Reduced trabecular meshwork (TM) cellularity is observed with age and correlates with increased outflow resistance. Stem cells from TM are multipotent, can become phagocytic TM cells. These stem cells offer a potential for development of a novel stem cell–based therapy for glaucoma.
Abstract
Purpose.
To isolate and characterize stem cells from human trabecular meshwork (TM) and to investigate the potential of these stem cells to differentiate into TM cells.
Methods.
Human trabecular meshwork stem cells (TMSCs) were isolated as side population cells by fluorescence-activated cell sorting or isolated by clonal cultures. Passaged TMSCs were compared with primary TM cells by immunostaining and quantitative RT-PCR. TMSC purity was assessed by flow cytometry and TMSC multipotency was examined by induction of neural cells, adipocytes, keratocytes, or TM cells. Differential gene expression was detected by quantitative RT-PCR, immunostaining, and immunoblotting. TM cell function was evaluated by phagocytic assay using inactivated Staphylococcus aureus bioparticles.
Results.
Side population and clonal isolated cells expressed stem cell markers ABCG2, Notch1, OCT-3/4, AnkG, and MUC1 but not TM markers AQP1, MGP, CHI3L1, or TIMP3. Passaged TMSCs are a homogeneous population with >95% cells positive to CD73, CD90, CD166, or Bmi1. TMSCs exhibited multipotent ability of differentiation into a variety of cell types with expression of neural markers neurofilament, β-tubulin III, GFAP; or keratocyte-specific markers keratan sulfate and keratocan; or adipocyte markers ap2 and leptin. TMSC readily differentiated into TM cells with phagocytic function and expression of TM markers AQP1, CHI3L1, and TIMP3.
Conclusions.
TMSCs, isolated as side population or as clones, express specific stem cell markers, are homogeneous and multipotent, with the ability to differentiate into phagocytic TM cells. These cells offer a potential for development of a novel stem cell–based therapy for glaucoma.
Glaucoma is a leading cause of irreversible blindness worldwide and the second leading cause of blindness overall in the United States.1 Glaucoma damages the optic nerve leading to progressive visual field loss and eventual blindness. For most forms of glaucoma, elevated intraocular pressure (IOP) and aging remain the most important risk factors.2 Although the pathogenesis is multifactorial, optic nerve damage is strongly associated with increased IOP. Experimental animal models demonstrate that elevated IOP is sufficient to produce glaucoma-like optic nerve damage.3
The main outflow pathway for aqueous humor in the eye consists of a series of endothelial cell–lined channels in the angle of the anterior chamber comprising the trabecular meshwork (TM), Schlemm's canal, the collector channels, and the episcleral venous system. The TM, especially the juxtacanalicular region, and the inner wall of Schlemm's canal are thought to be the source of the most resistance to aqueous outflow. The cells lining the lamellae of the TM play two primary roles: secretion of specific enzymes and extracellular matrix and phagocytosis of debris in the aqueous humor.4 Both functions help maintain aqueous outflow over the trabecular lamellae.5 Reduced cellularity within the TM is observed with age and correlates with increased outflow resistance and elevated IOP.6–9 Current therapies for IOP control involve pharmacologic reduction of aqueous humor production and surgical enhancement of outflow. These therapies are effective but still have significant limitations; toxicity, side effects, complications, failure, and patient noncompliance are common.
Resident pools of somatic stem cells in many organs are responsible for tissue maintenance and repair.10 Many of these stem cells expanded in vitro exhibit effective tissue regeneration when introduced to pathologic tissues in vivo.11 Cell-based restoration of TM function to glaucomatous eyes is a potential therapy not yet explored due to lack of cells that maintain TM phenotype in vitro and home to the TM in vivo. Several studies have reported cells in TM which express properties of adult stem cells (Challa P, et al. IOVS 2003;44:ARVO E-Abstract 3164)12–14; however, characterization of the presumptive TM stem cells (TMSCs) is incomplete. Our present study demonstrates isolation of a multipotent population of adult stem cells from human TM, which can be greatly expanded in vitro while maintaining the novel ability to differentiate to TM cells.
Materials and Methods
Primary Cell Culture
Deidentified human corneas were obtained from the Center for Organ Recovery and Education (Pittsburgh, PA). Donor human corneas including scleral rim and TM not usable for transplantation were used for experiments. Cells from three donors at 23, 41, and 55 years of age were used in the experiments shown. For each cell population, every experiment was repeated at least once. After careful removal of the iris, a cut was made through the inner edge of Schwalbe's line and the TM tissue was peeled off. We processed TMSCs as either explant culture or dissociated cell culture. For explant culture, the tissue was cut into pieces and put in a 25-cm2 culture flask. Stem cell growth medium (SCGM) was added and the culture was left undisturbed for 10 to 14 days. For dissociated cell culture, the dissected TM tissue was digested in 0.3 mg/mL collagenase type L (Sigma-Aldrich, St. Louis, MO) in Dulbecco's modified Eagle's medium (DMEM) at 37°C for 20 to 22 hours. After digestion, the cells were filtered through a 70-μm mesh and washed twice with DMEM. Cells were seeded at 2 × 104 cells/cm2 in SCGM. For both cultures, cells were passaged at 80–90% confluency by trypsinization and seeded at 2 to 5 × 103 cells/cm2 in SCGM or seeded for clonal expansion by limiting dilution at 30 cells in a 96-well plate (0.3 cells/well). On average, approximately 1% to 10% of the 96 wells had clones with small cells, which were picked up for subcultivation at 2 to 3 weeks after seeding. Among them, approximately a third to a half could be continuously passaged up to 30 to 50 population doublings. At least six clones from three donors were used and repeated in this study. SCGM was modified from a corneal endothelial cell culture medium15 containing multipurpose reduced-serum media (OptiMEM-1; Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS; Hyclone, Logan, UT), 10 ng/mL EGF (Upstate Biotechnologies, Billerica, MA), 100 μg/mL bovine pituitary extract (Biomedical Technologies, Stoughton, MA), 20 μg/mL ascorbic acid, 200 μg/mL calcium chloride, 0.08% chondroitin sulfate (Sigma-Aldrich), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 50 μg/mL gentamicin (Sigma-Aldrich). Primary TM cells were cultured in DMEM without FBS or any growth factors.
