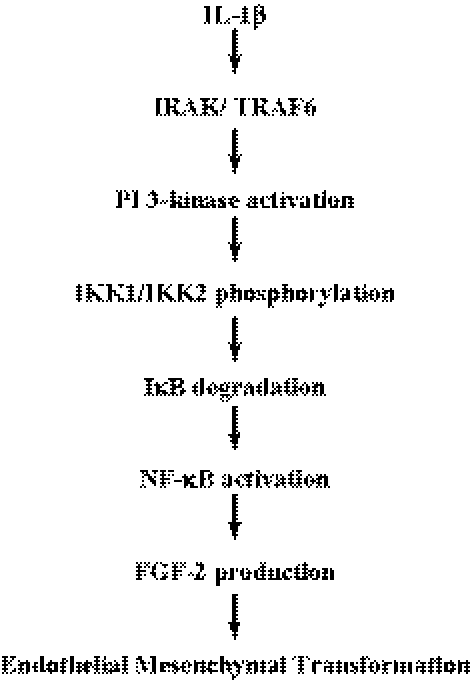
IL-1β signaling combines the canonical pathway and the PI 3-kinase signaling to upregulate FGF-2 production through NF-κβ, a transcription factor of FGF-2 gene.
Abstract
Purpose.
To determine the role of nuclear factor-κB (NF-κB) during FGF-2–mediated endothelial mesenchymal transformation (EMT) in response to interleukin (IL)-1β stimulation in corneal endothelial cells (CECs).
Methods.
Expression and/or activation of IL-1 receptor–associated protein kinase (IRAK), TNF receptor–associated factor 6 (TRAF6), phosphatidylinositol 3-kinase (PI 3-kinase), IκB kinase (IKK), IκB, NF-κB, and FGF-2 were analyzed by immunoblot analysis. Cell proliferation was measured by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay. NF-κB activity was measured by NF-κB ELISA kit, while binding of NF-κB to the promoter region of FGF-2 gene was determined by chromatin immunoprecipitation.
Results.
Brief stimulation of CECs with IL-1β upregulated expression of IRAK and TRAF6 and activated PI 3-kinase; expression of IRAK and TRAF6 reached maximum within 60 minutes, after which the expression disappeared, while PI 3-kinase activity was observed up to 4 hours after IL-1β stimulation. Use of specific inhibitor to PI 3-kinase or IRAK demonstrated that IRAK activates PI 3-kinase, the signaling of which phosphorylates IKKα/β and degrades IκB, subsequently leading to activation of NF-κB. The induction of FGF-2 by IL-1β was completely blocked by inhibitors to NF-κB activation (sulfasalazine) or PI 3-kinase (LY294002), and both inhibitors greatly blocked cell proliferation of CECs. Chromatin immunoprecipitation further demonstrated that NF-κB is the transcription factor of FGF-2 as NF-κB binds the putative NF-κB binding site of the FGF-2 promoter.
Conclusions.
These data suggest that IL-1β signaling combines the canonical pathway and the PI 3-kinase signaling to upregulate FGF-2 production through NF-κB, which plays a key role as a transcription factor of FGF-2 gene.
The retrocorneal fibrous membrane (RCFM), first described by Fuchs in 1901,1 has been observed in various clinical conditions associated with disease and damage of the corneal endothelium.2–4 The presence of RCFM (or posterior collagenous layer) posterior to the Descemet's membrane is thought to represent an end-stage disease process of the corneal endothelium, which results in functional alteration of the corneal endothelium and leads to corneal opacity and blindness. An in vitro model to elucidate the molecular mechanism of RCFM formation led us to the finding that fibroblast growth factor 2 (FGF-2) is the direct mediator of endothelial mesenchymal transformation (EMT) observed in RCFM: first, FGF-2 signaling directly regulates cell cycle progression by degrading p27Kip1 (p27), leading to a marked stimulation of cell proliferation5–7; second, FGF-2 signaling upregulates the steady state levels of α1(I) collagen RNA by stabilizing the message and subsequently facilitates synthesis and secretion of type I collagen into the extracellular space8; and third, FGF-2 signaling induces a change in cell shape from a polygonal to a fibroblastic morphology through regulation of the Rho family of small GTPases and subsequent reorganization of actin.9,10 We also reported that interleukin (IL)-1β exerts a critical role as a switch of the FGF-2–mediated EMT; IL-1β signaling greatly upregulated FGF-2 production through phosphatidylinositol 3-kinase (PI 3-kinase)/p38 pathways in corneal endothelial cells (CECs).11,12 We further confirmed such is the case in vivo, in which polymorphonuclear leukocytes (PMNs) infiltrating the anterior chamber are a major source of IL-1β.13
IL-1β, a potent proinflammatory cytokine, plays an important role in acute and chronic inflammatory diseases14–16 and a crucial role in the regulation of inflammation and wound healing on the ocular surface.17–19 Numerous studies have reported that IL-1α and IL-1β both orchestrate the inflammatory process by inducing the production and release of secondary cytokines; IL-1β stimulates the expression of a variety of genes necessary for the wound repair processes.20–22 Both IL-1α and IL-1β markedly stimulate synthesis and release of FGF-2 in a variety of cell types.23–25 Likewise, CECs both in vivo and in vitro produce all isoforms of FGF-2 in response to IL-1β stimulation through PI 3-kinase/p38 signaling.11–13 A recent study demonstrated that PI 3-kinase/AKT-dependent pathway upstream to the transcription factor nuclear factor (NF)-κB is partly involved in IL-6 gene transcription in response to IL-1.26 It is likely that the induction of FGF-2 by IL-1β is mediated by NF-κB in CECs. Activation of NF-κB by IL-1β requires the type I IL-1 receptor (IL-1R1), which recruits specific cytoplasmic proteins to transmit its signals, such as IL-1 receptor–associated protein kinase (IRAK) and TNF receptor–associated factor 6 (TRAF6).27,28 There is evidence that activation of PI 3-kinase in response to IL-1β depends on the presence of IRAK-1.28 A recent study showed that IL-1β–induced IL-6 production is mediated by both PI 3-kinase and IRAK-4.29 Thus, PI 3-kinase is involved in IL-1β–induced NF-κB activation signaling.
