This study showed that VEGF-C may potentially serve as an important target in corneal transplant by modulating corneal alloimmunity at three different levels. Anti-VEGF-C inhibits the ingrowth of lymphatic and blood vessel, reduces the trafficking of APC, and, interestingly, inhibits APC maturation.
Abstract
Purpose.
To investigate the role of anti–vascular endothelial growth factor (VEGF)-C therapy in corneal graft survival and concomitant suppression of hem- and lymph-angiogenesis.
Methods.
Corneal suture model in BALB/c mice was placed and immunohistochemical staining was performed with CD31/PECAM-1 and LYVE-1 to quantify the level of blood and lymphatic vessels. Corneal transplants were done in BALB/c mice from C57BL/6 mice donors; grafts were subsequently scored for opacity. VEGF-C was blocked in the angiogenesis and transplant model using neutralizing monoclonal anti-VEGF-C (VGX-100) by intraperitoneal injection. To determine the function of VEGF-C in maturation of antigen-presenting cells (APCs), bone marrow–derived dendritic cells were generated and matured in the presence or absence of VEGF-C.
Results.
VEGF-C expression was demonstrated to be markedly upregulated in corneal graft rejection. VEGF-C blockade, through administration of a VEGF-C blocking monoclonal antibody, suppresses corneal angiogenic responses, inhibits trafficking and maturation of APCs, and significantly improves allotransplant survival.
Conclusions.
These data suggest VEGF-C as a potentially important target in corneal transplant pharmacotherapy and immunobiology.
The Eye Bank Association of America reports that >40,000 corneal transplants are performed annually in the United States.1 However, survival of corneal transplants is severely compromised when grafts are placed in vascularized and inflamed so-called high risk host beds.2–5 In fact, recipient vascularization has been identified as a critical proximal cause for early and fulminant rejection episodes in corneal transplantation.4–7 Moreover, postoperative growth of blood and lymphatic vessels into the avascular recipient bed is a strong promoter of subsequent immune rejection, even in “normal risk” corneal transplants.8 Ingrowth of lymphatic neovessels enables efficient access of donor and host antigen-presenting cells (APCs) and antigenic material to regional lymph nodes, and accelerates host sensitization to graft antigens.3 Therefore, suppression of neovascularization in the setting of corneal transplantation has been a core area for many investigators interested in the immunobiology of corneal transplantation.
Members of the vascular endothelial growth factor (VEGF) family are critical modulators of endothelial cell proliferation and migration,9–11 and are key regulators of angiogenesis through three receptors (VEGFRs) including: VEGFR-1 (Flt-1), VEGFR-2 (KDR), and VEGFR-3 (Flt-4).12 Given the potent proangiogenic functions of VEGF-A, its blockade has been widely adopted to inhibit pathologic angiogenesis.13–15 Bevacizumab, a recombinant humanized monoclonal antibody approved by the U.S. Food and Drug Administration as a first-line treatment for metastatic colorectal cancer, inhibits angiogenesis by blocking VEGF-A binding to its receptors, VEGFR-1 and VEGFR-2.16 Lymphangiogenesis, however, is considered to be regulated by different members of the VEGF family, that is, VEGF-C and VEGF-D, through their high-affinity binding to VEGFR-3.17,18 However, it is noteworthy that proteolytically processed VEGF-C binds to VEGFR-2 and subsequently induces hemangiogenesis in the cornea.19,20 However, there are sparse data evaluating the expression levels of individual members of VEGF ligands and receptors, and their function in particular in the context of immunity, after corneal transplantation.
Failure of the immune privileged state of the cornea as a result of heme- and lymph- angiogenesis is associated with a significant deterioration of graft outcome.21 Here, we hypothesized that anti-VEGF-C therapy improves corneal graft survival by concomitant suppression of hem- and lymph-angiogenesis and alloimmune responses. Our data demonstrate that VEGF-C blockade, through administration of a VEGF-C blocking monoclonal antibody, suppresses corneal angiogenic response, inhibits trafficking and maturation of APCs, and significantly improves transplant survival.
Materials and Methods
Animals
Male, 6- to 8-week old, BALB/c or C57BL/6 mice were commercially purchased (Taconic Farms, Germantown, NY). Animals were anesthetized with intraperitoneal injection of ketamine (120 mg/kg) and xylazine (20 mg/kg) before any surgery and were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments described herein were conducted under institutional animal care and use committee approval.
