensory nerves. Dev Biol. 2001;239:323–337 [DOI] [PubMed] [Google Scholar]
- 2. Clarke ND, Bee JA. Innervation of the chick cornea analyzed in vitro. Invest Ophthalmol Vis Sci. 1996;37:1761–1771 [PubMed] [Google Scholar]
- 3. Lwigale PY, Bronner-Fraser M. Lens-derived Semaphorin3A regulates sensory innervation of the cornea. Dev Biol. 2007;306:750–759 [DOI] [PubMed] [Google Scholar]
- 4. Riley NC, Lwigale PY, Conrad GW. Specificity of corneal nerve positions during embryogenesis. Mol Vis. 2001;7:297–304 [PubMed] [Google Scholar]
- 5. Kubilus JK, Linsenmayer TF. Developmental corneal innervation: interactions between nerves and specialized apical corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:782–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conrad AH, Strafuss JM, Wittman MD, Conway S, Conrad GW. Thyroxine increases the rate but does not alter the pattern of innervation during embryonic chick corneal development. Invest Ophthalmol Vis Sci. 2008;49:139–153 [DOI] [PubMed] [Google Scholar]
- 7. Kubilus JK, Linsenmayer TF. Developmental guidance of embryonic corneal innervation: Roles of Semaphorin3A and Slit2. Dev Biol. 2010;344:172–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lwigale PY, Bronner-Fraser M. Lens regulates sensory innervation of the cornea via Semaphorin3A. Dev Biol. 2007;306:294–295 [DOI] [PubMed] [Google Scholar]
- 9. Schwend T, Lwigale PY, Conrad GW. Nerve repulsion by the lens and cornea during cornea innervation is dependent on Robo-Slit signaling and diminishes with neuron age. Dev Biol. 2012;363:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Landmesser L, Dahm L, Tang JC, Rutishauser U. Polysialic acid as a regulator of intramuscular nerve branching during embryonic-development. Neuron. 1990;4:655–667 [DOI] [PubMed] [Google Scholar]
- 11. Seki T, Arai Y. Distribution and possible roles of the highly polysialylated neural cell-adhesion molecule (Ncam-H) in the developing and adult central nervous system. Neurosci Res. 1993;17:265–290 [DOI] [PubMed] [Google Scholar]
- 12. Hildebrandt H, Muhlenhoff M, Weinhold B, Gerardy-Schahn R. Dissecting polysialic acid and NCAM functions in brain development. J Neurochem. 2007;103:56–64 [DOI] [PubMed] [Google Scholar]
- 13. Monnier PP, Beck SGM, Bolz J, Henke-Fahle S. The polysialic acid moiety of the neural cell adhesion molecule is involved in intraretinal guidance of retinal ganglion cell axons. Dev Biol. 2001;229:1–14 [DOI] [PubMed] [Google Scholar]
- 14. Jungnickel J, Bramer C, Bronzlik P, et al. Level and localization of polysialic acid is critical for early peripheral nerve regeneration. Mol Cell Neurosci. 2009;40:374–381 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Yeh J, Richardson PM, Bo XN. Cell adhesion molecules of the immunoglobulin superfamily in axonal regeneration and neural repair. Restor Neurol Neurosci. 2008;26:81–96 [PubMed] [Google Scholar]
- 16. Mehanna A, Mishra B, Kurschat N, et al. Polysialic acid glycomimetics promote myelination and functional recovery after peripheral nerve injury in mice. Brain. 2009;132:1449–1462 [DOI] [PubMed] [Google Scholar]
- 17. Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9:26–35 [DOI] [PubMed] [Google Scholar]
- 18. Ronn LCB, Hartz BP, Bock E. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol. 1998;33:853–864 [DOI] [PubMed] [Google Scholar]
- 19. Kulahin N, Walmod PS. The neural cell adhesion molecule NCAM2/OCAM/RNCAM, a close relative to NCAM. In: Berezin V, ed. Structure and Function of the Neural Cell Adhesion Molecule NCAM. New York, Springer; 2010;403–420 [DOI] [PubMed] [Google Scholar]
- 20. Filiz S, Dalcik H, Yardimoglu M, Gonca S, Ceylan S. Localization of neural cell adhesion molecule (N-CAM) immunoreactivity in adult rat tissues. Biotech Histochem. 2002;77:127–135 [PubMed] [Google Scholar]