NF-κB signaling pathway functions in essentially all mammalian cell types and regulates genes involved in the inflammatory and immune responses.30–32 NF-κB is kept inactive in the cytoplasm through association with an inhibitory protein of the IκB family. In response to multiple stimuli, IκB becomes phosphorylated and polyubiquitinated, subsequently leading to degradation by ubiquitin-proteasome complex. As a consequence, the released free NF-κB is further activated and enters the nucleus to activate transcription of its target genes. We, therefore, hypothesized that one of the target genes of NF-κB is FGF-2, in response to IL-1β stimulation. In the present study, we showed that the IL-1β/IL-1R signaling for FGF-2 production employs PI 3-kinase signaling as part of the canonical NF-κB signaling: IRAK and TRAF6 activate PI 3-kinase, which is subsequently involved downstream of the canonical NF-κB signaling to activate IκB kinase (IKK) and NF-κB. We further demonstrated that NF-κB directly binds to the putative κB binding site of the promoter region of human FGF-2 gene. It is the first demonstration that NF-κB is indeed the transcription factor of FGF-2, the direct mediator of endothelial to mesenchymal transformation observed in RCFM.
Materials and Methods
Materials
Anti-Akt, phospho-Akt (Ser473), IRAK, TRAF6, IκB kinase α (IKKα), phospho-IKKα/β antibodies, and anti–phospho-p65 (Ser236) antibody were purchased from Cell Signaling Technology (Danvers, MA). Sulfasalazine was obtained from Calbiochem (San Diego, CA). IL-1β, LY294002, 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT), anti–β-actin antibody, and peroxidase conjugated secondary antibodies were obtained from Sigma-Aldrich (St. Louis, MO). Anti–FGF-2 antibody was purchased from Upstate (Charlottesville, VA). Anti-p65 antibody was obtained from Invitrogen (Camarillo, CA).
Rabbit Corneal Endothelial Cell Culture
Rabbit eyes were purchased from Pel Freez Biologicals (Rogers, AR). Isolation and establishment of rabbit CECs were performed as previously described.33 Briefly, the Descemet's membrane complex was treated with 0.2% collagenase and 0.05% hyaluronidase (Worthington Biochemical, Lakewood, NJ) for 90 minutes at 37°C. Primary cultured cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY) supplemented with 15% fetal bovine serum (Omega Scientific, Tarzana, CA) and 50 μg/mL of gentamicin (DMEM-15) in a 5% CO2 incubator. For subculture, confluent primary cultures were treated with 0.05% trypsin and 5 mM EDTA in phosphate-buffered saline (PBS) for 5 minutes. First passage CECs maintained in DMEM-15 were used for all experiments. For serum starvation, culture medium was changed to DMEM and cultures were maintained for 24 hours. In some experiments, pharmacologic inhibitors were used: LY294002 (PI 3-kinase inhibitor: 20 μM) or sulfasalazine (inhibitor for degradation of IκB: 2 mM).
Human Donor Corneas
Human donor corneas were obtained from National Disease Research Interchange (NDRI; Philadelphia, PA) and stored in storage media (Optisol-GS; Bausch & Lomb, Rochester, NY) at 4°C. Donor confidentiality was maintained by NDRI and this laboratory in accordance with the tenets of the Declaration of Helsinki. We accepted corneas only if the donor history and condition of the corneas suggested no damage to the corneal endothelium and only with cell numbers larger than 2300/mm2. The information about corneas used in this study is shown in Table 1.
Table 1.
Donor Information
| Donor Age | Cells (n)* | Cause of Death | Experiment |
|---|---|---|---|
| 61 | 2507 | Metastatic pancreatic cancer | Western blot analysis |
| 64 | 2791 | Renal failure | Western blot analysis |
| 69 | 2433 | Cerebrovascular accident | Western blot analysis |
| 70 | 2491 | Cerebrovascular accident | Western blot analysis |
| 72 | 2304 | Small cell carcinoma | Western blot analysis |
All corneas were used within 3 days after preservation in storage media (Optisol-GS; Bausch & Lomb) at 4°C.
Average number of CECs per mm2 of OD and OS.
Isolation and Growth of Human Corneal Endothelial Cells (hCECs)
Isolation and establishment of hCECs were performed according to previously published protocols34 with some technical modifications. Briefly, corneas stored in storage media (Optisol-GS; Bausch & Lomb) were washed several times with reduced serum media (OptiMEM-I; Gibco-BRL) containing 50 μg/mL gentamicin. After dissection of the Descemet's membrane and endothelium complex in small pieces, these were incubated overnight in reduced serum media (OptiMEM-I; Gibco-BRL) supplemented with 8% fetal bovine serum (Omega Scientific) to stabilize the cells before culture. After centrifugation, the Descemet's membrane and endothelium complex was treated with 0.2% collagenase type II and 0.05% hyaluronidase (Worthington Biochemical) for 90 minutes at 37°C. After centrifugation, the primary cells were resuspended in culture medium: reduced serum media (OptiMEM-I; Gibco-BRL) supplemented with 8% fetal bovine serum, 5 ng/mL epidermal growth factor (Upstate Biotechnologies, Lake Placid, NY), 20 ng/mL nerve growth factor (Biomedical Technologies, Stoughton, MA), 100 μg/mL bovine pituitary extract (Biomedical Technologies), 20 μg/mL ascorbic acid (Sigma-Aldrich), 200 mg/mL calcium chloride, 0.08% chondroitin sulfate (Sigma-Aldrich), 50 μg/mL gentamicin, and antibiotic-antimycotic solution (Sigma-Aldrich) diluted 1:100 and then plated in precoated 24-well tissue culture plates with coating mix (FNC; Biological Research Faculty & Facility, Inc., Ijamsville, MD). For subculture, confluent primary cultures were treated with 0.05% trypsin and 5 mM EDTA in PBS for 5 minutes. Second passage CECs were used for all experiments. For serum starvation, culture medium was changed to DMEM and cultures were maintained for 24 hours. LY294002 was used for inhibition of PI 3-kinase (20 μM).
Human Retina Pigment Epithelial (hRPE) Cell Culture
The hRPE cell stocks were a gift from Steve Ryan (Doheny Eye Institute, Los Angeles, CA); they were isolated from human fetal eyes of >22 weeks' gestation (Advanced Bioscience Resources, Inc., Alameda, CA).35 The stock cells were thawed at room temperature for 15 minutes and maintained in DMEM-15 in a 5% CO2 incubator. For subculture, confluent primary cultures were treated with 0.05% trypsin and 5 mM EDTA in PBS for 5 minutes. First passage hRPE cells maintained in DMEM-15 were used for all experiments. For serum starvation, cells were maintained for 24 hours in DMEM. Pharmacologic inhibitors used were LY294002 (PI 3-kinase inhibitor: 20 μM) and sulfasalazine (inhibitor for degradation of IκB: 2 mM).