Isolation of Stem Cells by Side Population Cell Sorting
Side population (SP) cell sorting was carried out as previously described16–18 (DyeCycle Violet [DCV] dye; Invitrogen) with minor modifications. After 2 to 3 passages, 5 × 105 to 2 × 106 trypsinized cells were incubated at 1 × 106 cells/mL in prewarmed DMEM with 2% FBS and 10 μM DCV for 100 minutes at 37°C. To inhibit DCV efflux, 1 × 105 to 5 × 105 cells were preincubated for 20 minutes with 25 μg/mL fumitremorgin C (a gift from Susan Bates, National Institutes of Health) before DCV incubation. After staining, the cells were washed twice in Hank's balanced salt solution with 2% FBS and stored on ice. Immediately before sorting, 2 μg/mL propidium iodide (PI) was added to identify nonviable cells. Cells were analyzed on a flow cytometer high-speed cell sorter (FACSAria; BD Biosciences, San Jose, CA), using 405-nm excitation. Cells showing reduced fluorescence at both blue (450 nm) and red (670 nm), designated SP cells, were collected. A small proportion of non-SP cells were collected separately as control. Dead cells stained with PI were omitted from the population. The sorted SP and non-SP cells were cultured and passaged without cloning for further studies.
Flow Cytometry
Clonal passaged TMSCs were trypsinized and cell suspensions were passed through a 40-μm filter-cap tube to remove debris. Antibodies used are listed in Table 1. For cell surface marker staining, fluorescent-conjugated antibodies (CD73-PE, CD90-Alexa 647, CD166-FITC) or appropriate isotype controls were incubated with cells on ice for 30 minutes followed by washing in 1% bovine serum albumin (BSA) in PBS once and resuspended in the same buffer for flow analysis using a flow cytometer (FACSAria; BD Biosciences). PI (2 μg/mL) was added to identify nonviable cells. For unconjugated antibody staining (Bmi1, AQP1, CHI3L1), cells were fixed in 1% paraformaldehyde on ice for 30 minutes and permeabilized with 0.1% Triton X-100 for 10 minutes. Cells were blocked in 1% BSA and stained with primary antibodies followed by secondary antibodies, 30 minutes on ice each. After being washed, cells were resuspended in 1% BSA for flow analysis.
Table 1.
Primary Antibodies Used for Immunostaining, Western Blotting, and Flow Cytometry
| Antibody | Type | Source | Catalog Number |
|---|---|---|---|
| ABCG2 | Mouse monoclonal | Chemicon (Billerica, MA) | MAB4146 |
| Mucin 1 | Mouse monoclonal | Santa Cruz (Santa Cruz, CA) | Sc-7313 |
| Ankyrin G | Mouse monoclonal | Santa Cruz | Sc-12719 |
| AQP1 | Rabbit polyclonal | Santa Cruz | Sc-20810 |
| MGP | Mouse monoclonal | Santa Cruz | Sc-81546 |
| CHI3L1 | Goat polyclonal | R&D Systems (Minneapolis, MN) | AF2599 |
| TIMP3 | Mouse monoclonal | Santa Cruz | Sc-101578 |
| MYOC | Rabbit polyclonal | Santa Cruz | Sc-20976 |
| OCT-3/4 | Rabbit polyclonal | Santa Cruz | Sc-9081 |
| Notch1 | Mouse monoclonal | BD Pharmingen (San Diego, CA) | 552466 |
| CD73 | PE-conjugated | eBioscience (San Diego, CA) | 12–0731-81 |
| CD90 | Alexa Fluor 647-conjugated | Biolegend (San Diego, CA) | 328115 |
| CD166 | FITC-conjugated | MBL International (Woburn, MA) | K0044–4 |
| Bmi1 | Mouse monoclonal | Millipore (Billericia, MA) | 05–637 |
| Neurofilament | Mouse monoclonal | Sigma (St. Louis, MO) | N0142 |
| β-Tubulin III | Mouse monoclonal | Chemicon | MAB1637 |
| GFAP | Mouse monoclonal | Chemicon | MAB360 |
| Keratan sulfate | Mouse monoclonal | Kind gift from Bruce Katerson | |
| Keratocan | Goat polyclonal | Kind gift from Winston Kao | |
| α-Tubulin | Mouse monoclonal | Sigma | T5168 |
Multipotential Differentiation of Clonal Isolated TMSCs
Neural Differentiation.
Clonal TMSCs were seeded onto coated 35-mm dishes (FNC Coating Mix; AthenaES, Baltimore, MD) at 1 × 104 cells/cm2 in neural differentiation medium (NDM) containing commercial medium (Advanced DMEM; Gibco, Carlsbad, CA) with 10 ng/mL EGF, 10 ng/mL FGF2, and 1 μM all-trans-retinoic acid.16 The medium was changed every 3 days and fresh 1 μM all-trans-retinoic acid was added each time. The cells were cultured for 1 to 2 weeks for neural induction.
Adipogenic Differentiation.
Adipocytes were induced as previously described,19 with minor modifications. TMSCs were seeded onto 1% gelatin-coated (Sigma-Aldrich) 35-mm dishes at 2 × 104 cells/cm2 and cultured in adipogenic differentiation medium (ADM) for 7 days, switched to adipogenic maintenance medium (AMM) for 4 days, then cycled again through ADM (7 days) and AMM (4 days) before fixation for histology or lysis for RNA. ADM consists of DMEM with 10% FBS, 1 μM dexamethasone, 0.5 mM methyl-isobutylxanthine, 10 μg/mL recombinant human insulin, and 200 μM indomethacin. AMM contains DMEM with 15% FBS and 10 μg/mL insulin.
Corneal Keratocyte Differentiation.
Keratocyte differentiation was carried out as previously described.18 In brief, 3 × 105 TMSCs were collected in a conical-bottom 15-mL tube and centrifuged at 1500 rpm (400g) for 5 minutes to form a pellet. The pellets were cultured in SCGM for 3 days and then changed into keratocyte differentiation medium (KDM) (Advanced DMEM with 10 ng/mL fibroblast growth factor 2 and 0.5 mM ascorbic acid; Invitrogen), which was changed every 3 days for up to 3 weeks. Pellets cultured in SCGM served as control.
TM Cell Differentiation.
Bovine aqueous humor (AH) was collected from enucleated bovine eyes by inserting a 27-gauge needle through the corneal limbus. AH was pooled and centrifuged at 10,000g for 1 hour at 4°C followed by filtering through 0.22-μm filter units (50 mL Steriflip Filter Units; Millipore, Billerica, MA). The AH was aliquoted and stored at −80°C for later use. TM cell differentiation was induced by culturing TMSC 35-mm dishes in three different conditions: 50% AH in SCGM, 100% AH, or DMEM/F12 plus 10% FBS. The media was changed every 3 days for up to 10 days.
