Histone deacetylation is associated with early onset silencing of normal gene transcription, prior to execution of the apoptotic program in ganglion cells after acute optic nerve injury. Now, evidence shows that a similar mechanism is active in dying ganglion cells in a mouse model of glaucoma.
Abstract
Purpose.
Downregulation of normal gene expression in dying retinal ganglion cells has been documented in both acute and chronic models of optic nerve disease. The authors examined the mechanism and timing of this phenomenon in DBA/2J mice, using genetically modified substrains of this inbred line.
Methods.
DBA/2J mice, doubly congenic for the Bax mutant allele and the ganglion cell reporter gene Fem1cRosa3 (R3), were evaluated to elucidate the timing of loss of normal gene expression during the apoptotic process. The localization of histone deacetylase 3 (HDAC3) and nuclear histone H4 acetylation were examined by immunofluorescence in dying cells. The role of HDACs in gene silencing during glaucoma was interrogated using the global HDAC inhibitor trichostatin A (TSA).
Results.
Silencing of the R3 allele occurred in Bax−/− ganglion cells, indicating that this process preceded the committed step of the intrinsic apoptotic pathway. Weekly TSA treatment, between the ages of 6 and 10 months, was able to attenuate the loss of R3 expression in the retina, but had no effect on optic nerve degeneration. Dying cells in aging DBA/2J mice exhibited nuclear localization of HDAC3 and a decrease in the level of H4 acetylation.
Conclusions.
Retinal ganglion cells exhibit a loss of normal gene expression as an early (pre-BAX involvement) part of their apoptotic program during glaucomatous degeneration. This process can be ameliorated, but not completely blocked, using HDAC inhibitors. Epigenetic changes to active chromatin, such as deacetylation, may be mediated by HDAC3 in dying neurons.
Axonal degeneration and retinal ganglion cell body atrophy and death are the fundamental characteristics of glaucomatous optic neuropathies.1 The identification of intrinsic apoptosis in glaucomatous ganglion cell somas2 has led to the hypothesis that disruption of this program may be a viable target to prevent ganglion cell neurodegeneration and, combined with conventional pressure-lowering therapy, may yield more effective preservation of vision. Substantial focus, therefore, has been given to obtaining a better understanding of the temporal sequence of events being executed in apoptotic ganglion cells, particularly to the early events that may critically activate subsequent and irreversible steps in the apoptotic cascade.1,3
One of the early events associated with ganglion cell death is the silencing of normal gene expression. This phenomenon has been documented in both acute and chronic (experimental ocular hypertension) models of optic nerve disease.4–8 These studies have typically reported an exponential decay in the mRNA abundance of genes that are normally expressed in ganglion cells. In all studies to date, the loss of mRNAs occurs well in advance of a measurable loss of ganglion cell bodies. Our initial characterization of this phenomenon was a quantitative analysis of the transcript abundance for the Thy1 gene. Thy1 mRNA becomes depleted before cell loss in mouse models of optic nerve crush, and intravitreal injection of the glutamate analog, N-methyl-d-aspartate. Additionally Thy1 transcripts were depleted in advance of cell loss in a rat model of experimental glaucoma.4,5 Loss of this mRNA was also observed in Bax-deficient retinal ganglion cells after optic nerve crush, even though these cells were completely arrested in the apoptotic program. Similar kinetics of decay of other ganglion cell–specific genes, including Brn3b,9 Nrn1, and Sncg6,7 have now been reported.
Collectively, these data indicate that downregulation, or the silencing of normal gene expression, is an early event in the apoptotic program, occurring before the point at which BAX is activated. The global nature of the effect suggests a coordinated mechanism that can simultaneously affect multiple actively expressed genes. One mechanism that could account for this effect is rapid modification of histones in the promoters of genes in an “open” conformation, particularly by histone deacetylation, which has been described as a principal epigenetic change that can rapidly silence active genes.10 Using the mouse optic nerve crush model to activate ganglion cell apoptosis, we observed that histones in the promoters of actively expressed genes were rapidly deacetylated, which was associated with the translocation of histone deacetylase 3 (HDAC3) from the cytoplasm to the nuclei of dying cells. Moreover, the downregulation of the Rosa3 ganglion cell reporter gene (Fem1cR3)11 could be attenuated in mice pretreated with the broad-spectrum HDAC inhibitor trichostatin A (TSA).8
Here, we report that DBA/2J mice, carrying the Fem1cR3 reporter gene, exhibit loss of expression of this gene before glaucomatous ganglion cell loss, similar to reports made for other genes in this mouse strain.7 This downregulation preceded the function of the proapoptotic BAX protein. Evaluation of dying cells in DBA/2J mice indicated both the nuclear accumulation of HDAC3 and deacetylation of histone H4, suggesting an epigenetic mechanism affecting gene transcription similar to that reported after acute optic nerve damage. Continuous treatment of mice with TSA was able to ameliorate the silencing of Fem1cR3, and therefore somewhat preserve retinal integrity. This effect was not translated to optic nerve axons, however, which still exhibited a similar level of damage compared with control treatment groups.
Materials and Methods
Experimental Animals
All animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research using protocols approved by the Animal Care and Use Committee of the University of Wisconsin. Mice were housed in microisolator cages and kept on a 12-hour light/dark cycle. They were maintained on a 4% fat diet (8604 M/R; Harland Teklad, Madison, WI). DBA/2JR3/R3 mice were used as a chronic disease model of retinal ganglion cell loss. These mice develop a spontaneously occurring secondary glaucoma as they age. These mice also carry the βGeo reporter trap gene in the first intron of the Fem1c gene (Fem1cR3). Expression of this allele produces a fusion protein (βGEO), which has β-galactosidase activity and can process X-Gal as a substrate. Expression from this gene decreases as these mice age and the disease progresses.12 In addition, a second substrain of DBA/2J mice, containing a mutant Bax allele (DBA/2JBax−/−,R3/R3), was used to examine the timing of gene silencing in retinal ganglion cells unable to execute a full apoptotic program.