Cytoplasmic and Nuclear Fractionation, Protein Preparation, Protein Assay, SDS-Polyacrylamide Gel Electrophoresis, Western Blot Analysis, and Immunofluorescent Analysis
All details of methods and procedures have been presented previously.5,7 The following gel concentrations were used to separate proteins: 15% for FGF-2, 12.5% gel for IκB, 10% gel for Akt, TRAF6, p65, and β-actin, and 8% gel for IRAK and IKKα.
Cell Proliferation Assay
MTT assay was used to measure cell proliferation as previously described.11 Briefly, cells were seeded in 96-well tissue culture plates at a concentration 4 × 103 cells per well. When cells reached approximately 70% confluence, the medium was changed to DMEM for serum starvation and maintained for 24 hours. The serum-starved cells were then maintained for 24 hours in each culture condition. At the end of culture, the medium was replaced with medium containing MTT (50 μg/mL) and further maintained for 2 hours at 37°C. The MTT-containing medium was discarded and 100 μL of undiluted dimethyl sulfoxide was added to the cells. After a 30-minute incubation at room temperature, absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 650 nm, using a spectrophotometric plate reader (Benchmark Plus Microplate Spectrophotometer, Bio-Rad Laboratories, Inc., Hercules, CA).
NF-κB ELISA
The nuclear fractions from the cells maintained in each culture condition were used to measure the NF-κB activity. NF-κB ELISA was performed using NF-κB ELISA kit (Rockland Immunochemicals Inc., Gilbertsville, PA) according to the manufacturer's protocol. Briefly, the 50 μg of nuclear fraction was mixed with transcription factor binding buffer supplied by the manufacturer and then applied to the each well of 96-well plate covered with oligo-DNA fragment containing consensus NF-κB binding sequence. After incubation for 16 hours at 4°C without agitation, the wells were washed five times with 200 μL PBS containing 0.05% Tween 20 (PBS-T). After the final wash, 100 μL of diluted anti–NF-κB (p65) antibody (1:100) solution was added to each well except the blank wells and the plate was incubated for 1 hour at room temperature without agitation. Each well was washed again using PBS-T and then incubated with 100 μL of diluted peroxidase conjugated secondary antibody (1:100) for 1 hour at room temperature without agitation. Each well was then treated with chemiluminescence developing solution. After a 30-minute incubation at room temperature with gentle agitation protected from light, 100 μL of the stop solution was added to each well and absorbance was measured at a wavelength of 450 nm using a spectrophotometric plate reader (Benchmark Plus Microplate Spectrophotometer, Bio-Rad Laboratories, Inc.). Cell extract of TNF-α–stimulated HeLa cell extract was also used as positive control for this assay.
Chromatin Immunoprecipitation
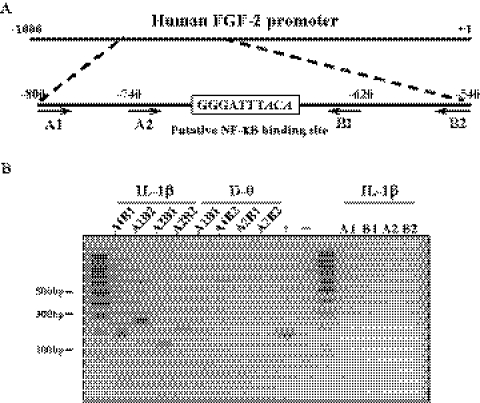
Human retina pigment epithelial cells (RPEs) were maintained with or without IL-1β for 10 minutes followed by further maintenance in serum free medium for 16 hours. After 16 hours, 2 × 107 cells were washed with PBS and cross-linked in 1% (vol/vol) formaldehyde for 10 minutes at room temperature. Chromatin immunoprecipitation (ChIP) was performed using an enzymatic ChIP kit (agarose bead; SimpleCHIP) from Cell Signaling Technology, according to the manufacturer's protocol. Briefly, nuclei were isolated by lysis of the cytoplasmic fraction, and chromatin was digested into fragments of 150 to 900 base pair (bp) by micrococcal nuclease for 30 minutes at 37°C, followed by ultrasonic disruption of the nuclear membrane using a standard microtip and a digital sonifier (Branson, Danbury, CT) with four pulses and 70% amplitude. For immunoprecipitation, three aliquots (10 μg each: 7 × 106 cell equivalents) of sheared and cross-linked chromatin were incubated with 5 μg of the mouse anti-p65 antibody, normal rabbit IgG or rabbit anti–histone H3 antibody at 4°C overnight, respectively. The normal rabbit IgG and anti–rabbit histone H3 antibody were used as negative and as positive control. After incubation with 30 μL of ChIP grade protein G-agarose beads for 2 hours at 4°C, antibody-DNA complexes were eluted from the beads and digested by 40 μg of proteinase K for 2 hours at 65°C, followed by spin column-based purification of the DNA. Transcription factor binding to FGF-2 promoter was finally assessed by PCR using the following primers: for forward, 5′-TAGGT-ACTCAATACATGCAA-3′ (A1: −800) and 5′-GCTATAT-CCTACTGAAAATT-3′ (A2: −740); for reverse, 5′-GACCTGGCATTTGCCCTAGC-3′ (B1: −620) and 5′-AATTAGACGAC-GCAGAAAGA-3′ (B2:−540). PCR conditions were as follows: 5 minutes at 94°C followed by 33 cycles of 30 seconds at 94°C, 30 seconds at 55°C, 30 seconds at 72°C, and a final extension for 2 minutes at 72°C. PCR products were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining.