Phagocytosis Assay.
Assessment of phagocytosis was performed following the procedures described by Zhang et al.20 with minor modifications. In brief, Alexa 488-conjugated Staphylococcus aureus bioparticles (from heat- or chemically killed S. aureus) were incubated with opsonizing reagent (purified rabbit IgG antibody; Invitrogen) at 37°C for 1 hour to enhance particle uptake. The cells were incubated with opsonized Alexa 488-conjugated S. aureus bioparticles at a ratio of 20 bioparticles per cell at 37°C for 1 hour. After incubation, the cells were fixed with 4% paraformaldehyde solution for 15 minutes at room temperature (RT) and incubated with Alexa 546 goat anti-rabbit IgG secondary antibody for 1 hour. The secondary antibody binds to the extracellular bioparticles opsonized with rabbit IgG, so the unphagocytosed bioparticles can be excluded when counting. Cell nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen) at 1 μg/mL for 10 minutes. Cellular phagocytosis of bioparticles was visualized and imaged with a confocal microscope (Olympus FluoView FV1000). The number of phagocytosed bioparticles was quantified by counting the cells and total bioparticles ingested by these cells. At least 10 individual views per condition were counted and averaged. The data were analyzed statistically by one-way ANOVA followed by the Tukey posttest to assess the significance of differences between all groups.
Quantitative Reverse Transcription–Polymerase Chain Reaction
Cells were lysed with RLT buffer (RNeasy mini kit; Qiagen, Valencia, CA) and RNAs were isolated following the manufacturer's instructions, including treatment with DNase I (Invitrogen) and concentration by ethanol precipitation. cDNAs were transcribed from the RNAs using reverse-transcriptase (SuperScript II; Invitrogen). Quantitative reverse transcription–polymerase chain reaction (qRT-PCR) of cDNAs was performed by direct dye binding (SYBR Green; Fermentas, Thermo Fisher, Pittsburgh, PA) as previously described.16 Primers (for SYBR assays) were designed using online software (Primer3; http://fokker.wi.mit.edu/primer3),21 with the sequences shown in Table 2. Amplification of 18S rRNA was performed for each cDNA (in triplicate) for normalization of RNA content. A negative control lacking cDNA was also included in each assay. Relative mRNA abundance was calculated as the Ct for amplification of a gene-specific cDNA minus the average Ct for 18S expressed as a power of 2 (2−ΔΔCt). Three individual gene-specific values thus calculated were averaged to obtain mean ± SD.
Table 2.
Primer Sequences Used in Quantitative RT-PCR
| Gene Name | DNA Sequence |
|---|---|
| 18S Ribosomal RNA | Forward: CCCTGTAATTGGAATGAGTCCAC |
| Reverse: GCTGGAATTACCGCGGCT | |
| ABCG2 | Forward: TGCAACATGTACTGGCGAAGA |
| Reverse: TCTTCCACAAGCCCCAGG | |
| Pax6 | Forward: CAATCAAAACGTGTCCAACG |
| Reverse: TAGCCAGGTTGCGAAGAACT | |
| Notch1 | Forward: AGTCTCTGCAGTGCTGGAAGTA |
| Reverse: CTTGCAGTACTGGTCGTACAGG | |
| Muc1 (Mucin 1) | Forward: CCATTCCACTCCACTCAGGT |
| Reverse: CCACATGAGCTTCCACACAC | |
| AnkG (Ankyrin G) | Forward: CATTCCTCCACGCAAGTGTA |
| Reverse: GTGGGTTGGCCAGTTTATGT | |
| AQP1 (Aquaporin 1) | Forward: CTGCACAGGCTTGCTGTATG |
| Reverse: TGTTCCTTGGGCTGCAACTA | |
| MGP | Forward: GCCGCCTTAGCGGTAGTAAC |
| Reverse: TCTCTGCTGAGGGGATATGA | |
| CHI3L1 | Forward: CCTTGACCGCTTCCTCTGTA |
| Reverse: GTGTTGAGCATGCCGTAGAG | |
| MYOC (Myocilin) | Forward: AAGCCCACCTACCCCTACAC |
| Reverse: TCCAGTGGCCTAGGCAGTAT | |
| ELAM1 | Forward: ACACCTCCACGGAAGCTATG |
| Reverse: AATTGCAACCAGGTGTGTGTA | |
| MMP1 | Forward: TGGACCTGGAGGAAATCTTG |
| Reverse: AGAATGGCCGAGTTCATGAG | |
| Neurofilament | Forward: GAGGAACACCAAGTGGGAGA |
| Reverse: CTCCTCCTCTTTGGCCTCTT | |
| GFAP | Forward: ACTACATCGCCCTCCACATC |
| Reverse: CAAAGGCACAGTTCCCAGAT | |
| ap2 | Forward: CATGGCCAAACCTAACATGA |
| Reverse: AATTCCTGGCCCAGTATGAA | |
| Leptin | Forward: TCCTGGATTCCTTTCCTTCA |
| Reverse: CAATCGAGGAGGGCAGAATA | |
| KERA (keratocan) | Forward: ATCTGCAGCACCTTCACCTT |
| Reverse: CATTGGAATTGGTGGTTTGA | |
| ALDH | Forward: CATTGGCACCTGGAACTACC |
| Reverse: GGCTTGAGGACCACTGAGTT |
Histology
Cells cultured directly on 35-mm tissue-culture dishes were rinsed briefly in PBS, fixed in 4% paraformaldehyde at RT for 15 minutes, rinsed in PBS, and stored at 4°C in 50% glycerol and 50% PBS (v/v) until staining. Cells cultured as pellets were rinsed and fixed in 4% paraformaldehyde and embedded in optimal cutting temperature (OCT) embedding compound (Tissue-Tek OCT; Electron Microscopy Sciences, Hatfield, PA), cut into 8-μm sections, and stored at −20°C until staining. Nonspecific binding was blocked with 10% heat-inactivated goat serum. Sections were incubated overnight at 4°C with primary antibodies (shown in Table 1). After three washes, anti-mouse Alexa 488 or 546, anti-rabbit Alexa 546 or 647 secondary antibodies, and nuclear dye DAPI were added and incubated for 2 hours at RT. Samples were imaged using a confocal microscope (Olympus) with a 40× oil objective.