Intraocular pressures (IOPs) were measured by applanation tonometry (Tono-Lab; Colonial Medical Supply, Franconia, NH), on mice that were anesthetized with ketamine (6 mg/mL) and xylazine (0.4 mg/mL). Measurements were taken 5 to 10 minutes after anesthesia, when normalized pressures under this anesthetic are most similar to IOPs in awake mice.13 Buphthalmia was scored as a presence or absence of eye bulging, also while the mice were anesthetized. Transillumination defects were scored on a 4-point scale, with 1 representing no defect and 4 representing complete loss of pigment from the iris.
Histochemical Staining for βGEO Activity
Ganglion cells expressing βGEO were identified histochemically in DBA/2J mice 10 to 22 months of age by staining retinal preparations with X-Gal, followed by whole mounting as previously described.11 Briefly, the mice were euthanized and the eyes were collected and fixed in 4% paraformaldehyde in PBS at room temperature for 30 minutes. The eye was then rinsed in PBS, and the anterior portion of the eye was removed and discarded. The resulting eyecup was washed in PBS containing 2 mM MgCl2 and 2 μM CaCl2 and stained by incubation in 1 mg/mL X-gal solution at 37°C for 18 hours. After staining, the retina was dissected from the eyecup and whole mounted on glass slides. The mounted retinas were then Nissl-stained with cresyl violet to enable counting of all cells in the ganglion cell layer.14 The slides were examined and photographed using a light microscope (Olympus BX40; Olympus America Inc., Center Valley, PA) and a digital camera attachment.
Double-stained cells were counted by overlying (×40) digital images with a 100 × 100-μm grid. Total cell number was determined by counting Nissl-stained cells exhibiting large round nuclei with prominent nucleoli. This metric essentially estimates all the neurons in this layer, including both ganglion and amacrine cells. After counting of all cells, the percentage of cells positive for X-Gal deposits was recorded. Regions with intense X-Gal staining were scored as 100% since it was not possible to sort positive from negative staining cells. Numbers of eyes examined were 18 each for Bax+/+ and Bax+/− mice, and 14 for Bax−/− mice. A minimum of 10 fields were scored for each eye.
Immunofluorescence
Immunofluorescence was performed on retinal cryosections, which were prepared in the following manner. Whole eyes were fixed in PBS (150 mM NaCl, 100 mM NaH2PO4, pH 7.4) containing 4% paraformaldehyde for 1 hour at room temperature. The anterior chamber, including the lens, was removed, and the eyecup was then stored in 0.4% paraformaldehyde in PBS at 4°C. Before embedding the tissue, the eyecup was placed in 30% sucrose overnight at 4°C. The tissue was embedded in optimum cutting temperature specimen matrix for cryostat sectioning (Tissue-Tek OCT compound; Fisher Scientific, Pittsburgh, PA), flash frozen using dry ice, and sectioned in 5-μm slices in the midregion of the retina containing the optic nerve. Antibody labeling and washes were done as described previously.8,15 Primary rabbit polyclonal antibodies were used for HDAC3 (Santa Cruz Biotechnology, Santa Cruz, CA) and acetylated histone H4 (Millipore Inc., Billerica, MA). The primary antibody for γH2AX was a mouse monoclonal (Millipore). All primary antibodies were used at a 1:100 dilution. Secondary antibodies used were goat anti-rabbit with FITC label (1:100) and goat anti-mouse with a Texas Red label (1:100) (both from Jackson ImmunoResearch Laboratories, West Grove, PA). The images were obtained using an imaging microscope (Zeiss Axioplan 2; Carl Zeiss Microimaging Inc., Thornwood, NY) and processed by extended focusing with application software (AxioVision 4.6.3.0 software; Carl Zeiss Microimaging).
Cells undergoing apoptosis in aged DBA/2J retinas were identified by staining them for a phosphorylated histone H2 variant (γH2AX). This variant becomes concentrated in nuclei of dying cells, coinciding with DNA damage that precedes mitochondrial involvement in apoptosis.16 After optic nerve crush, we previously reported that γH2AX exhibited three distinct stages of localization, including minimal expression except for a small concentration of protein near the nucleolus (stage I), the formation of a perinuclear ring of protein early during the apoptotic process (stage II), and, finally, concentration of γH2AX within the nucleus (stage III).8 The localization patterns of this histone variant also exhibited distinct patterns of colocalization with both HDAC3 and acetylated histone H4. HDAC3 distribution shifted from cytoplasmic to nuclear in stage II γH2AX-labeled cells, and eventually colocalized with this histone variant in the nuclei of stage III cells. Conversely, stage II cells nearly always exhibited prominent acetylation of H4, whereas stage III cells featured dramatic loss of acetylation of H4. A flow chart of the changes in distribution of HDAC3 and acetyl H4 with γH2AX labeling has been published elsewhere as a supplemental figure.8
For immunofluorescent labeling experiments, a total of 35 eyes, from mice between 10 and 12 months of age, were analyzed (minimum of two sections each eye). Assuming uniform loss of 60,000 ganglion cells per eye, over a period of 4 months (9 to 12 months of age), we could predict a loss of approximately 500 cells per day. The frequency of finding cells in stage III labeling was low (<1%) using longitudinal sections in this study, consistent with the predicted rate of cell loss.
In Vivo HDAC Inhibitor Studies
Systemic TSA treatments were used to inhibit HDAC activity in the retina of DBA/2JR3/R3 mice. TSA was delivered via intraperitoneal (IP) injections given as 1 mg/kg TSA in dimethyl sulfoxide (DMSO). Vehicle injections consisted of an equal volume of DMSO. Injections were given weekly beginning at 6 months until 10 months, when the animals were euthanized and the retinas were collected. Three cohorts of mice were followed for this experiment, including animals receiving TSA (25 mice, 50 eyes), DMSO (24 mice, 48 eyes), or no treatment (23 mice, 46 eyes). Since disease develops independently in each eye of DBA/2J mice,12 each eye was considered an independent sample for analysis.