Results
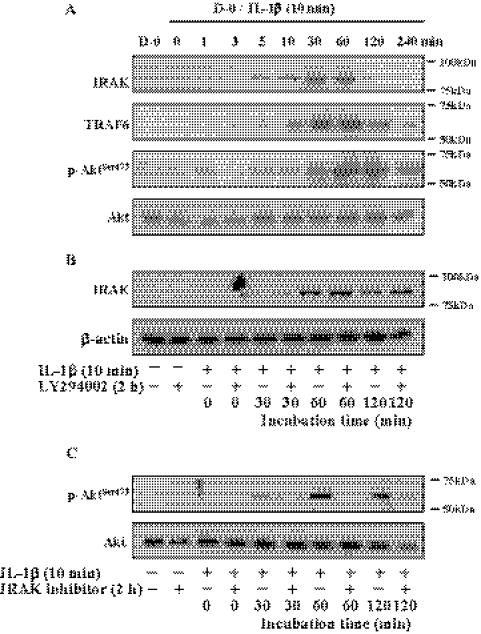
Temporal Expression of IRAK and TRAF6 and Activation of PI 3-kinase by IL-1β
We reported that PI 3-kinase is a major signaling pathway for the inductive activity of IL-1β on FGF-2 production. Because IL-1R1 signal transduction depends on downstream activation of IRAK and TRAF6, we attempted to determine the corelationship between the canonical NF-κB pathway (IRAK and TRAF6) and PI 3-kinase pathway. The central question was whether the two signaling pathways operate in parallel or are operated in a cascade of events to activate NF-κB. Serum starved CECs were stimulated with IL-1β for 1 minute to 240 minutes; immunoblotting analysis was performed to determine the expression levels of IRAK and TRAF6, and phosphorylation of Akt at Ser473 to measure PI 3-kinase activity. Expression of IRAK was observed within 5 minutes of stimulation with IL-1β, with the maximum expression reached in 30 to 60 minutes, after which its expression ceased (Fig. 1A). Expression of TRAF6 showed similar kinetics but at a little slower rate than that of IRAK; its expression was observed in 10 minutes, reaching maximum expression in 60 minutes, after which a gradual decrease in its expression was observed up to 4 hours after IL-1β stimulation. On the other hand, the high level of phosphorylation of Akt at Ser473 was maintained for up to 2 hours after IL-1β stimulation. To determine whether there was hierarchy, specific inhibitors to PI 3-kinase (LY294002) and to IRAK were employed to block the downstream signaling. After pretreatment of cells with LY294002 for 2 hours, cells were stimulated with IL-1β for 10 minutes then further maintained in serum-free medium for 30, 60, or 120 minutes. LY294002 did not hamper the transient expression of IRAK by IL-1β (Fig. 1B), while IRAK inhibitor was able to block phosphorylation of Akt at Ser473 residue after IL-1β stimulation (Fig. 1C). These findings suggest that PI 3-kinase is a part of the canonical pathway triggered by IL-1β and that IRAK is upstream to PI 3-kinase signaling.
Figure 1.
Effect of IL-1β on expression of IRAK pathway and IL-1β–mediated PI 3-kinase activation through IRAK. (A) When cells reached approximately 70% confluence, they were starved of serum for 24 hours. The serum-starved cells were treated with IL-1β (5 ng/mL) for 10 minutes and then maintained in DMEM for the designated time. At the end of treatment, cells were lysed with radio immunoprecipitation assay (RIPA) buffer and the cell debris was eliminated by centrifugation. After centrifugation, the supernatant was immunoblotted with the respective antibody. The serum-starved cells were pretreated with LY294002 (B) or IRAK inhibitor (C) for 2 hours and then treated with IL-1β for 10 minutes. The IL-1β–treated cells were maintained in DMEM for the designated time. At the end of treatment, cells were lysed and then immunoblot analysis was carried out with the respective antibody. Total Akt and β-actin were used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments. D-0, DMEM without serum.
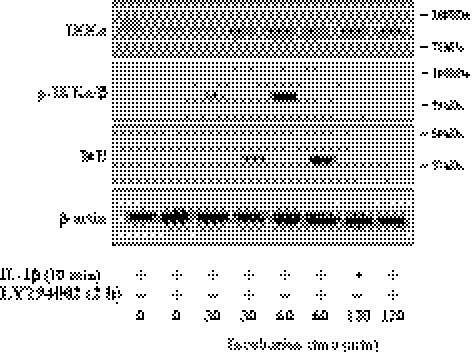
Involvement of PI 3-kinase in the Canonical NF-κB Activation Pathway
Activation of NF-κB is mediated by its IKK complex, which is composed of IKKα (IKK1) and IKKβ (IKK2) catalytic subunits and the IKKγ (NEMO) regulatory protein. On stimulation, the IKK complex phosphorylates IκB, which triggers a cascade of events, ubiquitination and proteasomal degradation of IκB, resulting in the activation of NF-κB. We, therefore, investigated whether the IRAK-activated PI 3-kinase was able to activate the IKK complex, leading to degradation of IκB. Phosphorylation of IKKα/β was observed in the cells maintained for 60 minutes after the brief stimulation with IL-1β. Pretreatment of cells with LY294002 completely abolished the activation of IKK1/2 (Fig. 2). On the other hand, expression of IκB was observed in the cells simultaneously treated with IL-1β and LY294002, suggesting that PI 3-kinase facilitates degradation of IκB, the inhibitor of NF-κB activation (Fig. 2). PI 3-kinase is further involved in phosphorylation of p65 subunit of NF-κB complex; Figure 3A showed that LY294002 completely blocked phosphorylation of p65 subunit of NF-κB at Ser236 residue. The involvement of PI 3-kinase for the NF-κB activation was further confirmed using NF-κB ELISA assay kit. Because the activated NF-κB is translocated into the nuclei, the nuclear fraction was used for this assay. In the presence of IL-1β stimulation, the NF-κB activity of the nuclear fraction was greatly upregulated, while cells treated with IL-1β and either LY294002 or sulfasalazine demonstrated the basal level of NF-κB activity (Fig. 3B).
Figure 2.
Involvement of PI 3-kinase in IL-1β–induced IκB degradation pathways. When cells reached approximately 70% confluence, they were starved of serum for 24 hours. The serum-starved cells were pretreated with LY294002 for 2 hours and then treated with IL-1β for 10 minutes. The IL-1β–treated cells were maintained in DMEM for the designated time. Total cell protein preparation and immunoblot analysis were performed as previously described in Figure 1. β-actin was used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments.
Figure 3.
Involvement of PI 3-kinase in NF-κB activation induced by IL-1β. The serum-starved cells were pretreated with LY294002 or sulfasalazine for 2 hours and then treated with IL-1β for 10 minutes. The IL-1β–treated cells were maintained in DMEM for 16 hours. (A) Cell lysate preparation and immunoblot analysis were performed as described Figure 1 with the designated antibody. (B) The serum-starved cells were pretreated with LY294002 or sulfasalazine for 2 hours during serum starvation before stimulation with IL-1β for 10 minutes. Cells were further maintained in DMEM for 16 hours and nuclear fractions were prepared. NF-κB activity was measured using NF-κB activity assay kit and quantified with a spectrophotometric plate reader at wavelengths of 450 nm. For positive control (+), cell extract from TNF-α–stimulated HeLa cell was used. Data were normalized to the nuclear fraction of serum-starved DMEM-maintained cells. These data were representative of three experiments. IL, IL-1β; LY, LY294002; Sul, sulfasalazine.