Oil red O stain for adipogenic differentiation (Sigma-Aldrich) was prepared at 0.5% in isopropanol, diluted to 0.3% in water, and filtered before use. Cells were stained with oil red O for 20 minutes and rinsed with 60% isopropanol followed by hematoxylin stain for nuclei. Brightfield micrography was performed with a 40x objective.
Immunoblotting
Cells were lysed directly in 1 × SDS sample buffer, heated at 95°C for 5 minutes, and sonicated until solubilized. Protein concentration was determined by a commercial protein assay (Bio-Rad DC Protein Assay; Bio-Rad, Hercules, CA) and then β-mercaptoethanol was added to a final concentration of 1% to the lysates and heated at 70°C for 20 minutes. An equal amount of protein was loaded to precast 4–20% gradient (Bio-Rad) and electrophoresis was performed for 1 hour at 200 V. Protein was transferred to a polyvinylidene difluoride membrane (Millipore) and blocked for 1 hour at RT in blocking buffer (1% gelatin in PBS). Membranes were incubated with primary antibodies diluted in blocking buffer with 0.1% Tween-20 followed by incubation with goat anti-mouse, goat anti-rabbit, or donkey anti-goat secondary antibodies (IRDye 680LT, IRDye 800CW, and IRDye 800CW, respectively; Odyssey Infrared Imaging System; LI-COR Biosciences, Lincoln, NE). The fluorescent signal was visualized with a commercial substrate followed by detection and capture of 16-bit images with an infrared imager (Odyssey Infrared Imager; LI-COR Biosciences).
Results
SP Cells Express Stem Cell Markers
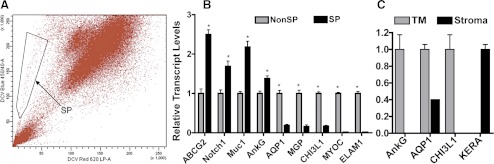
We previously isolated SP cells from human corneal stroma using a DNA-binding dye (Hoechst 33342; Molecular Probes, Carlsbad, CA),16,22 which is excited by ultraviolet light. Here we used a commercial dye (DyeCycle Violet Dye), which is excited by violet light rather than ultraviolet laser to isolate SP cells from TM tissue. Both dyes can be actively effluxed by ATP-binding cassette transporter proteins and will generate SP cell population.17,23 To confirm the dissected TM tissue was not contaminated with adjacent corneal stromal tissue, the expression of TM-specific markers AnkG (Challa P, et al. IOVS 2003;44:ARVO E-Abstract 3164) and CHI3L1 (Liton PB, et al. IOVS 2009;50:ARVO E-Abstract 4859); corneal stromal marker keratocan (KERA)24; and AQP1, expressed in both keratocytes25 and TMs,26 was compared on the TM tissue and the adjacent stromal tissue by qRT-PCR. Figure 1A shows that unfractionated cells from TM contain a small but significant side population that was collected by fluorescence-activated cell sorting (FACS). The percentage of SP cells ranged from 0.1 to 1.5% of the total population across multiple sorts. The isolated SP and non-SP cells were further expanded in SCGM and at passage 8, gene expression was analyzed by qRT-PCR (Fig. 1B). The expression of stem cell genes ABCG2, Notch1, MUC1, and AnkG of SP cells was approximately 1.5- to 2-fold higher than that of non-SP cells. The expression of TM genes AQP1, MGP, and CHI3L1 was approximately 4-fold lower in SP cells compared with non-SP cells. SP cells had almost no MYOC or ELAM-1 expression. The differences between SP and non-SP cells are statistically significant (P < 0.05). Figure 1C confirms that the isolated TM tissue, which generated the SP cells, was TM and not cornea. The isolated TM expressed AnkG, AQP1, and CHI3L1 but not the corneal gene KERA. In contrast, corneal stroma expressed AQP1 and KERA, but not AnkG nor CHI3L1.
Figure 1.
Isolation of TMSC as SP cells. (A) SP cells were isolated by FACS from passage 3 human TM cells (using DyeCycle Violet Dye; Invitrogen). Cells showing reduction of both blue and red fluorescence (SP cells) were collected as defined by the box outlined on the left (arrow). (B) The sorted SP and non-SP cells were passaged and assessed for differential gene expression by qRT-PCR. Error bars show SD of triplicate analyses. *P < 0.05 (n = 3, Student's t-test). (C) RNAs extracted from dissected TM tissue and the adjacent corneal stroma tissue were compared on the expression of TM markers AnkG, CHI3L1; stromal marker KERA; and the common marker for both TM and stroma AQP1 to assess if the dissected TM tissue contains corneal tissue.
Clonal Cultured TMSCs Are Distinct from Primary TM Cells
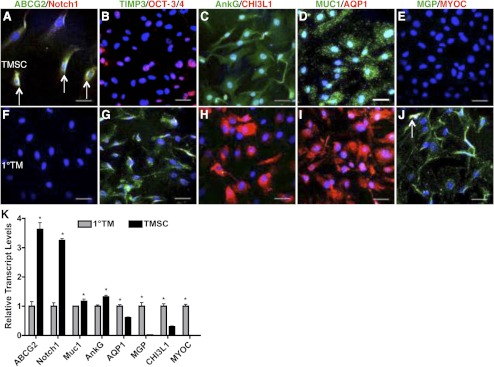
TMSCs isolated by clonal growth and expanded in SCGM were initially compared with unfractionated primary TM cells cultured in medium without FBS or any growth factors. Immunofluorescent staining shows clonal TMSCs were positive for stem cell markers ABCG2, Notch1, OCT-3/4, AnkG, MUC1; but not TM cell markers TIMP3, CHI3L1, AQP1, MGP, or MYOC (Figs. 2A–E). In contrast, primary TM cells expressed these TM cell markers but not stem cell markers (Figs. 2F–J). The qRT-PCR result (Fig. 2K) shows clonal TMSCs, compared with primary TM cells, have significantly increased expression of stem cell genes (ABCG2, Notch1, MUC1, AnkG), decreased expression of TM genes (AQP1, MGP, CHI3L1), and decreased expression of MYOC. The differences of each transcript between the two groups are statistically significant (P < 0.05).
Figure 2.