The level of Fem1cR3 expression was quantified as a function of βGEO enzyme activity assessed by β-galactosidase solution assay (Promega, Madison, WI). Briefly, dissected retinas were individually homogenized in 400 μL 1 × reporter lysis buffer. After a 15-minute incubation at room temperature, the samples were vortexed for 15 seconds and centrifuged to pellet cellular debris. A portion of the resulting supernatant (50 μL/well), along with the prepared standard curve (10–50 μL of a 1:104 dilution of the β-galactosidase provided in the kit), was plated in duplicate for the assay. After a 1-hour incubation at 37°C, the plates were read with a microplate reader (ELX800; Bio-Tek Instruments Inc., Winooski, VT). Background levels of β-galactosidase activity were determined from age-matched DBA/2J mice not carrying the R3 allele. Total protein in each sample was determined using a bicinchoninic acid protein assay (BCA; Thermo Fisher Scientific, Rockford, IL). The total enzyme activity measured in the samples was normalized to the amount of protein in each sample, minus the background level.
Evaluation of Optic Nerve Degeneration
Optic nerve degeneration in 10-month-old mice was scored using a modified mild, moderate, or severe grading scale previously described.17,18 Optic nerves from each eye used for βGEO activity assays were harvested, fixed in 4% paraformaldehyde, and embedded in glycolmethacrylate (JB-4 Plus; Polysciences, Inc., Warrington, PA). Sections (1 μm) were cut from the postlaminar region, where the axons are myelinated, and stained with a chicken polyclonal antibody against βIII tubulin (Chemicon International, Temecula, CA) and a rabbit anti-chicken IgG conjugated to rhodamine as the secondary antibody (Chemicon International). Optic nerve sections were scored by three masked observers. Discrepancies in scores were rectified by consensus among the three observers. Nerves with ≥95% of their area positive for βIII tubulin were considered to be mild, nerves with 95%–50% staining were considered moderate, and nerves with <50% staining were considered as severe.
Statistical Analyses
Statistical evaluation of means between two data sets was performed by Student's t-test. Comparisons of multiple data sets were conducted by one-way ANOVA. Statistical evaluation of optic nerve damage was performed by χ2 test. Significant difference among groups was set at P ≤ 0.05.
Results
Gene Silencing Occurs in Protected Retinal Ganglion Cells in a Glaucoma Model
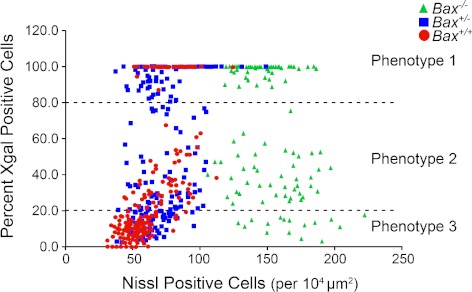
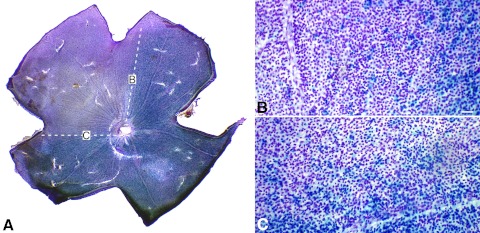
DBA/2JR3/R3 mice, with different genotypes for a functional Bax allele, were aged between 10 and 22 months and euthanized. Previously, we had observed that neither the Fem1cR3 nor the mutant Bax allele prevented the development of elevated IOP and glaucomatous optic nerve disease in DBA/2J mice.12,17 By combining these alleles on the DBA/2J genetic background, we were able to more precisely investigate the timing of ganglion cell gene silencing in glaucoma. Isolated retinas were double-labeled for total cells present in the ganglion cell layer and the cells positive for the βGEO reporter protein. Morphometric analysis of randomly selected areas of 100 × 100 μm revealed roughly three distinct phenotypes in these mouse retinas when stratified by the percentage of βGEO expression and cell density (Fig. 1, Table 1). In wild-type mice, regions with phenotype 1 (100–80% of the cells expressing βGEO) exhibited the highest density of neurons in each region. This phenotype was also typically observed in young mice before the development of disease (Schlamp et al.12 and data not shown). Phenotype 2 (80–20% of the cells expressing βGEO) exhibited cell densities similar to those of the phenotype 1 group (78 ± 2.3 cells/region vs. 84 ± 1.6 cells/region). Phenotype 3 (<20% cells expressing βGEO) exhibited the lowest density of neurons in each selected region (52 ± 0.7 cells per region, P ≪ 0.001; Fig. 1, Table 1). Using the level of βGEO expression as the principal criterion for each phenotype, we then examined regions in Bax-deficient mice. Bax+/− mice exhibited cell densities similar to those of wild-type animals for phenotypes 1 and 2, whereas density in phenoytpe 3 regions was also significantly decreased (67 ± 1.4 cells/region, P ≪ 0.001; Fig. 1, Table 1). Mice completely null for a functional Bax gene contained nearly twice the density of neurons than that of Bax+/+ and Bax+/− mice (Table 1), consistent with previous reports that the absence of Bax prevents normal programed cell death of this layer during development.19 Even though Bax-deficient ganglion cells are resistant to apoptosis in glaucoma,2 there are still regions with nearly complete loss of βGEO activity, indicative of silencing of the Fem1cR3 reporter gene, but no significant cell loss (P = 0.108; Fig. 1, Table 1). Additionally, in some DBA/2JBax−/−,R3/R3 mice, loss of βGEO activity occurred in distinct wedge-shaped regions extending from the optic nerve head (Fig. 2), typical of the pattern of cell loss observed in wild-type mice with glaucomatous retinal degeneration.
Figure 1.
Scatterplot of total neuron counts relative to percentage of βGeo expression in aged DBA/2JR3 mice with different Bax genotypes. Randomly selected 100 × 100-μm fields were evaluated in double-labeled retinal whole mounts of mice 10 months of age. Each field was also designated as one of three phenotypes based on the apparent percentage of cells expressing βGEO based on X-gal staining. Wild-type (red circles) and Bax+/− (blue squares) mice exhibited similar cell densities for phenotype regions 1 and 2, but a lower density of cells in phenotype 3 regions. Bax−/− mice (green triangles) exhibited approximately twice the density of cells, which did not decrease in regions with phenotype 3. See Table 1 for quantitative values and statistics.
Table 1.