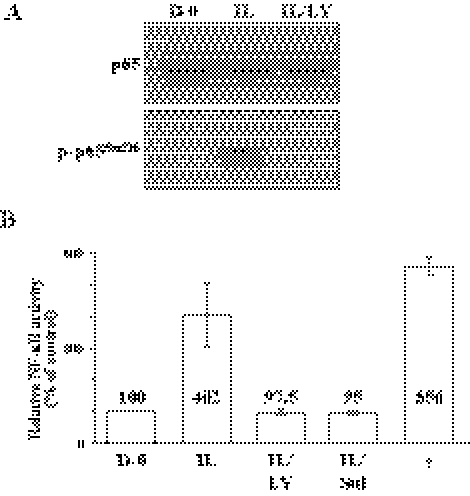
Involvement of NF-κB on the IL-1β–Mediated FGF-2 Production
We reported that IL-1β greatly upregulated FGF-2 production in CECs through PI 3-kinase/p38 pathways both in vivo and in vitro.11–13 The findings shown in Figure 1 and Figure 2 suggest that the inductive activity of IL-1β on FGF-2 production may be facilitated by the activated NF-κB signaling. When serum-starved cells were stimulated with IL-1β, there was a marked increase of FGF-2 production (Fig. 4A), whereas both sulfasalazine and PI 3-kinase inhibitor completely abolished the inductive activity of IL-1β on FGF-2. Because the IL-1β–induced FGF-2 is the direct mediator of EMT observed in CECs, we further determined whether the activated NF-κB signaling was able to stimulate cell proliferation of CECs, one of the major events observed during EMT process. Figure 4B showed that IL-1β greatly stimulated cell proliferation of CECs, while both sulfasalazine and LY294002 are able to block the proliferation of CECs. These data suggest that the induction of FGF-2 by IL-1β is promoted by NF-κB activated by the cascade activation of the signaling proteins, IRAK, TRAF6, PI 3-kinase, IKK, and IκB.
Figure 4.
Effect of NF-κB activity induced by IL-1β on FGF-2 production and inhibitory effect of LY294002 and sulfasalzine on cell proliferation stimulated by IL-1β treatment. (A) The serum-starved cells were pretreated with LY294002 or sulfasalazine for 2 hours during serum starvation before stimulation with IL-1β for 10 minutes. Cells were further maintained in DMEM for 16 hours. The cell lysate preparation and immunoblot analysis were performed as described Figure 1 with the anti–FGF-2 antibody. β-actin was used to control protein concentration on immunoblot analysis. The results represent data obtained in three independent experiments. (B) Cell proliferation was determined by MTT assay. The serum-starved cells were pretreated with LY294002 or sulfasalzine for 2 hours and then stimulated in DMEM with IL-1β for 10 minutes. Cells were further maintained in DMEM for 24 hours. At the end of incubation, MTT was added for 2 hours and intracellular purple formazan, the MTT metabolic product by the action of dehydrogenase enzymes of metabolically active cells, was quantified with a spectrophotometric plate reader at dual wavelengths of 570 and 650 nm. Data were normalized to DMEM-treated cells. The results represent data obtained in three independent experiments. DS, dimethyl sulfoxide used to dissolve sulfasalazine.
IκB degradation allows NF-κB to translocate to the nucleus and bind DNA to exert its role as a transcription factor of its target genes. It is, therefore, likely that FGF-2 is a target gene of NF-κB. We chose ChIP assay to prove this, but there were technical problems: the promoter sequence of FGF-2 gene in rabbit is not fully identified; and use of hCECs was not feasible because insufficient hCEC cell numbers were available for the ChIP assay. Nonetheless, the promoter of the human FGF-2 gene contains a consensus sequence of κB binding site with two mismatches (GGGATTTACA; mismatches in italic). Therefore, the first, second, and third passage hRPE cells were used. We first confirmed that hRPE cells upregulated FGF-2 production in response to IL-1β stimulation regardless of the passage number: a brief stimulation of hRPE cells with IL-1β markedly upregulated FGF-2 production (Fig. 5). ChIP assay was then performed with the nuclease-treated cross-linked chromatin obtained from the first passage hRPE cells stimulated with IL-1β; immunoprecipitation was performed with either anti-p65 antibody, normal IgG (negative control), or anti–histone H3 antibody (positive control). Binding of NF-κB to FGF-2 promoter was assessed by PCR using a combination of four primers to generate four DNA products containing κB-binding DNA sequences (Fig. 6A). The stimulated cells with IL-1β showed the expected PCR products of 120, 180, 200, or 260 base pairs (Fig. 6B), despite the fact that κB binding site of human FGF-2 gene has two mismatches. On the other hand, hRPE cells in the absence of IL-1β stimulation failed to generate the PCR products, suggesting a lack of binding of NF-κB to the putative κB-binding DNA sequences of FGF-2 gene (Fig. 6B).
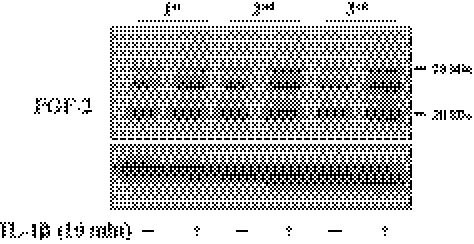
Figure 5.
Inductive activity of IL-1β on FGF-2 production in hRPE cells. When hRPE cells reached approximately 70% confluence in each culture passage, they were starved of serum for 24 hours. The serum-starved cells were treated with IL-1β (5 ng/mL) for 10 minutes and then maintained in DMEM for 16 hours. The cell lysate preparation and immunoblot analysis were performed as described in Figure 1 with the anti–FGF-2 antibody. β-actin was used to control protein concentration on immunoblot analysis. 1st, 2nd, and 3rd, designate the first, second, and third passage cells. The results represent data obtained in three independent experiments.
Figure 6.