- 21. Dalseg AM, Linnemann D, Bock E. Soluble neural cell-adhesion molecule in brain, cerebrospinal fluid and plasma in the developing rat. Int J Dev Neurosci. 1989;7:209–217 [DOI] [PubMed] [Google Scholar]
- 22. Krog L, Olsen M, Dalseg AM, Roth J, Bock E. Characterization of soluble neural cell-adhesion molecule in rat brain, CSF, and plasma. J Neurochem. 1992;59:838–847 [DOI] [PubMed] [Google Scholar]
- 23. Nybroe O, Linnemann D, Bock E. Heterogeneity of soluble neural cell-adhesion molecule. J Neurochem. 1989;53:1372–1378 [DOI] [PubMed] [Google Scholar]
- 24. Ibsen S, Berezin V, Norgaardpedersen B, Bock E. Quantification of the D2-glycoprotein in amniotic fluid and serum from pregnancies with fetal neural tube defects. J Neurochem. 1983;41:363–366 [DOI] [PubMed] [Google Scholar]
- 25. Rutishauser U, Thiery JP, Brackenbury R, Sela BA, Edelman GM. Mechanisms of adhesion among cells from neural tissues of chick embryo. Proc Natl Acad Sci USA. 1976;73:577–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gower HJ, Barton CH, Elsom VL, et al. Alternative splicing generates a secreted form of N-Cam in muscle and brain. Cell. 1988;55:955–964 [DOI] [PubMed] [Google Scholar]
- 27. Olsen M, Krog L, Edvardsen K, Skovgaard LT, Bock E. Intact transmembrane isoforms of the neural cell-adhesion molecule are released from the plasma-membrane. Biochem J. 1993;295:833–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diestel S, Hinkle CL, Schmitz B, Maness PF. NCAM140 stimulates integrin-dependent cell migration by ectodomain shedding. J Neurochem. 2005;95:1777–1784 [DOI] [PubMed] [Google Scholar]
- 29. Hinkle CL, Diestel S, Lieberman J, Maness PF. Metalloprotease-induced ectodomain shedding of neural cell adhesion molecule (NCAM). J Neurobiol. 2006;66:1378–1395 [DOI] [PubMed] [Google Scholar]
- 30. Hubschmann MV, Skladchikova G, Bock E, Berezin V. Neural cell adhesion molecule function is regulated by metalloproteinase-mediated ectodomain release. J Neurosci Res. 2005;80:826–837 [DOI] [PubMed] [Google Scholar]
- 31. Kalus I, Bormann U, Mzoughi M, Schachner M, Kleene R. Proteolytic cleavage of the neural cell adhesion molecule by ADAM17/TACE is involved in neurite outgrowth. J Neurochem. 2006;98:78–88 [DOI] [PubMed] [Google Scholar]
- 32. Toikka J, Aalto J, Hayrinen J, Pelliniemi LJ, Finne J. The polysialic acid units of the neural cell adhesion molecule N-CAM form filament bundle networks. J Biol Chem. 1998;273:28557–28559 [DOI] [PubMed] [Google Scholar]
- 33. Nakata D, Troy FA. Degree of polymerization (DP) of polysialic acid (PolySia) on neural cell adhesion molecules (N-CAMs)-Development and application of a new strategy to accurately determine the DP of polySia chains on N-CAMs. J Biol Chem. 2005;280:38305–38316 [DOI] [PubMed] [Google Scholar]
- 34. Bruses JL, Rutishauser U. Roles, regulation, and mechanism of polysialic acid function during neural development. Biochimie (Paris). 2001;83:635–643 [DOI] [PubMed] [Google Scholar]
- 35. Johnson CP, Fujimoto I, Rutishauser U, Leckband DE. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J Biol Chem. 2005;280:137–145 [DOI] [PubMed] [Google Scholar]
- 36. Fujimoto I, Bruses JL, Rutishauser U. Regulation of cell adhesion by polysialic acid. J Biol Chem. 2001;276:31745–31751 [DOI] [PubMed] [Google Scholar]
- 37. Gluer S, Schelp C, Madry N, von Schweinitz D, Eckhardt M, Gerardy-Schahn R. Serum polysialylated neural cell adhesion molecule in childhood neuroblastoma. Br J Cancer. 1998;78:106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hildebrandt H, Becker C, Gluer S, Rosner H, Gerardy-Schahn R, Rahmann H. Polysialic acid on the neural cell adhesion molecule correlates with expression of polysialyltransferases and promotes neuroblastoma cell growth. Cancer Res. 1998;58:779–784 [PubMed] [Google Scholar]