βGEO Activity Is Depleted Prior to Cell Loss in Aged DBA/2JR3 Mice
| Phenotype |
Bax+/+ |
Bax+/− |
Bax−/− |
|||
|---|---|---|---|---|---|---|
| Percentage of Cells βGEO Positive | Nissl-Stained Cells* | Percentage of Cells βGEO Positive | Nissl-Stained Cells | Percentage of Cells βGEO Positive | Nissl-Stained Cells | |
| 1 | 99.58 ± 0.31 | 78 ± 2.3 | 97.15 ± 0.51 | 81 ± 2.0 | 98.63 ± 0.33 | 145 ± 1.7 |
| 2 | 36.94 ± 2.26 | 84 ± 1.6 | 52.04 ± 2.69 | 75 ± 3.2 | 39.28 ± 1.75 | 155 ± 3.8 |
| 3 | 12.27 ± 0.77 | 52 ± 0.7 | 16.79 ± 0.75 | 67 ± 1.4 | 11.74 ± 1.39 | 158 ± 5.9 |
DBA/2JR3 mice ≥10 months of age were examined by double labeling for ganglion cell gene activity and total cell number. Cell counts were obtained in sample areas of 100 × 100 μm randomly placed on whole-mounted mouse retinas (10–15 fields per retina) double labeled for βGEO enzyme activity (ganglion cells) and Nissl substance (all neurons). Each sample was assigned a phenotype principally based on the percentage of cells expressing βGEO and secondarily the overall density of total neurons present. These phenotypes were first designated for wild-type mice. The criteria for βGEO expression were then applied to both the Bax+/− and Bax−/− mice. Phenotype 1 contained 80–100% expressing cells, phenotype 2 80–20% expressing cells, whereas phenotype 3 contained fewer than 20% expressing cells. All data are shown as mean ± SE. Retinas from both wild-type (n = 18 eyes) and Bax+/− (n = 18 eyes) mice show regions of normal cell concentration (P = 0.07), but significantly reduced βGEO activity when assigned to phenotype 2 (P << 0.001, compared with phenotype 1). The nearly complete loss of βGEO activity (phenotype 3), however, is associated with a significant decrease in cell density relative to phenotypes 1 and 2 (P << 0.001). If cell death is prevented in Bax−/− mice (n = 14 eyes), the loss of βGEO activity is not associated with cell loss in any phenotype group (P = 0.108), indicative of silencing of the Fem1cR3 gene early in the apoptotic pathway.
Cells counted per 10,000 μm2.
Figure 2.
Regional loss of βGEO activity occurs in 10-month-old Bax−/− DBA/2JR3/R3 mice. Cells with βGEO activity appear blue, whereas Nissl-stain appears violet. (A) Whole-mounted retina, showing a wedge-shaped region devoid of βGEO activity (defined by white hashed lines). Regions (B) and (C) in borders of the wedge are shown in higher magnification. Although cells have stopped expressing βGEO, they are still present in the wedge region. Scale bar for (B) and (C): 50 μm.
Nuclear HDAC3 Localization and Histone H4 Deacetylation Are Associated with Apoptotic Cell Death in Aging DBA/2J Mice
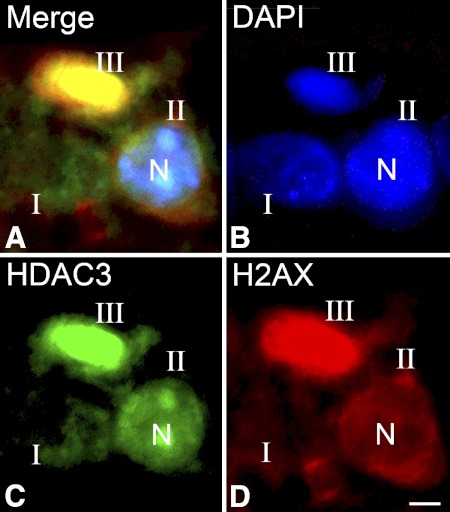
Previously, we reported that apoptotic retinal ganglion cell death after optic nerve crush was associated with the nuclear accumulation of HDAC3 and the deacetylation of histone H4.8 To investigate if similar events occur in dying cells in this glaucoma model, frozen sections from DBA/2J mice at different ages were stained to examine changes in both HDAC3 localization and nuclear acetylation. Staining for γH2AX was used to identify cells in early or later stages of apoptosis. Double-label immunostaining for γH2AX and HDAC3 on sections of DBA/2J mouse retinas revealed that dying cells with either stage II or stage III γH2AX localization exhibited increasing concentration of nuclear HDAC3 localization. Relatively normal appearing cells (stage I γH2AX localization) exhibited minimal labeling HDAC3 (Fig. 3).
Figure 3.
HDAC3 concentrates in nuclei of apoptotic cells. Immunofluorescent double labeling of cells in the ganglion cell layer of a DBA/2J mouse. Frozen sections were double labeled with antibodies against the histone variant γH2AX to identify dying cells, HDAC3, and counterstained for DNA using DAPI. (A) Merged image of all three labels in a representative section. (B–D) Individual channels for DAPI, HDAC3, and γH2AX. Based on the γH2AX labeling, the nuclei in the section were identified as stages I, II, or III. Stage II and stage III cells exhibit nuclear localization of HDAC3. Stage II and stage III cells also appear to contain fragmented or highly condensed chromatin, respectively. N, representative nucleus. Scale bar: 5 μm.
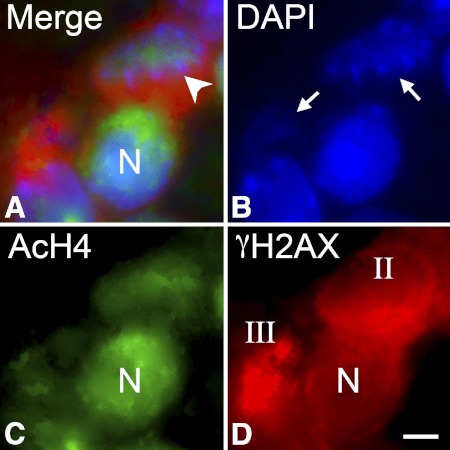
Similarly, colocalization of acetylated histone H4 (AcH4) and γH2AX showed that normal-appearing (stage I) cells had robust AcH4 labeling (Fig. 4). Stage II cells had variable staining for AcH4, whereas stage III cells typically had little to no staining for AcH4. DAPI counterstaining of cells also revealed that stage III cells with minimal AcH4 staining also exhibited abnormal nuclear structures indicative of chromatin condensation.