Direct interaction of NF-κB to the human FGF-2 promoter. (A) Schematic illustration of a proposed NF-κB binding site of human FGF-2 promoter. The DNA sequence that is different from conserved NF-κB binding sites (GGGATTTCCC) is designated with italic. A1, A2 and B1, B2 indicated the location of DNA oligo PCR primer used in ChIP assay. (B) The serum-starved human RPEs were stimulated with IL-1β (5 ng/mL) for 1 hour. The cross-linked cell lysates were treated with nuclease and immunoprecipitated with anti–NF-κB antibody. The purified DNA samples were subjected to PCR using the primer sets as designated. The expected PCR product sizes are: A1–B1, 180 bp; A1–B2, 260 bp; A2–B1, 120 bp; A2–B2, 200 bp. Anti–histone H3 antibody was used for positive control (+) and normal rabbit IgG was used for negative control (−). The results represent data obtained in three independent experiments.
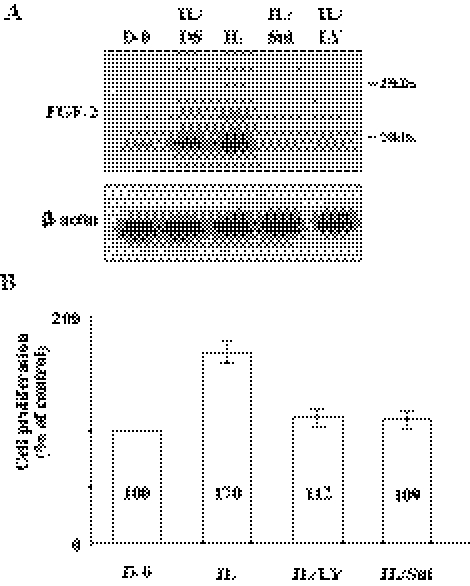
Involvement of PI 3-kinase for FGF-2 Production in hCECs
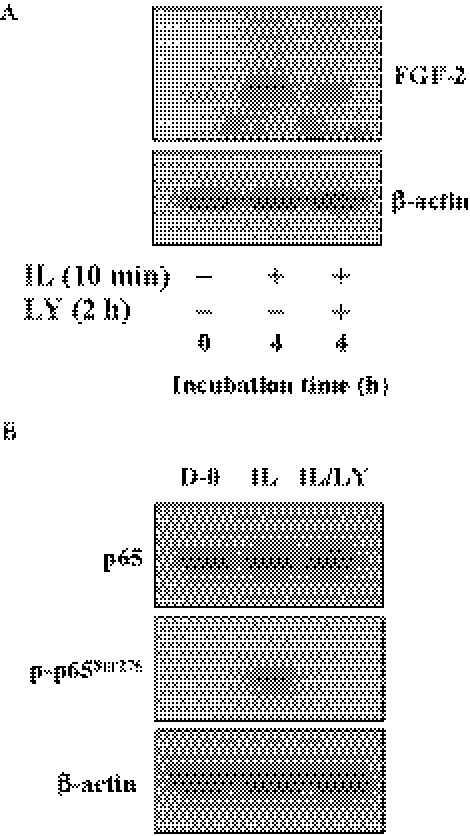
Finally, we confirmed that upregulation of FGF-2 triggered by IL-1β through PI 3-kinase pathway in hCECs was identical with that observed in rabbit CECs. When hCECs were stimulated with IL-1β for 10 minutes, FGF-2 production is greatly upregulated within 4 hours. Pretreatment of hCECs with LY294002 markedly hampered FGF-2 production (Fig. 7A). We also confirmed that IL-1β signaling facilitated phosphorylation of p65 subunit of NF-κB through PI 3-kinase (Fig. 7B). These data indicate that both CECs, regardless of species, employ identical signal transduction (PI 3-kinase/NF-κB pathway) for the inductive activity of IL-1β on FGF-2.
Figure 7.
Activation of NF-κB induced by IL-1β through PI 3-kinase in human CECs. (A) The serum-starved human CECs were pretreated with LY294002 for 2 hours and then treated with IL-1β (5 ng/mL) for 10 minutes. The IL-1β–treated cells were maintained in DMEM for 4 hours and immunoblot analyses were performed with anti–FGF-2 antibody. (B) The serum-starved human CECs were pretreated with LY294002 for 2 hours and then treated with IL-1β (5 ng/mL) for 10 minutes. The IL-1β–treated cells were maintained in DMEM for 16 hours. The cell lysate preparation and immunoblot analysis were performed as described Figure 1 with designated antibody. These results indicated that PI 3-kinase also involved activation of NF-κB stimulated by IL-1β in human CECs. β-actin was used to control protein concentration on immunoblot analysis. These data were representative of three experiments.
Discussion
Human CECs remain arrested at G1 phase of the cell cycle throughout their lifespan.36,37 Such characteristic behavior of cell proliferation dictates the regenerative (physiological) wound healing process occurring in the corneal endothelium: CECs do not use cell division to replace the lost cells; instead, they use migration and attenuation to cover the denuded area. On the other hand, in the nonregenerative (pathologic) wound healing process, CECs are transformed into mesenchymal cells that subsequently produce fibrillar extracellular matrix (ECM) in the basement membrane environment. Thus, corneal fibrosis represents a significant pathophysiological problem—one that causes blindness by physically blocking light transmittance. One clinical example of corneal fibrosis observed in corneal endothelium is the development of a RCFM in Descemet's membrane.1–4 It has been known that RCFM production can have epithelial, keratocytic, and endothelial origins.4 We have investigated the molecular mechanism of RCFM production derived from endothelial origin. An in vitro model to elucidate the molecular mechanism of endothelial to mesenchymal transformation observed in RCFM led us to the finding that FGF-2 is the direct mediator of EMT (cell proliferation, loss of contact inhibited monolayer, and production of fibrillar ECM proteins).5–13,38 Our in vitro and in vivo studies demonstrated that IL-1β induces FGF-2 production in CECs through PI 3-kinase and p38 pathways.11–13
In the present study, we further explored the mechanism of the inductive activity of IL-1β on FGF-2 production. We showed that temporal expression of IRAK and TRAF6 (the two major early signaling proteins of the canonical pathway) takes place before the activation of PI 3-kinase and that PI 3-kinase is the downstream effector protein to the canonical NF-κB pathway (Fig. 1). Of great interest, PI 3-kinase signaling subsequently phosphorylates IKKα/β leading to degradation of IκB protein (Fig. 2). Our study also showed that IRAK is essential for the IL-1β–induced activation of PI 3-kinase. Of interest, the PI 3-kinase activated by the canonical signaling directly involves the activation pathway of NF-κB; PI 3-kinase inhibitor inhibits NF-κB activation (Fig. 3). At present, the mechanism by which IRAK facilitates PI 3-kinase activation is not known. Earlier studies demonstrated that the p85 regulatory subunit of the PI 3-kinase directly interacts with the activated IL-1R complex.39,40 It is likely that such interaction may take place in CECs stimulated with IL-1β. Degradation of IκB leads to NF-κB activation; and the activated NF-κB is then translocated into the nuclei and binds directly to the promoter of FGF-2 gene (the κB binding site), suggesting that NF-κB is the transcription factor of FGF-2 gene (Figs. 6A, 6B). The IL-1β–upregulated FGF-2 via NF-κB signaling then exerted its action for endothelial to mesenchymal transformation5–13,38 (Fig. 8). This is the first demonstration that FGF-2 is the target gene of the transcription factor NF-κB in response to IL-1β stimulation. We previously showed that p38 is the downstream effector molecule in PI 3-kinase pathway stimulated by IL-1β.28,39–41 Therefore, further examination is needed to determine whether p38 is also involved in PI 3-kinase–mediated NF-κB activation.