Suture-Induced Angiogenesis
To quantify the effect of anti-VEGF-C therapy on corneal lymph and blood vessel formation, a corneal suture model was used as described previously. In brief, three stromal interrupted 11-0 sutures were inserted in the cornea of BALB/c mice and left in place for 1 week. This procedure induces inflammatory corneal neovascularization, associated with noticeable lymphangiogenesis.22 After 7 days, five mice per group were euthanized and enucleated eyes were prepared into corneal flat mounts.
Orthotopic Corneal Transplantation
Orthotopic penetration23 was performed as described previously. Briefly, donor corneas (central 2-mm diameter) were excised from C57BL/6 mice, using laboratory microscissors (Vannas; Storz Instruments, El Segundo, CA) and placed in commercial storage media (Optisol GS, College Station, TX). The recipient graft bed was prepared by excising a 1.5-mm site in the central cornea of BALB/c hosts. The donor button was then placed onto the recipient bed and secured with eight interrupted 11-0 nylon sutures. Antibiotic ointment was placed on the corneal surface and the eyelids were closed with an 8-0 suture. Grafts were examined twice a week for 8 weeks by slit-lamp microscopy and scored for opacity, as described previously.23Grafts were defined as rejected when they became opaque and iris details could not be recognized clearly using a standardized opacity-grading (ranges, 0–5) scheme.
Immunohistochemistry and Morphometry
Immunohistochemical staining was performed as described previously.24 Briefly, corneas from either suture-induced angiogenesis or transplanted mice were excised and fixed with ethanol for 5 minutes at room temperature. Staining against FITC-conjugated rat anti-mouse antibody (CD31/PECAM-1; Santa Cruz Biotechnology, Santa Cruz, CA) and goat anti-rabbit antibody (lymphatic endothelium-specific hyaluronic acid receptor [LYVE-1]; Abcam, Cambridge, MA) was performed with purified antibody followed by rhodamine-conjugated secondary antibody. To quantify the level of blood (CD31high/LYVE-1low) and lymphatic (CD31low/LYVE-1high) vessel formation, low magnification (×2) micrographs were captured, and the area covered by vessels was measured (ImageJ software 1.34, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) and expressed as the percentage of total corneal area.
VEGF-C Neutralization with Blocking Antibody
VEGF-C was blocked using neutralizing monoclonal anti-VEGF-C (VGX-100).25 VGX-100 was commercially supplied (Vegenics; Circadian Technologies Ltd, Melbourne, Australia), based on a signed material transfer agreement; and according to the manufacturer, VGX-100 binds to and precipitates all forms of VEGF-C including full-length, partially processed, and mature forms in both the human and mouse. To block VEGF-C, 20 mg/kg (in total 0.1 mL volume) of VGX-100 was injected from the day before surgery, through the intraperitoneal route and on alternate days subsequently up to 2 weeks. Control mice received 0.1 mL of normal saline intraperitoneally.
Quantitative Real-Time PCR
RNA was isolated from normal, accepted, and rejected corneas (RNeasy Micro Kit; Qiagen, Valencia, CA) and reverse transcribed (Superscript III Kit; Invitrogen Life Technologies, Carlsbad, CA). Quantitative real-time PCR (qRT-PCR) was performed (TaqMan Universal PCR MasterMix; Applied Biosystems, Foster City, CA) and primers (Applied Biosystems) were preformulated for VEGF-A (assay ID: Mm00437304_ml), VEGF-C (assay ID: Mm00437313_ml), VEGF-D (assay ID: Mm00438965_ml), VEGFR-1 (assay ID: Mm00438994_m1), VEGFR-2 (assay ID: Mm00440086_m1), VEGFR-3 (assay ID: Mm00433337_ml), LYVE-1 (assay ID: Mm00475056_m1), and GAPDH (assay ID: Mm99999915_gl). Results were derived from the comparative threshold cycle method and normalized by GAPDH as an internal control. Values obtained from normal corneas were then used to calculate relative mRNA expression for experimental corneas.
Generation and Treatment of Bone Marrow–Derived Dendritic Cells
To determine the function of VEGF-C in maturation of APCs, we generated bone marrow–derived dendritic cells (DCs), as described previously, and matured them in the presence or absence of VEGF-C.26 In brief, 6- to 8-week-old mice were euthanized and femurs were isolated. Bone marrow cells were collected by flushing the femurs with 28G syringes and were seeded (2 × 106) in 10 mL petri dishes of cultured medium (RPMI-1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, 50 mM 2-ME) containing 20 ng/mL of granulocyte-macrophage colony-stimulating factor (peproTech, Rocky Hill, NJ). Cultures were fed again on days 3 and 6 and nonadherent and loosely adherent immature DCs were collected on day 8. To induce maturation, 100 ng/mL of lipopolysaccharide (LPS) (R&D Systems, Minneapolis, MN) was added 24 hours before cell harvesting. In a separate experiment, 10 ng/mL of VEGF-C (R&D Systems) was concurrently added with LPS, 24 hours before harvesting to evaluate its effect on maturation.