Figure 4.
Apoptotic cells exhibit reduced staining for acetylated histone H4. Immunofluorescent double labeling of cells in the ganglion cell layer of a DBA/2J mouse. Frozen sections were double labeled with antibodies against the histone variant γH2AX to identify dying cells, acetylated histone H4 (AcH4), and counterstained for DNA using DAPI. (A) Merged image of all three labels in a section. (B–D) Individual channels for DAPI, AcH4, and γH2AX, respectively. Representative stages I, II, or III nuclei (N), based on the γH2AX labeling, are indicated. Stage I cells exhibit strong labeling for AcH4, whereas the single stage III cell in the image has virtually no AcH4 present. Stage II cells show some AcH4 staining, but it is typically intermediate between stage I and stage III labeling. Scale bar: 5 μm.
Inhibition of HDAC Activity Attenuates Fem1cR3 Silencing in Aged DBA/2J Mice
To establish that HDAC activity was linked to the downregulation of gene expression in glaucomatous mice, we treated DBA/2JR3/R3 mice once per week with the broad-spectrum HDAC inhibitor TSA. To mimic a potential treatment paradigm for human glaucoma, we initiated TSA treatment in mice that were 6 months old, and continued this once every week until mice reached 10 months of age. This period coincided with the reported longitudinal progression of disease in this model, when mice first begin to show elevation of IOP, to a point where the majority of mice exhibit some level of retina degenerative effects.12,17 At 10 months of age, all eyes in the study were also scored for IOP, iris pigment dispersion and transillumination defects, and buphthalmia (Table 2). Eyes from mice treated with TSA showed, on average, statistically equal elevations of IOP and anterior chamber defects as those of both the DMSO and nontreated mice (P = 0.307), whereas these metrics were significantly increased in all treatment groups relative to 6-month-old mice (P ≪ 0.001). TSA-treated mice also exhibited relatively high levels of buphthalmia. Collectively, these results indicated that TSA did not interfere with the development of the characteristic features of anterior chamber pathology and elevation of IOP typical for this strain.
Table 2.
TSA Treatment of DBA/2JR3 Mice Does Not Affect Development of Ocular Hypertension
| Treatment | n (Eyes) | Mean IOP (mm Hg ±SD)* | IOP Range (mm Hg) | Mean AC Score (± SD)† | Buphthalmia‡ |
|---|---|---|---|---|---|
| DMSO | 46 | 20.56 ± 7.82 | 9–40 | 1.2 ± 0.7 | 26.1% |
| TSA | 50 | 19.88 ± 5.96 | 7–35 | 1.6 ± 0.9 | 42.0% |
| No injection | 48 | 20.13 ± 6.43 | 11–41 | 1.6 ± 0.7 | 22.9% |
Mean intraocular pressure (IOP) in 6-month-old mice is 12.5 ± 3.0 mm Hg (range, 8–20, n = 18). Note that IOPs were taken under ketamine and xylazine anesthesia, which may yield artificially low values, particularly in ocular hypertensive mice.13 There was no statistical difference between IOP levels of all treated groups (ANOVA, P = 0.307), but all treated groups were significantly different from 6-month-old mice (P << 0.001). These values are also comparable to the mean IOPs reported for age-matched DBA/2J animals that do not carry the R3 allele.17
Anterior chamber (AC) score was based on a qualitative evaluation of iris pigment dispersion and transillumination defects by two observers. Scores were made on a 1 to 4 scale, where 1 is no damage and 4 is severely damaged, and the average taken. All treated groups exhibited significantly more AC defects than 6-month-old mice (P < 0.003), but there was no significant difference among treated groups (P > 0.32).
Buphthalmia, which is characterized by bulging of the eye from the orbit as a consequence of elevated IOP, scored as + or − based on agreement between two observers. The value given is the percentage of eyes in the cohort that were scored as “+.”
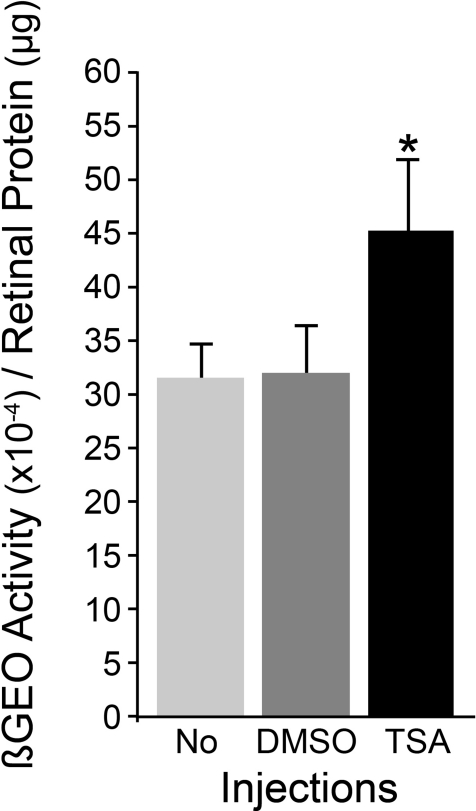
The effect of TSA treatment on the silencing of the Fem1cR3 reporter gene, as a function of βGEO enzyme activity, was measured from retinal homogenates of eyes harvested at 10 months of age. TSA-treated mice (n = 50 eyes) retained significantly more βGEO enzyme activity/μg of total protein in retinal homogenates than did either nontreated (n = 48) or DMSO-treated (n = 46) animals (P = 0.032 and P = 0.049, respectively; Fig. 5). TSA treatment, however, appeared to only partially prevent the decrease in βGEO activity, which was approximately 80% of the mean level detected in 6-month-old animals. This difference was not statistically significant (P = 0.314). No treatment, or DMSO injections alone, resulted in an approximate 50% decrease in βGEO activity relative to younger mice (P = 0.021 and 0.044, respectively). Some mice were also aged to 12 months, with weekly injections of TSA. In these mice (n = 6 eyes), βGEO activity was nearly at undetectable levels (6% of levels found in young mice), suggesting that TSA was only able to delay the progressive damage to ganglion cells, leading to gene silencing. The small sample size for the 12-month-old animals combined with the high degree of variability for disease progression in these mice warrant further investigation of the effects of HDAC inhibitors on long-term glaucoma.