Figure 8.
Schematic presentation of the inductive activity of IL-1β on FGF-2, the direct mediator of EMT. On stimulation of cells with IL-1β, the canonical signaling components, IRAK and TRAF6, are transiently expressed. IRAK activates PI 3-kinase, which subsequently phosphorylates IKK complex, causing degradation of IκB. Removal of IκB from the cytoplasmic NF-κB/IκB complex leads to phosphorylation of NF-κB, which is translocated into the nuclei. The transcription factor NF-κB then binds to the κB binding site of the promoter in FGF-2 gene. Such induced FGF-2, then, exert EMT process, leading to irreversible fibrosis, RCFM.
Because RCFM production begins with injury-mediated inflammation, our findings indicate a linkage between the injury-mediated inflammation stage (IL-1β pathway) and the subsequent EMT process (FGF-2 pathway) mediated by PI 3-kinase. Our previous data demonstrated that IL-1β activates PI 3-kinase and p38 in a biphasic fashion; the first wave of activation is triggered by IL-1β and is subsequently involved in the inductive activity of IL-1β on FGF-2 production; the IL-1β–induced FGF-2 then initiates the second wave of activation, which induces cell migration during the course of EMT. Our findings further suggest that NF-κB, acting as a transcription factor, is the key signal to trigger the cellular responses mediated by FGF-2. Thus, blockade of NF-κB activation is sufficient to inhibit cell proliferation (one of the major phenotypes altered during EMT) stimulated by the IL-1β–induced FGF-2 (Fig. 4B). Our long journey to elucidate the molecular mechanism of the endothelial originated RCFM in vivo has come to the conclusion that the extent of inflammation predicts whether the corneal endothelium may face the nonregenerative wound repair process. Namely, continuous inflammatory responses cause persistent infiltration of PMNs, which continuously release IL-1β into the anterior chamber (and/or cornea). The cytokine then triggers the canonical NF-κB pathway, which includes PI 3-kinase as a signaling component, to activate the transcription factor NF-κB. The activated NF-κB binds to κB DNA elements of FGF-2 gene and turns on the gene expression of FGF-2. The amount of FGF-2 is also proportional to the degree of the inflammation and the presence of IL-1β. The persistent production of FGF-2 causes endothelial to mesenchymal transformation at the cellular level, leading to the final stage of irreversible fibrosis (RCFM). The whole spectrum of the complex mechanism involved in RCFM formation has been extensively identified from this laboratory, and such information allows us to appropriately design blockade of RCFM production at multiple levels. When and where to stop and how to block can be predicted from the known signaling pathways of the injury-mediated inflammation and EMT. Thus, there will be many therapeutic approaches depending on the stage of the endothelial pathology.
Footnotes
Supported by National Institutes of Health/National Eye Institute Grants EY06431 and EY03040; and Research to Prevent Blindness, New York, New York.
Disclosure: J.G. Lee, None; E.P. Kay, None
References
- 1. Fuchs E. On keratoplasty. Z Augenheilkd. 1901;5:1–5 [Google Scholar]
- 2. Rodrigues MM, Waring GO, Laibson PR, Weinreb S. Endothelial alterations in congenital corneal dystrophies. Am J Ophthalmol. 1975;80:678–689 [DOI] [PubMed] [Google Scholar]
- 3. Waring GO, Laibson PR, Rodrigues M. Clinical and pathologic alterations of Descemet's membrane: with emphasis on endothelial metaplasia. Surv Ophthalmol. 1974;18:325–331 [Google Scholar]
- 4. Jakobiec FA, Bhat P. Retrocorneal membranes: a comparative immunohistochemical analysis of keratocytic, endothelial, and epithelial origins. Am J Ophthalmol. 2010;150:230–242 [DOI] [PubMed] [Google Scholar]
- 5. Lee JG, Kay EP. Two populations of p27 use differential kinetics to phosphorylate Ser-10 and Thr-187 via phosphatidylinositol 3-kinase in response to fibroblast growth factor-2 stimulation. J Biol Chem. 2007;282:6444–6454 [DOI] [PubMed] [Google Scholar]
- 6. Lee JG, Kay EP. Involvement of two distinct ubiquitin E3 ligase systems for p27 degradation in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2008;49:189–196 [DOI] [PubMed] [Google Scholar]
- 7. Lee JG, Kay EP. PI 3-kinase/Rac1 and ERK1/2 regulate FGF-2-mediated cell proliferation through phosphorylation of p27 at Ser10 by KIS and at Thr187 by Cdc25A/Cdk2. Invest Ophthalmol Vis Sci. 2011;52:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ko MK, Kay EP. Regulatory role of FGF-2 on type I collagen expression during endothelial mesenchymal transformation. Invest Ophthalmol Vis Sci. 2005;46:4495–4503 [DOI] [PubMed] [Google Scholar]
- 9. Lee JG, Kay EP. Cross-talk among Rho GTPases acting downstream of PI 3-kinase induces mesenchymal transformation of corneal endothelial cells mediated by FGF-2. Invest Ophthalmol Vis Sci. 2006;47:2358–2368 [DOI] [PubMed] [Google Scholar]
- 10. Lee JG, Kay EP. FGF-2-induced wound healing in corneal endothelial cells requires Cdc42 activation and Rho inactivation through the phosphatidylinositol 3-kinase pathway. Invest Ophthalmol Vis Sci. 2006;47:1376–1386 [DOI] [PubMed] [Google Scholar]
- 11. Lee JG, Kay EP. Common and distinct pathways for cellular activities in FGF-2 signaling induced by IL-1β in corneal endothelial cells. Invest Ophthalmol Vis Sci. 2009;50:2067–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee HT, Lee JG, Na M, Kay EP. FGF-2 induced by interleukin-1β through the action of phosphatidylinositol 3-kinase mediates endothelial mesenchymal transformation in corneal endothelial cells. J Biol Chem. 2004;279:32325–32332 [DOI] [PubMed] [Google Scholar]