Differential expression of stem cell and TM markers between TMSCs and primary TM cells. Clonal passaged TMSCs (A–E) and primary TM cells (F–J) were double-stained with stem cell markers ABCG2 (green), Notch1 (red), OCT-3/4 (red), AnkG (green), MUC1 (green); TM markers TIMP3 (green), CHI3L1 (red), AQP1 (red), MGP (green); and MYOC (red). Arrows in (A) point to the ABCG2 and Notch1 double-positive cells. Arrow in (J) points to the MGP and MYOC double-positive cell. DAPI stains nuclei blue. Bars: 50 μm. (K) Expression of stem cell and TM markers from primary TM cells and clonal TMSCs at passage 4 was quantified by qRT-PCR. Error bars show SD of triplicate analyses. *P < 0.05 (n = 3, Student's t-test).
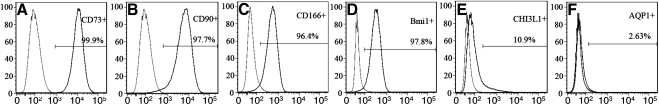
Flow cytometry was used to assess the purity of clonal passaged TMSC. Figure 3 revealed passage 4 TMSCs enriched with stem cell markers CD73, CD90, CD166, and Bmi1 (>95%) and lowered in TM markers AQP1 and CHI3L1 (<10%). This experiment was repeated on passage 2 and passage 6 TMSCs with similar results.
Figure 3.
Flow analysis of passage 4 TMSCs to assess the cell purity. Stem cell markers CD73, CD90, CD166, and Bmi1, and TM cell markers CHI3L1 and AQP1 were analyzed by flow cytometry. The peak on the left in each histogram represents the isotype control for comparison with the cells labeled for each marker (on the right).
Passaged TMSCs Are Multipotent
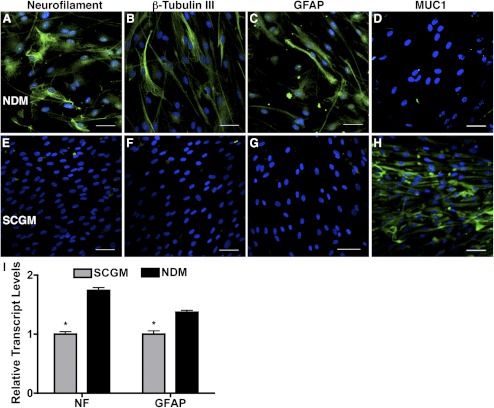
One of the characteristics of adult stem cells is multipotency, the ability to differentiate into a number of different cell types. We examined this property of the cloned TMSCs by culturing them under different conditions. When TMSCs were cultured in neural differentiation medium containing EGF, FGF, and retinoic acid, expressions of neurofilament protein, β-tubulin III, and GFAP were observed (Figs. 4A–C), whereas MUC1 expression disappeared (Fig. 4D). In contrast, TMSCs cultured in SCGM were MUC1 positive (Fig. 4H) without neural marker expression (Figs. 4E–G). Analysis of mRNA levels (Fig. 4I) confirmed significantly upregulated transcript of both neurofilament and GFAP after induction (P < 0.05).
Figure 4.
Induction of neural cell differentiation from TMSCs. Immunofluorescent staining on neural markers neurofilament, β-tubulin III, GFAP, and TMSC marker MUC1 shows different expression between TMSCs in NDM (A–D) for neural induction and in SCGM (E–H) to stem cell maintenance. DAPI stains nuclei blue. Bars: 50 μm. (I) Neural markers NF and GFAP from TMSCs in SCGM and in NDM for neural induction were quantified by qRT-PCR. Error bars show SD of triplicate analyses. *P < 0.05 (n = 3, Student's t-test). GFAP, glial fibrillary acidic protein; NF, neurofilament.
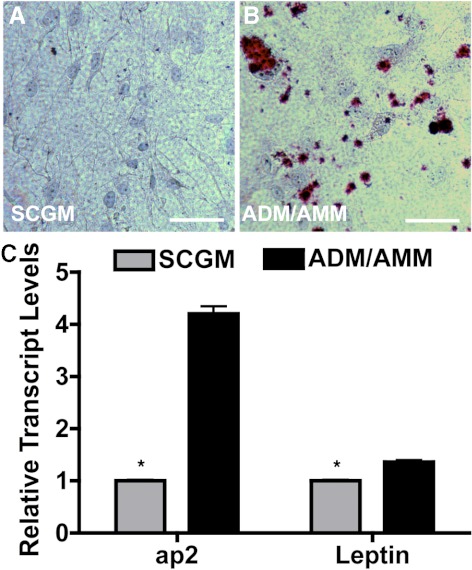
Under conditions inducing adipocytes, oil red O–stained distinct intracellular lipid deposits in the induced TMSCs (Fig. 5B), which were not present in TMSCs cultured in SCGM (Fig. 5A). mRNA levels for adipocyte markers ap2 and leptin were significantly increased (P < 0.05) after induction in ADM/AMM compared with TMSCs in SCGM (Fig. 5C).
Figure 5.
Induction of adipocyte differentiation from TMSCs. TMSCs were cultured in ADM and AMM alternately for adipogenic induction. Oil red O (red) staining was compared on TMSCs in SCGM (A) and in ADM/AMM (B). Hematoxylin stains nuclei blue. Bars: 50 μm. (C) Adipocytic markers ap2 and leptin from TMSCs in SCGM and induced cells in ADM/AMM were quantified by qRT-PCR. Error bars show SD of triplicate analyses. *P < 0.05 (n = 3, Student's t-test). ap2, adipocyte protein 2.