Figure 5.
TSA treatment attenuates the downregulation of Fem1cR3 expression. Mice treated weekly with vehicle (DMSO), or TSA, between 6 and 10 months of age, were compared with age-matched mice that had no treatment (No). The level of Fem1cR3 gene expression was assessed as a function of the amount of βGEO fusion protein activity relative to total protein in retinal homogenates. TSA provided a significant level (asterisk) of attenuation of the loss of βGEO enzyme activity observed in both noninjected and DMSO-injected mice (P = 0.032 and P = 0.049, respectively). The preservation of βGEO activity afforded by TSA is only partial, however, and represents approximately 80% of the level observed in young DBA/2J mice without disease.
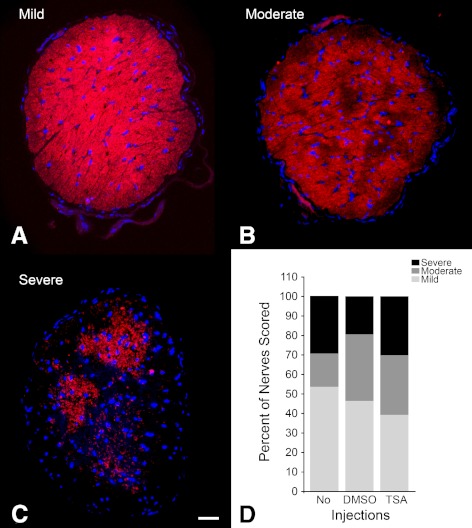
Several groups have now determined that axonal damage and degeneration precede the activation of apoptosis in ganglion cells in these mice.12,20–23 To determine whether TSA treatment was also able to attenuate optic nerve degeneration, we examined every nerve from each 10-month-old mouse for βIII tubulin immunoreactivity and scored them for mild, moderate, and severe disease (Figs. 6A–C). Nerves in TSA-treated eyes (n = 43) exhibited the same frequency of severe and moderate disease as DMSO-treated (n = 41) and untreated (n = 41) mice (χ2 test, P = 0.207 vs. DMSO, and P = 0.051 vs. no injection) (Fig. 6D).
Figure 6.
Optic nerve scores for treated 10-month-old mice. Optic nerves for each eye harvested for βGEO activity (Fig. 5) were also examined for optic nerve degeneration. Sections were immunostained for βIII tubulin and counterstained with DAPI. Digital images were scored by three masked observers for (A) mild, (B) moderate, and (C) severe damage, based on the extent of positive staining for βIII tubulin. The frequency of each category, for each treatment group, was then graphed and evaluated (D). TSA treatment provided no statistical difference in the frequencies of mild, moderate, or severe damage to the optic nerves of mice, compared with either noninjected mice (No), or vehicle-injected mice (DMSO) (χ2 test, P = 0.051 and P = 0.207, respectively). Scale bar for (A–C): 100 μm.
Discussion
Loss of βGEO Expression During Glaucomatous Degeneration
Three phenotypes, based on the level of βGEO expression and cell density, were defined in randomly selected 100 × 100-μm regions of retinal whole mounts of aged DBA/2JR3/R3 mice. The most informative phenotype was in regions of retinas that exhibited a decrease in βGEO activity without a concomitant decrease in cell density (phenotype 2). This phenotype was characteristic only of elderly mice, at ages where we, and others, have observed retinal damage as a result of glaucomatous damage.12,17,20,24,25 Regions with phenotype 2 cells are consistent with ganglion cells that have become damaged and are suspected of downregulating the expression of normal gene expression before the completion of the apoptotic program observed previously in this model.7 In mice, wild-type for a functional Bax gene, some regions with substantially decreased βGEO activity (phenotype 3) also had significantly fewer cells, which we interpret as regions that have lost retinal ganglion cells due to completion of the apoptotic program.
To confirm that downregulation of the Fem1cR3 gene occurred early in the process of ganglion cell apoptosis, we also observed loss of expression in cells deficient for the Bax gene. In both Bax+/− and Bax−/− mice, regions with phenotype 3 still exhibited a high density of cells remaining, although some cell loss was detected in Bax+/− animals. In these mice the onset of cell death may be delayed or retarded by the reduction in BAX protein levels, similar to findings in heterozygous mice after acute optic nerve crush.26 Further evidence that the silencing of Fem1cR3 was a characteristic event of ganglion cell damage was noted in the formation of entire regions of affected cells forming a wedge-shaped pattern radiating from the optic nerve head. This same pattern has been observed for cell loss in both the DBA/2J mouse model20,21 and rat models of experimental glaucoma,27 indicating that gene silencing is an early stage of ganglion cell pathology leading to soma loss in this disease.
Gene Silencing and HDAC3 Activity
In the mouse optic nerve crush model of ganglion cell death, the downregulation of gene expression is associated with the nuclear translocation of HDAC3 and deacetylation of histone H4. Inhibition of HDAC activity is able to partially attenuate this silencing phenomenon and delay cell death. Importantly, in these more acute studies, temporal observation of cells indicated that HDAC3 translocated early in the apoptotic process, and was followed by global histone deacetylation in the nuclei of damaged cells.8 Determining the same role for HDAC3 in ganglion cells affected by glaucoma is technically more challenging due to the chronic nature of disease progression. Using γH2AX expression as a marker of dying cells, however, allowed us to evaluate HDAC3 translocation and histone deacetylation at the resolution of different stages of the apoptotic process in individual cells. Similar to dying cells in the acute model, HDAC3 localized to the nuclei of cells with stage II γH2AX staining, whereas deacetylation was more common in stage III labeled cells. The timing of these changes in dying cells supports the role for HDAC3 in establishing early epigenetic modifications in apoptotic nuclei, first suggested in the acute injury model. Furthermore, TSA treatment was also able to attenuate the loss of Fem1cR3 gene expression in this glaucoma model, confirming a link between HDAC activity and the silencing of normal ganglion cell expression.