Flow Cytometry
The single-cell suspensions obtained from either submandibular or cervical lymph nodes samples on days 1, 3, 7, and 14 post-transplant or bone marrow–derived DCs were blocked with anti-Fc receptor monoclonal antibody for 30 minutes at 4°C in 1% BSA/0.02% NaN3/PBS. Cells were then stained with the following antibodies for 45 minutes at 4°C: PE-conjugated anti-CD11b, FITC-conjugated anti-IAb (MHC class II of ‘donor' APCs), FITC-conjugated anti-IAd (MHC class II of ‘host' APCs), and their isotype-matched mouse PE-conjugated IgG2a and FITC-conjugated IgG2b were purchased from BD Biosciences (San Jose, CA). Finally, cells were washed and analyzed on a flow cytometer (BD LSR II Analyzer; DB Biosciences).
Statistical Analysis
Student's t-test was used for comparison of mean between the groups. Kaplan–Meier analysis was adopted to construct graft survival curves and the log-rank test was used to compare the rates of corneal graft survival. Data are presented as mean ± SEM and P < 0.05 was considered statistically significant.
Results
Angiogenesis and Expression of VEGF Ligands and Receptors after Corneal Transplant
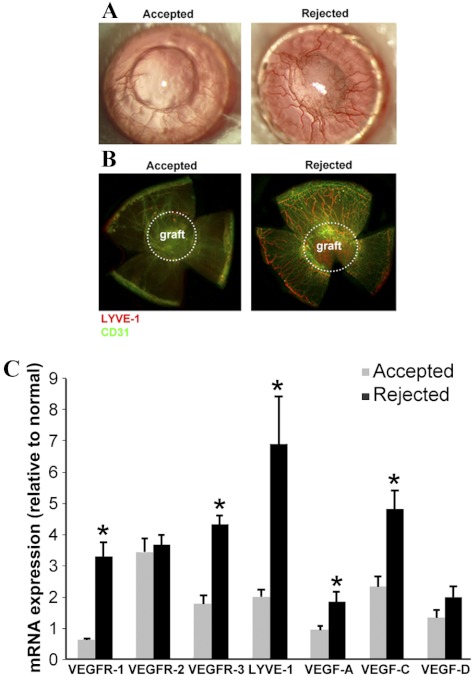
As illustrated by the color photographs taken 3 weeks after transplantation (Fig. 1A) and immunofluorescent staining of corneal flat mounts (Fig. 1B), transplant rejection was associated with extensive development of blood (CD31high/LYVE-1low) and lymphatic (CD31low/LYVE-1high) neovessels, mostly localized around the graft area. The extent of lymphatic vessel formation as well as expression levels of VEGF receptors and ligands were quantified by qRT-PCR in rejected and accepted corneas. As shown in Figure 1C, qRT-PCR results demonstrated a marked increase in RNA expression of LYVE-1 in rejected compared with accepted corneas (6.90- vs. 1.97-fold increase compared with nontransplanted corneas, P = 0.006). Expression of VEGF-A (1.84- vs. 0.95-fold increase compared with normal corneas, P = 0.020), VEGF-C (4.81- vs. 2.34-fold increase compared with normal corneas, P = 0.002), VEGFR-1 (3.28- vs. 0.63-fold increase compared with normal corneas, P = 0.0001), and VEGFR-3 (4.33- vs. 1.77-fold increase compared with normal corneas, P < 0.0001) were also markedly higher in rejected corneas compared with accepted corneas (compared with normal corneas). Despite the marked increase in the expression of VEGFR-2 in transplanted corneas, there was no significant difference in VEGFR-2 expression between accepted and rejected corneas.
Figure 1.