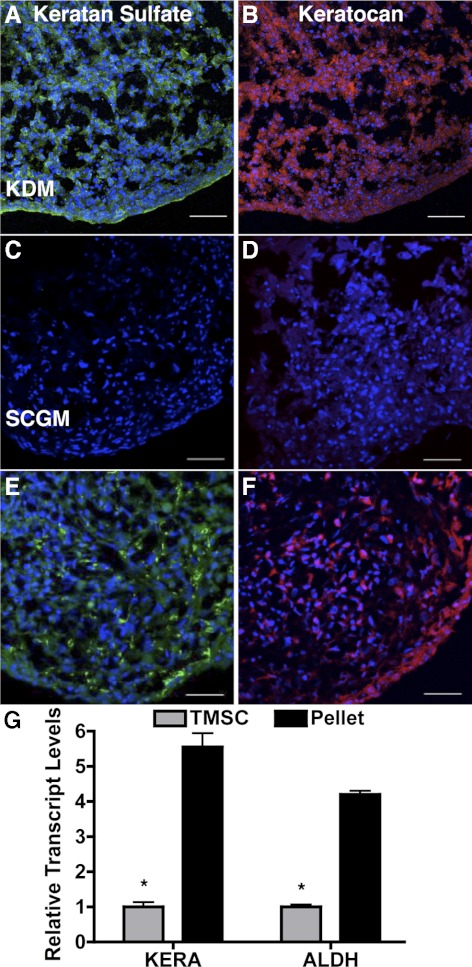
When TMSCs were cultured as pellets in a medium shown to induce keratocyte phenotype,18 TMSCs secreted corneal stromal-specific extracellular matrix components keratan sulfate (Fig. 6A) and keratocan (Fig. 6B), similar to the pellets from human corneal stromal stem cells (Figs. 6E, 6F). When TMSCs were cultured as pellets in SCGM, however, they formed fragile aggregates, a little extracellular matrix, and did not stain for keratan sulfate and keratocan (Figs. 6C, 6D). Figure 6G shows increased mRNA expression of keratocan (KERA) and ALDH after keratocyte induction. The upregulation is significant (P < 0.05).
Figure 6.
Induction of corneal keratocyte differentiation from TMSCs. Cryosections of TMSCs cultured as pellets in KDM (A, B) or in SCGM (C, D) were stained with keratan sulfate (A, C) and keratocan (B, D). Human corneal stromal stem cells cultured as pellets in KDM, serving as positive control, were also stained with keratan sulfate (E) and keratocan (F). DAPI stains nuclei blue. Bars: 50 μm. (G) Keratocytic markers KERA and ALDH from TMSCs in SCGM and TMSCs cultured as pellets in KDM were quantified by qRT-PCR. Error bars show SD of triplicate analyses. *P < 0.05 (n = 3, Student's t-test). KERA, keratocan; ALDH, aldehyde dehydrogenase.
TMSCs Differentiate into TM Cells with Phagocytic Function
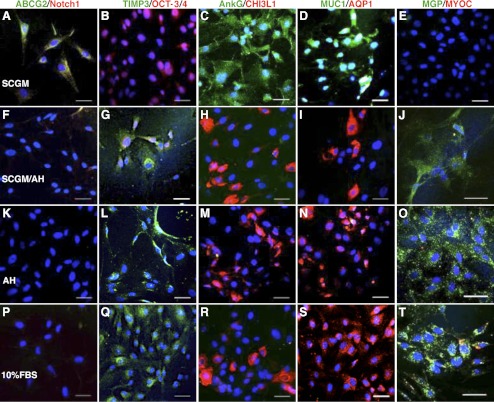
TM cells function to maintain aqueous humor outflow and proper IOP with phagocytic activity and secretion of specific enzymes and extracellular matrix.4 TM cell number decreases with age and affects IOP.6–9 We examined if TMSCs can differentiate into TM cells. Clonal TMSCs were incubated in several media to induce TM cell differentiation: 50% bovine aqueous humor in SCGM (SCGM/AH), 100% bovine aqueous humor (AH), or DMEM/F12 containing 10% FBS. The expression of stem cell– and TM-cell markers was compared by immunofluorescence, qRT-PCR, and Western blotting. Figures 7A–E show TMSCs cultured in SCGM retained expression of stem cell markers ABCG2, Notch1, OCT-3/4, AnkG, MUC1, but not TM differentiation markers TIMP3, CHI3L1, AQP1, MGP, and not MYOC. When cultured in SCGM/AH (Figs. 7F–J), in AH (Figs. 7K–O), or in 10% FBS (Figs. 7P–T), TMSC lost expression of stem cell markers but expressed the TM markers. Some induced TM cells were found to become MYOC positive (Fig. 7T, red).
Figure 7.
Different protein expression of TMSCs and induced TM cells. Immunofluorescence compares different expression of stem cell markers ABCG2, Notch1, OCT-3/4, AnkG, MUC1; TM markers TIMP3, CHI3L1, AQP1, MGP; and MYOC of TMSCs cultured in SCGM (A–E), in 50% AH in SCGM (SCGM/AH) (F–J), in AH (K–O), or in 10% FBS (P–T). Double-stain model is the same as Figure 2 and the markers are labeled as green/red on top. DAPI stains nuclei blue. Bars: 50 μm.
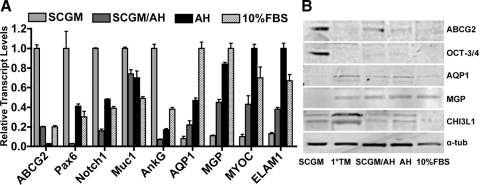
qRT-PCR (Fig. 8A) revealed expression of ABCG2, Pax6, Notch1, MUC1, and AnkG to be decreased, whereas expression of AQP1 and MGP increased on TMSC differentiation in SCGM/AH, AH, or 10% FBS. The expression of MYOC and ELAM-1 also increased after induction. Expression differences of all genes between TMSC in SCGM and induced cells in AH or in 10% FBS were statistically significant (P < 0.05, one-way ANOVA followed by Dunnett's posttest). The differences between cells in SCGM and SCGM/AH were not consistently statistically significant. There were no statistical differences between cells in AH and 10% FBS.
Figure 8.
Different gene expression of TMSCs and induced TM cells by qRT-PCR and Western blotting. (A) mRNA pools for stem cell makers ABCG2, Pax6, Notch1, MUC1, AnkG; TM markers AQP1, MGP; and MYOC and ELAM1 were quantified by qRT-PCR to compare the differences between TMSCs and TM cells induced in different media. Error bars show SD of triplicate analyses. (B) Western blotting compares expression of stem cell markers ABCG2, OCT-3/4, and TM cell markers AQP1, MGP, and CHI3L1 on passage 4 TMSCs in SCGM, 1°TM cells, induced TM cells in SCGM/AH, AH or 10% FBS. α-Tubulin serves as loading control. 1°TM, primary trabecular meshwork cells.