- 39. Michalides R, Kwa B, Springall D, et al. NCAM and lung cancer. Int J Cancer Suppl. 1994;8:34–37 [DOI] [PubMed] [Google Scholar]
- 40. Durbec P, Cremer H. Revisiting the function of PSA-NCAM in the nervous system. Mol Neurobiol. 2001;24:53–64 [DOI] [PubMed] [Google Scholar]
- 41. Gascon E, Vutskits L, Kiss JZ. Polysialic acid-neural cell adhesion molecule in brain plasticity: from synapses to integration of new neurons. Brain Res Rev. 2007;56:101–118 [DOI] [PubMed] [Google Scholar]
- 42. Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164 [DOI] [PubMed] [Google Scholar]
- 43. Durbec P, Gennarini G, Goridis C, Rougon G. A soluble form of the F3 neuronal cell-adhesion molecule promotes neurite outgrowth. J Cell Biol. 1992;117:877–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomaidou D, Coquillat D, Meintanis S, Noda M, Rougon G, Matsas R. Soluble forms of NCAM and F3 neuronal cell adhesion molecules promote Schwann cell migration: identification of protein tyrosine phosphatases zeta/beta as the putative F3 receptors on Schwann cells. J Neurochem. 2001;78:767–778 [DOI] [PubMed] [Google Scholar]
- 45. Thiery JP, Duband JL, Rutishauser U, Edelman GM. Cell-adhesion molecules in early chicken embryogenesis. Proc Natl Acad Sci USA. 1982;79:6737–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rothbard JB, Brackenbury R, Cunningham BA, Edelman GM. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982;257:1064–1069 [PubMed] [Google Scholar]
- 47. Langley OK, Ghandour MS, Gombos G, Hirn M, Goridis C. Monoclonal-antibodies as neural cell-surface markers. Neurochem Res. 1982;7:349–362 [DOI] [PubMed] [Google Scholar]
- 48. Balak K, Jacobson M, Sunshine J, Rutishauser U. Neural cell-adhesion molecule expression in Xenopus embryos. Dev Biol. 1987;119:540–550 [DOI] [PubMed] [Google Scholar]
- 49. Rosner H, Moller W, Wassermann T, Mihatsch J, Blum M. Attenuation of actinomyosinII contractile activity in growth cones accelerates filopodia-guided and microtubule-based neurite elongation. Brain Res. 2007;1176:1–10 [DOI] [PubMed] [Google Scholar]
- 50. Liu P, Johnson RL. Lmx1b is required for murine trabecular meshwork formation and for maintenance of corneal transparency. Dev Dyn. 2010;239:2161–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Foets BJJ, Vandenoord JJ, Volpes R, Missotten L. In situ immunohistochemical analysis of cell-adhesion molecules on human corneal endothelial cells. Br J Ophthalmol. 1992;76:205–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Foets B, Vandenoord J, Engelmann K, Missotten L. A comparative immunohistochemical study of human corneotrabecular tissue. Graefes Arch Clin Exp Ophthalmol. 1992;230:269–274 [DOI] [PubMed] [Google Scholar]
- 53. Conrad AH, Albrecht M, Pettit-Scott M, Conrad GW. Embryonic corneal Schwann cells express some Schwann cell marker mRNAs, but no mature Schwann cell marker proteins. Invest Ophthalmol Vis Sci. 2009;50:4173–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Koo SJ, Clark-Alderfer JD, Tanaka H, et al. Species-specific immunostaining of embryonic corneal nerves: techniques for inactivating endogenous peroxidases and demonstration of lateral diffusion of antibodies in the plane of the corneal stroma. J Neurosci Methods. 1998;85:63–71 [DOI] [PubMed] [Google Scholar]
- 55. Dillon TE, Saldanha J, Giger R, Verhaagen J, Rochlin MW. Sema3A regulates the timing of target contact by cranial sensory axons. J Comp Neurol. 2004;470:13–24 [DOI] [PubMed] [Google Scholar]
- 56. Angata K, Suzuki M, McAuliffe J, Ding YL, Hindsgaul O, Fukuda M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide accepters by three distinct alpha 2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J Biol Chem. 2000;275:18594–18601 [DOI] [PubMed] [Google Scholar]
- 57. Franceschini I, Angata K, Ong E, Hong A, Doherty P, Fukuda M. Polysialyltransferase ST8Sia II (STX) polysialylates all of the major isoforms of NCAM and facilitates neurite outgrowth. Glycobiology. 2001;11:231–239 [DOI] [PubMed] [Google Scholar]