Rejected corneas are infiltrated by both blood and lymphatic vessels. (A) Representative photographs of accepted (left) and rejected (right) corneas at 3 weeks after transplantation demonstrating extensive neovessel formation. (B) Immunostaining of flat mounts of accepted and rejected corneas at 3 weeks after transplantation demonstrates distinct increase in hemangiogenesis (CD31high/LYVE-1low) and lymphangiogenesis (CD31low/LYVE-1high). White dotted circles define the borders of the grafted cornea. (C) mRNA levels of VEGF receptors and ligands and LYVE-1 are determined in normal, accepted, and rejected corneas, at week 3 post-transplant, by qRT-PCR. VEGF ligands (VEGF-A and -C), receptors (VEGFR-1 and R-3), and the lymphatic marker (LYVE-1) expression levels are markedly increased in rejected compared with accepted corneas. Data are expressed as the fold-change compared with normal nontransplanted corneas (defines as =1). Graphs represent mean ± SEM of five samples per group (*P < 0.05 vs. accepted group).
VEGF-C Blockade Effectively Inhibits Corneal Heme- and Lymph-Angiogenesis
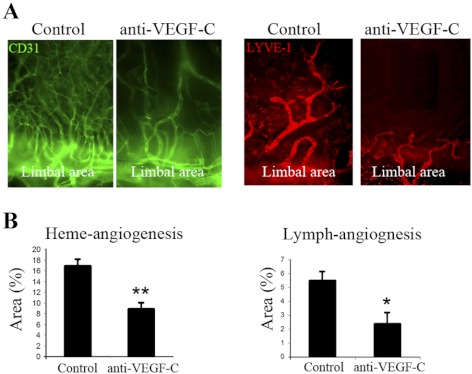
Suture placement upregulates VEGF ligand expression and induces a robust corneal inflammatory response associated with marked heme- and lymph-angiogenesis.27 To further study the in vivo function of VEGF-C on blood and lymphatic vessel growth, we investigated the effect of VEGF-C blockade on suture-induced lymph- and heme-angiogenesis. Immunostaining (Fig. 2A) and morphometric measurements at day 7 after the operation (Fig. 2B) demonstrated significant reductions in the percentage area of the cornea covered by both blood (control: 16.8 ± 0.9% vs. anti-VEGF-C–treated: 8.9 ± 1.1; P < 0.01) and lymphatic (control: 5.5 ± 0.6% vs. anti-VEGF-C–treated: 2.3 ± 0.8; P < 0.05) vessels in mice treated with anti-VEGF-C antibody.
Figure 2.
Corneal blood and lymphatic vessel growth are suppressed by VEGF-C blockade. (A) Fluorescent microscopic images (×40 magnification) of immunohistochemical staining of flat-mount corneas reveal reduced densities of blood and lymphatic vessels in anti-VEGF-C–treated mice. Growth of the vessels can be seen from the limbal area toward the sutures. (B) Quantitative comparison demonstrates significant reductions in the percentage of total corneal area covered by blood and lymphatic vessels after VEGF-C blockade. Graphs represent mean ± SEM of 10 mice in each group. *P < 0.05, **P < 0.01.
Anti-VEGF-C Therapy Blocks Post-transplant Migration of APCs to Draining Lymph Nodes
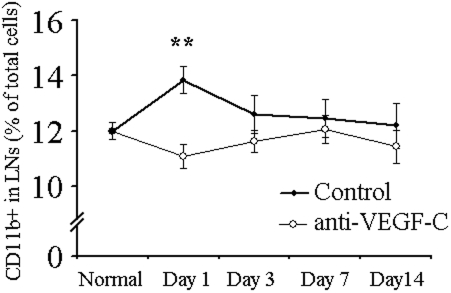
Corneal APCs are capable of migration to lymphoid tissues (e.g., lymph nodes) through lymphatic vessels and are critically involved in induction of the post-transplant allostimulatory response.28 We designed a series of experiments to determine the effect of VEGF-C blockade on the kinetics of CD11b+ cell trafficking to regional lymph nodes after corneal transplantation. We observed an early increase in the CD11b+ cell content of ipsilateral (but not contralateral; data not shown) draining lymph nodes at day 1 after corneal transplantation, which returned to normal by day 3 (Fig. 3). Anti-VEGF-C administration significantly blocked the early accumulation of these APCs in ipsilateral draining lymph nodes (Fig. 3). It is noteworthy that the significant majority of the CD11b+ cells in the lymph nodes are resident cells, not draining from the graft site per se. Thus, even a small change in APC content of the lymph node reflects a dramatic change in cell trafficking from the transplant site.
Figure 3.
Trafficking of APCs to draining lymph nodes after corneal transplantation is suppressed by anti-VEGF-C. Percentage of CD11b+ APCs in ipsilateral draining lymph nodes of transplanted corneas evaluated at different time points (days 1, 3, 7, and 14 post-transplant) by flow cytometry. APC content of the draining lymph nodes is significantly increased at day 1 after transplantation and returns to normal levels by day 3. Administration of anti-VEGF-C antibody significantly blocked the early rise in the APC content of draining lymph nodes. Results represent mean ± SEM of five samples per group (**P < 0.01 compared with control).