At this point, there is no direct evidence linking HDAC3 activity as a central molecule in the apoptotic pathway executed by retinal ganglion cells. Recently, however, Bardai and D'Mello28 reported that forced expression of exogenous HDAC3 in a variety of neurons was toxic, whereas similar overexpression in nonneuronal cells had no effect. Silencing of Hdac3 expression using short hairpin RNAs increased resistance of neurons to damaging stimuli. Additionally, selective HDAC3 inhibitors have been found to be therapeutic in a mouse model of Friedreich's ataxia,29,30 principally by reversing the silencing of frataxin expression.
A major contribution of HDAC3 in the process of cell death may also be in the formation of heterochromatin and nuclear condensation. Histone deacetylation is a fundamental characteristic of heterochromatin,10 which in turn is associated with transcriptionally silent gene activity.31,32 One of the earliest steps in the apoptotic program of retinal ganglion cells is the atrophy and shrinkage of the cell nucleus (Janssen KT, et al., manuscript submitted, 2012). This process occurs within 2 hours after acute optic nerve damage and is associated with the deacetylation of histone H4 and the formation of heterochromatin. Additionally, this process occurs in the absence of BAX activation. Ultimately, the condensation of chromatin is considered a hallmark of the apoptotic process.33 Although some have suggested that the formation of heterochromatin is dependent on caspase activation,34,35 this may be more likely a secondary process associated with DNA fragmentation that is a consequence of caspase activity.36 It is possible heterochromatin formation is a multistep process, requiring both early deacetylation of histones, followed by caspase-mediated activation of apoptotic endonucleases. Certainly, it is plausible that cellular mechanisms designed to facilitate soma and nuclear condensation and shrinkage may also secondarily affect active gene transcription through the process of histone modification.
Inhibition of HDAC Activity and Attenuation of Neuronal Apoptosis
There is growing evidence that HDAC inhibitors, in general, have a protective effect on neurons in several animal models of chronic neurodegeneration.37,38 Several studies, in fact, have documented neuroprotection of retinal ganglion cells treated with the HDAC inhibitors valproic acid, sodium butyrate,39,40 and TSA.8,41 Importantly, these previous studies examined the effects of HDAC inhibitors in more acute models of ganglion cell death or on isolated ganglion cells in culture. Using a continuous paradigm of TSA treatment, our experiments document a similar protective effect, specifically on the ganglion cell gene expression, in a chronic model of secondary glaucoma. Although we did not explicitly examine ganglion cell somas after treatment, we interpret the relative preservation of Fem1cR3 gene expression as an indicator that ganglion cell somas were not only present, but somewhat less affected in disease. This effect was apparently not translated to the axons in the optic nerve, however, which still exhibited a similar level of degenerative effects compared with DMSO-injected and noninjected control groups. This observation suggests that the principal effects of an HDAC inhibitor on neuronal survival are restricted to the soma, possibly by directly influencing progression of the apoptotic program. An analogous finding was made in Bax-deficient DBA/2J mice with glaucoma,2 and is consistent with the concept that soma damage is the downstream effect of initial axonal damage.
Although HDAC inhibitors are now being recognized as a potential therapeutic to prevent ganglion cell death, the mechanism of their protective function is still being elucidated. Our studies suggest that one mechanism may be ameliorating the process of gene silencing in affected retinal ganglion cells. This could provide cells with a more stabilized molecular environment, allowing them a better chance to recover from damaging insults. Alternatively, HDAC inhibitors may also influence the expression of other genes that help to prevent apoptosis. For example, valproic acid and TSA have been reported to stimulate the upregulation of antiapoptotic members of the Bcl2 gene family.42,43 Preliminary data from our laboratory suggest that TSA may similarly enhance BclX expression in retinal ganglion cells (Pelzel HR, et al., manuscript in preparation). It is likely that the combination of these two effects provides an important advantage to neurons under stress conditions.
Acknowledgments
The authors thank Joel Dietz for maintaining the mouse colony and husbandry to develop the double congenic DBA/2J substrain and Simon John (Jackson Laboratory, Bar Harbor, ME) for originally providing the Bax-deficient DBA/2J mice.
Footnotes
Supported by National Eye Institute Grant R01 EY012223 (RWN) and Vision Science CORE Grant P30 EY016665 (Department of Ophthalmology and Visual Sciences, University of Wisconsin), and unrestricted funding from Research to Prevent Blindness, Inc. (Department of Ophthalmology and Visual Sciences, University of Wisconsin).