- 58. Angata K, Suzuki M, Fukuda M. Differential and cooperative polysialylation of the neural cell adhesion molecule by two polysialyltransferases, PST and STX. J Biol Chem. 1998;273:28524–28532 [DOI] [PubMed] [Google Scholar]
- 59. Angata K, Fukuda M. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie (Paris). 2003;85:195–206 [DOI] [PubMed] [Google Scholar]
- 60. Sunshine J, Balak K, Rutishauser U, Jacobson M. Changes in neural cell-adhesion molecule (NCAM) structure during vertebrate neural development. Proc Natl Acad Sci USA. 1987;84:5986–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Geyer H, Bahr U, Liedtke S, Schachner M, Geyer R. Core structures of polysialylated glycans present in neural cell adhesion molecule from newborn mouse brain. Eur J Biochem. 2001;268:6587–6599 [DOI] [PubMed] [Google Scholar]
- 62. Hirn M, Ghandour MS, Deagostinibazin H, Goridis C. Molecular heterogeneity and structural evolution during cerebellar ontogeny detected by monoclonal antibody of the mouse cell surface antigen Bsp-2. Brain Res. 1983;265:87–100 [DOI] [PubMed] [Google Scholar]
- 63. Gennarini G, Rougon G, Deagostinibazin H, Hirn M, Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM - a monoclonal antibody recognizing a cytoplasmic domain and evidence for the presence of phosphoserine residues. Eur J Biochem. 1984;142:57–64 [DOI] [PubMed] [Google Scholar]
- 64. Edelman GM, Chuong CM. Embryonic to adult conversion of neural cell-adhesion molecules in normal and Staggerer mice. Proc Natl Acad Sci USA. 1982;79:7036–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morgan CW, Nadelhaft I, Groat WCD. Anatomical localization of corneal afferent cells in trigeminal ganglion. Neurosurgery. 1978;2:252–258 [DOI] [PubMed] [Google Scholar]
- 66. Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea - a retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30:461–472 [PubMed] [Google Scholar]
- 67. Hildebrandt H, Becker C, Murau M, Gerardy-Schahn R, Rahmann H. Heterogeneous expression of the polysialyltransferases ST8Sia II and ST8Sia IV during postnatal rat brain development. J Neurochem. 1998;71:2339–2348 [DOI] [PubMed] [Google Scholar]
- 68. Reyes AA, Small SJ, Akeson R. At least 27 alternatively spliced forms of the neural cell-adhesion molecule messenger-RNA are expressed during rat heart development. Mol Cell Biol. 1991;11:1654–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rutishauser U, Goridis C. NCAM - the molecule and its genetics. Trends Genet. 1986;2:72–76 [Google Scholar]
- 70. Winter C, Pawel B, Seiser E, et al. Neural cell adhesion molecule (NCAM) isoform expression is associated with neuroblastoma differentiation status. Pediatr Blood Cancer. 2008;51:10–16 [DOI] [PubMed] [Google Scholar]
- 71. Quantock AJ, Young RD. Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn. 2008;237:2607–2621 [DOI] [PubMed] [Google Scholar]
- 72. Mirsky R, Jessen KR, Schachner M, Goridis C. Distribution of the adhesion molecules N-CAM and L1 on peripheral neurons and glia in adult rats. J Neurocytol. 1986;15:799–815 [DOI] [PubMed] [Google Scholar]
- 73. Landmesser L, Dahm L, Schultz K, Rutishauser U. Distinct roles for adhesion molecules during innervation of embryonic chick muscle. Dev Biol. 1988;130:645–670 [DOI] [PubMed] [Google Scholar]
- 74. Rutishauser U, Landmesser L. Polysialic acid on the surface of axons regulates patterns of normal and activity-dependent innervation. Trends Neurosci. 1991;14:528–532 [DOI] [PubMed] [Google Scholar]
- 75. Franz CK, Rutishauser U, Rafuse VF. Polysialylated neural cell adhesion molecule is necessary for selective targeting of regenerating motor neurons. J Neurosci. 2005;25:2081–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]