VEGF-C Is a Potent Chemoattractant for DCs and Promotes DC Maturation
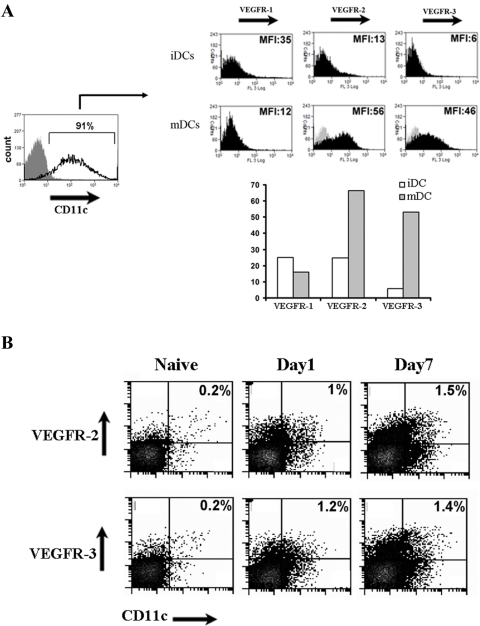
We showed that VEGF-C blockade resulted in significant reduction in APC content of draining lymph nodes early after corneal transplantation (Fig. 3). We thus investigated whether this reduction is due solely to the chemoattractant role of VEGF-C on mature APCs generated during their recruitment or whether VEGF-C could directly influence APC maturation. First, we evaluated the expression levels of VEGF receptors by flow cytometry on mature and immature bone marrow–derived DCs (Fig. 4A). Our results demonstrated that mature DCs expressed high levels of cell surface VEGFR-2 and VEGFR-3, but expressed lower levels of VEGFR-1, compared with immature DCs (Fig. 4A). To determine whether this finding also translated to the in vivo setting of corneal allografts, we evaluated the ipsilateral lymph nodes after corneal transplantation. Seven days after corneal grafting, the population of DCs expressing VEGFR-2 or R-3 was increased more than sixfold compared with lymph nodes in naïve mice (Fig. 4B).
Figure 4.
(A) Flow cytometric studies demonstrate that expressions of VEGFR-2 and VEGFR-3 are significantly higher in mature bone marrow–derived DC (mDCs) compared with immature DC (iDCs). Results are shown as mean ± SEM. Data are from single experiments (MFI, mean fluorescent intensity). (B) Increased level of VEGFR-2 and VEGFR-3 positive dendritic cells (CD11c+) were observed on days 1 and 7 in ipsilateral lymph nodes of corneal transplanted mice evaluated by flow cytometry.
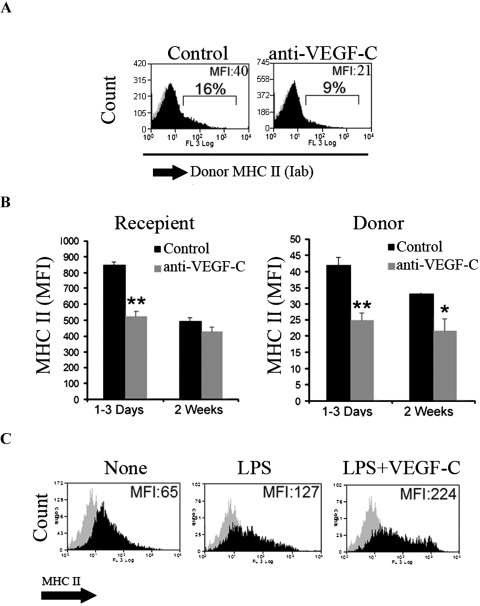
The majority of corneal APCs are normally highly immature in the uninflamed eye.29 Maturation of APCs is associated with upregulation of MHC class II and is critical in their priming of T cells and regulation of T-cell–mediated immune responses.30 We thus examined whether VEGF-C blockade suppresses APC maturation after corneal transplantation. Anti-VEGF-C significantly reduced MHC class II expression in donor and recipient APCs in ipsilateral lymph nodes after corneal transplantation, as reflected by a reduction in mean fluorescent intensity (Fig. 5). Interestingly, donor, but not recipient, APCs of anti-VEGF-C–treated animals continued to express low levels of MHC class II, even at 2 weeks after transplantation (Fig. 5B). To determine whether these observations are specific to the function of VEGF-C in transplantation, or represent a biological function of VEGF-C in regulating APC maturation, we evaluated the effect of VEGF-C on LPS-mediated maturation of C57BL6 bone marrow–derived DCs. Our results demonstrated that coincubation of DCs with VEGF-C significantly augmented LPS-induced maturation (Fig. 5C), suggesting that VEGF-C can enhance DC/APC function in alloimmunity not merely by permitting their recruitment, but also their maturation.