Disclosure: H.R. Pelzel, None; C.L. Schlamp, None; M. Waclawski, None; M.K. Shaw, None; R.W. Nickells, None
References
- 1. Nickells RW. From ocular hypertension to ganglion cell death: a theoretical sequence of events leading to glaucoma. Can J Ophthalmol. 2007;42:278–287 [PubMed] [Google Scholar]
- 2. Libby RT, Li Y, Savinova OV, et al. Susceptibility to neurodegeneration in glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitmore AV, Libby RT, John SWM. Glaucoma: thinking in new ways—a role for autonomous axonal self-destruction and compartmentalised processes? Prog Retin Eye Res. 2005;24:639–662 [DOI] [PubMed] [Google Scholar]
- 4. Schlamp CL, Johnson EC, Li Y, Morrison JC, Nickells RW. Changes in Thy1 gene expression associated with damaged retinal ganglion cells. Mol Vis. 2001;7:192–201 [PubMed] [Google Scholar]
- 5. Huang W, Fileta J, Guo Y, Grosskreutz CL. Downregulation of Thy1 in retinal ganglion cells in experimental glaucoma. Curr Eye Res. 2006;31:265–271 [DOI] [PubMed] [Google Scholar]
- 6. Yang Z, Quigley HA, Pease ME, et al. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48:5539–5548 [DOI] [PubMed] [Google Scholar]
- 7. Soto I, Oglesby E, Buckingham BP, et al. Retinal ganglion cells downregulate gene expression and lose their axons within the optic nerve head in a mouse glaucoma model. J Neurosci. 2008;28:548–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pelzel HR, Schlamp CL, Nickells RW. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis (Abstract). BMC Neurosci. 2010;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weishaupt JH, Klocker N, Bahr M. Axotomy-induced early down-regulation of POU-IV class transcription factors Brn-3a and Brn-3b in retinal ganglion cells. J Mol Neurosci. 2005;26:17–25 [DOI] [PubMed] [Google Scholar]
- 10. Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Ann Rev Biochem. 2007;76:75–100 [DOI] [PubMed] [Google Scholar]
- 11. Schlamp CL, Thliveris AT, Li Y, et al. Insertion of the βGeo promoter trap into the Fem1c gene of ROSA3 mice. Mol Cell Biol. 2004;24:3794–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schlamp CL, Li Y, Dietz JA, Janssen KT, Nickells RW. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ding C, Wang P, Tian N. Effect of general anesthetics on IOP in elevated IOP mouse model. Exp Eye Res. 2011;92:512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Semaan SJ, Schlamp CL, Nickells RW. Dominant inheritance of retinal ganglion cell resistance to optic nerve crush in mice. BMC Neurosci. 2007;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlamp CL, Nickells RW. Light and dark cause a shift in the spatial expression of a neuropeptide processing enzyme in the rat retina. J Neurosci. 1996;16:2164–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreau K, Castedo M, Perfettini JL, et al. Preapoptotic chromatin condensation upstream of the mitochondrial checkpoint. J Biol Chem. 2004;279:55937–55945 [DOI] [PubMed] [Google Scholar]
- 17. Libby RT, Anderson MG, Pang I-H, et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637–648 [DOI] [PubMed] [Google Scholar]
- 18. Howell GR, Macalinao DG, Sousa GL, et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J Clin Invest. 2011;121:1429–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mosinger Ogilvie J, Deckwerth TL, Knudson CM, Korsmeyer SJ. Suppression of developmental retinal cell death but not photoreceptor degeneration in Bax-deficient mice. Invest Ophthalmol Vis Sci. 1998;39:1713–1720 [PubMed] [Google Scholar]
- 20. Jakobs TC, Libby RT, Ben Y, John SWM, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005;171:313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Howell GR, Libby RT, Jakobs TC, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J Cell Biol. 2007;179:1523–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buckingham BP, Inman DM, Lambert W, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crish SD, Sappington RM, Inman DM, Horner PJ, Calkins DJ. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci USA. 2010;107:5196–5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danias J, Lee KC, Zamora MF, et al. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: comparison with RGC loss in aging C57BL/6 mice. Invest Ophthalmol Vis Sci. 2003;44:5151–5162 [DOI] [PubMed] [Google Scholar]
- 25. Filippopoulos T, Danias J, Chen B, Podos SM, Mittag T. Topographic and morphologic analyses of retinal ganglion cell loss in old DBA/2NNia mice. Invest Ophthalmol Vis Sci. 2006;47:1968–1974 [DOI] [PubMed] [Google Scholar]
- 26. Semaan SJ, Li Y, Nickells RW. A single nucleotide polymorphism in the Bax gene promoter affects transcription and influences retinal ganglion cell death. ASN Neuro. 2010;2:e00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soto I, Pease ME, Son JL, et al. Retinal ganglion cell loss in a rat ocular hypertension model is sectorial and involves early optic nerve axon loss. Invest Ophthalmol Vis Sci. 2011;52:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bardai FH, D'Mello SR. Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3β. J Neurosci. 2011;31:1746–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rai M, Soragni E, Chou CJ, et al. Two new pimelic diphenylamide HDAC inhibitors induce sustained frataxin upregulation in cells from Friedreich's ataxia patients and in a mouse model. PLoS One. 2010;5:e8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandi C, Pinto RM, Al-Mahdawi S, et al. Prolonged treatment with pimelic o-aminobenzamide HDAC inhibitors ameliorates the disease phenotype of a Friedreich ataxia mouse model. Neurobiol Dis. 2011;42:496–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. O'Neill LP, Turner BM. Histone H4 acetylation distinguishes coding regions of the human genome from heterochromatin in a differentiation-dependent but transcription-independent manner. EMBO J. 1995;14:3946–3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pazin MJ, Kadonaga JT. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–328 [DOI] [PubMed] [Google Scholar]
- 33. Kerr JFR, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolf CM, Reynolds JE, Morana SJ, Eastman A. The temporal relationship between protein phosphatase, ICE/CED-3 proteases, intracellular acidification, and DNA fragmentation in apoptosis. Exp Cell Res. 1997;230:22–27 [DOI] [PubMed] [Google Scholar]
- 36. Kitazumi I, Tsukahara M. Regulation of DNA fragmentation: the role of caspases and phosphorylation. FEBS Lett. 2011;278:427–441 [DOI] [PubMed] [Google Scholar]
- 37. Saha RN, Pahan K. HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ. 2006;13:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biermann J, Grieshaber P, Goebel U, et al. Valproic acid–mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:526–534 [DOI] [PubMed] [Google Scholar]
- 40. Biermann J, Boyle J, Peilen A, Lagreze WA. Histone deactylase inhibitors sodium butyrate and valproic acid delay spontaneous cell death in purified rat retinal ganglion cells. Mol Vis. 2011;17:395–403 [PMC free article] [PubMed] [Google Scholar]
- 41. Crosson CE, Mani SK, Husain S, Alsarraf O, Menick DR. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest Ophthalmol Vis Sci. 2010;51:3639–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sinn DI, Kim SJ, Chu K, et al. Valproic acid-mediated neuroprotection in intracerebral hemorrhage via histone deacetylase inhibition and transcriptional activation. Neurobiol Dis. 2007;26:464–472 [DOI] [PubMed] [Google Scholar]
- 43. Cui XS, Xu YN, Shen XH, et al. Trichostatin A modulates apoptotic-related gene expression and improves embryo viability in cloned bovine embryos. Cell Reprogram. 2011;13:179–189 [DOI] [PubMed] [Google Scholar]