Western blotting (Fig. 8B) identified ABCG2 and OCT-3/4 expression in clonal TMSCs but not in primary TM cells. A low level of ABCG2 was detected in cells cultured in SCGM/AH but not in cells in AH or 10% FBS. AQP1 and MGP were expressed in primary TM cells and induced cells but not in TMSCs. CHI3L1 appeared as a doublet migrating at approximately 39 kDa in primary and induced TM cells and weakly in TMSCs. The lower molecular weight band may represent a glycosylation variant of the protein.27
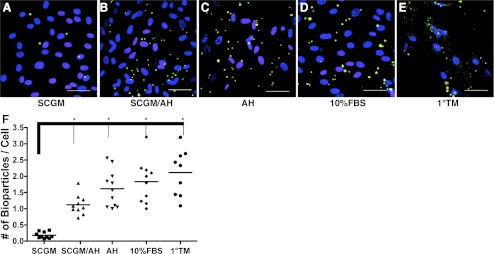
TM cells have phagocytic activity that eliminates debris, pigment, and other materials from the aqueous to maintain the outflow pathway.20,28,29 The phagocytic ability of TMSCs was assessed using fluorescently tagged S. aureus bioparticles comparing clonal TMSCs, induced and primary TM cells (Figs. 9A–E). Ingested S. aureus bioparticles appear green. On average, 0.18 green particles per cell were ingested by TMSCs in SCGM (Fig. 9F). In contrast, TMSCs induced in SCGM/AH ingested 1.12 particles per cell; AH, 1.61; 10% FBS, 1.83; and primary TM cells had 2.11 particles per cell. ANOVA followed by Dunnett's test shows the increase in phagocytic activity by TSMCs to be significant (P < 0.0001) under all conditions, although none of the induced TSMCs differed significantly from the activity of primary TM cells.
Figure 9.
Phagocytosis. (A–E) Phagocytosis assay to compare TMSCs and both induced and primary TM cells. Green dots are phagocytosed fluorescently conjugated S. aureus bioparticles. Yellow dots are unphagocytosed free bioparticles labeled with red secondary antibody. DAPI stains nuclei blue. Bars: 50 μm. (F) The number of phagocytosed bioparticles per cell shows on the y-axis. The green particles and the nuclei in each view were counted and at least 10 different views were counted in each condition. *P < 0.0001 between TMSCs in SCGM and induced TM cells in different media and 1°TM (n = 10, one-way ANOVA followed by the Tukey posttest).
Discussion
In this report we describe the isolation and characterization of a population of stem cells from human TM. These cells can be isolated as a side population by FACS or by clonal growth. In culture they present a homogeneous population displaying antigenic markers previously characterized for mesenchymal stem cells (ABCG2, CD73, CD90, CD166, and Bmi1) as well as expressing gene products associated with pluripotent stem cells (Notch1, OCT-3/4). Their stem cell character was confirmed by the ability of these cells to display phenotypic properties of cells from several different developmental lineages (neural, adipose, cornea) under culture conditions known to induce differentiation of multipotent stem cells. These cells are capable of differentiating into TM cells with phagocytic function and expressing TM markers AQP1, MGP, CHI3L1, and TIMP3 in the presence of aqueous humor or 10% serum. All these support the hypotheses that these cells represent a resident population of adult stem cells in the human TM.
Our results confirm and extend conclusions of previous studies suggesting stem cells in human TM. Challa and colleagues (IOVS 2003;44:ARVO E-Abstract 3164) described “novel ” cells in primary TM cultures expressing MUC-1 and AnkG. Kelley et al.14 confirmed the expression, suggesting it might be associated with a stem cell population. Gonzalez et al.12 found cultured TM cells capable of forming free-floating neurospheres, a function associated with neural stem cells. More recently, McGowan et al.13 observed cells expressing Oct-3/4, nestin, telomerase, PAX6, and Sox2 in the peripheral endothelium and TM of human corneas. Our data confirm the presence of a stem cell population in TM, which expressed MUC1, AnkG, PAX6, and Oct-3/4. Expression of these markers clearly distinguishes TMSCs from typical mesenchymal stem cells. PAX6 is a homeobox gene essential to ocular development and is present in some adult ocular tissues but not generally present in TM.30 PAX6 is present in corneal stromal stem cells16,31 but is not expressed by mesenchymal stem cells.32 MUC1 is a cell surface mucin associated with breast and other epithelial cancers.33 AnkG was recently described as essential for production of new neurons in the brain34 and was described with higher expression in Schwalbe's cells that have been postulated to be responsible for cell regeneration in the TM (Challa P, IOVS 2003;44:ARVO E-Abstract 3164). The expression of these three genes in the TMSC thus defines markers distinguishing these cells from bone marrow–derived mesenchymal stem cells.
Similarly, the ability to differentiate to functional TM cells is a novel and, at the present time, unique property of this cell population. The observation that TMSCs differentiate to TM cells in the presence of fetal bovine serum suggests that differentiation to TM is the default lineage for these cells, implying that they are indeed a specialized population of stem cells, not mesenchymal stem cells from the vasculature or other tissues. The identification of cells that naturally differentiate to TM in vitro can be useful as a research tool to better understand steps in the developmental lineage of these rare cells. The ability to expand the numbers of TMSCs provides access to a large number of homogeneous TM cells for study in vitro, a facility not previously available from such a small tissue.
Our data show that TMSCs express characteristic TM proteins after induction. These markers have essential roles in TM function and help to establish that TMSCs can reestablish primary TM functions maintaining aqueous outflow. The water channel aquaporin 1 (AQP1) has been detected in the TM in vivo26 as well as in cultured human TM cells and plays an important role in modulation of aqueous outflow.5 Matrix Gla protein (MGP) has the ability to function in the TM as a calcification inhibitor35 and may be a key contributor to IOP homeostasis by regulating calcification and hardening of the TM.36 Aqueous humor contains chitinase 3-like 1 (CHI3L1), which has a protective role against inflammation, ECM remodeling, and cell death in the outflow pathway (Liton PB, et al. IOVS 2009;50:ARVO E-Abstract 4859). Myocilin expression in TM cells is induced on treatment with dexamethasone, TGF-β, or mechanical stretch and may lead to impaired outflow resistance.37 TM cells induced from TMSCs with 10% serum also showed increased myocilin expression (Fig. 8). This may be a result of the steroid content of fetal bovine serum or simply reflect the difference in myocilin expression between TMSCs and TM cells (as observed in Fig. 2). It will be of interest to investigate in future studies whether TMSCs respond to stressors in a manner similar to that of TM cells.
TM cells have phagocytic activity that is essential in maintaining normal aqueous outflow. We report here that TMSCs after induction demonstrated phagocytic function almost as strong as that of primary TM cells. These are important findings that provide a biological source of differentiated TM cells for stem cell–based therapy on glaucoma.