Figure 5.
VEGF-C promotes DC maturation. (A, B) Recipient (Iad) and donor (Iab) APCs from ipsilateral draining lymph nodes express significantly lower levels of MHC class II after anti-VEGF-C blockade (day 1 to day 3 after corneal transplantation. Anti-VEGF-C administration significantly reduced MHC class II expression by both donor and recipient after transplantation. (C) Bone marrow–derived DCs were matured either by LPS alone or LPS in the presence of 10 ng/mL VEGF-C for 24 hours. LPS-induced DC maturation was associated with overexpression of MHC class II, which was augmented in the presence of VEGF-C. Data are representative of three different experiments.
VEGF-C Blockade Promotes Corneal Transplant Survival
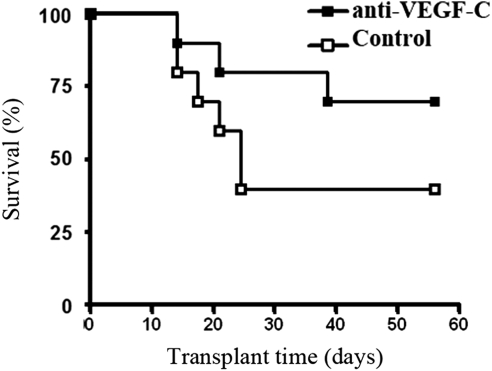
The above-cited data clearly demonstrated that corneal transplant rejection results in a robust heme- and lymph-angiogenic response associated with significant upregulation of VEGF ligands and receptors, in particular VEGFR-3, VEGF-A, and VEGF-C. We also showed that anti-VEGF-C therapy significantly reduced suture-induced corneal angiogenesis. Finally, we examined whether VEGF-C blockade for 2 weeks after transplantation could promote corneal graft survival. As shown in Figure 6, transplant survival was significantly enhanced in recipient mice treated with anti-VEGF-C antibody throughout the follow-up period compared with the control group (Fig. 6) (P < 0.05, n = 20).
Figure 6.
VEGF-C blockade promotes corneal allotransplant survival. In a masked fashion, orthotopically transplanted corneas from C57BL/6 (Iab) to BALB/c (Iad) mice were examined biomicroscopically and scored using a grading system designed to detect rejection for 8 weeks after transplantation. The fate of corneal graft is reported by Kaplan–Meier survival curves. VEGF-C blockade reduced the graft rejection rate and markedly improved graft survival from 33% in the control group to 66% in the anti-VEGF-C–treated group (n = 20 per group; *P < 0.05).
Discussion
Corneal transplantation is the oldest and most common form of solid organ/tissue transplantation. Although outcomes in first-time recipients with nonvascularized (normal) host beds are quite good (e.g., survival rates approximately 90%), vascularized and inflamed host bed predisposes corneal grafts to a very high rate of rejection, often well over 50%.31 However, there have been very few changes in the pharmacotherapy of corneal transplantation over the past half-century, and many subjects undergo repeat grafting for recurrent graft rejections.
Much attention has been focused on downmodulation of angiogenic responses, mediated by VEGF-A and VEGFR-2, including use of anti-VEGF-A antibodies (e.g., bevacizumab), given the association between corneal angiogenesis and corneal graft rejection, to promote transplant survival.8,14,15 However, in comparison, much less has been studied about the molecular regulation of lymphangiogenesis and leukocyte trafficking in the transplant setting, in particular by non-VEGF-A species. Herein we demonstrate that corneal graft rejection is associated with a robust overexpression of the endothelial mitogen VEGF-C (4.8- and 2.0-fold increase compared with nontransplanted and accepted allografts, respectively). VEGF-C plays a critical role in the proliferation and survival of lymphatic endothelial cells during embryogenesis and in adult life through interaction with its receptor VEGFR-3 (Flt-4).32–34 In addition to its role in lymphatic vessel development, VEGFR-3 is also highly expressed in blood vessel angiogenic sprouts.35 Moreover, VEGF-C binds to and activates another member of the VEGF receptor family, VEGFR-2,19 which is a potent angiogenic receptor and appears to mediate most of the known intracellular responses to VEGF-A. Parallel with the increased expression of VEGF ligands, we demonstrate that corneal graft rejection is associated with a marked upregulation of VEGFR-3. Moreover, we show that VEGF-C blockade significantly reduces both blood and lymphatic vessel growth. Although it is difficult to experimentally establish the contribution of different receptor subtypes to the observed effects of VEGF-C blockade, its potential to limit both the afferent (lymphatic) and efferent (blood vessel) arms of the immune system suggests VEGF-C blockade as an attractive candidate for VEGF-targeted therapy of corneal transplant immunity.