- 13. Song J-S, Lee JG, Kay EP. Induction of FGF-2 synthesis by IL-1β in aqueous humor through PI 3-kinase and p38 in rabbit corneal endothelium. Invest Ophthalmol Vis Sci. 2010;51:822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241 [DOI] [PubMed] [Google Scholar]
- 15. Dinarello CA. Blocking interleukin-1β in acute and chronic autoinflammatory diseases. J Int Med. 2011;269:16–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dinarello CA. A clinical perspective of IL-1 as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217 [DOI] [PubMed] [Google Scholar]
- 17. Moore JE, McMullen TCB, Campbell IL, et al. The inflammatory milieu associated with conjunctivalized cornea and its alteration with IL-1 RA gene therapy. Invest Ophthalmol Vis Sci. 2002;43:2905–2915 [PubMed] [Google Scholar]
- 18. McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ. Defensin expression by the cornea: multiple signaling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Djalilan AR, Nagineni CN, Mahesh SP, Smith JA, Nussenblatt RB, Hooks JJ. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea. 2006;25:709–714 [DOI] [PubMed] [Google Scholar]
- 20. Jung YD, Liu W, Reinmuth N, et al. Vascular endothelial growth factor is upregulated by interleukin-1β in human vascular smooth muscle cells via the p38 mitogen-activated protein kinase pathway. Angiogenesis. 2001;4:155–162 [DOI] [PubMed] [Google Scholar]
- 21. Im H-J, Li X, Muddasani P, et al. Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J Cell Physiol. 2008;215:452–463 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Watari K, Nakao S, Fotovati A, et al. Role of macrophages in inflammatory lymphangiogenesis: enhanced production of vascular endothelial growth factor C and D through NF-κB activation. Biochem Biophys Res Commun. 2008;377:826–831 [DOI] [PubMed] [Google Scholar]
- 23. Cronauer MV, Stadlmann S, Klocker H, et al. Basic fibroblast growth factor synthesis by human peritoneal mesothelial cells: induction by interleukin-1. Am J Pathol. 1999;155:1977–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sobue T, Zhang X, Florkiewicz RZ, Hurley MM. Interleukin-1 regulates FGF-2 mRNA and localization of FGF-2 protein in human osteoblasts. Biochem Biophys Res Commun. 2001;286:33–40 [DOI] [PubMed] [Google Scholar]
- 25. Hayashi T, Matsuoka K, Saitoh M, Takeda S, Kimura M. Influence of α-tumor necrosis factor and β-interleukin-1 on production of angiogenetic factors and thymidine phosphorylase activity in immortalized human decidual fibroblasts in vitro. J Obstet Gynaecol Res. 2006;32:15–22 [DOI] [PubMed] [Google Scholar]
- 26. Cahill CM, Rogers J. Interleukin (IL) 1β induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IκB kinase α pathway targeting activator protein-1. J Biol Chem. 2008;283:25900–25912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamazaki K, Gohda J, Kanayama A, et al. Two mechanically and temporally distinct NF-κB activation pathways in IL-1 signaling. Sci Signal. 2009;2:ra66. [DOI] [PubMed] [Google Scholar]
- 28. Neumann D, Lienenklaus S, Rosati O, Martin MU. IL-1-induced phosphorylation of PKB/AKT depends on the presence of IRAK-1. Eur J Immunol. 2002;32:3689–3698 [DOI] [PubMed] [Google Scholar]
- 29. Eda H, Burnette BL, Shimada H, Hope HR, Monahan JB. Interleukin-1β-induced interleukin-6 production in A549 cells is mediated by both phosphatidylinositol 3-kinase and interleukin-1 receptor-associated kinase-4. Cell Biol Int. 2011;35:355–358 [DOI] [PubMed] [Google Scholar]
- 30. Chen LF, Greene WC. Shaping the nuclear action of NFκB. Nat Rev Mol Cell Biol. 2004;5:392–401 [DOI] [PubMed] [Google Scholar]
- 31. Kim HJ, Hawke N, Baldwin AS. NF-κB and IKK as therapeutic targets in cancer. Cell Death Differ. 2006;13:738–747 [DOI] [PubMed] [Google Scholar]
- 32. Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132:344–362 [DOI] [PubMed] [Google Scholar]
- 33. Kay EP, Smith RE, Nimni ME. Basement membrane collagen synthesis by rabbit corneal endothelial cells in culture. Evidence for an alpha chain derived from a larger biosynthetic precursor. J Biol Chem. 1982;257:7116–7121 [PubMed] [Google Scholar]
- 34. Lee JG, Song J-S, Smith RE, Kay EP. Human corneal endothelial cells employ phosphorylation of p27Kip1 at both Ser10 and Thr187 sites for FGF-2-mediated cell proliferation via PI 3-kinase. Invest Ophthalmol Vis Sci. 2011;52:8216–8223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Senoo T, Joyce NC. Cell cycle kinetics in corneal endothelium from old and young donors. Invest Ophthalmol Vis Sci. 2000;41:660–667 [PubMed] [Google Scholar]
- 37. Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95:16–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee JG, Ko M, Kay EP. Endothelial mesenchymal transformation mediated by IL-1β- induced FGF-2 in corneal endothelial cells. Exp Eye Res. 2012;95:35–39 [DOI] [PubMed] [Google Scholar]
- 39. Reddy SAG, Huang JH, Liao WS-L. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NF-κB and AP-1 activation. J Biol Chem. 1997;272:29167–29173 [DOI] [PubMed] [Google Scholar]
- 40. Marmiroli S, Bavelloni A, Faenza I, et al. Phosphatidylinositol 3-kinase is recruited to a specific site in the activated IL-1 receptor 1. FEBS Lett. 1998;438:49–54 [DOI] [PubMed] [Google Scholar]
- 41. Wang KZQ, Wara-Aswapati N, Boch JA, et al. TRAF6 activation of PI 3-kinase–dependent cytoskeletal changes is cooperative with Ras and is mediated by an interaction with cytoplasmic Src. J Cell Sci. 2006;119:1579–1591 [DOI] [PubMed] [Google Scholar]