Our present study provides new tools to design experiments to test the role of the TMSCs in cellular dynamics of normal and pathologic TM. Pharmacologic stimulation of differentiation of existing TMSCs to new functional TM cells is one avenue in which these cells might be useful in restoration of normal outflow. We confirmed that the TMSCs are exclusively from TM and not corneal stroma, although the potential of the stem cell population from corneal stroma to differentiate to phagocytic TM cells is worthy of exploration. It may open a wider pharmacologic and clinical application on TM restoration.
Another potential use of TMSCs is developing a cell-based therapy for glaucoma. We are exploring the feasibility of TMSCs to be introduced into the anterior chamber and their ability to locate in the TM and adopt a TM phenotype. These data will support the idea that TM damaged by high IOP or inflammation may be restored via a cell-based–therapy approach.
Acknowledgments
The authors thank Nancy Zurowski for help in cell sorting and flow cytometry and Kira Lathrop for help in imaging.
Footnotes
Supported in part by National Institutes of Health/National Eye Institute Grants EY016415 (JLF) and P30-EY008098; Eye and Ear Foundation (Pittsburgh, PA); Research to Prevent Blindness; and an anonymous philanthropic donation (YD).
Disclosure: Y. Du, None; D.S. Roh, None; M.M. Mann, None; M.L. Funderburgh, None; J.L. Funderburgh, None; J.S. Schuman, None
References
- 1. Gupta D. Glaucoma Diagnosis and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2004 [Google Scholar]
- 2. Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci. 2003;44:3783–3789 [DOI] [PubMed] [Google Scholar]
- 3. Levkovitch-Verbin H. Animal models of optic nerve diseases. Eye. 2004;18:1066–1074 [DOI] [PubMed] [Google Scholar]
- 4. Buller C, Johnson DH, Tschumper RC. Human trabecular meshwork phagocytosis. Observations in an organ culture system. Invest Ophthalmol Vis Sci. 1990;31:2156–2163 [PubMed] [Google Scholar]
- 5. Stamer WD, Seftor RE, Snyder RW, Regan JW. Cultured human trabecular meshwork cells express aquaporin-1 water channels. Curr Eye Res. 1995;14:1095–1100 [DOI] [PubMed] [Google Scholar]
- 6. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–727 [PubMed] [Google Scholar]
- 7. He Y, Leung KW, Zhang YH, et al. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49:1447–1458 [DOI] [PubMed] [Google Scholar]
- 8. Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4 [DOI] [PubMed] [Google Scholar]
- 9. Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–579 [DOI] [PubMed] [Google Scholar]
- 10. Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548 [DOI] [PubMed] [Google Scholar]
- 11. Verfaillie CM. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 2002;12:502–508 [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez P, Epstein DL, Luna C, Liton PB. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp Eye Res. 2006;82:959–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGowan SL, Edelhauser HF, Pfister RR, Whikehart DR. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis. 2007;13:1984–2000 [PubMed] [Google Scholar]
- 14. Kelley MJ, Rose AY, Keller KE, Hessle H, Samples JR, Acott TS. Stem cells in the trabecular meshwork: present and future promises. Exp Eye Res. 2009;88:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mimura T, Joyce NC. Replication competence and senescence in central and peripheral human corneal endothelium. Invest Ophthalmol Vis Sci. 2006;47:1387–1396 [DOI] [PubMed] [Google Scholar]
- 16. Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Telford WG, Bradford J, Godfrey W, Robey RW, Bates SE. Side population analysis using a violet-excited cell-permeable DNA binding dye. Stem Cells. 2007;25:1029–1036 [DOI] [PubMed] [Google Scholar]
- 18. Du Y, Sundarraj N, Funderburgh ML, Harvey SA, Birk DE, Funderburgh JL. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant. 2008;17:303–311 [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Ognibene CM, Clark AF, Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp Eye Res. 2007;84:275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, eds. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000:365–386 [DOI] [PubMed] [Google Scholar]
- 22. Du Y, Carlson EC, Funderburgh ML, et al. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu CY, Birk DE, Hassell JR, Kane B, Kao WW. Keratocan-deficient mice display alterations in corneal structure. J Biol Chem. 2003;278:21672–21677 [DOI] [PubMed] [Google Scholar]
- 25. Ruiz-Ederra J, Verkman AS. Aquaporin-1-facilitated keratocyte migration in cell culture and in vivo corneal wound healing models. Exp Eye Res. 2009;89:159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamer WD, Snyder RW, Smith BL, Agre P, Regan JW. Localization of aquaporin CHIP in the human eye: implications in the pathogenesis of glaucoma and other disorders of ocular fluid balance. Invest Ophthalmol Vis Sci. 1994;35:3867–3872 [PubMed] [Google Scholar]
- 27. Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. J Biol Chem. 2005;280:41213–41221 [DOI] [PubMed] [Google Scholar]
- 28. Bill A. The drainage of aqueous humor (Editorial). Invest Ophthalmol. 1975;14:1–3 [PubMed] [Google Scholar]
- 29. Johnson DH, Richardson TM, Epstein DL. Trabecular meshwork recovery after phagocytic challenge. Curr Eye Res. 1989;8:1121–1130 [DOI] [PubMed] [Google Scholar]
- 30. Collinson JM, Quinn JC, Hill RE, West JD. The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev Biol. 2003;255:303–312 [DOI] [PubMed] [Google Scholar]
- 31. Funderburgh ML, Du Y, Mann MM, SundarRaj N, Funderburgh JL. PAX6 expression identifies progenitor cells for corneal keratocytes. FASEB J. 2005;19:1371–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagai A, Kim WK, Lee HJ, et al. Multilineage potential of stable human mesenchymal stem cell line derived from fetal marrow. PLoS One. 2007;2:e1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mukhopadhyay P, Chakraborty S, Ponnusamy MP, Lakshmanan I, Jain M, Batra SK. Mucins in the pathogenesis of breast cancer: implications in diagnosis, prognosis and therapy. Biochim Biophys Acta. 2011;1815:224–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paez-Gonzalez P, Abdi K, Luciano D, et al. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron. 2011;71:61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xue W, Comes N, Borras T. Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Invest Ophthalmol Vis Sci. 2007;48:3184–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004;201:126–137 [DOI] [PubMed] [Google Scholar]
- 37. Tamm ER. Myocilin and glaucoma: facts and ideas. Prog Retin Eye Res. 2002;21:395–428 [DOI] [PubMed] [Google Scholar]