The lymphatic system serves as the sensitization (afferent) arm of the immune response by enabling efficient trafficking of APCs to regional lymph nodes.36 The critical role of ocular APC trafficking to draining lymph nodes in generating corneal alloimmune responses has been established by the demonstration that lymphadenectomy leads to universal and indefinite allograft survival, even without the use of immunosuppressants.37 Here we show that DC maturation is associated with upregulation of cell surface receptors of VEGF-C (i.e., VEGFR-2 and R-3). This upregulation renders DCs more responsive to the VEGF-C gradient induced by inflammation. Consistent with this finding, our data show that the APC content of the draining lymph nodes increases as early as 1 day after transplantation and this early APC trafficking is almost completely blocked by anti-VEGF-C therapy. This is before any significant effect on lymphatic vessel development, which further supports the direct chemoattractant role of VEGF-C on APC trafficking. VEGF-C is also known to enhance chemoinvasion toward lymphatics by inducing higher levels of lymphatic CCL21, and thereby increases trafficking of CCR7+ tumor cells and potentially APCs to lymphoid tissues.38 Interestingly, we also found that VEGF-C promotes in vitro maturation of bone marrow–derived DCs. Consistent with this finding, in vivo anti-VEGF-C treatment reduced the number of mature donor and recipient APCs in draining lymph nodes. The mechanisms by which VEGFR-mediated signaling regulates APC maturation are not clear, and clearly constitute an area of further investigation, which could shed considerable light on the link between angiogenesis and immune amplification in both transplant and tumor immunity.
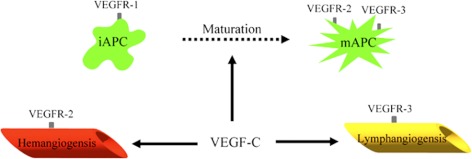
In summary, our results demonstrate that corneal alloimmunity can be amplified by VEGF-C at least at three different levels (Fig. 7). First, VEGF-C blockade inhibits the ingrowth of both lymphatic and blood vessel into the graft. Second, it reduces the trafficking of corneal APCs to draining lymph nodes. Finally, it promotes graft survival by inhibition of APC maturation. These data suggest that VEGF-C may potentially serve as an important target in corneal transplant pharmacotherapy.
Figure 7.
Schematic diagram summarizing the mechanisms via which anti-VEGF-C therapy suppress alloimmunity. VEGF-C binding to VEGFR-2 and VEGFR-3 induces hemangiogenesis and lymphangiogenesis, respectively. VEGF-C also promotes maturation and migration of APC to lymphoid tissues. iAPCs, immature antigen-presenting cells; mAPCs, mature antigen-presenting cells.
Footnotes
Supported in part by National Institutes of Health/National Eye Institute Grant EY-R01-12963 (RD) and Vegenics, Circadian Technologies Ltd. (Melbourne, Australia).
Disclosure: A.R. Hajrasouliha, None; T. Funaki, None; Z. Sadrai, None; T. Hattori, None; S.K. Chauhan, None; R. Dana, None
References
- 1. Eye Bank Association of America Statistical Report on Eye Banking Activity for 2008. Birmingham, AL: Eye Bank Association of America; 2009:1–25 [Google Scholar]
- 2. Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173:4464–4469 [DOI] [PubMed] [Google Scholar]
- 3. Fink N, Stark WJ, Maguire MG, et al. Effectiveness of histocompatibility matching in high-risk corneal transplantation: a summary of results from the Collaborative Corneal Transplantation Studies [in Czech]. Èeskoslovenská Oftalmol. 1994;50:3–12 [PubMed] [Google Scholar]
- 4. Maguire MG, Stark WJ, Gottsch JD, et al. Risk factors for corneal graft failure and rejection in the collaborative corneal transplantation studies. Collaborative Corneal Transplantation Studies Research Group. Ophthalmology. 1994;101:1536–1547 [DOI] [PubMed] [Google Scholar]
- 5. Williams KA, Roder D, Esterman A, Muehlberg SM, Coster DJ. Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology. 1992;99:403–414 [DOI] [PubMed] [Google Scholar]
- 6. Hamrah P. High-risk penetrating keratoplasty [in Spanish]. Arch Sociedad Espanola Oftalmol. 2005;80:5–7 [PubMed] [Google Scholar]
- 7. Volker-Dieben HJ, D'Amaro J, Kok-van Alphen CC. Hierarchy of prognostic factors for corneal allograft survival. Aust N Z J Ophthalmol. 1987;15:11–18 [DOI] [PubMed] [Google Scholar]
- 8. Bachmann BO, Bock F, Wiegand SJ, et al. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation. Arch Ophthalmol. 2008;126:71–77 [DOI] [PubMed] [Google Scholar]
- 9. Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor Flt-1. Blood. 1996;87:3336–3343 [PubMed] [Google Scholar]
- 11. Zittermann SI, Issekutz AC. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J Leukoc Biol. 2006;80:247–257 [DOI] [PubMed] [Google Scholar]
- 12. Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000;19:5598–5605 [DOI] [PubMed] [Google Scholar]
- 13. Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol. 2009;6:507–518 [DOI] [PubMed] [Google Scholar]
- 14. Chen WL, Lin CT, Lin NT, et al. Subconjunctival injection of bevacizumab (avastin) on corneal neovascularization in different rabbit models of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:1659–1665 [DOI] [PubMed] [Google Scholar]
- 15. Lee SY, Kim DK, Cho JH, Koh JY, Yoon YH. Inhibitory effect of bevacizumab on the angiogenesis and growth of retinoblastoma. Arch Ophthalmol. 2008;126:953–958 [DOI] [PubMed] [Google Scholar]
- 16. Goodin S. Development of monoclonal antibodies for the treatment of colorectal cancer. Am J Health Syst Pharm. 2008;65:S3–S7; quiz S22–S24 [DOI] [PubMed] [Google Scholar]
- 17. Ji R-C. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol Life Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Su JL, Yen CJ, Chen PS, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007;96:541–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung ES, Chauhan SK, Jin Y, et al. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol. 2009;175:1984–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptor-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10:813–815 [DOI] [PubMed] [Google Scholar]
- 21. Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67:1503–1508 [DOI] [PubMed] [Google Scholar]
- 22. Cursiefen C, Maruyama K, Jackson DG, Streilein JW, Kruse FE. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25:443–447 [DOI] [PubMed] [Google Scholar]
- 23. Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice—evidence that the immunogenetic rules of rejection do not apply. Transplantation. 1992;54:694–704 [DOI] [PubMed] [Google Scholar]
- 24. Zhang H, Grimaldo S, Yuen D, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011;52:6529–6535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch Ophthalmol. 2012;130:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92 [DOI] [PubMed] [Google Scholar]
- 27. Cursiefen C, Chen L, Saint-Geniez M, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci USA. 2006;103:11405–11410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise. The Cogan lecture. Invest Ophthalmol Vis Sci. 2004;45:721–727 [DOI] [PubMed] [Google Scholar]
- 29. Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70 [DOI] [PubMed] [Google Scholar]
- 30. Shen L, Barabino S, Taylor AW, Dana MR. Effect of the ocular microenvironment in regulating corneal dendritic cell maturation. Arch Ophthalmol. 2007;125:908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams KA, Esterman AJ, Bartlett C, Holland H, Hornsby NB, Coster DJ. How effective is penetrating corneal transplantation? Factors influencing long-term outcome in multivariate analysis. Transplantation. 2006;81:896–901 [DOI] [PubMed] [Google Scholar]
- 32. Lymboussaki A, Partanen TA, Olofsson B, et al. Expression of the vascular endothelial growth factor C receptor VEGFR-3 in lymphatic endothelium of the skin and in vascular tumors. Am J Pathol. 1998;153:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Makinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Veikkola T, Jussila L, Makinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tammela T, Zarkada G, Wallgard E, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660 [DOI] [PubMed] [Google Scholar]
- 36. Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yamagami S, Dana MR, Tsuru T. Draining lymph nodes play an essential role in alloimmunity generated in response to high-risk corneal transplantation. Cornea. 2002;21:405–409 [DOI] [PubMed] [Google Scholar]
- 38. Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor-C and C–C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69:349–357 [DOI] [PubMed] [Google Scholar]